Note: Descriptions are shown in the official language in which they were submitted.
WO 93/14215PCr/US92/10189
2127~7
Pi~OCESS AND USES FOR
4 5-DIHYDROGELDANAMYCIN AND ITS HYDROQUINONE
Background of the Invention
5This invention is coneerned with a new proeess for the preparation of 4,5-
dihydrogeldanamyein and its hydroquinone by fermenting the mieroorganlsm
Streptomyces hygroscopicus, Pfizer cuiture collection number FD 29068, a derivative
by subcuiture from NRRL 3602, now deposited as ATCC 55256, using standard
fermentation methods and conditions, followed by isolating the compounds of this10 invenUon using standard separation methods. The hydroquinone ean also be
chemically synthesked from 4,~dihydrogeldanamyein.
Both 4,5-dihydrogeldanamyein and its hydroquTnone are ehemieai
eompounds belonging to the ansamycin benzoquinone family of anUbiotics. 4,5-
Dihydrogeldanamycin and its hydroquinone are eonsidered to be derivatives of
15 geldanamycin, a well-known member of the ansamycin ben oqulnone family.
Geldanamyein itself is obtained by fermenting the mieroorganism Streptomyees
hyaroseopieus, NRRL 3602, and separating it out from the fermentation broth.
Geldanamyein is known to be useful against eerWn mieroorganisms, primarily yeastand fungi. Geldanamycin's preparation and utility are diselosed in U.S. Patent
3,595,955. 4,5 Dihydrogeldanamycin has previously been syntheslzed by
catalytieally hydrogenating geldanamycin. Referenee to the semisynthesis of 4,5-dihydrogeldanamyein is found in Progress in the Chemistry of Organie Natural
Produets, Chemistrv of the Ansamyein Antibioties, 33, 1976, p. 278. As of this date,
no utility for 4,5-dihydrogeldanamycin has been diselosed in the art.
Semisynthetic d~riva~ives of geldanamyein and thelr use as antitumor agents
are described in Derwent abstracts B2-983OOE, 81-70796D, 80-72388C and 8
627~0C.
WO 93/14215 PCI`/US92~10189
212 7 ~ 5 summary o; the Invention
This invention provides a process for preparation ot 4,Wihydrogeldanamycin
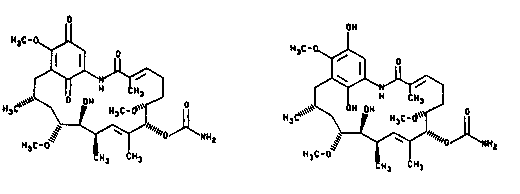
which has the chemical formula 1, and the hydroquinone of 4,~dihydrogeldanamycinwhich has the chemicaJ tormula 11, below.
~
, ~ ~oJ~lluz
l~C H~ CH~
(I) .~,:
OH
OJ~NH2
H3~:-0 ~ ¦
C H~ CH3
(Il)
The process comprises the submergad aerobic propagation in aqueous nutrient
media ~f the microorganism Streptomyces hyqroscopicus, ATCC 5525B, followed by
isolation of tine compounds of formulae I and 11. The inventors have discovered that
4,5~iihydrogeldanamycln and its hydroquinone can be obtained by ferrnenting
30 Streptomvces hv~rosconicus, ATCC 55256, a microorganism not previously known
to produce the compounds ot thls Invention. 4,5-Dihydrogeldonamycin is ~iso
shown to have v~uable utility in treating proliferative disorders, especiaily cancer, in
munmais and, more specfflcaily, in humans. 4,~Dihydrogeldanamycin is also
expected to be usehl in trcating certain gram-positive and -negative bacteriai
2127~S~ `
WO 93/14215 - PCI`/US92/10189
-3-
infecUons; useful as a virucidai agent and as a herbicide and possess antifungai and
anUprotozoai properties. Because the eompound aiso possesses
immunosuppressive properties, it is expected to be usetul in treaffng a vuiety ot
autoimmune diseases inciuding but not limited to rheumatoid uthritis and ~rdt `
5 versus host diseases.
The hydroquinone ot 4,5 dihydrogeldanamycin is a novel eompound that can
be isolated from a natural source or chemicaily synthesked. The hydroquinone eanbe isolated from the fermentaUon broth of Streptomyees hygroseopicus in essenUa~~ly
.
the same manner thd geldunumyein und 4,5-dihydrogeldanamyein is isolated. The
10 hydroquinone can also be ehemicaily synthesked by redueing 4,5- ;`
dihydrogddulamycin ~dth a ehernieal redueing agent. The hydroquinone of 4,6
dihydrogeldununycin b expectod to be usehl against ail ot the sune diseases as
Iisted above for 4,S-dihydrogeldunumycin. The instunt invention provides a new
proeess for the prepuation of 4,5 dihydrogeldanamyein and 4,5-
15 dihydrogeldanamycin hydroquinone from a natural source, namely StreDtomycos ?
hvaroseodeus, ATCC ~5256, und provides new uses tor the eompounds of this
invenffon. Aiso, the ehemTe~i synthesis ot the hydroquinone trom 4,S-
dihydrogeldunumyein is provided
Detailed Deseriptlon of the Invention
- 20 Streptomyees ~oseopieus, NRRL 3602, aiso referred to as i~liz euiture
eolleetion number FD 29068, has been deposited under the brms ot th- Budapest
Treaty in the Ameriean Type Cuiture Colleetion, Roekville, Maryland, a reeognkeddepository affording permanence of the deposits and ready aceessibility thereto by -
the public if a patent is granted on this application. It has been given the
designatTon Streptomyces hyaroscopicus ATCC 55256. The deposit is available
during pendency of this applicaUon to one determined by the Commissioner ot the
United States Patent and Trademark Office to be entitled ther~to under 37 CFR 1.14
and 35 USC 122, and in aeeordance with foreign patent laws in eountries wherein
eounterparts ot this applleatlon, or its progeny, are tiled. All restrictlons on the
30 availability to the public of the mlcroorganism deposlted will be irrevocably removed
on June 2, 1993 or upon grantlng ot the patent, whiehever is eariier.
Compounds (I) and ~Il) are natural produets whieh can be isolated trom the
fermentation broth ot Streptomvces hvaroseoDicus, ATCC 552S6. Th method of
propagating StreDtomyces hyaroseopieus, ATCC 55256, to obtain 4,5-
,
WO 93/14215 PCr/US92/1018g
2127!1S7
dihydrogeldanamycin and its hydrc- ~one is the same as for propagating it to - -
obtain geldanamycin. The methoo ropagation is standard In the art and can be
found in U.S. Patent 3,595,955, the teachings ot which are Incorporated herein by
reference. The Streptomyees hyaroscoDious, ATCC 55256, cuituro can be ~rown at -
5 24 to 36C under submerged condiUons with agitaUon and aeration on media
consisting of carbohydrab sourees such as sugars, starches, glycerol; organlc
nitro~en sourees such as soybean meal, casamino acids, ye~st extract; growth
subdanee such as grain solubles, flsh meal, eotton seed meal; mineral saits
eontaining traee elements sueh as iron, eobait, eopper, zinc, etc. Inoeulum is
10 p~pu by seraping VegetatiNe eells from slants inoeulated with the ATCC 55256euiltwe. A suitable solid medlum tor hitial growth on slants is ATCC no. 172, the
eomponents of whieh are Usted b-low.
ATCC #172 GramsAiter
Glucose 10
lS Soluble Starch 20
Yeast Extract 5
~iNZ Amlne A 5
Calcium Carbonab
Agar 20
'Added dlsUlled water to 1000 ml and adJusted the pH to 7.0 with
KO11.
~NZ Amine A is a regTstered trademark of Kratt, Inc., Product ot auest
Intemational (Shemeld Products).
Cuitlvatlng Streptomyces hygroscoplcus, ATCC 55256, and isolating the
25 compounds ot tormulae I and ll is conducted in a similar manner to those employed
in previous fermentations yielding geldanamycin. See, for example, U.S. Patent
Number, 3,595,955. CuRivation preferably takes place in aqueous nutrient medla
under submerged aerobic conditions with agitation at a temperature ot 24 to 36C.
Nutrient media useful for cuitivation include a source of assimilable carbon such as
30 sugars, starches and glycerol; a source of organic nitrogen such as soybean meal,
casamlno acTds and yeast extracts. A source ot growth substances such as grain
solubles, flsh meal and cotton seed meai as well as minerai saits such as sodiumchloride and trace elements such as Iron, cobait, copper and zinc. Buttering agents
sueh aa calclum carbonab and phosphates are used as well~ if excessive foamlng .
SS ts encountered during fermentation, antifoam agents such as vegstable oils orsilbones may be added to the fermenbtion medium. Aeration of the medium in
WO 93/14215 2 1 2 7 4 ~s 7 - pcr/us92/lo189
~ .
tanks for submerged growth is preterably maintained at the rate of about 1/2 to 2
volumes ot sterile free air per volume ot fermentation broth per minute foreed hto ~ -
the broth through a sparger. Agitation may be maintahed by means of agitators
generally familiar to thos skilled in the fermentation art. The rate of agitation
5 depends on the type of agitator employed. A shaker flask is usuaily run at 150 to
200 eyeles per minute whereas a fermentor is usually run at 300 to 1700 revolutions `~
per minute. Aseptie eonditions must, of eourse, be maintained through the transfer
of the organism and throughout its growth.
Inoeulum for the prepa aUon of the anUbioties aeeordlng to thls hvention
10 may be obtained by employing growth from a slant of the eulture or Roux botties
inoeulated ~Ath the euitur, A solid medium suitable for initial growth d th-
organism on dants and in Roux botties is ATCC medium no. 172. Th- growth may
be used to inoeulate either shaker flasks or inoeulum tanks or the inoeulum tanks
may be seeded from the shaker flasks. Growth in shaker flasks will generaily have
15 reaehed its maximum in 4 to 5 days whereas inoculum in submerged inoeulum
tanks will usuaily be in the most favorable period in 2 to 3 days.
Nigerein and daiophtlin, whieh are aetive against gram-posRive and negative
mieroorganisms, are eoprodueed In the fermentation broth and are good indbators
of growth assoeiated wRh the prociuetion of 4,5 dihydrogeldanamyeln. Therefore,
20 the bioaetivity of the ferrnentaUon broth ean be monltored by blologlcai assay ot the
broth employing a sensitlve strain ot Staphyloeoeeus raureus ATCC 6538P or
Badllus subtilis ATCC 6633~ Standard plate assay teehniques are employed in
whieh the zone of inhlbition surrounding a fiiter paper disc saturated with the broth is
used as a measure ot anUbiotic potency. Aiso, thin-layer ehromatography
25 employing siliea gel is a useful tool for deteeting the antibioties produeed in the
ferrnent~tion media and anaiyzing the eomposition of the erude and purHied
materiais extracted from the fermentation broths. The ehromatograms are
deveioped with ethyl acetate and the antibiotic compounds ue visuaiized by
spraying with vanlllin reagent and heatlng the TLC plate at 80C. The developed
30 plate ean aiso be overiayed with agar seeded with either S. aureus or B. subtilis and
Ineubated at 37C for 16 hours to visuallze the antibloties.
Compounds of lormulae I and ll produeed by fermentaffon of Streptom~ees
hygroseopieus, ATCC 5S256, may be separateci and reeovered by eonventionai
methods, e.g~, extraeting the whole, unfiitered fermentation broth with an organie
WO g3/14215 PCl /US92/10189
21274~7 -6-
solvent such as chloroform, ethyl acetate, methyl isobutyl ketone or butanol at the
naturally prevailing pH. Altematively, the mycelium ean be separated after growth
has been eompleted and the myeeliwm extraeted with an organie soivent. The
solvent extraet ean tnen be eoncentrateci to a thin syrup and the pure antibiotie
5 obtained by ehromatography.
A typieal method of separation and reeovery of the eompounds ot formulae I
and ll is as follows. The whole broth from fermentation ot Streptomyees
hygroseoDieus, ATCC 55256,is extraeted with methyl isobutyl ketone. The soivent
is evaporated to yield a thin syrup. The syrup is dissoived in methyiene chloride,
10 loaded onto a siliea gel eolumn and eluted with a gradient of neat methylene
ehiloride to neat ethyl aeetate. The eluates are examined by thln~iayer
ehromatography. Fraetions eontaining eompound I are eombined and evaporated
, . to dryness. Fraetions eontaining eompound ll are eombined and evaporated to
dryness. The produets may be hrther purifled by erystallkation or by eolumn
15 ehromatography N desired.
The eompounds of formulae I and ll are expeeted to be usehl against eertain
genera of hngal plant pathogens, gram-positive and negative baeteria and eertainparasitie mleroorganisms. 4,5-Mhydrogeldanamyeln and its hydroquinone ean be
testeci for use against the above mentioned mieroorganisms using the method
- 20 discioseci In U.S. Patent3,595,955.
The eompound ot tormula I also inhibits the growth ot eertain human
c~reinoma eells. The In vitro activity ot 4,~dihydrogeldanamycin was determined
accordlng to the method contained in M.C. Alley et ai. Cancer Research 48,58
601,Feb.1,1988, and using SKBR3 and MCF7 cell lines. Therefore, 4,~
dihydrogeldanamycin is puticularly vaiuable in treating eancer, and especlaily
breast, ovarhn and gastric cancer in humans. The eompound ot formula ll is
expected to be useful for the same purposè.
4,~Dihydrogeldanamycin also has potent immunosuppresshJe effects as
determined by methods well known to those skilled In the ut~ This activity can be
30 convenlently determlned by assessing the inhlbition ot T-cell proliteration stimulated
by IL2 and phorbol 12-myristate 13-acetate (PiVlA) as measured by a reduction inuptake ~f tritiated thymldlne relatlve to non-drug treated eontrols. Compound ll is
also expected to have immunosuppressive effects.
W 0 93/14215 21 2 7 4 S 7 P ~ /US92/10189
-7-
When the compounds of formulae I or ll are to be used as an antiproliferative
agent, such as an anticancer agent, it can be administered to a mammalian subJect
either alone or, preferably, in combination with pharmaceuticaily-aeeeptable carriers
or diluents in a pharmaceutical composition according to standard pharmaceutleai5 practice. The compounds can be administered oraily or parenterally. Parenteraiadministration includes intravenous, intramuscular, intraperitoneai, subeutaneous
and topieai administraUon.
For orai use of a eompound ot formula I or ll ot this invenUon, the eompound
ean be administered, for exarnple, in the torm of tablets or eapsules, or as an
10 aqueous solution or suspension. In the ease of tablets for orai use, eaniers whieh
are eommonly used Inelude laetose and eom starch, and lubrieaUng agents~ sueh a
magnesium stearab, are eommonly added. For orai administration in eapsule form,
useful diluents are laetose and dried com starch. When aqueous suspenslons are
required for orai use, the aetive ingredient is combined with emulsifying and
15 suspending agents. if desired, eertain sweetening and/or flavoring ~gents can be - -
added. For intramuscular, intraperitoneai, subcutaneous and intravenous use, sterile
solutions of the active ingredient are usually prepared, and the pH of the solutions
should be suitably adjusted and buffered. For intravenous use, the totai
eoneentration of solutes should be controlled to render the prep~tlon isotonie.
In a pharmaeeutica~ composiUon comprising the compound of tormula I or ll
the weight ratio ot carrier to acUve Ingredient will normally be Tn the range from 1:10
to 10:1. However, in any given case, the ratio chosen will depend on such factors
as the solubility of the active component, the dosage contemplated and the precise
route of administration.
When the compound of formula I or ll is used in a human subject, the daily
dosage will normaily be determined by the prescribing physician. Moreover, the
dosage will vary according to the age, weight and response of the individuai patient,
as well as the severity of the patient's symptoms and the potency of the particul~r
compound being administered. However, an effective dose In most instances will
be 0.01 to 0.5g as needed (e.g., every four to six hours). For chronic
admlnistratTon, in most instanees, an eftective dose will be from 0.01 to 1.0 g per
day, and preferably 20 to 250 mg per day, in single or divlded doses. On the other
hand, it may be necessary to use dosages outside these limits in some cases.
WO 93/14215 - PCI`/US92/10189
21274~7
-8-
The following examples are being provided solely ~or the purpose of further
illustration.
EXAMPLE 1
1. Preparation of Inoculum
A solid medium suitable for initial growth on slants and Roux botUes is ATCC
medium No. 172. To 300 ml shaker flasks, 100 ml of medium was distributed, then
the shaker nasks were sterilized at 120C and 15 p.S.i. for 30 minutes. Afler
10 cooling, the medium was inoculated with a vegetative cell suspensbn from the
Streptomyces hygroscopicus, ATCC 55256, culture grown on ATCC no. 172 medium
agar. The nasks were shaken at 28C on a shaker having a displacement ot 1-1/2
to 2-1/2 inches and 150 to 200 cycles per minute (CPM) for 3 to 5 days.
Shaker 11asks are prepared using one of the following media:
~JDYTT Grams/liter - `
Cereiose 10
Com Starch 5
Com Steep Uquor 5 `
NZ Amine `frT 5
Cobait Chloride 0.002
Calcium Carbonate 3
pH to 6.9--7.1
2S
C' GramsJter
Cerelose 10
Soy Flour 10
Com Fermentation Products 5
Com Starch l O
Sodium Chloride 5
3S Cobait Chloride 0.002
Caicium Carbonate
pH to 7~0- 72
WO 93/14215 PCI/US92/10189
2127~ ~
g
2. Fermentation and isolation of 4,~Dihydrogel~anamycin
One shaker tlask is used to inoculate a ffve-liter fermentation vessel
containing three liters of one of the following media:
HER~F Gramsliiter
Cerelose 25
Ammonium Sulfate 5
Soybean Flour 10
Yeast i~xtract 2.5
Potassium Chloride 4
Meat Extract 1
Cobait Chloride 0.002 - -
Caicium Carbonate 3
pH to 7.1-7.3
HER~F2 Grams/iHer
Cerelose 1 0
Com Starch 40 - :-
. Cotton Seed Meai 4 -~
Cobalt Chloride 0.002
Caicium carbonate 6
BrewersYeast 2
Sodium Chloride 2
Magnesium Sulfate-7H20 0,5
Ammonium Nitrate 2
pH to 6.9~ 7.2
MACB-M _ Grams/iiter
Glycerol 1 0
Yeast Extract 10
Sodium Nitrate 2
Cobait Chloride 0.002
Magnasium Suifate-7H2O 0.50
Potassium Dibasic Phosphate
Potassium Chloride 0.S
Ferrous Suitate 0.01
pH to 6.9- 7.2
To each vessel was added 1 ml ot P2000 (silicone) as an antitoarnlng ngent, thenthe vessels were sealed and sterilized at 120C and 15 p.s.i. for one hour. Then,
the pots were inoculated with one (ca. 3% Inoculum) tlask, ferrnented for 72-12040 hours a~ 2BC and stirred at 1700 revolutions per minute with an air rate ot one
volume of air per volume of liquid per minute.
The bioactivity of the broth and subsequent recovery strearns was followec
by using a sensitive strain of Bacillus subtilis ATCC 6633 or Staphylococcus aureus
WO 93/14215 21 ~ 7 1 5 7 PCI/US92/10189
-10-
ATCC 6538P. The components in the broth and recovery streams were visualked
by using silica gel plates in the following system: neat ethyl acetate. The
developed plates were sprayed with vanillin reagent (3 g vanillin dissolved in 75 ml
of ethanol and diluted to 100 ml with 85% phosphoric acid) then heated to 80C.
5 The antibiotics appear as a deep blue/purple coloration. The developed thin-layer
chromatography (TLC) plate was also visualized by viewing in a dark box with 254~um light. When fermentation was completed the termenters were stopped. The
whole broth was extracted with 1/3 volume of methyl isobutyl ketone at broth pH,separated on a DeLaval separator and the solvent phase concentrated in vacuo to
10 an oil. The oil was subjected to a 3 tube countercurrent using hexane/acetonitrile
10:1. The lower phase (CH3CN) containing 4,~dihydrogeldanamycin and its
hydroquinone was separated, combined and concentrated in vacuo. The residue
was redissolved in methylene chloride, treated with Darco G60 (activated carbon),
filtered and concentrated. The concentrate was added to a Waters Prep 500 silicagel column in.methylene chloride, then subjected to a gradient of neat methylenechloride to neat ethyl acetab. Fractions enriched wi~h 4,5 dihydrogeldanamycin,
R,=2.5-3 in 9:1 CHClJAcetone, were combined and subjected to repeated
chromatographies until pure 4,5 dihydrogeldanamycin was isolated. 4,~
Dihydrogeldanarnycln was crystallized from hot isopropyl ether and dried In a
- 20 vacuum oven at 50C ovemight~ m.p. 221-222C.
Fractions enriched with the hydroquinone R,=1-1.5 in 9:1 CHClJAcetone, ot
formula ll were combined and subjected to repeated chromatographTes until pure
hydroquinone was isolated.
EXAMPLE 2
Chemical Svnthesis of 4.~dihydroaeldanamycin hydroquinone
The reaction was commenced by mixing 10 grams of sodium hydrosu~ite
dissolved in 100 ml of water with 100 ml of ethylacetate, then 200 mg of 4,5-
dihydroge!danarnycin was added to the above solutlon. The reaction was followed
by tlc using Analtech silica gel plates and neat ethyl acetate as the system. The
reaction was observed under UV light (254 ~um) and by vanillin spray. The reaction
was almost complete after 20 minutes.
The aquQous layer was extracted twice with ethyl acetate. Then, the solvent
layw was back extracted with pH 7.0 phosphate buffer. The solvent was dried overanhydrous Na2SO~ and concentrated to a gum.
WO 93/14215 PCr/VS92/10189
i 2127457
-11-
The residue was taken up in isopropyl ether (IPE), heated to a boil and then
stirred for 3 hours. The isopropyl ether solution was filtered and the solid dried on -
the filter. The dried 4,~dihydrogeldanamycin was kept under house vacuum at
50C.