Note: Descriptions are shown in the official language in which they were submitted.
~ 093/14198 2 1 2 ~ 5 5 0 P~T/US93/0~82
MULTIFUNCTIONAL SURFACE PROTEIN OF STREPTOCOCCI
.. . ,. , , . ~
Thi~ invention was made with goYernment support
under Grant Number AI-11822 awarded by the National
Institutes of Health. The GoYernment ha~ certain rights
in the invention.
REL~TED APPLICATTON
. . _
This application is a continuation in part of
commonly owned and cop~nding application ~erial number
07/913,732 filed July 15, 1992 which is, in tu~n, a
continuation of applica*ion ~erial number 07/818,~70
filed January 8, ~992. The latter applicatlon is now
abandoned.
Thi~ invention relates to a surfac~ protein of
streptococci which is involved in early c~lonization of
the pharyng~al mucosa. More sp~cifically, it relate~ to
a ~ultifunctional p~otein which is on the surface of
: streptococci, includin~ pathogenic streptocosci, such as
Streptococcus pyoqenes and is particularly characteriæed
`` ~ by its ability to bind fibronectin, lysozyme, and the
~o cyclosketal prot~in myosin and ac~in as well a~ by its
enzy~e activity, specific~lly dose dependent
dehydrogenase activity with gly~eraldehyde-3-phosphatP
~G~DPH~. The molecu~e also functions ~s an ADP-
ribosylating enzy~e and as an ~DP ribosyl transferase.
.
2 5 I It is concerned also wi~h thexapeutic compositions
and uses of the surf ace protein including, ~or example
vaccines prepared ~from the: whole protein and segments
thereof, E~articularly~ conserved segments ha~ring ac:tivity
similar to that of ehe protei-. ~ .
WO93/14198 P~T/US93/ ~ 82
2127~50
~2
BACKGROUND OF THE INVENTION
Mammalian diseases, especially human di~eases caused
by streptococcal infection with bacteria ~uch as
Streptococcus pyogenes are a ~ignificant health problem.
In the United States alonej 25 to 35 million cases of
group A strep~ococcal inf~ctions, which primarily afflict
school age children are reported annually (l). The high
inciden~e and potential severity of str~ptococcal
infections provide impetu~ ~or development of an
e~fective and safe vaccine to prevent streptococcal
related infec~ions.
It has now been discovered that there is a
streptococcal surface dehydrogenase (SDH) protein on the
surface of streptococci from several serological groups
such as ~roup A type 6 strep~ococci which has both
`~ enzymatic activity and a binding capacity for a variety
of proteins. In the earlier applications in this series,
this ~Ur~aze protein was referred to as MF6, that b~ing
the laboratory code designation assigned to it when it
was initially isolated, purified and characterized. It
is now referred to as SDH ~ince one of its principal
characteristics is that it is Streptococc~l Surface
Dehy~rogenase.~ ~ore specifical~y, it is a member of the
clas of proteins which manife~t glyceraldehyde-3-~
~5 phosphate de~ydrogenase (GAPDH) activity.
G~PDH proteins,~as~the name implies, are a class of
dehydrogenase enzymes in~imately involved in ma ~ alian
physiological r~actions. Generally, mem~ers of thelclass
are~found in the~cytoplas~r but some have been found
~ associated with membranes and cellular cyclosketal
: structur~s o~ eukaryotes. Th~ g~ycolytic enzyme of this
::
~: :~ : : :: : :
,
WO93/14t98 ~12 7 53 Q PCT/US93/~082
invention is believed to be unigue ln that it is a
sur~ace protein of prokaryot~s. No other such G~PDH
prot~in has previously b~en described.
The GAPD~ protein of this invention ha~ some
structural characteristics similar to other proteins of
the GAPDH family~ For example, over 80% of the NH2-
terminal 18 of 39 amino acids are identical to the GAPDH
family of enzymes. ~o~ever, it dif~ers in many other
respects, as will be explained hereinafter~ It is,
therefore, a novel product which has not hereto~ore been
isolat~d and characterized.
A detailed characterization of puri~ied SDH has
disclos~d that its native conformation is probably a
tetramer wlth a molecular weight of about 156 kDa~ The
molecular weight of the protein by mass pectrometric
analysis is about 35.~ kDa. By sns PAGE, it is about
3g.2 kDa.
~; :
; The prot~in has been identified on the sur~ace of
Group~ A, B, C, E~ G, ~ and L streptococci utiliæing
~o affinity purified anti-SD~ antibodies. The protein
:: :
exhibited a dose dependent dehydrogena~e actiYity on
glyceraldehyde-3-phosphate (G-3~P) i n the presence of.
bet~nicotinamide adenine dinucl~otide (N~D). The
~ultifun¢tional~ activi~y of SDH was revealed by its
ability to b$nd~fibronec~in::~and lysozyme as well as the
ytoskeletal proteins myosin and actin. The binding
acti:~ity o SDH~to~myo6in;was found to be localized to
t~e globular heavy merom~o~in domain. SDH did not bind
to~streptococcal M protein,~tropomyosin: or the coiled~
~coi~l ~omain of myoæin:~ me multiple binding capacity of
SDH e pecially in connection with:~ycloskeletal proteins,
in~conjunction with its GAPDH~acti~ity indicates a role
W093/14198 PCT/US93/OQ~82
in the coloniæation, in~ernalization and the subsequent
proliferation of streptococci. Trypsin tr~atment of
whole streptococci result~d in a marked reduction in
their reactivi~y to SDH antibodi~s. Th2 inability to
remove SD~ from the streptococcal surface after washing
in 2 M NaCl or 2% SDS indicates that the protein is not
p~ripherally associated but tightly bound to the cell.
These data all indicatQ *hat the prok~in i~ a sur~ace
G~PDH mol~cule on the streptococcal ¢~
The novel SDH is obtained by solubilizing the
selected streptococcus with lysin to produce a mixture
containing SDH~ The 5DH may be isolated from the mixture
by any of a number of con~enient methods known to the
skilled artisan including the method illustrated below.
It may al50 be produced by tranæformin~ an organism
such as E. coli with an appropriate gene so that the E.
coli will express SDH.
The following abbreviations are employed in the
description oP this invention: ~
NAD: beta nicotinamide adenine dinucleotide
: PVDF: polyvinylidine difluoride
~DT~: Eyhylenediamine tetra acetic acid
: P~SF: Paradimethyl sulfonyl fluoride
TLCK: ~-p-tosyl~L-lysine chloro methyl ke~one
2~ SDS: Sodium~dodecyl sulfate
: Mono Q FPLC: Mono Q(Trade Name) Fast protein liquid
chromatography
Super~se 12 FPLC: Superose-l2(Trade Name~ Fast protein
liquid hromatography
TSK-Phenyl HPLC: TSK-Ph~nyl lTrade Name) high
performance/pre~sure liquid
: ~
~W093/1~198 ~ 2 7 S ~ ~ PCT/US93/ ~ 82
chromatography
NADH: beta-nicotlnamide ad~nined~nucl20tide, reduced
ELISA: Enzyme linked immunosorbent assay
ELIDA: Trad~ name of Physica Inc.
Sephadex ~-25 PD-10: G-25 PD-10: trade name of
Pharmacia-LKB Inc.
HEPES: ~N-[2-hydroxyethyl]piperizine-N'~2-ethansulfonic
acid~)
RGDS: Arginine~Glycine-A partic acid-Serine (Axg~-Gly-
~sp-Ser)
G-3-P: Glyceraldehyde-3-phosphate
G~PDH: Glyceraldehyde-3~phosphate dehydrogenase
THE FIGURES
There follows a description of ~he figures.
Fig. 1: SDS-polyacrylamide gel (10%) analysis o~
SDH protein ~rom M6 e~reptococci. Lane a: Lysin extra~t
of D47~ streptococci. Lane b: Precipikate of 65%
(N~4)2S04 satu~ation o~ ~he;ly~in extract. Lane c:
Precipitate of 85% (NH4~2S04 saturation o~ ~he
: 20 supernatant after 65% precipitati~n. Lane d: Pooled
Mono Q fractions at 0028 M gradient elution. Lane e: ~
; : Partially purified::SDH fxom the Superose 12 col ~ . ~ane
f: Puri~ied SDH~from Ph~nyl ~SR::aolumn. Arrow marks on
lan~ a and b at 50 kDa indicate the position of M
protein~ Prestained marker protein~mixtur~ ~ith
: ~ molecular mass:as indica~ed on the left margin. 200
k~a:~yosin(H-ch~in)~, 97.4 kDa-: Phosphorylas~ b, 68
kDa:bo~ine~j~serum albumin, 43 kDa: ~valbumin, 29 kDa:i
: ca~bonic~anhydrase~, ~18.0 kDa: lactoglobulin, 14.3 kDa:
~ 30~ egg-white ly80z~me.
:: : : ~
:
WO93/14198 PCT/US93/~82
~ 1 2 ~ 5 5 6
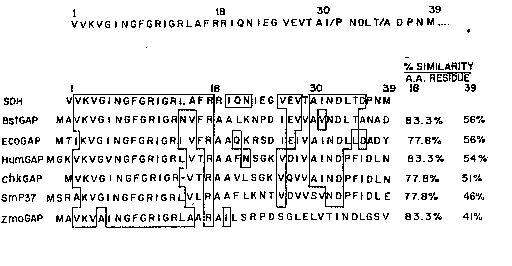
Fig. 2: (a) The NH2-terminal sequence of SDH.
(b) Comparison of the NH2-terminal amino acid seguence of
SHD with the amino acid sequences o~ the known GAPDH
molecules obtained frm the translated Genbank database.
BstG~P- Bacillus stearothermoPhilus GAPDH, EcoGAP-
Escherichia coli GAPDH, ~umGAP- h~man GAPDH, G~PDH,
ChkGAP- Chicken GAPDH, SmP37- 5chi~tosomia manæoni 37 kDa
.
protein (GAPDH) (54)~ ZmbGAP- zYmomon2s mobilis GAPDH.
Numb~rs on the right side of the figure indic~te the
percentage similarity of SDH with other GAPDH molecules
with residues 1-18 and 1-39. The gap(-) between the 14th
and 15th residue of the chicken GAPDH se~uence was
introduced to maximize homology.
Fig. 3: Lin~weaver-Burk's double reciprocal
kinetic analysis of GAPDH acti~ity of SHD. 25 ug o~ SDH
was assayed as function of G-3-P in the presence of NAD
~: (lOOuM) in triethanolamine-phosphate-EDTA-D~T buffer at
: pH 8.6. The Km fo~ G-3-P wa~ estimated to be 1.33 uM,
Vmax: 0.487 X 10 3 M NADH min-l, intercept Y axis
(l/Vmax): 2.05 and slope (Rm/Vmax): 2.73. The inset
shows the analysis based on ~ichaelis-Menten~. Km: 1.22
mM and Vmax: 00 466 X 10 3 M NADH min 1, (b) Lineweaver-
Burk's double reciprocal kinetic analysis o~ G~PDH
activity of the 39~kDa protein. 25 ug of SDH was assayed
as a function of ~AD in the presence of G-3-P (2 mMj in
: the buffer system as described in (a). The ~m for NAD
was esti~a~ed to be 156.7 uM, Vmax:~.459 X 10 3 M NADH
min 1, Intercept::on Y axi~ Vmax) 2.18, and slope
(Km/Vmax~ 341.74. K~ ;for NAD by the m~thod of
. 3Q Nichaelis-M~nten as shown in :the inset was estimated!to
be 148.86 uM and Vmax:~ 0.445 X lO 3 M ~ADH min 1.
`
:
,~.~93/14198 212 7 5 5 a PCT/US93/00082
~ .
Fig. ~: (A) Coomassie Blue stain o~ SDS~gel and (B)
Western-blo~ analysis of SD~ with affinity purified
anti-SDH antibodies sugge~ting a multimeric structure for
the SDH molecule. ~ane~ a and d: Crude ly~in extract.
Lanes b and e: Puri~ied 5DH. Lanes c and f: Unboiled
purified SDH in sample buffer without SDS and æaturated
wit NAD. Arrow mark indicateæ the position of a molecule
of the size consistant with a tetrameric fcrm of SDH. MW
markers are indicated on the le~t margin (Detail~ on each
marker-see Fig. 1).
Fig. 5: Dot blot i~munoanalysis to locate SDH on
the streptococcal ~urface. The assay determin2s the
extent of reactivity o~ a~f inity purified anti-SDH
antibodies to surface ~xposed protein before and after 2%
SDS, 2M ~aCl and trypsin treatments. Dot blots were
treated with LumiPhos-530 substrate (41) and de~eloped on
X ray filmO Densitometric reading of the image obtained
o~ the X-ray film;was expressed a~ an optical density in
t~rms of arbit~ary units~m~asured on an image analyzer
using the ~umas progra~:(Drexel University, Philadelphia,
US~). An internal }inear standard curve for~the optical
density ~0.008 to 1.333 was:obtained ~ox final
: de~sitometric ~nalysis of the:dot blot. Each bar
representes the mean of four~to eight separa~e readings
S.D. ~ ~
Fig. 6: G ~ DH~activity of whale streptococci. (a)
: ~ The GAP~H acti~lty was observed at 340 mn of whole M6
:
:: s~reptococci by detennining the conversion of 2~AD to NADH
. inl the presence o* G-3-P. I:setails of the bu~fer cys~em
is deæcri~ed in materials ~and m~thods. (b) Activity of
tryp inized M6~:streptococci. (c)~ I~hi~ition of enzyme
:
' ~ ;
`: ~
'
WO93/14198 8 PCT/US93/~82
~, ~ 2 7
activity of affinity purifi~d anti SD~ antibodies (1:30
of 0.5 mg/ml for 3 hr at room temperature). Each bar
represent the mean o~ three separate readings ~ S~Do
Fig. 7: (A) Western-blot analysis of lysin extract
s of various strep~ococcal ~ types with affinity purfied
anti-SDH antibodies at a 1:2000 dilution of 0.5 mg/ml
stock. Purified SDH ~nd an ~ nega~ive (M ) streptococci
are also incuded in the analysis. (B) Western-blot
analysis of mutanolycin ~xtract of various gxouping
strains of streptococci using anti-SDH antibodies as
described in (A).
Fig. 8: Competition kinetic enzyme-linked
immunosorbent assays tkELISA) with immobilized SDH.
Commercially a~ailable purified GAPD~ from B.
stearothermop~llus, human erythrocytes and rabbit
skeletal muscle were used to compete for the binding of
affinlty purified anti-SDH antibodies (1:1000 dilution of
0.5 mgtml stock). Each curve repre~ents the mean of
three ~epara~e experiments with le s than 5% standard
deviaiton ~not shown). Ins~t ~hows the W~stern blot of
~: the r~act.i.~ity of af~init~ purified anti-SDH antibodies
: with (a~ streptococcal SDH and GAPDHs of (b) bacterial '
(B. stearothermophilus), (c) rabbit skeletal mus~le and
(d) human erythrocytes.
'
~5 Fig. 9: Binding o~ 125I-SDH to cytos~eletal
proteins. (A) ~oomassie Blue s~ained~SDS-PAGE gel (10%~
containing 5 ug protein of various cytoskeletal proteins,
ysozyme ahd M6 pro~ein (43~. Lane a: rabbit skeletal
myosin. Lane b: heaving meromyosinO Lane c: light
m@romyosin. Lane d: actin~ Lane e: M6 protein. Lane f:
S-2 ~ragment of heavy meromyosin. Lane g: egg WhitP
lysozyme. (Bj autoradiograph of proteins in a duplicate
~ :
WO 93/1419g 212 7 5 !j Q PC~/US93/00082
9 .
i
gel after transfer onto nitrocellulose and incubation
with the 12~1-S~H. Tha proteins in eac:h lane are as
describe~ in Fig. 9 (A) . MW markers on the lert margin
(Detail of each marker - ee Fig. l).
Fig. lO: Binding activity of SDH to fibronectin.
(A) Coomassie stain of an SDS gel containirlg 5 ug of SDH
~nd 13SA. (B) Western-blot analysis of a duplicate gel
showing the binding of fibrone::tin followed by anti-
~ibronectin to the SD~ ~ol~cule. (C) Autoradiograph of a
similar W~stern blot showing the binding o~ l25I-
~ibronect.i n to the SDH protein . Lanes a, c & e - SDH.
Lanes b, d, & f - BSA.
Fig . ll: ADP-ribosylation o~ SDH . Purif ied SD~I
( lanes l , 5 and 9 ), crude streptococcal cell wall extract
~lanes 2 , ~ and lO), cytoplasm (lanes 3 , 7 and ll) and
membrane (lanes 4, 8 and 12~ fractions were incubated
with ~32P~NAD in ~PR buffer. The proteins were then
parated on a 12~6 ~ SDS gel and stained with tA~ Coomassîe
blue. (B~ We~;tern blot analysis of a duplicate gel
reac~ed with af~inity purified anti-SDH antil~odies and
(C~ autoradiography of a si~ilar Western blot. MW marker
is indicated on the le~t side.
Fig . 12: ADP-ribosyl transf exase activity of SD~I .
SDH and various~ bindig proteins (~740~ were incubated
~; 25 tolaether in the presence of ~32P]N~D. Proteirl mixtures
:~ w~re precipit~:ted, washed and r~solved on 12~ SDS-PAGE:
and stained with Coomassie;blue and a duplicate gel was
;: dried and autoradiographed. SDH was incubated with actin
~(lanes, 1 an~ 6~, chioken egg white Lysozyme (lanes, 2
and 7~, Sol fragment of myosin ~lanes, 3 and 8),
: fib~onec~in :~lanes~ 4 and 9), and:plasmin (lanes, 5 and
~ lO)o ~W markers are indicated~o~ the left side~
: :
W~ 93/14198 PCr/US93/~2
The f ollowing Materials and Methods section is
provlded f or convenience and ease o~ understanding the
invention .
MAT13RIA~S AND METHODS
5 Materials:
.. ..
n f ibronectian was ob~ain~d ~om Boehringer
~lannheim ~ Goat anti-human :~ibronectin and af f inity
purif ied rabbit anti-goat I~; coupled to alkaline
phosphata~3e wer~ obtained from Sigma. Pre stained
molecul~r w~ight standards w~re purcha~;ed from Bethesda
Research Laboratories. PVDF meDIbrane ~Immobilon-Pn was
~r~m Millipore. Na125I was from New England Nu~l~ar. All
other ch~micals and reagents unles; otherwise indicated
were purchased from Sigma.
Bact~ria:
Bac:teria. Group A ~ hemolytic streptoaoccal
strains o~ variou ~ 3s ~and standard stra~ins used for
streptococc:al ~grouping w~re from The P~ocksfeller
Universi~:y cultur~ colIection~ (New York, NY~ and are
~a listad ~ as foll~ow~;: M2 (I: 626~ 4 tD896), M5 (~an~rando), .
M6(~4~71), ~24(CS241,~M29(DZ~3~, M41(ClOl/~03/4),
M57:(A995), 2~58(D774), M60(1:398), ~ (T28/51/4); group A,
: J17~4 ~:an M- strain)~, group ~ A va~iant, A486var; group Br
: : :: : :` ::
; 09OR; group C,: C74;~: group :~, D76; group E, Kl3l; group F,
~: 25 F68C; ~group G,~ D166B;; grou~ :H, ~F9OA; group L, Dl67:B; and group N,~:C~59~
:: : : :
` ~
: :
~: :
: :
,~O9~/14198 21 ~ 7 5 ~ a PC~/US93t~082
Lysin_extraction and locat on of_SDH protein:
A crude extract containing the major surface
proteins was prepared using the procedure of lysin
extraction ~o remove the streptococcal cell wall as
described before (2). Esæentially, bacteria washed in 50
mM sodium acetate buffer, pH 5.5 were suspended in the
same buffer containing 30% ra~finose and 5 NM EDTA.
Lysin is added to the su~;pen~ion (l: loo dil; 360 Urlits
and incubated ~or 90 min at 37C with end-to-en~ slow
rotation. The resulting pr~toplasts ~edimented at 15,000
X g ~or 30 min in a Sorvall centrifuge. The supernatant
was saved, dialyzed again~t 25 mM Tris/HCl, pH 8.5,5mM
EDT~, concetrated on ~micon PM-10 membrane (Amicon Corp)
and used for further puri~ication.
lS After lysin extraction, the pelleted prot~plasts
were resuspended and lysed in hypotonic buffer (2 MM
sodium ac~tate, pH 5.5, ~ontaining 2 mM P~SF~ 1 MM TLCK,
10 MM MgC12 and 10 ug/ml DNAse~ followed by three
fre~ze/thaw cycles. ~he membranes were sedimented at
100,000 x g ~or 45 min at 4C. The me~brane~pellet and
cytoplasmic extract in the supernatant were analyzed with~
: Coomassie blue stain~a~ter separation on SDS gel.
Membranes were further treated with 1.5 M sodium ~hloride
or 100 mM sodium carbonate, pH 11. 3 to determine lthe
natura of associa~ion o~ SDH protein with the membran~.
To determine whether this protein is surf ace
exposed, lysin extra~tion:of trypsinized bacteria was
rried outias:déscribed earlier~3). Bri fly, washed
: bacteria were suspended in 100 mM NH4HC03 and digested
: 30 : with trypsin ~250 ug/ml) at 37C ~or 3 hr, after which
the trypsin was inactivated by thP addition of soybean
trypsin inhibitor (200~ug~ml). Lysin extracts of
WO93/14198 ~1 2 PCT/US93/~82
trypsinized and control non-trypsinized bacteria were
compared for the loss or reduc~ion in the size of SDR
protein.
Purification of S~H:
Lysin extraction waæ used as the starting material
for the purification o~ the SDH. The dialyzed,
concentrated, lysin extract wa~ precipi~ated at 60%
saturation o~ ammonium sulfate at 4C. The precipitates
were centrifug d at 6,000 X g for 20 min and the
~upernatant was brought to 85% satur~tion o~ ammonium
sulfate. The re~ulting precipitate was dialyzed against
~5 mM Tris/HCl bu~fer pH 8.5, 5 mM ~DTA and passed over
to Mono Q FPLC column (Pharmacia LKB Biot~chnology Inc.)
e~uilibrated wi~h t~e same dialyzing buffer. After`the
col~mn was washed with 5 column ~olumes of starting
buffer, bound proteins were eluted with a 50 ml linear
NaCL gradient from O to 300 mM. Fractions containing 5DH
were pooled and~dialyzed against the 35 ~M Tri~/~Cl/EDTA
buffer and rechroma~ographed on th2 ~ono Q column.
Fractions containing SDH w~re then pool~d, and
coneen~rated ~o a volume l.O ml cn~Centricon
: concentrator (cuto~ mw lOjOOO kDa). The concentrated
sampl~ was applied:~o a Superose 12 FPLC column
(Pharmacia ~RB Inc): pr~-e~uilibrated with 50 ~M Tris/~Cl
~: : 25 pH 8.5:containing 0.3 M:NaCl and 5 mM EDTA. Fractions
: ~ ~ containing SDH~protein were pooled~ dialyzed against
0.025 N Tris-tHCl buffer pH 8.5 containing l.O M (NH4)2S04
and applied to a~TSK-phenyl HPLC column ~Bio-Rad
aboratories,~Richmond~CA) preequilibrated in the same
buff:~r. Theprotein was~ elu~ed by a decr~asing linear
: gradient of ~H~zS04 from 1.0 M to 9.0 M~ The purity of
the ~inal pr~duct;:was determined by Coomasie hlue stain
of the purified~protein on SDS gel and ~y the analytical
:: : ~:
::
:
,-~093/1~1~8 2 1 ~ 7 ~ 5 o PCT/US93/00~2
procedures. Purified material wa~ stored at 4C after
dialyzing against 0.025 M ~ris/HCl pH 8.5 ~or various
protein binding experiments or at -70C ~or longer
storage.
Anal~tical Procedures (NH -terminal se~q~ e and amino
acid com~osition~:
NH2-terminal amino acid sequence was determined
according to the method of ~atsudaira et al ~4~.
Briefly/ the purified SDH was separated on a pre-
electrophorese~ 10% ~crylamide-SDS gel under non-
denaturing condition and th2n transferred to PVDF
Immobilon-P filter pre-wetted in methanol. Protein was
visualized by ooos% Coomassie ~lue in 50:40 methanol,
water, acetic acid solvent mixture. The blots w~re
destained in methanol: water: acetic acid ~50:40:lO).
The portio~ of the membrane containing the SDH band was
exciced and 5ub; ected to automated Edman degradation on
an ~pplied Biosystem model A470 sequenator. Each band
contained about 2-3 ug protein as determined by BCI
pxotein e~timation method (Pierce). ~or ami~o acid
composition, the PVDF membr~ne containing the SDH was
s~ained with 0.1% Ponceau-S (Sigma~ in 1% acetic acid.
The ~ection of me~brane containing the prot in ~and was
exci ed and destained~with water~ This ~ection ~f
: 2~ m~mbrane was hydrolyzed:in 6N HCljpheno7 at llOC for 22
hr. Amino acids were separated on Waters No~apek C~
column analyæed with;Waters Maxima so~tware, 510 pump and
490 de~ector. Cy~teine content was analyz~d also from
the PVDF boun~ carboxyamide methylated protein as
des~ri~ed by Cres~field ~(53. All analyses were performed
by Protein Biotechnology Facility of the Rockefeller
~:; University~ ~
;
W~93/14198 ,1 4 PCT/US93/0~2
olecular Mass Determination:
~ olecular ma~s of the puri~ied protein was
determined in the department of ~ass Spectrometry and Gas
Phase ion chemistxy of The Rockefeller University using
the modified method of matrix-a~sisted laser desorption
technique (6).
Glyceraldehyde-3-phosphate D~ydr~ nase (G~PDH~
Activity: .
GAPDH assay wa carried out according to the method
originally describ~d by Ferdinand (7) with a minor
modifications. Since GAPDH catalyzes the oxidative
: phosphorylation of D-G 3 P to form 1,3-diphosphoglycerate
in the present of NAD+ and inorganic phosphate, the as~ay
solution was made of t~iethanolamine (40 mM~/ Na2HP04 (50 ~ 15 m~) and EDTA (5 mM). Disposable semi-micro 1.5 ml
capacity spectrophotom~ter cuvette (VWR) contained 7ul
G-3-P (Sigma, 49 mg/ml), 100 uM~NAD ~Boehringer Mannheim~
and as.ay buffer to a final volume of 1.0 ml after the
a~dition o~ ~nz ~ e source with pH of th~ mixture being
:20 8.6. Different:c:oncsntrations of SDH were used to plot
the standard curve ~or~he absorbance at 340 nm per q
~; minute as a measure of conversion:of~NAD to NADH using.
; ~ : Spectronic 3000 speotrophotometer tMilton Roy).
: Enzy~e Kinetlcs~
Kinetics ~of enzymatio~r~action of SDH were made with
v~rying;concentra~ions of NAD and a ~ixed concentration
of~G-3-P and~vice:versa to determine respectively the Km
and Vmax ~or NAD and G-3-P. The results were recorded as
rate~analysis o~ NADH;releas~ at:~every half second for a
period of:l min at 340 nm. The molar extinction
: `
,-3~,0 93/14198 1 ~, 2 1 ~ 7 .rj 5 QPCr/VS93/00082
coefficient of NADH 6.22 X 103 (8) was used to convert
absorbance (340 nm~/min to NADH ~/min. The kinetic co-
e~ficient were estimated from the ~econdary plots of
intercept of primary Lineweaver-Burke plot~ with respect
to each sub~trate. The ~pecific activity of th0 enzyme
(units/mg) was measured using the equation:
Sp activity = v(l~m/S)NAD(l+Km/S)~ 3 pwhere ~ = u
moles NADH/min/mg of enzyme.
Specific activity of GAPDH activity for 5DH wa~
10 measured in the lysin extract, ammonium sulphate
precipitate and pooled ~raction at Yarious purification
stages.
Rabbit Immunization and affinity purification of immune
sera:
Naw Zealand white rabbits were immunized
~ su~cutaneously with 200 ug:of purified SDH emulsified in
;:~ Fr~und's compl~te ~djuvant (1:1) at multiple sites.
Rabbi~s:were boosted once with 200 ug of this protein in
Freund's inc~mplete ad~uvant~ 1). All ra~bits were
bled 3 w~eks after the first and 10 days after the secon~
` - : immuniza~ion. All sera were filter:~cterilized and stor~d
. .
~ at 4C. ~ ~
- : :
To purify~SDH specific antibodies from the
: polyclonal sera,~2.0:mg:of purified:SDH was linked
: 75 co~alently with~free àmino group:of gluteraldehyde-
ac~i~ated a~finity adsorbent 2S described before (9).
nti-:~DH: rabblt æera (2-3 ml):was adsorbed to and eluted
rom ~he:SDH-bea~ds column:,~ dialyz~d, concentrated and
stored~a~ describe~before~(9)0~ These antibodies wexe
~: :30 fùrther purified:on~:Pro~ein A column (Pharmacia LKB)
,
:
W093/~4198 1 ~ P~/USg3/~18:Z!
essentially using the same buffer described ~bove (9).
The monospecificity of anti SDH was first checked on
Western blot as described above.
Dot-Blot Immunoassay to determin2 location of SDH:
~he surf ace locati~n o~ SDH was determined with the
monospecific antibodieæ using a bacterial dot-blQt
immunoaæ~ay as pr~viously d~scribad (lO). E~sentially,
an overnight culture oP strain D471 was adjusted to OD650
nm 1.0 with 50 m~ Tris/HCl bufer, pH 8.5. Aliguots of
this suspension were centrifuged and resuspende~ to the
same volume of buffer containing either 2 ~ NaCl or 2%
SDS, and rotated at room temperature for 1 h,
centrifuged, and the respective supernatants were saved.
Aftex washing, the pellets were again adjusted to OD650
l; nm 1.O with 50 ~M Tris/HCl buffer, pH 8.5. In a separate
experiment, the bacterial suspension in the ~ris/HCl
buf*er was centrifuge~, and~the bacteria were suspended
in 100 mM NH4Hco3 to OD650 ~m 1.O and ~rea~ed with
trypsin (250 ug/ml~ for 3 h at 37C. Tryp~in activity
was i~hibit~ with t~ypsin inhibitor as described above,
, ~
~: ~ and the bacteria were pelleted and:resuspended in the
Tris/HCl buffer~to:;OD650~nm~l.0 50 ul of each bacterial .
suspe~sion was trans~erred to nitroc llulose paper using
dot-bl~t ass~mbly~(Bio-Rad Laboratories, ~ichmond, C~).
Reactivity of~ ~urface-exposed epitopes of the 39-kD
;protein Wa8 determined using àffinity-purified anti-S~H
: ~ protein ant:ib~odies (l:l,000 dilution of 0.5 mg/ml stock).
: For densitom~ric~analysis~of the dot blot, a duplicate
lot was:develop~d with Lumi-Pho6T~530 (Adamantyl-lt2-
; 30 dioxetane ph~nylphosphate; Lumigen Inc., netroit , ~I),
which under~o~s:enzyme:;(alk line~phospha~ase)-catalyzed
depho~phorylation:to~form a dioxetane anion that is
conver~ed ultimately into an excited~state o~ the methyl
:
,
:
~093/14198 212 7 ~ S Q PCT/US93/00082
17
meta-o~y~enzoate anion, ~he light emitter~ The developer
was then drained off, and the wet blot wrapped in Saran
Wrap was exposed to x ray film for 20 min and dev~loped
using conventional procedures. Densitometric analysis of
each spot on the x-ray ~ilm was carried out on an image
analyzer using the conventional procedures.
Densitometric an~lysis of each spot on the x--ray film was
caried out on an image analyzer using the Dumas program
(Drexel Univ~rsity, Philad~phia, PA) inter~aced with IBM
1 0 computer .
GAPDH en~ymatic activity of in.act_SDH protein on whole
streptococci:
A whol~ cell assay was developed to determine
whether SDH on the surface of streptococci ser~es as an
active GAPDH enzyme. Different concentrations of
trypsinized and non-trypsiniæed ctreptococci were
incubated with and without G-3~P in presence of NAD in
triethanolamine-phosphate-EDT~-DTT buffer as described
above in a ~inal volume of l.0 ml for a period of 2 min
~ 20~ at room .témperature ~nd centrifuged to pellet ou the
:~ : bacteria. The supexnatants were analyzed for the
conversion of NAD to N ~ by:recording absorbance at 340
nm. This enz~mati~ activity was also determined on
streptococci preinoubated with 1:50 dilution (l mg~ml) of
purified anti-SDH:antibodies as prepared above to
:~ ~ : determine specific inhibit:ion of enzymatic activity.
:
Po1yacrylamide-~qel elec~roPh-oresis and _ :
: ~ Electrophoresis, Western blotting o~ lysin
extraction and protein samples at di~ferent purification
: stages:werie carried out as de~cribed earlier t2,3).
Spe~ific prote:ins:~bound to the nitrocellulose membrane
WO9~/14~98 PCT/US93/OQ~
~ ~ 5~ ~ 18
were pro~ed and visualized with affinity purified anti~
SDH antibodies (1:2000, 0.5 mg/ml) as d~scribed
previously ~2,3).
Presence of SDH_on heteroloqous streptococcal M
serotypes:
Ly~in extracts of M serotypes 2, 4, 5, 6, 24, 29,
4l, 57, 5~, ~0, and ~ were prepared as descri~ed (2).
The muralytic enzyme mut~nolyæirl (20 ug/ml; siqma
Chemical Co.) was used to prepare cell wall extracts of
each grouping str~in suspended in 50 mM Tris/HCl bu~r,
pH 6~8, containing 5 mM EDTA, 5 mM MgC12, and 30%
raffinose, and incubated at 37C for 60 min under .
constan~ end-to-end rotation. Proteins in all the
extracts were sep~rated on SDS-PAGE and transferred to
nitro~ellulose. The blots were probed with affinity-
purified anti-SDH protein antibodies as described above.
Relationshi~ o~ ~PHDs from bace~rial and ~ammalian
origins with SD~:
The cross reactivity of GAPDHs isolatad from rabbit
skele al mu:scl~,~human erythrocytes and
B.~tearothermoE_i-us were ~ete~mined both on Western blot
.. . .
and comp~titi~e ELISA as described~below.
: :
~ ELISA and Competitive i~ibltion:
: : ~ :
:A~inity purified antibodies~were ~djusted to a
dllution that:;ga~e an ELISA reading of l.0 at 405 nm
aft~r 60 min. ELISA was performed following standard
: proc~dures except that:ELISA:plates were coated with lO0
; ul/well of l ug/ml SDH f~r 3 hr at 37C followed by
overnight at 4~C.
: ~ ,
: ~
~w~ g3,l4l9~ 2 1 2 7 ~ 5 ~ PCT~US93/~0~2
19
Competition of GAPDH from different bacterial as
well as mammalian origin containing cross reactive
epit~pes ~or the binding of Anti SDH antibodie~ was
per~ormed as describ~d pr~viously ~0~. Briefly, ~LISA
plates were c~ated as described above with SDH. Optimum
dilution of affinity purified antibodies as determined
above wa~ u~ed. Competing GAPDH were ~erially diluted in
antibody dilu~ing bu~er containing 0~05% Brij-35 p~ 7.4
(10) at decreasing molar excess rel~tive to SDH starting
with 100 X molax exces O Anti-SDH antibodies were then
added in ~ach w~ll and the plates were processed and
~inally developed and binding of these proteins was
determin~d by kinetic ELISA as de~cribed (11) u~ing ELIDA
5 microtitre plate reader Physics Inc. ~20) at 405 nm.
Radioiodination of Proteins:
SDH was lab~led with 125I by the chloramine~T method
using Iodobeads (Pierce Chemical C~.). The labeled
protein was s~parated from free:iodine by filtration over
a column of Sephadex G-25 (PD-10, Pha~acia LKB Biotech
;~20 Inc)~and collected in 10 mM HEPES buffer saline pH 7.4
containing 10 m~ MqCl2, 2 mM CaCl , 50 mM KCl and 150 mM
NaCl. The lab~led pxotein wa~ stored at -20C in
aIiquots containing:0.02% NaN3. Fibronectin and plasmin
` ~ were labeled~essentially:by the same m~thod. The:: 25 ~peci~ic activities of SDH, fibronectin and plasmin were,
: ; re:sp~ctively~, 2X1~05, 1:.0X106 and 1.21X106 CPM/mg.
,
Bindinq Studies:
:The Binding acti~ity of~SDH and fibrone~tin was
:~ determin~d by:the~use of radioaative proteins. Egg
: white-lysozym~ and/or cytoskeletal protei~s (myosin,
heaYy~meromyosin~(HMM), light chain myosin (LMM3,
.
WOg3/1419X PCT/US93/~ ~2
tropomyosin, and actin) all of which ~btained ~rom sigma,
were electrophoresed on 10% SDS PAGE gels and
electroblo~ted on nitroce~lulo~e paper. The blot~ were
blocked in 10 mM H~PES buffer containing 15 mM NaCl, 0.5%
Tween-20, 0.04% NaN3 and 0.5% ~SA pH 7.4 for 2 3 hr at
room temperature and probed for 3-4 hr at room
tempera~ure in th~ same buf~er containing 1~5I-
fibxonectin, 125I-pla min at 3X105CPM/ml~ The probed
blots were then washed 3-4 times wi~h bloaklng bu~fer.
Autoradiography were prepared b~ exposing the dried
nitrocellulose ~lots to Kodak Blue Brand film with an
int~nsifying screen for 3~-48 hour at -70~.
Lysin trac~ion~eion of Stre~tococ i for ribosylation
study:
An overnight culture of streptococci was washed and
the cell wall was digested using the amidase enzyme lysin
in 30% raffinose at pH 6.1 as described t2,3). After
lysin extraction, which represents the cell wall fraction
of ~he str~ptococci, the resulting protoplasts were
further fractionatQd into cytoplasm and membrane after
lyisng in a hypotonic buffer cont~ining 1~ mM MgC12 and
DNAse (250 uglml~as described (3). :Membranes were then -
separated from the cyt~plaamic fraction by
ultracen~rifuga~ion (100,00 X:g, 45 minn, 4C~.
~: 25 ADP-Ribosylation of SDH: ~ ~
: ` :~ :
~: ~he ADP-ribo~ylation of SDH was performed as
dels~ribed (15~ with slight modification. Briefly, the
standard reaction mixture (0~2 ml~) contained 100 mM
TrisfH~l at pH 7.:4,~ 10 mM dithiothreitol, 1 mM NADP~ 10
:~ 30 mM::th~midine (ADPR~bu~Per~ ;A~ter the addîtion of 10 uM
~lpha 32P]N~D and 20 ug of:purified SD~, the reaction
-~ : :
~ :: : : :
.
_.WO93/1419~ 21 2 7 5 ~ ~ PCT/US93J00082
21
mixture was incubated for 1 hour at 37C. The reaction
was then stopped by the additicn of 50 ul of 100% (w/v)
chilled trichloroacetic acid (TCA), and allowed to stand
for 30 minute~ on ice after which time the precipitat~d
pr~teins were separated by centrifugation ~16,000 X g, 5
minutes at 4C~. The protein pellet was wash~d in
absolute alcohvl containing 1% of 5 M sodium acet~te and
dri~d in a Speedvac (Savant~ to remove remaining TC~.
The dried precipitates w~re di~sclved in 50 ul of sample
buf~er and then subjected t~ SDS PAGE tl2%
polyacrylamide) as described (2,3~. The gel was dried,
and autoradiograms wsre made with Kodax X-omat film using
an intensifying screen at -80C.
GAP~H ACtlV ty of ADP-ribosylated SDH:
,
The GAPDH activity of purified SDH ~nd the ~DP-
ribosylat~d SDH was measured by the method originally
described by Ferdinand (7~ and modified as described
16). ~riefly,:the rsaction was performed in a final
volume o~ 1 ~1 containing 809-850 ul o~ buffer (40 mM
triethanolamine, 50 mM Na2HP04, 5 ~ EDTA, p~ 8.6),
100 uM NAD and~the enzyme source (ADP ribosylated and
non-ADP-rihosylat~d SDH, :5 ug~ ~dispensed in a 1.5 ~1
capacity microcu~ette.~: The reaction was initiated with .
the addition:of ~7 ul glyceraldehyde:(49 mg/ml) 7
~ sorbanc~ at~A340~ nm~showing the conversion of N~D to
~ NADH was recorded o~er~a period of 2 m.in.
:
Eff
SDH~
: Sodium nitroprusside~was fre~hly diluted in ADPR
30: ~ ~uffer (200~ul) ~o a final concentration of 2 mM and
preincubated for:2 minutes at:~room t~perature bef~re the
~:
,
: : :
: ~ :
WO93/i4198 PCT/lJSg3~0~82
~ r 0 22
a addition of 30 ug of SDH and [32p] NAD to start the ADP-
ribosylation reaction. At different time intervals~ 40
ul aliguots were removed and precipitated with TC~ A
parallel control repres~nting the same quantity of S~H
and ~32P]-NAD were incubated in the absence of sodium
nitriprusside and aliguots were taken at the same time
interval~ as the test samples. Precipitat~d proteins
were ~eparated on SDS gel and autoradiographed. In a
similar set of experime~ts, ADP-rib~ylation was also
performed u~ing ~00 ul of a ~treptococcal ly~in extrac~
in ADPR buf~er incubated in the presence and absence of 2
mM sodium nitroprussi~e.
The results o~ the foregoing procedures are
summarized below.
Purif1cation of SDH ~rotein r~gEt~r:
SDH protein, was precipitated from the lysin extract
by fir~t preaipitating non~specific proteins at 60%
. saturation o a ~ onium sulfate ~ollowed by 85%
saturation. The SD~ was found in the 85% ammonium
sulfate precipitate (Fig, l~. The dialyzed precipitate
was appli~d to a Mono Q FPLC column and the proteins
elut~d with an NaCl gradient~ from 0 mM to 300 mM. SDH
eluted at a salt~concentration of about 280 mM.
~:~ Fractions with fibroncctin binding activity were pooled,
dialyzed and~urther purified on a Superose-l2 FPLC
molecular sieving co}~n. The small amount of
contaminating~proteins was removed by hydrophobic
chromatography using a TSK-pheny} column. SDS-PAGE of
the final preparation~reveal~d a h~mogeneous pro~ein with
~ -30 a molecular weight~of 39 kDa. The total yield of
: puri~ied protei~ from f:our liters of culture representing
~: : 6-8 gms wet weight of bacteria:was ~00 ug.
~: ;
:
.
WO 93/14198 21 2 7 5 ~ ~ P~/US93/OOOX2
N--terminal Seauence an~ amino acid coml~osition anal~rses
NH2-terminal amino acid sequence analysi; o the
purif ied sDH c:onf irmed the homogeneity of the preparation
resulting in a single amino ac:id at nearly all positions
(Fig. 2a3. Ex~ept ~or positions 31 and 35, a single
amino acid wa~3 id~nti~i~d in the ~irst 35 residues with
the remaining~ f our tentativel}r id~ntif ied .
The am~no acid co~po~ition of the puri~ied protein
indicated a high content of Asp/Asn (12 . 1%~, followed by
Ala (10.7~6), Gly (10.3%), Val (10.2%~, and Glu/Gln
(8.4%). The ma~3s s~ the puri~ied protein ~35,~82
daltons ) as determirled by laser desorption ma~s-
spectrometry was used to more precisely assign the number
of residue~/mol (Table 1).
Amino Ac:id Sequen e and Composition Comparison:
:
,~
When the sequence of the f irst 3 9 ~mino acids of SD~I
was cs:~mpared to known æequences i n the translat:ed Gen-
Bank data~ase; (Fig, 2b~, significant identity was ~ound
,~
: with: bact rial and e~aryotic: GAPDHs. The identity
within the first 18 residlaes was 77-83% with bacterial~
eukaryotie,~: or fungal G~PDHs. This strong homoïogy
decreas~ed ov~ the remaining 21 residues with an overall
idQntity of :from 41-56% ~(Fig. `2b)~,
; ~ en the~amino~ acid compo itions of the Yarious
: ~ 25 GAPDHs w~re~ c:o31lpared, th~ methionine content of SDH was
ound to~ be signi~ia~antly low (I.8 residue/mol) with
relation to the eukaryotic ( 8 . 4 residue jmol ) or other
baclterial ~DHs ~(~7;~ residues/mol) ~;(Table 1). Although
~: ~: the a~in~ aci~l~compo :itions :of the rem;~inirlg residu~s of
30 ~ SDN ;were found~ to: be rèlatively close to that of the
1` ~
~093/14198 PCT/US93~ ~ 2
other GAPDHs, sufficient d~ff~rences were found that
suggest that, except for the NH2-terminal sequence, SDH
is di~ferent from other repoxted G~PDHs.
GAPDH activity of SDH ~rotein:
In the pr~sence of G-3-P in triethanolamine buffer
at pH 8.6, SDH ~howed a do~e dependent conversion of N~D
to N~DH a~ ob~erved by absorbanc~ of the lattex at 3~0
nm. Using 30 ug of puri~ied SDH, ~ariation of enæyme
reaction rates with varying concentrations of G-3 P and
1 a N~D was detexmined. ThP results were analyzed bo~h as
Michaelis~Menten plots aæ well as double reciprocal plots
according to Lineweav@r~Burk (13) as shown in Figs. 3a
and b. From the e plots the Km for G-3-P and N~D was
estimated to be l.33 m~ Vmax 0.487 X lO 3 M NADH min l.
Fig. 5 shows the analysis based on the method of
Micha~lis-~ent~n. ~ , 1.22 m~; and Vmax, 0.466 x lO 3 M
: ~ NADH/min. ~b) 25 ug of the 3~-kD protein was assayed as a
~: function of NAD in the pr~sence of G-3-P (2mM) in ~he
buffer y~tem described above. The Km for NAD was
estimated ~ be 156.7 ~ ; Vmax, 0.459 x l~ 3 ~ NADH/min;
~-: intercept on y-axis~1/Vmax), 2.l8; and slope (Km/Vmax),
34~.7~ Km for N~D by the meth~d of Michaelis-Menten as q
: shown in the inset was estimated to be 148.86 uM; and
-3
~ Vmax, 0~445~x::10 ~ ~:N~H/min.
: ~ ~ 25 Determina~lon of~:loca~ion of SD~ on cell:
: Antibodies~to SDH wer~af~inity puri~ied on SDH-
~ b~und to activa~ed gluteraldehyde beads ~ollowed by~a
: prot~:in A column. ~he resultant purified anti-SDH IgG
: r~cognized only ~he SD~ protein band~ig. 4). Dot-~lot
30~ immunoa~say was::applied to determine the location of SDH
on ~treptococcal~-ur~ace. Reiults revealed tha~ trypsin
::
: :
~_W0 93/14198 2 ~ i 5 a PCr/U~3/00082
treated streptococci were markedly reduced in their
reactivity to anti-S~H IgG; (Fig~ 5~. To determine if the
SDH protein is peripherally bound to the cell wall or
tightly bound, the streptococGal cellæ w~re washed wi1:h
2M NaCl and 2% SDS. The results revealed that the SDH
was not extractable by the high s~lt or ionic detergent.
_urface En~e activi~ of streptococci:
To d¢termille if the G~PDH enzymati~ activity f ound
with the puri~ied SD~ protein was also present on the
stxepto~:os:cal surface, enzymatic studies were carried out
using wh~le streptococci. The same concenkration of
substrates (G 3-P and NAD) used with the purif ied SDH
were us~d with whole streptococci.
Data presQnted în FigO 6 revealed a dose dependent
GAPI~H ac:ti~rity catalyzed by the whole organi~3m~:. As
found with the purified SDH, the intact b~c~eria also did
not catalyze the reaction in absene:e of th~ specif ic
substrates Go3-P and ~AD (Fig. 6a). The enzymatic
activi~:~ on the whole org~ ;ms was also f ound to be
partially: (30%) but specifically inhibitable by anti-SDH
IgG (~ig. 6c). ~ Enzymatic activity was ~ound to be
d~cEea ed by B0% when trypsinized bac:teria were used in
the reaation mixture (Fig. 6b). The background 20%
acti-.rity ~;uggested an incompl~te digestion of SDH protein
by ;trypsin
Preval~nc_ of SDH ~teil~ In oel~r I( serotypes:
The ubi~itous nature of the SDH protein in
different ~;treptococs::al M serotypes was determined by
Western blot analysis of lysin extrac~s using affinity
puri~ied anti-SDH: IgG. As ~hown in Fig~ 7, SDH pro~ein
~ ' -
.
W093/14198 P~T/US93/0~82
was found in several ~erotypes. Furthermore, all were
found to be of same molecular weight without any
indication of ~ize variati~n.
Relationship of SDH with GAPDHs of bacterial, animal and
human ori i~:
The relationship of SDH with known GAPDHs was
determined ~y both Western blot and competitive kin~tic
(k) E~ISA using af~inity-p~rified anti-SDH antibodies.
Western blot (Fig. 8, insert) analysis with with SDH-
specific antibodies revealed that GAPDH from bacillus,
human RBCs, and rabbit muscle reacted weakly or not at
all~ This ~indin~ wa~ further confirmed by competitoin
ELIS~ ~Fig. 83 showing that only a maximum of 20 25~
inhibition of binding of anti-SDH antibodiee to SHD,could
be achieved with 100 molar exce~s of these proteins~ with
the rabbit muscle~ GAPDH exhibiting the least actiYity.
The fact that almost 20% inhibition is o~served wikh 20
times mola~ excess~of bacillus and h~man GAPDH may
reflect the 3e~uence homology ob~erved at the Nff2 termini
~:20 ~ ~ of these mole~les~(Fig. 2jO : r
~: Bindi~nq ~ro~rt~_o~ SDH with lysoz ~ e and cytoskel~tal ,
roteins: ~ . ;: : : :
:: Sinc~:many:~glycolyti~ enzymes have been shown to
bind cytoske~etal:proteins a d~termination was made as ~o
;~ 25 whether SD~ ~as:a~similar property.: 1~5I-SDX w~s used to
pr~be a:We~tern~blo~:containing~severa:l cytosk~lçtal
~ prstein The results rev~aled that SDH bi~ds to myosin
:~: :: : and~its globular~domain (heavy~meromyosin) and actin but
not to~the ~-helica~ d~main o~myosin ~light meromyosin)
~ or tropom~osin.~(Figs.~9a:and b): :
,
`: :
'
,_~093/14198 2 1 2 7 ~ ~ ~ PCT/US93/OOOX2
27
Binding of fibronectin to the SDH prot~in:
The fibronectin binding activity of SDH was
determined both by using l25I~labeled fibronectin or
fibronectin-an~i-fihronectin on a West~rn blot. The
results revealed that the SDH protein wa8 able to bind
fibronectin in both assay (Figs. lOb and c).
Based on the result~ of all of the ~boYe, it has
b~n determined that SDH i~ a major ~urace pro~ein of
str~ptococci, including group A streptococci and has both
enzyme activity and multipl~ binding activity. No su h
protein has previously been detected, isolated and
charact~rized. The novel surface protein is principally
characterized by its ability to bind fibronectin,
lysozyme a~d cyclo~keletal protein as well as by i~s
enzymatic activity a-~ a GAPDH. Its molecular weight is
approximately 39 kDa. The first fifteen amino acid
r~sidues at the amino te~minal are:
~ Val-Val-Lys-Val-Gly-Ile-Asn-Gly-Arg~ Gly-Arg-Leu
: -Ala-Phe
Theæe flrst ~i~teen amino acid residues mani~est
100% homolo~ with the bacterial form of GAPDH and 80-9096
. . - ~
~: homology with eukaryo c~or ~ungal GAPDX. 5DH is,
: however, significant1y difSer~nt from preYiously
recorpt~d GAPDHs, because the high homol~y of the first
: 25~: fifteen amino acid residues is no~ pxeserved ~owards the
: car~oxy end of the molecule and th~ amino acid
omposition varies appr~ciably from other GAPDHs.
SDH also functions as an~ADP-ribosylating enzyme
:~ which, in the presence of NAD, is auto-ADP-ribosylated.
~ : 30 It has been found that in a crude lysin extract of group
:
W093/14198 PCT/US93/0~2
A streptococci containing a mixture of cell wall
associat~d ~olecules, SDH is the only molecule that is
ADP-ribo~ylated. Treatment of ~DP-ribosylated SDH with
the cytopla~mic fraction of Group A removed the ADP-
ribosye of SDH which indicates the pre~ence of SDH
specific ADP-ribosyl hydrolase in the cytoplasmic
compartment. Treatment of purified SD~ or the crude
lysin extract with sodium nî~roprusside, which
spontaneously generate nitric oxide, was found to
stimulate the ADP-ribo~ylation of SDH in a time dependent
manner. Both ADP-ribosylation and nitric oxide treatment
inhibited the ~lycer~ldehyde-3-phosphate dehydrogenase
activity of SDH. In addition to its auto ADP-
ribosylation activity, either purified SDH or whole
streptococci with surfac~ SDH were able to ADP-ribosylate
specifically both chicken and human lysozyme, strong SDH
binding proteins. These data show that SDH has both
autoribosylation and ADP-ribosyl trans~era6e acti~ities.
SDH, as will be reco~nized by those skilled in the
art, does not represent~a single protien, but rather a
class of surface~proteins of streptococci, ~ll of which
have simil~ properties.: The protein is involv~d in the
~ coloniæation and probably:in the int~rnalization and
: proliferation of:group A~streptococci. One of the
: 25 initial steps in~th~ colonization of mucosal tissue by
~; streptococci and ~ubsequent infection by this bacteria,
;: ~ is the binding of the~bacteria to fibxonectln~ Lysozyme
: is alæo believed to~ be involved in this binding step.
The enzyme activity of SDH may be involved in the
' I' . i ` I ` ~ ,. .
binding of the bacteria to endothelial cells by reaction
o~ an aldehyde reduction product;of teichoic acid which
: : :
, ~ 93~1~198 2 1 2 7 ~ ~ ~ PCT/US93/~82
29
is a polyglycerol phosphate. The aldehyde function could
bind the bacteria to the tissue surface by reaction with
~mino group ~n that surfaae.
Inhibition of this initial binding is, therefore, a
major fuction in inhibiting ~r preventi~g streptococcal
infectionO Accordingly, antibodies to SDH which
successfully ~ompete with ~i~ronectin and ly30zyme for
binding sites on the bacteria will inhibi~ the
colonization of the pharyngeal muaosa by group A
tréptoGocci. Therefor~ the SDH and amino acid segments
o~ the protein containing the appr~priate antigenlc
determlnanks, for example those containing from about 6
to about 20 amino acid residues, are useful to inhibit
streptococcal infection o~ ~a~mals, including humans, by
administering an amount of the selected product which
will be effectiv~ to inhibît fibronectin binding and
thereby inhibit colonization of~the pharyngeal mucosa.
:: :
The proteins, pol~ eptides and peptides of this
invention may be obtained by any::of a number of known
: : 20 processes. : -
The protein can be~isolated~as described above.
; Alternativley, rthe protein or se~ments thereof can be
prepared by~recQmbinant~DNA t~chniques. For example, the
:~ : g~ne for the prot~in or an oli~onucleo~ide for the
~25 desired eg~ent~can be inserted into a plasmid and the
plasmid:used~to transf~rm E. coli so that the ba teria
will ~xpresæ:~he desired product.
Polypeptide and~eptides within the scope of the
invention c~ntaining, for example~rom abou~ 6 to 20 or
more amino aci~ ~eg~ents, may be sy~thesized by standard
~ ~ solid phase procedures with appropriate ~mino acids using
:~: : ::
W093/14198 PCT/US93/O,Q.~.8Z
~r~ 30
the protection, deprotec~ion and cleavage techniques and
reagents appropriate to ~ach speciPic amino acid or
peptide. A combination of ~anual and auto~ated (e.g.,
Applied Biosystem 430A) solid phase techniques can be
u~ed to synthesize the novel peptides of this invention.
Altough less convenient, classical methods of peptide
synthesis can also be emplo~ed. For background on solid
phase techni~ues, rePerence is made to Andreu, D~,
Merri~i~ld, R.B.~ Steiner, H. and Boman, ~I.G., (1983)
Proc~ Natl. Acad. Sci US~ 80, 6475-6479; ~ndreu, D.,
Merrifield, R~B., St~iner, H. and Boman, H.G./ (1985)
Biochemistry 24, 1683-1688; Fink, J., Boman, A., Boman,
H . G., and Merrifield, R~B., (June l~9) Int~ J0 Peptide
Protein Res. 33, 412-42~; Fink, J., Merrifield, R.B.,
Boman, A. and ~oman, H.G., (1989~ J. Biol. Chem. 264-
6260-6267; each of which is hereby incorporated herein by
reference.
~, :
~: The products ~f the invention are amphoteric. They
can exist and be utilized as free bases or as
~: ~ 20 pharmaceutically acceptabl~ metallic or acid addition
alts. Suitable metallic salts include alkali and
;` : alkaline earth~etal salts, preferably sodium or
~: ~ potassium sal~s.~ Acid addition salts may be prepared
from a wise va~iety~of organic and inorganic acids
including mineral acids, for example ci~ric, lacti~,
maleic, tartaric, phosphor;ic:and hydrochloric acids.
: These sal~s~can be pr~pared~by procedures well known to
those skilled in~the~art.~
` ~
For use~as:a vaccine, it i8 presently pre~erred to
admini~ter the selected p~oduct conjugat~d to a carrier
such as cholera~toxi~ B~: Methods for preparing such
~ ; ~
: ~
WO93/141g8 ~12 7 ~ ~ Q PCT/US93/00082
31
co~juga~es are known. One procedure is de~cribed by
B~ssen and Fischetti ~14~. Oth~r carriers can be
employed or the products can be used without a carrier.
The protein or segmen~ thereof may al~o be
administered as a hybrid protein expres~d on a
streptococcal surface utilizing ~h~ procedure of Pozzi et
al (17,18).
Mice or other m ~ als including human6 wh~n
immunized parenterally or orally have significant
resistance to subsequent streptococcal challenges.
The presently preferr~d method for the
administration of the vaccines of the invention is by the
intranasal route, but the invention i~ not so limited.
Other parenteral or oral prcoedures may be employed.
Typicallyt the patient to be protected will be
tr~ated with an a~ount o~ 5DH or other product of the
invention which is e~ective to elicit a protective
: immune response.: The sel~cged agen~ may b~ a~ministered
alone or in a pharmaceutically acceptabl~ liguid or solid
~ carri~r in which~it;may~be:dispersed, dissolved or
~: suspend~d. If, for~xample, the patie ffl is to be treated
intravenously,~he p~ptid ay be ~u~pended as a free
: bas@ or dis olved ~as a: metallic salt in i50tonic a~ueous
~ uffer. Other methods:of:treatment and pharmaceuti~ally
:~ 25 ~acceptable carriers will be apparent to the skilled
artisan.
:
Ths proteins,:~polypeptida~ and peptides of this
invention an~:the~ genes or oligonucleotides which are
employed in their expression~are useful as probe~ ~or
~30 genes and protein~ ~:They~ ~re~also ~seful to rai~e
~ ,
:~:~ : : :
WO 93/141~8 PCI/lJS93/0~0~2
32
antibodies by which specif ic strains of ~;treptococci can
be identif ied . For example in t2g;ts f or mammalian
inf ection~ .
~,,
:,~ , : , :
.
, ~: : :: : : :~
: ~ :
wo 93/l4lg8 2 i 2 7 ~ 5 ~ PCTJUS93/~082
~BLE 1
Comparison of Amino ~cid Composition of SDH Protein from
M Type 6 Streptococci wi~h That of GAPDH from Different
Species
~ ~ _ . _ __ _
No. o~ residues/mol
.... _ . _ . ... .. . ... ....... ., . , , . _
S~ BSt Tha~ RSM
~sn/Asp 43.3 41 36 35~5
G1u/G1n 29.9 26 24 18.7
Ser 16.~ 17 13 1~.7
G1y 36.6 24 25 31.7
~is 7.2 9 10 9.8
Arg ~5.5 15 15 10.1
Thr 27.0 ~ 22 21.6
Ala 3~.1 38 41 3~.6
Pro 13.6 11 12 11.9
: Tyr ~ 9.1 ~ 10 8.6
2a~ Val 36.5 43 ~9 ~31.
M~t 1.8~ : 7 7 8.4
~ Ile~ ~ 22.4 19 ~ :22 lS.3:~ Leu ~ ~23~.4 ~ 26 30 17.8
: ~ Phe ;~13.~ ~ ~ 7 12.9 -
2S ~ ~Ly8 21.:4 :~: 23 23 23.9
C~sS~ : 3:.1~ ;: 2~ .0
Trp~ ND ~ 2: ~ 3 ND
~ Bst, B.stearothe~ophi1us (19~; Thaq, Th~rmus aquaticus
: 30~ ~52); RSM, Rab~it:~skeletal:;mu~c1~e~(203.
* Nean~of threè~determinati~ns.
Mo1ecu1ar ~ass of~SDH:protein ~35,882~ was measured by
:
WO93/~4198 P~T/US93/00082
. ..
15~ laser desorption ma~s ~pectrometry.
Determined by carboxy amidomethylation ~e hod (5).
The publications id~ntified in thi~ ~pecification
are all incorporated herein by r~ference.
~EFER~NCES
1. Wannamaker, L. W. 1973. The chain~ that link the
throat to the beart. Circulation 48:9.
2. ~ancholi, V. and V.A. Fischetti. 1989.
Identif~catiDn o~ an endogeneous m~mbrane anchor-cleaving
~nzyme for group A streptococcal M protein. J. Exp~ Med.
: 17~:211g.
3. Pancholi, V. and V.A. Fishcetti. 19~8. Xsolation and
characterization of the c~ ass~ciat~d r~gion of group A
streptococcal M6 protein~ J. Bact~riol. 170:2618.
I5 4. Matsudaira, P. 19~7. Sequence ~rom picomole
; quantities of prot~ins ~lectroblotted onto polyvinylidene
difluoride:me~brane~. J. Biol. Chem. 26~:10~035.
5. Cre tfiPld, A.M., S. Moore, and W.H. Stein. 1963. ,
: ~ The preparation and~enæymatic hydrolysi~ of reduced and
~ S.: carboxy~ethylated proteins~J. Biol. Ch~m: 238:~22.
; 6. ~Beavis~ R.C.:~and B. ~. Chait. 1990. High aacuracy
ma~s~de~rmination of proteins:using matrix assisted
: ~ ~ lasor~desorption~mass spectrometry. Anal. ~iochem.
2:1~36.
: ~ Ferdinand,~;W. 1964.~ The:isolation~and specific
activity of rabbit-muscle glyceraldehyde phosphate
dehydrogena e.~Biochem. J 9~:578.~: ~
: -
. :
WO 93/1419B 2 ~ ~ 7 ~ 5 G PCl/VS~3/00082
.
8. Horecker, Bo L., and ~. Kornberg. 1948. The
extinction co f f icient of the reduced band of pyridine
nucleotides ., J. Biol . Chem. 175: 385 .
9. Jones, K. F. and V.A. Fischetti. 1988. The
S importance of the location of antibody binding on the M6
protein for opsonization and phagocytosi~i o~ group ~ M6
streptoc:occi. J. Exp. M~d. 167: lll4 .
10. Joale;, K.F., S.A. ~, B.W. Erickson, S~X.
Hollingshead, J.R. Scott, and ~I.A. Fischetti. 1986.
Immunochemic:al localization and amino acid sequence of
cross-reac:tive ~pitope~; within the group A streptocoGcal
M6 protein. J., Exp. Med. l64: l226.
11. Fischetti,: V.~. and M. Windels. 19~8. Mapping the
i~munodeterminants of the comple~e strepts:~coccal M6
l 5 protein molecule: Identif ication of an immunodominant
region. J. Immunol. ~ 41: 3592 .
2 . Beavis, R. C:~, and B. T. Chait. l990 . l~apid, sensitive
analysis of protein mixkure by mass spectro~n~try. Proc.
Na~}. Acad. Sai. U~ S.A. 87: 6873 .,
~ ~ 9
13. Lineweaver, H. and D. ~urk. 1934. The det~rmination
of enzy~e dissociat~ on constants, J. Am. Chem. So~:.
5~:658. ~:
, :
~: ~ 14. ~Be sen~ D. and Y.A. ~Fischetti:. l988. Influerlce of
` int~anasal immNnization with synt:hetic peptides
2 5 clorrespo~ding to aonserved epitope~; of M protein on
mucosa~ colonization by group A streptococci. Infect.
Immun. 56:2666. ~ ~
: ~`: : ` : :
:
`:` ~ : : :
: : :
WO 93/14198 PCI~/US93/00082
~! '"
36
15. Kot~, ~.Y., Skurat, A.~r., Sergienko, E.A.,
Bulargirla" T.V. & Severin, E.S. (1992) FEBS Lett. 300,
9-12 .
16. Pancholi" V. & Fi~;chetti, V.P.. 1992. A l~ajor
Surf ace Protein On Group A Streptococc~ A
Glyceraldehyd~-3~Pho~phate-Dehydrogenase with Multiple
Binding Activity. J. Exp. Med. 176~ 415~42~o
17. Pozzi9 G., M. Contorni, M.R. Oggioni~ R. Manganelli,
M. Tomma~ino, F. Cavalieri, and V.A~ Fischetti. 1992.
The delivery and expression o~ a heterologous antigen on
th~ surface of streptococcî. Infect. Immun. 60:1902-
19~7.
18. Pozzi/ G., ~. Contorni, M.R. Oggioni, R. Manganelli,
and V~A. Fischetti. 1992. Expression of ~6 protein gene
of Streptococcus pyo~enes in Streptococcus gordonii after
chromosomal integration and transcriptional fusion. Res.
Microbiol. 143:449-45~. :
: 19. Harris, J.I., J.D. ~oc~ing, M.J. ~unswick, K.
Suzuki, and J.E. Walker. 1980. D-glycer~ldehyde-3-
phosphate dehydrogenase: the purification ~nd
char~cteriz~tion of the enzyme from the thermophiles
13acillus tearothermophiluc and ~hermus aquatic:us. Eur.
J. Biochem. 108: 535.
: 20. Caswell, A~H., and ~.M. Corbett. 1985. Interaction
of glyceraldehyde-3-phosphate dehydrogenase with isolated
micros~mal subfractions of skeletal muscl~. J. Biol.
Chem. 260: 6892 .
.