Note: Descriptions are shown in the official language in which they were submitted.
93/14389 ~ ''~ PCT/US93/00234
1
METHOD AND APPi~RATUS FOR PERFORMING AND UNIVERSALLY
DETECTING CAPILLARY ISOELECTRIC FOCUSING WITHOUT
MOBILIZATIODT USIN(= CONCENTRATION GRADIENT IMAGING SYSTEMS
Background of the Invention
Fie d: The invention is in the field of capillary
electrophoresis, detection methods for capillary
electrophorEasis a:nd apparatus based on such methods, and
on Schlieren optics.
State of the Art: It has been known for some time
that a refractive index gradient such as produced by a
concentration gradient in a fluid such as a gas, liquid,
or supercrii~ical fluid, will cause deflection of light
passed through t:he gradient. The optical method of
observing anal mea~;uring the deflection of light caused by
refractive index gradient fields is generally referred to
as Schliere:n optics. In the past, Schlieren images
resulting from light deflections have been recorded on
photographic plates and the plates then analyzed for light
intensity d:istrib~ution using densitometers. Recently,
evaluation of the photographic images has been done by
computer. These methods are useful in studying plasmas
where very complicated toroidal and parabolic shapes are
generated.
United ;States Letters Patent No. 4,547,071 discloses
a sensor for :measuring density gradients in a
nonhomogeneous fluid sample using Schlieren optics. In
such sensor, a laser light beam is directed through a
sample chamber and is moved along said chamber. A
quadrant light position sensor located on the opposite
side of the chamber detects the deflection of the laser
light beam as it is moved through the sample. The amount
of deflection indicates the density gradient at any point
in the sample. Rather than moving the laser beam along
the sample chamber" the beam can be held constant and the
sample chamber with sample therein moved. However, moving
a laser and detector together in relation to a sample
WO 93/14389 ~,1 ~ PCT/US93/00234
2
chamber and keeping the laser beam focused on the sample
chamber, even a relatively large chamber, is difficult, as
is moving a sample chamber through the laser beam so as to
keep the laser beam properly focused. Trying to do either
with a small capillary sample chamber is very difficult
and impractical.
My U.S. Patent ~Nos. 4,784,494, 4,940,333 and
4,993,832 show detectors that can be used to detect
concentration and thermal gradients in very small samples.
The detectors utilize a light source to generate one or
two probe beams of light that pass through the sample
having the gradient to be detected and the deflection of
the probe beam or beams is measured on a beam position
detector. Various light sources may be used to generate
the probe beam or beams, such as a laser or light emitting
diode (LED). These detectors, however, are designed
generally to be used where the gradients to be detected
move through the probe beam or beams of light.
It is also known that certain components of a sample
may absorb light of certain frequencies and that the
concentration of such components may be measured under
some conditions by measuring the amount of light from a
beam of light passing through the sample that is absorbed
by the sample. Such absorption detection is also
generally done by passing the sample through the light
beam of the detector.
Capillary electrophoresis has become an important
separation method in bioanalytical chemistry. Separation
and detection of very small amounts of biological samples,
about pL - nL volumes, can be achieved with capillary
electrophoresis. This is generally not possible with more
conventional methods of separation, even with high
performance liquid chromatography. There are several
capillary electrophoresis separation methods in use for
different kinds of samples. They include capillary zone
electrophoresis, moving boundary capillary
electrophoresis, capillary isotachophoresis, and capillary
CA 02127977 2004-03-29
WHO 93Jtd~9 PCIYtJSl3~O0i~1
3
isosimctric focusing. Capillary some eleotrophorosis,
aoving boundary caplliary elsotrophorwis, and
isotaohophoresis ail have the advantage that the sample
mops through a capillary sample separation chamber during
the a~eparatioa so can be used trith the detectors of ay
acted prior patents. Capillary sons alectrophoresiss and
aoving boundary capillary sieatxophorea~is are dynsaic
processes vhara separation occurs at nn instant in tire
and then the ~eones iaa~ediatelp begin to diffuse and
o disperse. The diffusion takes place as the samples move
through the sample chaaber to the detector. This wakes
deteatian of the various,sones more difficult and less
aaaurate than li~ay by desir~d_ Isotachophoresi: has the
advantage that the sores stay relatively sharp as the
saaple moves ttrraugh tba capillary, but isotachaphorasis
is w difficult process to ~rorlc with.
Isoalectric focusing has been eaploy~d for separation
of sample co~on~nts bassd on differences in their
isoelectric points. Recently, the development of
ao capillary vlactrophorssi~s techniques has geriersted
interest in praforming the isoalactrio focusing in
capillaries, since ellici~nt dissipation of Joule heat
from s io-:00 ptt diameter capillary eliainates cxmveation
alfeats which occur in larger sawple ohasbera and enables
highly aftioient separations. Capillaries v3th
microbores, i.e., with very wall inner diameter, also
require only small amts of sample, which is desirable
for analysis of biological matsrials, such ae mox~ocloaal
antibodies and other proteins. The capi7.lary isoalactria
3o foausit~g process involves establishing an electrical field
between the ends o! the capillary and establishing a
stable pH gradient inside the capillary using a mixture of
asopholytes. Jrt the arms time, an ampholytic analyte,
such as a protein, moves along this pH gradient and is
focused at the point where the pH is equivalent to its
isoalectric pofnt. 'fhe migration then ceases. Thus, a
stationary condition is reached and saintained in the
PCT/US93/00234
WO 93/14389 21
4
capillary. During this separation process, narrow
Gaussian bands are generated with high peak concentrations
which results in high separation resolution of the
analytes. In order to detect the now focused analytes
with available detectors, the focused zones must be moved
through a stationary detector which is usually located at
one end of the capillary. Thus, the focusing of the
sample in the capillary is followed by a mobilization
process. The commonly used mobilization process requires
addition of salt to the electrolyte at one end of the
capillary. The salt causes changes in pH at that end of
the capillary. Because of this pH shift, the analytes
focused in the capillary are no longer at their
isoelectric points and will consequently move or migrate
toward the end of the capillary and will pass through the
detector. During the mobilization process, distortion of
the focused zones and loss in resolution are unavoidable.
Further, the mobilization process also takes at least
about 15 minutes compared to the about five minutes
required for the focusing itself using commonly available
isoelectric focusing systems. Since the detection is
necessary, the required mobilization makes the isoelectric
focusing a relatively slow separation method thought to
have little advantage compared to other capillary
electrophoretic techniques. Therefore, it is necessary to
develop an on-line detection method to eliminate the
mobilization step and thereby improve the speed and
performance of detection using the isoelectric focusing
separation technique.
Several on-line scanning spectroscopic and
radiometric detection methods have been developed for
electrophoresis performed on slabs. However, such methods
cannot be satisfactorily used with electrophoresis carried
out in microbore capillaries because of their small size.
Recently, there have been attempts made to continuously
monitor capillary isoelectric focusing separation. In one
instance, photographs were taken of the focusing of blue
J 93/14389 ~ ~ ~ ~~ PCT/US93/00234
dye stained proteins inside a 0.4 - 0.6 mm i.d. capillary,
and the photographs used to study the zones of proteins.
However, this technique requires labeling of the proteins
and can not gave good quantitative information. In
5 another instance, the separation in the capillary was
monitored using chemical electrodes spaced along the
length of the saimple chamber. Although a complicated 100
chemical electrode array was used, the resolution obtained
in these experiments was very poor.
With currently available capillary electrophoresis
equipment, the capillary tube is generally about a meter
in length and each end must be manually positioned in a
container holding solute or sample to be separated. The
longer the capi7.lary tube, the longer the time necessary
for a sample to move through the tube. When a new sample
is to be separat:ed, one end of the capillary tube has to
be moved to another container which contains the new
sample. A:Lso, as the ends of the capillary are moved from
container to container, the electrodes necessary for
operation of the system must also be moved. Since
voltages up to about lOKV are required to operate the
system, the person moving the capillary tube and
electrodes from container to container may easily come
into dangerous contact with the electrodes.
One of the ;most promising applications for capillary
electrophoresis is for routine analysis in research
laboratories, ph<irmaceutical manufacturing facilities, and
hospitals. However, in many cases, relatively rapid
separation and accurate detection of samples is required,
because the feedback of the analyzed data is essential for
observing eaffectiveness of a therapy, adjusting drug doses
in treatment of patients in hospitals, or controlling
process conditions in industrial manufacturing. Also,
since different methods of capillary electrophoresis apply
to different types of samples and situations, it would be
convenient to be able to run different methods on the same
instrument. It is impossible for current commercial
CA 02127977 2004-03-29
WO 9~111~! PCPII,lS~3~01Z~
6
capillary electrophoresis instruwenta to change lro~n one
separation sethaa tv another. 8aoh instrument and
capillary cartridge is designed !or a particular typo of
separation, e.g., for capillary isotachophoresis, or for
aoving boundary capillary e~lectrophorasis. ~1 !urther
probles is that current oosssroially available capillary
electrophoresis iirstruaents lack sensitive, universal, and
i:~axpsnsive detectors. Rlthongh conventional absorption
spectrophotometxio de~teators can be universal, they are
xo not sensitive snouqt~ for capillary alactrophoresis using
narrow capillaries, and as expensive sonoehromator is
required. The finorosetric detevtors in use nvt only
heed expensive lasers and photomultipliers but also
require fluorescent derivatization !or most anaiytec. The
is comserdal capillary electrop~doresis instrursnts with such
detectors era usually expensive and large devices.
Also, it is sometimes desirable to separate and
identify salapla ccmpotients or detatsine if such coasponerita
era present in a sample, for caoasponents which do not lend
~fl themselves to separation by eleatrophoretic techniques.
Thus, other more complicated detectors and detection
a~etbods are required to detect these oampo=rents.
' S~s~r o! the inyention
Z$ According to one aspect of the invention
there is provided a capillary electrophoresis
system which includes a light transmitting
capillary tube having a capillary passage
therethrough is which separation of components of
. a sample take place, the capillary passage having
a diameter. Means is provided for introducing a
sample to be separated into the capillary passage.
Also provided is means for creating a separation
of a sample by electrophoresis within the
capillary passage along a predetermined length of
the capillary passage, the predetermined length
being substantially greater than the diameter of
CA 02127977 2004-03-29
-6a-
the capillary passage. This system also includes a
light source for generating a light beam and means
for forming the light beam into a sheet of light
having a width at least equal to the predetermine8
length of the capillary passage and a height no
greater than the diameter of the capillary passage
and for directing the sheet of light through the
capillary passage so that the light passes through
the predetermined length of the capillary passage,
said predetermined length being such as to include
separations of interest present in the capillary
passage. Detectors means located in the path of the
sheet of light after passing through the capillary
passage for detecting the intensity of light at
various positions along the predetermined length of
the capillary passage and to provide an output
representative of the light intensity at the various
positions along the predetermined length of the
capillary passage. The intensity of the light at
various positions along the predetermined length of
the capillary passage is indicative of the sample
separation along the predetermined length of the
capillary passage.
~t has been found that concentration gradient
detention is uniquely suited for use as a detection
means and method for detecting the various bands of
components as separated by capillary isoelectric
focusing techniques. It has also been found that
isoelectric focusing can be effectively accomplished
in relatively short capillary tubes, such as
capillary tubes with about ten centimeters overall
length, and that the actual separations take place
within an eves shorter portion of such tubes.
Furthers it has been found that a light beam can be
generated as a sheet or line of light, i.e., a beam
of predetermined width with very small height
dimension, that can be focused on the capillary
passage in
J 93/14389 ~ ~ ~ ~ ~ ~ ~~ ~ PCT/US93/00234
7
the capillary tube and the separated sample therein so as
to extend along the capillary passage through the portion
containing the separated sample. The light within the
light beam is deflected by the concentration gradients in
the sample along the width of the light beam to produce
variations in the intensity of the light beam along its
width which are representative of the concentration
gradients established in the capillary passage by the
isoelectric focusing. By monitoring the light beam after
passing through lthe capillary tube and sample therein, the
separation of i~he sample can be easily and quickly
determined.
Rather than using a light beam to detect
concentration gr<~dients directly through the deflection of
portions oi° the beam, the frequency of light in the beam
may be chosen so that light is absorbed by certain
components of the sample to be separated. In such
instance, light within the light beam is absorbed by
certain components in the sample along the width of the
light beam to again produce variations in the intensity of
the light beam along its width which are representative of
the concentration of the certain sample components in the
capillary passages that have been concentrated or separated
by the isoelectric focusing. Again, by monitoring the
light beam after passing through the capillary tube and
sample therein, the separation of the sample can be easily
and quickly detez~mined. It should also be noted that the
concentration and the concentration gradient are related
in that t:he concentration gradient is the second
derivative of they concentration. Thus, a measurement of
concentration is indicative of the concentration
gradients, and visa versa.
In a preferred embodiment of the invention, the
intensity of the light beam after passing through the
sample is m~onitor~ed by a light detector in the form of a
photodiode array extending along the portion of the
capillary tube where the expected separations take place.
WO 93/14389 21 ~~, r~ J ~ PCT/US93/00234
..
The photodiode array is made up of individual sensing
elements of a size small enough to be able to resolve and
detect the differences in light intensity caused by the
refraction of the light passing through the concentration
gradients in the sample. With such an arrangement, the
light source, detector, and sample chamber, i.e.,
capillary passage, are all fixed relative to one another
to maintain accurate light beam focusing and detection,
yet the measurements can be made with a stationary sample
separation in the capillary. It is not necessary to move
the sample through the light beam to obtain a measurement.
The entire sample of interest is comprehended by the light
beam passing through the sample. Further, in the
preferred embodiment where the concentration gradients are
measured directly, the light beam is generated by an
inexpensive He-Ne laser with a cylindrical lens to convert
the beam from the laser into a sheet of light and to focus
the sheet onto the capillary passage. Various other means
of producing the sheet of light could be used, however.
In an alternate preferred embodiment of the
invention, the intensity of the light beam after passing
through the sample is monitored by a light detector in the
form of a single photodiode having a narrow sensing area
compared to the width of the light beam to be detected so
that the detector senses only a small portion of the beam
at any one time. The photodiode is mounted for movement
along the sample chamber where the expected separations
take place. By moving the photodiode along the sample
chamber through the beam, the diode measures the light
intensity at the particular location it moves through and
thereby provides an output proportional to the intensity
of the beam at each point the photodiode moves through.
Since the light beam is not moved with the detector, i.e.,
the sample chamber and the light beam remain fixed in
relation to one another, the problem with maintaining
alignment of the light beam and sample chamber is not
present.
l 93/14389 - ~ ~ ~ ~ ~ r' ~ PCT/US93/00234
9
A simple and easy to use capillary electrophoresis
apparatus c:an be constructed with a first reservoir and a
second reservoir connected and held together with a sheet
of glass, such as a microscope slide. A capillary tube
secured to the :Aide extends between the reservoirs so
that the capillary passage through the capillary tube
extends between and connects the two reservoirs.
Electrodes are inserted into the reservoirs and the
reservoirs are configured to easily accept a tube
extending from a syringe to enable liquid to be easily
added to or withdrawn from a reservoir. Thus, the solute
or sample can lde easily changed in a reservoir by
withdrawing it from the reservoir with the syringe and
adding new solute: or sample, or mixture, with a syringe.
In such apparatus, the capillary passage will usually be
about ten centimeaers long and have a capillary diameter
of between ten and one-hundred microns. The reservoirs
have small capacity so that only small sample volumes are
needed. The vohume of the reservoir, however, will be
larger than the volume of the capillary so that enough
sample will be present to fill the capillary. With such
an arrangemE~nt, any method of electrophoresis may be used.
It is not necessary to use a different apparatus for each
different method of electrophoresis.
A method of the invention allows electrophoretic
separation, particularly using the isoelectric focusing
technique, for various sample components not otherwise
subject to such separation. The method involves selecting
a reagent capable of separation and having a high degree
of specificity for the component to be detected. The
reagent is .added to the sample, preferably in an amount
sufficient t:o inte:ract with all of the sample component to
be detected that might be in the sample. The reagent has
its own isoe~lectr:ic point and the product of reagent and
component has a different isoelectric point. Thus, with
isoelectric focussing, the reagent in the sample, and the
product of reagent: and component in the sample, will each
WO 93/14389 r PCT/US93/00234
212'797 l 10
be separated to form different bands in the sample which
can be detected by the detector of the invention.
The Drawings
The best mode presently contemplated for carrying out
the invention is shown in the accompanying drawings in
which:
Fig. 1 is schematic representation of a light beam
passing through a gradient;
Fig. 2, a schematic representation of a light beam
passing through a sample chamber, showing the deflection
angle produced by the presence of a refractive index
gradient in the chamber;
Fig. 3, a second schematic representation of a light
beam passing through a sample chamber with a concentration
gradient;
Fig. 4, a schematic representation of a light beam
passing through a sample chamber similar to Fig. 2, but
showing a wide beam with a gradient in part of the beam;
Fig. 5, a further schematic representation of a wide
light beam passing through a sample chamber with a
concentration gradient in a portion of the beam;
Fig. 6, a vertical section through the capillary
passage of an electrophoresis apparatus of the invention;
Fig. 7, a fragmentary horizontal section taken on the
line 7-7 of Fig. 6, but showing the light source, lenses,
and detector in top plan view;
Fig. 8 a fragmentary vertical section taken through
a capillary tube showing sample component separation
resulting from isoelectric focusing;
Fig. 9, a vertical section through a light beam
formed by the apparatus of the invention;
Fig. 10, a fragmentary vertical section taken on the
line 10-10 of Fig. 7;
Fig. 11, a fragmentary top plan view of an alternate
detector of the invention;
212"1977
93/14389 PCT/US93/00234
11
Fig. 12, a block diagram of a detector of the
invention;
Fig. 13, a curve representing the light intensity
profile of a probe light beam passing through a sample
separated by isoe:lectric focusing in the apparatus of the
invention a.nd produced as the output signal by a moving
detector;
Fig. 14, a .curve representing the integrals of the
curve of Fig. 13;
l0 Fig. 15, three curves representing the light
intensity profilea of a probe light beam passing through
a sample during separation by isoelectric focusing in the
apparatus c>f the invention and produced as the output
signals by a moving detector;
Fig. 16, i:hree curves representing the light
intensity profiles of a probe light beam passing through
a sample during separation by isoelectric focusing in the
apparatus o~f the invention and produced as the output
signal by a diode array detector;
Fig. :17, a fragmentary vertical section of an
alternate sample receiving reservoir usable with the
apparatus o:E Figs. 6 and 7;
Fig. 18, a schematic representation of light
intensity pnofile~s showing separations obtained through a
method of the invention;
Fig. 1.9, a curve representing the concentration
profile of a sample separated in the apparatus of the
invention a:nd mobilized to move through a detector and
produced as the output signal by the detector;
Fig. 20, curves showing the concentration gradient
profiles of the same sample separated using capillary zone
electrophorsais and moving boundary capillary
electrophoresis;
Fig. 2~., a curve showing the concentration gradient
profile for the same sample as used for Fig. 20, showing
the sample separaited by isoelectric focusing;
WO 93/14389 '~ 1 '~ ~ ~ r~ ~ PCT/US93/00234
_ 12
Fig. 22, a curve representing the light intensity
profile of a probe light beam passing through a hemoglobin
sample during separation by isoelectric focusing in the
apparatus of the invention wherein the curve represents
concentration of sample components; and
Fig. 23, a curve representing the light intensity
profile of a probe light beam passing through a hemoglobin
sample similar to that for Fig. 22, wherein the curve
represents the concentration gradients of the sample
components.
Detailed Description of the Preferred Embodiment
It is well known that light passing through a
refractive index gradient in a solution is deflected. The
physical reason for light deflection when passing through
this gradient lies in the relationship between the
refractive index and light propagation velocity.
Different parts of the light advance to a different degree
with time, which generates the phase shift. Thus, as
shown in Fig. 1, during a given time period t+dt, light at
the top of a light beam indicated by arrow 10 which is
passing through a solution with a refractive index of n+dn
will travel a distance of D1. The light at the bottom of
the light beam indicated by arrow ll which is passing
through a solution with a refractive index of n will
travel a distance D2. This results in a tilt of the light
wavefront and since light travels perpendicular to the
wavefront, the light beam is tilted as illustrated. In
Fig. 1, D2 is greater than D1 resulting in an upward tilt,
but depending upon the values of n and n+dn, the tilt
could be downward.
The light path through the refractive index gradient
can be calculated by using the Fermat principle that the
light path through the medium is such that the time
necessary for its traversal is minimum. The relationship
between the angle of deflection, 8, and the refractive
CA 02127977 2004-03-29
W0 93/14389 PCT/LIS93I00234
~3
index gradient normal t0 the light propnQation dn/dx and
path length through this gradient, D, can bs written as
tan 8 = sink (D/n)(dn/dx)~
(D/n) (dn/dx) + (dn/dx) ° (D'/na3 : ) + (dn/dx) s
' 8 (Ds/ns51 ) + .. .
where n is the refractive index of the tedium. In
situations where the sensor ox the invention will be used,
D and 8 are small. ws can then approximate:
8 = (D/n)(dn/dx)
to Fig. 2 illustrates the detection principle behind
this method. With a nonunifora distribution of a solute
in the sample ohambex shown schematically between sample
chamber walls 12 and 13 giving a sample chamber distance
D, a concentration gradient is established. This gradient
15 forces the corresponding refractive index gradient dn/dx R
(dn/dc)(da/dx), which then tilts or deflects the
propagating light beam by angle 9 ~ (b/n) (dn/dc)(dc/dx).
This deflection cat? be measured by measuring the position
of the light beam on the position detector 14. The
~o information produced during the measurewent of the
concnntration gradient relates to the universal property
of the solute -- refractive index n. Consequently, the
concentration gradient produced by any solute that has a
different n than the solvent will be detected by noting a
. 25 deflection or tilt in the light beaa.
Fig. 3 shows the same principle as Fig. 2, but
illustrates it somewhat differently. Thus, if a
concentration gradient represented by line 16 exists in a
sample in a sample chamber defined by walls 12 and 13, a
30 probe beam o! light 1~ directed through the sample will be
deflected as indicated above by an angle 6. This causes
the position of the beam to cove on the suxface pf the
' position sensor 14 as indicated above.
Fig. 4 is similnr to Fig. 2, but shows a wide light
15 bean with a concentration gradient 18 within the width of
the light bean. Thus, rather than the light beam being
uniformly deflected as shown in Figs. 1, Z, and 3, a
CA 02127977 2004-03-29
14
portion of the light beam 19, on one aide of the gradient
18, which does not pass through a gradient, passes
xtraight through the sample to detector 14. portion 20 of
the light bean passes through the gradient 18 and is
defleoted by angle A as shorn by the broken arrows, and
falls onto detector 14 partially overlapping portion 19.
Where the overlap ocGUrs on the detector, indicated ~xt 22,
the light striking detector 14 is brighter than in non
overlapping areas. Portion 21 of the light beans on the
other side of the gradient 18, again passes straight
through the sable and onto detector 14. As indicated in
Fig. 4, there will be an area Z3 Where little or no light
will fall. Thus, the single light beam after passing
through a sale with one or~aore gradients therein wi7.l
have varying intensity indicating the gradients present in
the sample. A gradient in the saapie will generally
result in a bright spot followed by a spot of very little
intensity, or, if just measuring intensity, a level
representing the light passing straight through the
ao sa~ople, an increased or positive signal (the increased
intensity), followed by decreased or negative signal (tbe
decreased intensity), followed again by the level
representing the light passing etxaight through the
sample.
.25 If the detector 14 is broken down into many small
detectors, such as an array of detectors, each detector
detecting the light from a s=ell portion of the beam, a
comparison or scanning o! the individual detectors will
produce an output signal representative of the intensity
30 of the light beam falling on the array along its length.
Alternatively, a single, small detector which detects only
a portion of the light beam could be positioned to be
roved through the beam along its width and measure the
light intensity as it is moved.
Fig. 5 ahorrvs the same principle as Fig. 4, but
relates it more directly to a s~rstem of the invention.
Thus, again, tire light beam say be considered as many
93/14389 21 ~'~ (~ ~ ~ PCT/US93/00234
parallel light rays 25. A gaussian refractive index
gradient produced by a similar gaussian concentration
gradient as, would appear in a sample in a sample chamber
is represented schematically by curve 26. Line 27
5 represents both t:he plane of the sample chamber where the
light beam passes through the sample and an axis
indicating length along the sample chamber for the
refractive index gradient curve 26. Axis n represents the
refractive index of the sample within the sample chambAr.
10 Line 28 represents the detector plane and also an axis
representing the intensity of light passing straight
through th~~ sam:ple chamber when no refractive index
. gradient is present. Curve 29 represents the intensity I
of the light striking the detector plane. When the light
15 beam passes; through the sample chamber, the individual
light rays :?5 are refracted and bent out of their original
path upon e~ncounitering the refractive index gradient 26
produced by a concentration gradient inside the sample
chamber. If the intensity of each light ray is constant,
equalling I,p, the relative changes of the light intensity
on the detector plane can be given by: .
~~_ L j ° ~ ~Ia9M~1 ~=L.d 1 den _~ 1 dn~~.
~o ° a~ ~~ dxz M~ ~
Here, x is the direction along the sample chamber, z is
the direction along the light beam, n is the refractive
index inside the sample chamber, d is the diameter ~f the
sample chamber, and L is the distance between sample
chamber and the detector plane. In this equation,
[1/n(x)] dn/dx corresponds to the light beam deflection
angle, and :is small. Its high power in the second term of
the equation can be neglected, compared with the first
term, then:
~- ~ d=n~
I° M~ dxz
which shows that the relative changes of the probe beam
intensity on the detector plane are proportional to the
WO 93/14389 PCT/US93/00234
16
second derivative of the refractive index inside the
capillary. The relationship between the magnitude of the
refractive index change and the sample's concentration is
approximately linear. Hence, the relative changes of
probe beam intensity on the detector plane are also
expected to be proportional to the second derivative of
the sample's concentration inside the capillary.
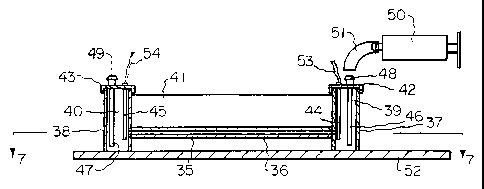
Figs. 6 and 7 show a capillary electrophoresis
apparatus of the invention. As shown, a capillary tube
35, preferably of glass, has a capillary passage 36
therein. Open containers 37 and 38, forming reservoirs 39
and 40, respectively, are secured to the ends of capillary
. tube 35 so that capillary passage 36 communicates with
reservoirs 39 and 40. The assembly is held together as a
unit by a member 41 to which the capillary tube 35 and
containers 37 and 38 are secured. If the capillary tube
is glass, it is preferred that member 41 also be glass and
that the capillary be secured thereto by epoxy so that no
refractive interface is formed between the two. The
containers 37 and 38 may be of any suitable material, such
as polyethylene, and secured to member 41 by any suitable
adhesive, such as epoxy. Containers 37 and 38 may be
provided with tops 42 and 43, respectively, which hold
electrodes 44 and 45 in position in reservoirs 39 and 40,
respectively. Tubes 46 and 47 extend through tops 42 and
43, respectively, to open near the bottom of reservoirs 39
and 40, respectively. Fittings 48 and 49 on the tops of
tubes 46 and 47 where they pass through the respective
tops are adapted to receive tubes from a source of, or
receptacle for, liquid to be added to, or withdrawn from,
the reservoir. Such means may conveniently take the form
of a syringe such as shown schematically as 50 with a tube
51 to connect it to the appropriate fitting 48 or 49. The
syringe may be manually operated or motor driven, or
various other types of pumps or delivery systems could be
used. Of course, electrodes 44 and 45 and tubes 46 and 47
could be positioned in the reservoirs by various other
_ 21279'7
J 93/14389 PCT/US93/00234
17
means and containers 37 and 38 could remain with open
tops. An advantage of providing containers 37 and 38 with
tops, and providing that the tops seal such containers, is
that the pres.:ure in the containers can then be
controlled. Thus, a syringe or other delivery system can
be used to pressurize a reservoir to force liquid into the
capillary passage, or can be used to draw a partial vacuum
in a reservoir to draw liquid from the opposite reservoir
into the capillary passage. The unit described may be
mounted on a base 52, if desired.
For tlae unit described, the capillary tube may vary
in length as dE~sired, but tube lengths between three
centimeter:a and fifteen centimeters have been found
satisfactory for various types of capillary electrophesis.
Further, the diameter of the capillary passage may also
vary as de:aired, with diameters of between 10 ~cm and 100
~cm having been found satisfactory. Either round or square
capillary passages may be used, but square passages have
been found particularly suitable for use with the detector
and detection mEahod of the invention. When a square
capillary i.s used, diameter of the capillary refers to the
length of a side of the square. The size of the reservoir
is not critical, although the volume of a reservoir must
be large enough so that the capillary passage can be
filled by the particular method being used to fill the
capillary. In addition, the electrodes must be in contact
with liquid in t:he reservoir. Thus, the volume of the
reservoirs will generally be larger than the volume of the
capillary passage:extending between them. For example, in
the config~u.ration shown in Figs. 6 and 7, reservoirs each
having a volume of about 0.2 milliliters has been found
satisfactory for various size capillary passages, such as
a fifteen centimeaer long passage of 20 ~,m diameter.
The electrodles 44 and 45 are connected to a source of
high voltage (not shown) by wires 53 and 54, respectively.
The voltage: will preferably be in the range of between 5
KV and 10 KV, depending upon the type of separation being
WO 93/14389 ~ PCT/US93/00234
2,12 9~
- 18
used. Generally, higher voltages will result in faster
separation times, but the voltage is limited by the
current flow through the sample in the capillary passage
which generates heat in the sample. The heat generation
must be kept below the level of heat that is readily
dissipated through the capillary tube or the capillary
tube may explode. The curtent flow is generally monitored
by monitoring current flow in an electrode, generally the
current flowing from the cathode. Such monitoring may be
with an ampmeter, not shown.
The electrophoresis apparatus shown allows
electrophoretic separation using all of the various known
electrophoresis separation techniques. These techniques
are capillary zone electrophoresis, moving boundary
capillary electrophoresis, capillary isotachophoresis, and
capillary isoelectric focusing. While with current
electrophoresis equipment, separate equipment is needed
for each technique, the apparatus described is truly
universal in that any of the techniques can be practiced.
This is an advantage because in some instances, it may be
advantageous to evaluate -a sample using two or more of the
methods since different methods rely on different
properties of the sample. For example, capillary zone
electrophoresis and moving boundary capillary
electrophoresis separate sample components based on
differences in the mobility of each component.
Isoelectric focusing separates components based on the
different isoelectric point of each component.
Capillary zone electrophoresis generally will require
only three steps using the apparatus of the invention.
The first is filling the capillary passage with buffer
solution. Generally, both reservoirs will be filled with
buffer solution. The capillary passage can be filled
hydrodynamically by filling one reservoir to a higher
level than the other and allowing the buffer from the
higher filled reservoir to run into the other reservoir
allowing their levels to equalize. Alternatively, if a
ry ) 93/14389 - ~ ~ ~ ~ ~ ~ ~ PCT/US93/00234
19
reservoir is fully enclosed, the reservoir can be
pressurized) to cause the buffer to flow into the capillary
passage. With buffer in both the reservoir and the
capillary passage, the buffer is then removed from the
anodic reservoir, i.e., the reservoir having the anode or
positive e7.ectrode therein, and is replaced with sample
solution. The high voltage is then turned on and the
sample is drawn into the capillary electrokinetically,
i.e., by action of the voltage across the capillary
passage. The length of the sample plug drawn into the
capillary i.s controlled by the time the sample solution
remains in the reservoir and the voltage across the
electrodes. When the desired sample plug is introduced
into the capillary passage, the sample solution is removed
from the reservoir and buffer returned to the reservoir.
The voltage remains across the electrodes. Sample
separation and movement through the capillary passage
continues under t:he influence of the voltage. After the
sample has moved through the passage, the voltage can be
disconnected.
Operation in the moving boundary capillary
electrophoresis mode is similar. Initially, both
reservoirs and the capillary are filled with buffer. In
this method, the high voltage can be applied at this
point. Th.e buffer is then removed from the anodic
reservoir a:nd replaced with sample solution. The sample
solution is drawn into the capillary passage
electrokinetically. When the desired amount of sample is
present in 'the capillary passage, the sample solution is
withdrawn from the reservoir and the buffer again placed
in the reservoir. This whole process is easily automated
by using two syringe pumps controlled by a computer. Each
pump could have its own tube, equivalent to tube 46 shown
in Figs. 6 and 7, extending into the reservoir, or the
pumps could be va:lved through an automatically controlled
valve to a :jingle tube in the reservoir.
WO 93/ 14389 ~ ~ ~ ~ ~ ~ ~ PCT/US93/00234
With capillary isotachophoresis, a first or leading
electrolyte is introduced hydrodynamically into the
capillary passage. This is followed by hydrodynamic
introduction of a sample plug into the capillary passage.
5 This would be achieved by removing the leading electrolyte
from the anodic reservoir and filling the reservoir with
sample to a higher level than the liquid in the cathodic
reservoir. When the desired sample plug flows into the
capillary passage, the sample is removed and the second or
10 tailing electrolyte is introduced into the anodic
reservoir and into the capillary passage. Thus, the
capillary passage includes a sample plug between leading
and tailing electrolytes. The high voltage is then
connected between the electrodes to perform the
15 separation.
With each of the three separation modes described
above, i.e., capillary zone electrophoresis, moving
boundary capillary electrophoresis, and capillary
isotachophoresis, as the separation takes place, the
20 sample naturally migrates through the capillary passage
from the anodic end to the cathodic end.
For capillary isoelectric focusing using the
described apparatus, the sample, which includes the sample
to be separated as well as carrier ampholytes, is
introduced into the capillary passage. This may be done
hydrodynamically or by placing the sample solution under
pressure in one reservoir. After the sample is introduced
into the passage, the anodic reservoir is filled with an
analyte and the cathodic reservoir filled with a
catholyte. The high do voltage is then connected. Under
the influence of the electric f field, the ampholytes are
arranged by their isoelectric points in order of
increasing isoelectric points from anode to cathode. The
sample components will migrate to the point in the
capillary passage where their isoelectric points are
equivalent to the pH established by the ampholytes, to
create narrow zones of the sample components. As shown in
2 ~ 2 7 9 7 7 PCT/US93/00234
J 93/14389
21
Fig. 8 components 55a, 55b, 55c are focused into scarp
narrow zones in the solute solution 55d within the
capillary passage of capillary tube 56. When separation
is complete, the separation remains as long as the voltage
remains. The separation process can be monitored by
monitoring the current f low through the capillary passage .
The current flow will reach a minimum and remain stable at
that minimum when separation has been achieved. The
actual separation will take place usually in about four to
seven minutes.
As indicated, the isoelectric focusing separates the
sample components into very narrow bands. These narrow
bands forms very high concentration gradients at their
boundaries. Thus, the isoelectric focusing forms very
high concentration gradients in the capillary even for low
concentrations of sample components. The detection of
concentration gradients, rather than detection of
concentration, is uniquely applicable to isoelectric
focussing and re:~ults in high sensitivity of detection and
high resolution" The ratio of the sensitivities of
detectors based on concentration gradient detection and
detectors based on concentration detection may be
expressed as a function of the zone or boundary width a'x:
where Q'x is the standard deviation of the concentration
distribution in the zone or boundary which is Gaussian.
The equation predicts that the sensitivity of a
concentration gradient detector increases more quickly
with a decreasing zone width than does the sensitivity of
detectors based on concentration detection, and that the
concentration gradient detector is more suitable than the
detectors x>ased on concentration detection for isoelectric
focusing 'which has self-concentration and focusing
properties ,.
WO 93/14389 ~ 1 ~ ~ ~ ~ PCT/US93/00234
22
If it is desired to mobilize the sample as focused in
the capillary passage, the catholyte is withdrawn from the
cathodic reservoir and replaced with a solution of
different pH. This causes the separated sample to migrate
through the passage.
Various concentration gradient detectors can be used
with the electrophoresis apparatus described. A single
light beam focused through the capillary passage at one
end of the passage to measure the deflection of the light
beam as the concentration gradients caused by the
electrophoretic separation pass through the light beam has
been found satisfactory and to have much better resolution
than other prior art detectors. This is because the
concentration gradient rather than merely concentration is
being measured. However, a detector which can measure the
separations along the length of the relevant portion of
the capillary passage and does not require movement or
migration of the separated sample in the capillary passage
is preferred for detection when using isoelectric focusing
which sets up a stationary separation in the passage since
such detector does not require mobilization of the
separated sample. By detecting a sample separated by
isoelectric focusing without having to mobilize the
separated sample, the results are obtained much more
quickly. While the focusing itself generally takes
between about four and seven minutes, the mobilization
usually takes between an additional fifteen to forty-five
minutes. By monitoring and detecting the stationary
sample, results are obtained as rapidly as the focusing
occurs. Further, excellent high resolution is maintained
between sample components because this resolution is not
lost or distorted through mobilization.
The basic concept of the detectors of the invention
is to focus a light beam which comprehends the entire
length of interest of the capillary tube in which
separations occur onto the capillary tube so that it
passes through the sample along the length of the sample
CA 02127977 2004-03-29
23
oontaining the separations of interest. This may range
frog a two cm le»gth along the capillary tube to ten or
mots eenti~ters. The intensity of the beam after passing
through the sample is sensed or detected in a way that the
variations in intensity along the width o! the bees which
has pas~xed through the desired length of sample is
determined. Thus, the width of tare beam has to be broken
down into iany wall segments, each indfvidnally sensed or
detected, in order to provide the desired output. In
io , preferred forms of the imertion, a wide but thin light
beam 59, Fig. 9 is generated and Focused onto the
capillary passage so that the vtidth of the bsal~ w will
. coaprehend the length along the capillary passage where
separations of interest have taken place, and so that the
height of the beam h is preferably smaller than the
diaaeter o! the capillary passage. Thus, the height h of
the bsato shown in Fig. 9 is somewhat exaggerated !or
purposes of illustration. There are several ways such a
light beam say be generated including the use of lenses
30 and the use of masks, i.s., an opaque sheet o! material
with a slit tdsrsirv. Further, the light !or the light
bsaa say originate from a laser, a light cititting diode
(LED), a laser diode, or an LED or laser diode array.
Preferred detectors of the invention are shown in
,25 pig's. ?, and 10-1~. As shown, a light source 60, such as
a lasex, or a laser diode, with appropriate lenses as
needed, is arranged to direct a light beam sl, of circular
cross section, toward capillary passage 36. ~ cylindrical
lens s2 in the path of light beam 61 focuses the circular
9o beau 61 into a thin bean or sheet of light 63 with a width
apprvxi.~sataly equal to the width or the circular bean.
This sheet of light is aligned~with and focused onto
capillary passage 36. I! the width of the beaa is shorter
than the entire length of the capillary passage, the beau
35 is set to pass through the length of the passage where it
is expected that separations of interest will take place.
The beam 63 ritay pass directly through the capillary
CA 02127977 2004-03-29
passage to a detector, or tray peas through the
capillary
passage to lens 64 vJhich expand the beam 63 before
it
reaabes the detector. The expanded laeam is labeled
65.
Such expansion can provide increased resolution
of
detection, particularly where the inareaents of
detection
are not as saall as desired.
J The preferred form of the invetion utilises two
alternate detecting or sensing techniqaea. one is
single detector, such as photodiode 66, ~oounted
to be
aoved linearly along the width of bean 65 to sense
the
inten*ity of light in light beaa 65 as a function
of the
linear position of photodiode 66. Since the light
beam
being sensed is very thin and the changes in intensity
occur in substantially a single dirsction, i.e.;
along the
. 15 width of the bear, accurate alignment of photodiode
66
i
with the beam from the standpoint of height of the
beam is
1 not necessary.
?hatodiode 66 may be moved along the width of beala
s3
in a variety of ways, and the way moveaent is obtained
is
o not critical. llny apparatus for moving the photodiode
say
be used as long ag the linear position of the diode
can be
kept track of. This may merely be a means that once
started, will ~rnrs~thraugh tire entire length o!
travel of
' interest at a constant rate. An sxa~ple of apparatus
for
" 25 moving diode 6s is shown in Figs. ? and s0. a track
70 is
mounted on base 52 arid receives slidably therein
a
carriage aaseably ?1. Carriage asseably ?1 includes
Teflone ar similar plastic bearinge~ ?2 which ride
against
track 70 to reducs friction and a threaded aeaber
73 which
30 accepts a threaded shaft 74 therethrough. ~1 platform
?5
with bracket 76 and photodiade 65 haunted thereon
is
seaurad to carriage 91. Shaft ~4, Fig. ?, is coupled
through sleeve ?e to the output shaft ?9 of stepper
motor
80. As threaded shaft 74 is rotated by stepper rotor
80,
3S carriage 71, platform 95, and photodiode 66 move
along
track ?o. The direction of travel depends upon the
direction of rotation of the stepper motor at~d
the speed
PCT/US93/00234
93/14389
of travel depends upon the speed of rotation of the
stepper motor. The speed of rotation, direction, and
amount of rotation of a stepper motor may be accurately
controlled in known manner so the rotation of the motor
5 and travel of photodiode 66 through the width of light
beam 65 can be accurately monitored and controlled.
During a scan of photodiode 66 along the width of light
beam 65, photodiode 66 will produce an output signal
proportiona:L to the light intensity of beam 65 striking
10 photodiode 66 and, thus, a signal proportional to the
light inten:aity air all points in the light beam along the
path of travel of the photodiode. If it is desired to
.further limit tlhe width of the segment sensed by
photodiode 66, a shield 81 may be mounted in front of
15 photodiode E>6 with a slit 82, Fig. 7, therein of desired
width. The output. of the photodiode is through leads 83.
The detector. electronics used would be standard and well
known.
An alternate detector is shown in Fig. 11 and
20 replaces the:moving photodiode detector of Figs. 7 and 10.
The detector- of F:ig. 11 is a photodiode array 85 mounted
in the path of light beam 63 after passage through the
sample or in the path of expanded light beam 65. Many one
dimensional or two dimensional photodiode arrays could be
.25 used, with the cost of the array balanced against the
desired resolution. The preferred arrays have very small
sensing elements spaced along the width of the array. For
example, arrays o~° charge coupled devices which are used
in television and video cameras having sensing elements
one one-thousandth of an inch in size are readily
available, but thE: larger of such arrays and arrays of a
grade with all elements working, are expensive. Arrays
with even ::mallet sensing elements are also becoming
available. Such .arrays may be obtained with as many as
1024 elementa in one dimension. Thus, the width of the
array and the width of the light beam comprehended thereby
would be broken down into 1024 individually sensed
WO 93/14389 PCT/US93/00234
26
~.~o~~~~ns. The electronics for scanning an array are
standard and well known. The connections between the
electronics and the array are through wires represented
schematically as 86.
An overall block diagram of a detector system is
shown in Fig. 12. The light source is represented by
block 90 and includes the necessary components, such as a
laser and lenses, to produce the necessary sheet of light,
indicated by broken line 91. Light beam 91 passes through
the capillary sample chamber 92 to the detector 93 which
measures the intensity profile of the beam after passing
through the sample. The output of the detector goes to
any necessary interface electronics 94 which processes the
signals from the detector and sends them to computer 95.
Signals from the computer needed to control the detector,
such as scanning signals or motor control signals, or
other control signals, are sent from computer 95, through
interface electronics 94, to detector 93. With such a
system, the intensity information obtained can be
displayed in real time on the computer monitor and stored
in memory for later display or processing. The computer
95 can also operate through interface electronics 96 to
operate any reagent pumps, voltage supplies, or other
equipment, indicated as block 97, to automate the
. 25 capillary electrophoresis separations conducted in the
capillary sample passage. This can allow coordination
between the conditions of the capillary electrophoresis
taking place in the sample chamber based upon the detected
results.
In a prototype of the detector, a 100 ~cm diameter,
6.5 cm long square capillary passage was used in the
apparatus shown for the isoelectric focusing. The
capillary tube was glass and was glued with epoxy between
two glass slides. The capillary passage walls were coated
with non-crosslinked acrylamide to eliminate
electroosmosis. The containers forming the reservoirs
were polyethylene. The apparatus was mounted on a two-
CA 02127977 2004-03-29
WO 93114389 PCT/US93100Z34
Z7
axis stage so the tilt angles in the borisental and
vertical planes were adjustable to aid in alignment of the
probe light beam with the capillary passage. A Ha-Ne
laser manufactured by UniphaseT" of Sea Jose, California was
toted to generate the probe light beam. The bean from the
laser was expanded to a 2 cn diameter beam and was then
focused into the capillary passage by a 6 am fooal length
aylindrival lens mounted on a three-axis stage. The
cylindrical lens produced a sheet of light of about x om
1o in width. After passage through the capillary passage,
the 2 ant beam was expanded to a ZO cm beam in the detector
plane by a 25 mat local length lens mounted behind the
capillaxy tube. In,thist way, 1 cm width in the detector
plane corresponded to a 1 mm length of ,the capillary
passage. This sakes it easier to aaasurs the intensity
profile of the bean. In so~te experiments, a single
photodfade with a shield with 0.1 bm slit therein was
mounted on a one-axis stage driven by the aoving part o!
a mode 3418 syringe pump trade by 4rion ~tessarah, Inc. of
Massachusetts. This was located so the photodiode scanned
in the detector plane. The scanning distance of the
photodiade so mounted vas about 150 mat which corresponded
to a 15 man length of the cetpillary passage. In other
experi.msnts, a one-dimensional, 128 element photodiode
a5 array was used to atonitor the intensity a! the beam in the
detector plane. This array was able to monitor a 3 MAm
length of the capillary passage. The whole system was
mounted on a vibration isolation table. The data obtained
by the detectors was collected through an IBM''" DACA board
in a PC-AT personal computer, using the ASYSTm software
supplied by Asyst Software Technology, Znc., Rochester,
New York.
~In the tests of the systems, all chemicals ears
reagent grads, and solutions were prepared using deionized
eater. 10 mM HzPO, and 20 m!1 HaON were used as the anolyte
and catholyte, respectively. NaOIM solution Bras degassed
before use, by sparging with helium. Samples used include
CA 02127977 2004-03-29
WO 9x111389 ~YUS93/007,34
28
~~-chymotrypsin (tYPe =I, Sigma), phosphorylase b (Sigma)
and ovalbumin (grade V, Sigma). semplss were ~al.xed with
ampholyte (pharmalyte pN 3 - io, sierra) solution Ior a
final concentration o! 2t nmpholyte. solutions ware
filtered using o.2 pm pore sire cellulose acetate filters
(Sartorius'~', Gottingen, Germany). The sample concentration
introduced into the capillary ranged from 0.3sg/mL to 1
The sample was introduced into the capillary passage
1o by pressure generated by a syringe. A plug of 1x agarose
gol i» the reservoir of the anadic end of capillary
(prepared in the anolyte, 10 mM H~p04) was used to avoid
hydrodynamic flow in the 100 um i.d. capillary. After
introduction of the sample into the capillary passage, a
5 1cV do voltage was applied arid current passing through
the capillazy was monitored to follow the focusing
process. Typically, the current dropped from 15 ~cA to
about 2.5 ~cA in 4-~ min, and then became stable for hours.
this minimum current flow indicates the isoelectric
focusing process has been completed.
Fig. 13 shorts the beau intensity profile detected by
the single photodiode scanned across the probe beam which
passes through the part of the capillary located 3.5 - 5
- cm distance from the anodic end. Two sharp, high peaks
100 dad 101 are observed in the probe beam intensity
profile shown in Fig. 13, which correspond to the
positions expecteA fox the focused phosphorylase b
(isoelectric point 6.3), and ovalbumin (isoeleCtric point
4.7), respectively. The concentrations of analytes are
3D about 1 mg/mL each. This result demonstrates that the
focused proteins inside the capillary can be detected by
this simple imaging system. The signal peak 100
corresponding to focused zone of phosphoryla$e b and its
integrals are illustrated in Fig, i4, which clearly shows
the second derivative characteristic of the detected
signal loo. since this imaging system is an on-line
detector, the isoelectric focusing process itself can be
O 93/14389 _ 212 7 9 7 7 PCT/US93/00234
29
monitored. Fig. 15 shows the focusing process of
phosphorylase b and ovalbumin. The concentrations of the
samples area 0.5 ~mg/mL, which corresponds to 3.4 pmole of
phosphorylase b and 7.2 pmole ovalbumin injected into the
6.5 cm lone square capillary. At the beginning (0 Min.)
of the focusing, as shown by curve a in Fig. 15, no sharp
peaks are observed. The detected signals are the probe
beam intensity profile after it passes through the
capillary. Many low peaks in Fig. 15 are generated by
refractive index defects in the capillary wall or coating
materials i.n the inner wall of the capillary, and their
positions do not. change with the time, which can be
observed in. curves .a, b, and c of Fig. 15. In curves b
and c, two aecond derivative peaks 102 and 103 appear and
become higher with longer focusing time, which correspond
to focused ;phosphorylase b and ovalbumin. In addition to
these two high peaks, other small peaks can be observed
and become higher with the time in the curves b and c of
Fig. 15. Those peaks are associated with the minor
components in the: samples. It should be mentioned that
the concentration gradients generated by the components of
carrier ampholytea can also be detected because of the
universal nature of the detector. The refractive index
fluctuations produced by the carrier ampholytes can be
noticed in 'the integral of the detected signals shown in
Fig. 14. lHowever the second derivative nature of the
imaging detector effectively reduces the amplitudes of low
frequency broad signals generated by the wide bands of the
carrier ampholytea. As shown in Fig. 13, high signal
peaks can only be observed for high concentration
gradients produced at the boundaries of narrow protein
zones.
Figs. 7.3 and 15 were obtained with the moving single
photodiode detector. Fig. 16 shows the focusing process
for a «-chymotrypsin sample and covers a 3 mm length of
the capillary passage. The curves of Fig. 16 were
obtained using the 128 element linear photodiode array
WO 93/14389 212 g~'~ PCT/US93/00234
described above. This clearly shows the practicality of
using sensor arrays in the detector of the invention.
Further, the results obtained show that the detectors of
the invention not only detect the focused analytes in the
5 sample, but can also be used for monitoring and studying
the dynamics of the isoelectric focusing process inside
the capillary passage.
Fig. 17 shows a second embodiment of a sample chamber
for the electrophoresis apparatus of the invention. Some
10 sample solutions, such as blood serum samples, contain a
high level of salts. This makes the sample have a high
degree of electrical conductivity. If such a sample was
introduced directly into a capillary passage, the voltage
applied for the separation would cause excessive current
15 flow through the sample, excessive heating, and could
result in explosion of the capillary tube. Thus,
preparation of a sample to remove the salts therefrom is
necessary prior to subjecting it to capillary
electrophoresis separation. The apparatus shown in Fig.
20 17 can be used to prepare the sample for electrophoresis
as part of the overall procedure.
The modification of the apparatus as shown in Fig. 17
is to provide a container 120 forming the sample reservoir
121 inside a second container 122. Sample container 120
25 is made of a porous membrane material such as a cellulose
acetate membrane material. Alternately, container 120
could be a combination of porous membrane material and
other material, such, for example, as a polyethylene
container with a porous membrane bottom. Container 122 is
30 made of normal non-porous material such as polyethylene or
glass. The capillary tube 123 passes through the wall of
container 122 in a sealed manner and is secured in sample
container 120 so that capillary passage 124 communicates
with sample reservoir 121. Generally, the illustrated
construction will be needed for only one of the reservoirs
as shown in the apparatus of Fig. 6, usually the anodic
reservoir. The sample 125 is introduced into sample
7 93/14389 ~ , ~- ~ 1,~,; PCT/US93/00234
reservoir 1.21, but will not initially flow into capillary
tube 124. Water, which may also contain the desired
ampholytes, is placed as solution 126 in container 122 so
as to surround a portion of container 120. It is
preferable to continually flow the water-ampholytes
solution through container 122 so for that purpose an
inlet tube 127 f~.~om a source of water-ampholyte solution
may be provided to introduce such solution to container
122 while an outlet 128 may be provided so that solution
may flow from container 122. With sample 125 in sample
reservoir 7.21, the salts therein will pass through the
porous membrane into the water solution 126 in container
122. Simultaneously, if the water also contains
ampholytes, the ampholytes will pass through the membrane
into the sample. Thus, the sample can be prepared by the
removal of salts and the addition of ampholytes while in
the sample reservoir. The sample 125 will remain in
sample reservoir 121 for the time required to exchange the
salts from the sample to the water through the membrane,
and to exchange the ampholytes from the water solution to
the sample. When the sample is properly prepared, the
sample will be moved into the capillary passage such as
through pre:asurizing the sample reservoir 121. The sample
reservoir will include a sealed top 129 to allow
pressurization oi: the reservoir to cause the sample to
flow into the capillary passage, the electrode 130, and
tube 131, a;s shown in the apparatus of Fig. 6.
The invention also includes a method of determining
the presence or concentration of a particular component
that does not have an isoelectric point so would not
normally be focused and detected by capillary isoelectric
focusing. The method includes the steps of adding a
reagent hav~.ng a urell-def fined isoelectric point and having
a high degree of specificity toward the component to be
detected to a sample which may have the component therein,
under conditions where the reagent and component will form
a combination or complex of the two which will have an
'~~,~ ~~~~~
", _ , ,
- , PGTiU ~ 9 3 / 0 0 2 3 4
03 Recd ~'~t/Ft~ 0 Z ~,U~ 1993.
32
isoelectric point different from the isoelectric point of
the reagent itself, and then detecting the presence of the
complex through iaoelectric focusing.
The meahod is useful in instances where it is
desirable to detect the presence of a component, for
example a toxin, in a sample, such as a body fluid, but
the toxin may not have an isoelectric point so would not
normally bas separated and detected by isoelectric
focusing. In the method of the inventi~, a reagent
having a high dE:gree of specificity toward a target
component or analyte is used. The reagent is a substance
which has a well-defined isoelectric point and forms a
sharp band inside the capillary which may be easily
detected through capillary isoelectric focusing and the
detectors of the invention as a second derivative of the
Gaussian. This :is shown by signal 135 in the curves
labeled a, b, and .c in Fig. 18. The reagent, for example,
may be proteain which is an antibody for the toxin being
looked for o~r could be a computer designed and laboratory
synthesized organic molecule, for example, a synthetic
cavity ligand. The component being looked for can be a
toxin or other mo7.ecule which generally will not have
an
isoelectric point:. The reagent, R, is picked to
specifically and strongly interact or react chemically
with the target component or analyte A, to form, for
example, the: product:
R + A = RA
The product of the: chemical process, RA, has a different
isoelectric point and therefore focuses in a different
part of the capillary then the reagent R. Thus, the
product is i.ndicat:ed as peak 1-36 in curve b of Fig.
18.
In addition, the hE:ight of the corresponding signal 136
is
proportionally related to the amount of the target
component present. The reagent can also be ~ie~= .-ned
to
react with several target analytes to produce several
different products. In such instance, several
corresponding signals 136, 137, and 138 may be obtained
as
SUBSTITUTE SHEET
.. 212977 ~ _ .. .
pCT~~.~~ 93 /00 23~+
33 03 Rec'c~ ~'~fT/~ '~ 0 2 ~~.~JG 1995
shown in curve c of Fig. 18. It will usually be desirable
to ensure that there is an excess of reagent added to the
sample so that all. of the component present in the sample
will react with reagent. In this way, the signal
representing the component-reagent product, i.e., peaks
136, I37, or :138, will be proportional to the
concentration of component in the sample. The presence of
a peak 135 for the reagent as well as a peak for the
product will. indicate this excess.
l0 Because: reagents having very high specificity for
components of a sample being looked for can be produced
to
therefore provide accurate detection and concentration
information, the method described using the simple and
relatively inexpensive detector of the invention has the
potential to replace many tedious analytical procedures
and expensive instrumentation.
As mentioned previously, the electrophoresis
separating apparatus of the invention could be used with
a detector ;simila:r to those shown in my prior patents
having a beam located at one end of the capillary passage
and mobilizing 'the sample separation obtained by
isoelectric focusing so that it flows through the
capillary and the: detector beam. Fig. 19 shows the
results obtained using capillary electrophoresis apparatus
similar to that shown but with a laser diode as the light
source for a light beam passed through one end of a 20 ~,m
capillary passage in a 12 cm long capillary tube. The
sample used contained 120 fmol of human hemoglobin
indicated by peak 140, myoglobin indicated by peak 141,
270 fmol of human carbonic anhydrase indicated by peak
142, 240 fmo~l of bovine carbonic anhydrase indicated by
peak 143, and 410 fmol of 8-lactoglobulin indicated by
peak 144. The con<:entration of the sample was 0.2 mg/mL.
Figs. 20 and ai show the difference in separation of
a sample b:y capillary zone electrophoresis, moving
boundary capillary electrophoresis, and isoelectric
focusing. :Figs. 20 and 21 show electropherograms of
~sUBSTITUT~ SHEE1°
CA 02127977 2004-03-29
WO 93114389 PC'F/US91l90I34
34
avalbu~ain separated by the three separation methods. The
ovalbumin was purified by the manufacturer with slab cone
electrophoresis whiatr is based ot~ a sample s mobility
differences. l~s expected, tt~e electropherogram of
capillary zone electrophoresis separation, curve a in Fig.
20, shows only one peak, 150. The elsctropherogram of
moving boundary capillary electrophoresis separation,
curve b in Pig. 20, also shaves one peak, lxl, since it is
based on the same separation principle, a difference in
component mobility, as capillary zone electrophoresis.
However, the electropharogra~a of the same sa~rple separated
by isoelectric focusing, Pig. 21, shows more than four
peeks. The highest peak 15Z, corresponds to ovalbumin,
and other peaks can be attributed to impurities or minor
components in the sample which have almost the same
mobilities as that of ovalbumin, but different iroelectric
points from that of avalbumin. xhe electropharagrams or
curves shown were obtained using the apparatus with
detector at the end thereof and mobilizing the sample
2o separation obtained by isoslectric focusing. This shows
the significant difference and improvement in separation
and sensitivity obtained by using isoelectric focusing.
The detector of the invention cnn also be used as an
absorbanca imaging detector to detect concentration of
separated sample components directly rather than x~easuring
concentration gradients. The only difference in such
case, is the nature of the light beam which provides light
of frequencies to be absorbed by sample components. The
light beam may be generated by ttn incoherent light source
such as a halogen lamp or may be generated by a laser ox
variable frequency laser. If from an incoherent light
source the light is preferably filtered to limit it to the
desired frequencies. The components o! the sample as
separated along the capillary passage will absorb some of
the light so the light intRnslty will vary along the width
of the light beam. The detector, as indicated above,
2127977
J 93/14389 PCT/US93/00234
detects the variation in light intensity along the width
of the light beam.
In an example of the absorbance imaging detector, the
light beam source was a halogen lamp. The light from the
5 lamp was collected by a paraboloidal ref lector which
reflected light onto a mirror. The light beam was
filtered by a color filter which was transparent in 400 nm
to 600 nm wavele:ngth range. The light beam was then
focused into a 200 ~m wide slit which was focused onto a
10 200 ~cm dia.meter capillary tube. The image of the
capillary illuminated by the light beam was projected by
a 10 cm focal length lens onto a 1024 pixel or sensing
element charge-coupled device (CCD) such as a S3903-1024Q
made by Hamamatsu,, Hamamatsu City, Japan, which had a 25-
15 mm X 0.5-mm sensing area. The changes in the light beam
intensity profile due to the refractive gradient inside
the capillary was eliminated by focusing the image of the
capillary onto the. detector plane, i.e., the sensor. The
data was collected by an IBM DACA board, in a PC-AT
20 personal computer. An averaging method was applied to
reduce the :random noise. - For each measurement, the CCD
was scanned ten times in 1 second and these scans were
averaged. The background image which was recorded before
the focusing voltage was turned on, was first subtracted
25 from the sample images, and the sample images were then
normalized by 'the background image to eliminate
fluctuation: created by the source beam intensity
distribution. ~, human hemoglobin sample from Sigma
Chemical Co., containing 75% methemoglobin, balanced
30 primarily 'with oxyhemoglobin, was separated using
isoelectric focusing. The electropherogram obtained is
shown in Fig. 22. Peaks 160 and 161 correspond to two
variants of hemoglobin; methemoglobin (pl 7.2, comprises
75% of the ;sample) and oxyhemoglobin (pl 7.0, comprises
35 less than 2_°°>% of 'the sample), respectively. Other peaks
may correspond to other variants of hemoglobin. The
CA 02127977 2004-03-29
1Ab 93114989
PCT/US93/ODZ~t
36
r~ssulta show effective detection through the absorbance
.
detection.
Figure Z3 shows the eleatropherogram of the sa~ae
hemoglobin sample es tbat in ~'ig. Z2, which is
focused in
a 100 pm diameter capillary and detected using
concentxatioa gradient iaaging. The resolution
of the
concentration gradient imaging shown in Fig. 23
is better
than that of the absorbance imaging due to the
second
derivative nature of the concentration gradient
imaging
l0 which eliminates broad bands and fluctuations.
Further,
when using an incoharet~t light source for the
absorbance
. detection, use of narrow capillaries is limited.
For
absorbance imaging using narr~ capillaries, such
ae
capillaries with loo ~m diaaater or lass, an adjustable
i5 wavelength laser is necessary, and !or use in detecting
proteins, an adjustable wavelength laser working
in the W
range is necessary since most proteins only have
ahsorbanca bands in the tN range. Thus, the concentration
gxadient imaging appears most practical fax small
diaseter
Zo aapillsry tubes. However, for larger diameter capi7.lary
tubes, the absorbance imaging can produce excalient
results.
iihereas this invention is here illustrated and
described with reference to e~obodimeats thereof
presently
Z5 contemplated as the best mode of carrying out such
invention in actual practice, it is to be understood
that
various changes clay be made in adapting the invention
to
different embodiments without departing from the
broader
inventive concepts disclosed herein and comprehended
by
3Q the claims that follow.