Note: Descriptions are shown in the official language in which they were submitted.
-\
:1 ~ ~ ~ ,r1!
v.~U~) d
°..'
- 1 -
TRIAZOLOPYRIMIDIN DERIVATTVES AS ANGTOTENSIN II
RECEPTOR ANTAGONISTS
05 ..
The present invention relates, by way of novel
products, to the triazolopyrimidine derivatives of
general formula (I) below and their tautomeric forms
and, where appropriate, their addition salts, in
particular the pharmaceutically acceptable addition
salts.
The compounds in question have a very valuable
pharmacological profile insofar as they possess anta-
gonistic properties towards the angiotensin II recep-
tors, and antiproliferative properties. They are
therefore particularly indicated for the treatment and
prevention of cardiovascular diseases and in particular
for the treatment of hypertension, for the treatment of
cardiac insufficiency and for the treatment and preven-
tion of diseases of the arterial wall, especially
atherosclerosis.
The present invention further relates to the
method of preparing said products and to their uses in
therapeutics.
These triazolopyrimidine derivatives and their
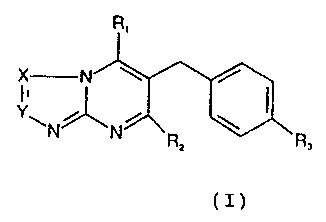
tautomeric forms have general formula (I):
R
X N \
Yw
R ~R~
x
Formula (I)
In formula ( T )
i ~7 1~
- 2 - ~ ~. r-,~ ~.~ u; ~ ~~l
- one of the radicals R1 and R2 is
- a lower alkyl radical having 1 to 6 carbon
atoms;
- an ether .radical of the formula -(CH2)pOR, in
05 which p is an integer from 1 to 6 and R is a lower
alkyl radical having 1 to 6 carbon atoms or a benzyl
radical; or
- an alcohol radical of the formula -(CHZ)pOH,
in which p is as defined above; and
- the other radical R1 or R2 is
- the hydrogen atom;
- a halogen atom;
- a lower alkyl radical having 1 to 6 carbon
atoms: or
- a radical selected from the group comprising
the radicals N3, ORQ, SR4, NR5R6 and NH(CH2)n-NRSR6,
in which:
RQ is
- the hydrogen atom;
- a lower alkyl radical having 1 to 6 carbon
atoms or a C3-C.,-cycloalkyl radical;
- a radical (CH2)m COOR', m being an integer
from 1 to 4 and R' being the hydrogen atom or a lower
alkyl radical having 1 to 6 carbon atoms; or
- a radical ( CH2 ) m-O-R' , m and R' being as
defined above;
R5 and R6, which are identical or different,
are
- the hydrogen atom: or
- a lower alkyl radical having 1 to 6 carbon
atoms or a C3-C.,-cycloalkyl radical; or
R5 and R6, taken together with the nitrogen
atom to wl2:ich they are attached, form a heterocycle
selected from morpholine, pyrrolidine or piperidine;
and
:'\
,n
~ ~ ~ ~ c~ '.)
- 3 -
n is an integer from 1 to 4:
- X and Y, which are different, are
- in one case the nitrogen atom: and
- in the other case a group C-R." in which R
05 is
- the hydrogen atom;
- a Lower alkyl radical having 1 to 6 carbon
atoms or a C3-C~-cycloalkyl radical;
- a radical (CHZ)n~OH, in which n' is an
integer from 0 to 4;
- a radical SR', R' being as defined above; or
- a radical NR5R6, in which R5 and Rg, which
are identical or different, are the hydrogen atom, a
lower alkyl radical having 1 to 6 carbon atoms or a 63
C.,-cycloalkyl radical; and
- R3 is a radical of the formula
R" R"
R,~
R" ~ or
~z z
in which:
- Z is CH or N or Z' is S or O;
- Ry1 is the hydrogen atom or a halogen atom:
and
- R12 is a tptrazole radical, CN, COOH or
CONH2.
The above-mentioned derivatives must also be
considered in their tautomeric form.
In the case where RZ is an azide group, the
compounds may be considered in the tricyclic tetrazolo-
[1,5-c]-1,2,4-triazolo[1,5-a]pyrimidine form according
to the equilibrium well known in the literature (cf.
Temple and Montgomery, J. Org. Chem., 30, 826 (1965)).
c ~~ :, n r7 ;~
1'J ~~
- 4 -
mhe above-mentioned derivatives can take the
form of addition salts, in particular the pharmaceuti-
cally acceptable addition salts.
Advantageously, the derivatives of the inven-
05 tion have general formula (I) criven above in which:
- one of the radicals R1 and R' is
- a lower alkyl radical having 1 to 6 carbon
atoms;
- an ether radical of the formula -(CH2)pOR, in
which p is an integer from 1 to 6 and R is a lower
alkyl radical having 1 to 6 carbon atoms or a benzyl
radical; or
- an alcohol radical of the formula -(CH2)pOH,
in which p is as defined above; and
- the other radical R1 or R2 is
- the hydrogen atom;
- a halogen atom;
- a lower alkyl radical having 1 to 6 carbon
atoms; or
- a radical selected from the group comprising
the radicals N3 , OR4 , SRS,, , NR5R6 arid NH ( CH2 ),_,-NRSR6 ,
in which:
RQ is
- the hydrogen atom; or
- a radical -(CH2)m-O-R' in which m is an
integer from 1 to 4 .and R' is a lower alkyl radical
having 1 to 6 carbon atoms;
R5 and R6, which are identical or different,
are
- the hydrogen atom; or
- a lower alkyl radical having 1 to 6 carbon
atoms; or
R5 and R6, taken together with the nitrogen
atom to which they are attached, form a heterocycle
selected from morpholine, pyrrolidine or piperidine;
,., .
-q . c . n
> ~ '7
~ ~F~~l) E ~)
- 5 -
and
n is an integer from 1 to 4:
- X and Y, which are different, are
- in one case the nitrogen atom: and
05 - in the other case a group C-R~ in which R~ is
- the hydrogen atoms
- a lower alkyl radical having 1 to 6 carbon
atoms;
- a radical (CHzJn~OH, in which n' is an
integer from 0 to 4;
- a radical SR', R' being as defined above; or
- a radical NR5R6 in which R5 and R6, which.
are identical or different, are the hydrogen atom or a
lower alkyl radical having 1 to 6 carbon atoms: and
- R3 is one of the following radicals:
N-N N-N
N'N ~ / HOOC / N~N ~ / N
H ~ ( ~ I H \ I
N-N
N-N N~N
H2NOC , N~N
H
~ I N
Br
In the description and the claims, lower alkyl
radical is understood as meaning a linear or branched
hydrocarbon chain having from 1 to 6 carbon atoms. A
lower alkyl radical is for example a methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, tert-butyl, pentyl,
isopentyl, hexyl or isohexyl radical.
C3-C~-Cycloalkyl radical is understood as
meaning a saturated cyclic hydrocarbon radical, pre-
rl .~ :~ n
~.~.~rl~~~~ v)
- 6 -
ferably a cyclopropyl, cyclobutyl, cyclohexyl or cyclo-
heptyl radical.
Halogen is understood as meaning a chlorine,
bromine, iodine or fluorine atom.
05 In one embodiment, R1 is an n-propyl group.
In another embodiment, R1 is an N-diethylamino
group.
In another embodiment, R1 is an n-butyl group.
In one embodiment, Rs is a hydroxyl group.
In another embodiment, RZ is an n-propyl group.
In another embodiment, R2 is an N-diethylamino
group.
In one embodiment, R3 is a 2-(1H-tetrazol-5-
yl)phenyl group.
In one embodiment, X is the nitrogen atom.
In one embodiment, Y is the group CH.
Tn another embodiment, Y is the group C-CH3.
In another embodiment, Y is the group C-NH2.
The particularly preferred compounds of the
invention are selected from the products of the formu-
lae
JH
<IMG>
.. ~ ~ L r) ~'
r ~ i7 l~
rN~
05 N
In general terms, the method of preparing the
compounds of formula (I) comprises:
a) preparing a compound of formula («):
B
X N \
Y~ ~ i \
N N A ~ R,
Formula (a)
in which:
- X, Y and R3 are as defined above; and
- A and B are in one case a hydroxyl group or a lower
alkyl radical having 1 to 6 carbon atoms and in the
other case a lower alkyl radical having 1 to 6 carbon
atoms or an ether radical of the formula -(CH2)p-OR, in
which p is an integer from 1 to 6 and H is a lower
alkyl radical having 1 to 6 carbon atoms or a benzyl
radical,
by condensing a 3-amino-1,2,4-triazole of formula (II):
.:yes
f~ ~ ~ ~
_ g _
~NHZ
N I
N
R ~N.~
H
05
Formula (II)
in which R~ is as defined above, with a derivative of
formula (,B)
Ra
Formula (,B)
in which R'1 is a lower alkyl radical having 1 to
6 carbon atoms or an ether radical of the formula
-(CHZ)p-OR, in which p is an integer from 1 to 6 and R
is a lower alkyl radical having 1 to 6 carbon atoms or
a benzyl radical, RS is a lower alkyl radical having 1
to 6 carbon atoms or a lower O-alkyl group having 1 to
6 carbon atoms, preferably methyl or ethyl, and R3 is
as defined above,
in an aprotic solvent such as dichloro- or trichloro
benzene, or in an acid solvent such as acetic acid, or
else in an alcohol in the presence of the corresponding
sodium or potassium alcoholate, or else in pyridine or
2-methyl-5-ethylpyridine in the presence or absence of
4-dimethylaminopyridine, at a temperature of between 50
and 200°C;
b) if appropriate, protecting the group carried by R3
:7 ~-) n r
~~ Y,. b to -0~ ;_~
- 10 -
using a method known per se;
c) heating the derivative thus obtained from the deri-
vative of formula (,e), when the latter is a ketoester,
in an appropriate reagent such as, for example, POC13,
05 to convert the hydroxyl group represented by A or B to
a chlorine atom;
d) heating this chlorinated derivative in the presence
of a nucleophile containing nitrogen, oxygen or sulfur,
under reflux in an alcohol or in an autoclave at 100°C,
in the presence or absence of a base such as, for
example, Na2C03, to give,a derivative of formula («) in
which A and B have the same meanings as R1 and RZ res-
pectively;
e) if appropriate, deprotecting the group carried by
R3 ;
e1) converting this group to an acid group, for example
by hydrolysis in the case where this group is a nit-
rile; or
ea) converting this group to a tetrazole group, for
example, in the case where this group is a nitrile, by
reaotion with a trialkyltin azide with heating in
toluene or xylene, followed by a treatment with gaseous
hydrochloric acid in tetrahydrofuran; or
e3) converting this group to an amide group, for
example, in the case where this group is a nitrile, by
reaction with sulfuric acid, or by reaction with hydro
gen peroxide, or else by reaction with polyphosphoric
;acid; and
f) if appropriate, converting the resulting derivative
to an addition salt, preferably a pharmaceutically
acceptable addition salt.
According to the invention, it will be possible
to synthesize the compounds of formula (I) in accor-
dance with the following reaction sequence:
Methods known per se, such as, for example, the
<~r,
~.~ ~ i ~j~i
- 11 -
Claisen reaction or the method using Meldrum's acid,
which methods can easily be found in the following
literature references:
- HAUSER C.R.; SWAMER F.W.; ADAMS J.T.; Org. Reaction,
05 vol. VIII, 1954, 59-196,
- HENNE A.L.; TEDDER J.M.; J. Chem. SOC., 1953, 3628,
- BRESLOW D.S.: BAUMGARTEN E.; HAUSER C.R.; J. Am.
Chem. Soc., 1944, 66, 1286,
- OIKAWA Y.; SUGANO K.; YONEMITSU O.; J. Org. Chem.,
1978, 43(10), 2087-88,
- WIERENGA W.; SKULNICK H.I.; J. Org. Chem., 1979, 44,
310,
- HOUGHTON R.; LAPHAM D.; SYNTHESIS, 1982, 6, 451-2,
- BRAM G.; VILKAS M.; Bull. Soc. Chim. France, 1964(5),
945-51,
- BALYAKINA M.V.; ZHDANOVICH E.S.; PREOBRAZHENSKII
N.A.; Tr. Vses. Nauchn. Issled. Vitam in. Inst.,
1961, 7, 8-16,
- RENARD M.; MAQUINAY A.; Bull. Soc. Chim. Belg., 1946,
55, 98-105,
- BRUCE F.W.; COOVER H.W.; J. Am. Chem. Soc., 1944, 66,
2092-94, and
- EBY C.J. and HAUSER C.R.; J. Am. Chem. Soc., 1957,
?9, 723-5,
will be used to prepare the compounds of formula (III):
R'; i -CHz C-R,
O O
Formula (III)
in which R'1 is a lower alkyl radical having 1 to
6 carbon atoms or an ether radical of the formula
-(CH2)p-OR, in which p is an integer from 1 to 6 and R
is a lower alkyl radical having 1 to 6 carbon atoms or
~A ø tl !'? '1 n
~ .~ E:;~ a L) ~ ~_i
- 12 -
a benzyl radical, and R8 is a lower alkyl radical
having 1 to 6 carbon atoms or a lower O-alkyl group
having 1 to 6 carbon atoms, preferably methyl or ethyl.
The compounds of the formula
05
~e
Formula (V)
:vill be obtained by benzylating the compounds of for-
mula (III) with compounds of formula (IV):
W
I
CH,
v o
Formula (IV)
in the presence of a base such as sodium or potassium
carbonate in acetone,~a sodium or potassium alcoholate
in an alcohol, or sodium or lithium hydride in solvents
such as tetrahydrofuran, dioxane or dimethylformamide,
for example, at a temperature of between 50 and 100°C,
or else in the presence of one equivalent of lithium
chloride or bromide and two equivalents of diisopropyl-
ethylamine in tetrahydrofuran under reflux, according
to the following reference:
- SUNG-EUN Y00; KYU YANG YI; Bull. Korean Chem. Soc.,
1989, 10(1), 112.
iy.~.r~t ~.~ri
- 13 -
These compounds of formula (V) can also be
obtained by condensation of an aldehyde of formula
(VI):
05 CNO
r
j
v
Formula (VI)
with the compounds of formula (III), followed by hydro-
genation in the presence of a catalyst such as Raney
nickel, palladium-on-charcoal or platinum oxide, in a
solvent such as an alcohol or tetrahydrofuran, under
pressure or at ordinary pressure if the substituents
present allow it.
In more general terms, methods of preparing the
compounds of formula (V) will be found in the following
references:
- DURGESHWARI P.; CHAUDHURY N.D.; J. Ind. Chem. Soc.,
1962, 39, 735-6,
- HEINZ P.; KREGLEWSKI A.; J. Prakt. Chem., 1963, 21(3-
4), 186-197,
- ZAUGG H.E.; DUNNIGAN D.A.; MICHAELS R.J.; SWETT L.R.;
J. Org. Chem., 1961 , 26, 644-51,
- KAGAN H.B.; HENG SUEN Y.; Bull. Soc. Chim. France,
1966(6), 1819-22,
- RATHKE M. W . ; DEITCH J . ; Tetrahedron Lett . , 1971 ( 31 ) ,
2953-6,
BORRIES KUBEL; Liebigs Ann. Chem., 1980, 1392-1401,
- MARQUET J.; MORENO-MAMAS M.; Chem. Lett., 1981, 2_,
173-6,
- IOFFE T.; POPOV E.M.; VATSURO K.V.; TULIKOVA E.K.;
.. q c7 f~ ;~ r n
~- ~ r,, ~ ~1 ~ ~_~
- 14 -
KABACHNIK M.I.; Tetrahedron, 1962, 18, 923-940, and
- SHEPHERD T.M.; Chem. Ind. (London), 1970, 17, 567.
In formula (IV), W is a halogen atom, prefer-
ably chlorine or bromine.
05 In the same formula:
V can be a group
R900C
Rg being a lower alkyl or benzyl radical, in which case
the compounds of formula (IV) are prepared by reacting
a magnesium compound of p-bromotoluene with a compound
of the formula
to give a compound of the formula
30
which is then hydrolyzed to give the compound of the
formula
~~'~~'~~3
- 15 -
05
Procedures for the three steps described above
will be found in the following reference:
- MEYERS A.I.; MIHELICH E.D.; J. Am. Chem. Soc., 1975,
97, 7383.
The acid is then esterified with an alcohol of
the formula RgOH, R9 being as defined above.
These derivatives are then brominated or
chlorinated, for example with N-bromosuccinimide, N
chlorosuccinimide or bromine, in a solvent such as
carbon tetrachloride, dibromoethane or dichloroethane,
to give the compounds of formula (TV) in which V is the
group
R9GaOC
V can be the group
NC \
in which case the compound
;~ rW ~~ r~ ~
~~JU
- 16 -
05
prepared above will be converted to the primary amide
by reacting the acid chloride, obtained with thionyl
chloride or phosphorus oxychloride, with aqueous ammo-
nia, and this amide will be converted to the nitrite by
reaction with phosphorus oxychloride in dimethylforma-
mide or with thionyl chloride. Likewise, the compound
CHI
N
'' I ~O
obtained above may be converted directly to tha carbo-
nitrile derivative by treatment in pyridine in the
presence of POC13. The resulting nitrite derivative of
the formula
35 wall then be brominated or chlorinated under the same
< j ';! '~ n
Gv i~ L! ~ ~~'
_ 1~ _
conditions as the above ester to give the compounds of
formula (IV) in which V is the group
05
NC
V can be a group
N
NC
in which case the corresponding compounds of formula
(IV) are synthesized in the following manner:
The magnesium compound
BrMg ~ \ CH20CH3
prepared by a conventional Grignard reaction, is
converted to the zinc derivative by reaction with
zinc chloride. This zinc derivative is condensed
with 2-chloronicotinonitrile, in the presence of
[Ni(P~3)]2C12, to give the derivative of the formula
N
CH30CH2 ~ \
NC~
This compound, treated with boron tribromide in chloro-
form, will give the compounds of formula (IV) in which
W is the bromine atom and V is the group
c :l t r~
7 CO :'
_ 1g - ~~a;.Ji.j '_~
N
r
NC
05 V can be a group
N
r
R900C
R9 being as defined above, in which case the correspon-
ding compounds of formula (IV) may be prepared from the
nitrite prepared above of the formula
CH30CH2
NCr
by conventional hydrolysis of the nitrite group fol-
lowed by esterification of the acid obtained, or direct
conversion of the nitrite group to the ester group by
the methods known to those skilled in the art, followed
by a treatment with boron tribromide in chloroform, to
give the compounds of formula (IV) in which W is the
bromine atom and V is the group
N
R900C \
V can be a group
r "1 1' ra
L
- 19 ° <~~..c=di~~ ~'~
NC
05 in which case the corresponding compounds of formula
(IV) are synthesized in the following manner:
The compound of the formula
to CHs ~ ~ C=C-CHz -CH2 C I
CI
will be obtained from 4-chloro-4'-methylbutyrophenone
of the formula
CH3 ~ ~ OU-CH2 CH2 CH2 C I
the preparation of. which is described in Belgian patent
577,977 of 15th May 1959, CA : 54, 4629 c, by treatment
with phosphorus oxychloride and dimethylformamide under
the conditions described in the following reference:
- VOLODINA M.A.; TENENT'EV A.P.; KUDRYASHOVA V.A.;
KABOSHINA L.N.; Khim. Geterosikl. Soedim; 1967, 5-8.
This compound is then treated with sodium
sulfide in a solvent such as tetrahydrofuran, under
reflux, to give the derivative
CH3 s
35 which is then converted in two steps to the nitrile
r'.._~~)
- 20 -
05
to
derivative by dehydration of the oxime formed from the
aldehyde and hydroxylamine. This dehydration may be
carried out for example with acetic anhydride to give
the nitrile compound
CH3 i
s S
NC
which may then be aromatized by treatment with bromine
in carbon tetrachloride to give the compound
15 C
s
NC
This compound can then be chlorinated or bro
minated with halogenating agents such as N-chlorosuc
cinimide or N-bromosuccinimide, in a solvent such as
carbon tetrachloride or dibromoethane, to give the
compounds of formula (IV) in which V is the group
S
V cam be a group
S
3 s R900C
G2 ,.~ :J ,-1 ;1 r' 1~
~. iii i) ~) ~ ~'i
- 21 -
R9 being as defined above, i:n which case the corres-
ponding compounds of formula (IV) may be prepared from
the nitrile prepared above of the formula
os CH3
to
by conventional hydrolysis of the nitrite group fol-
lowed by esterification of the acid obtained, or direct
conversion of the nitrite group to the ester group by
the methods known to those skilled in the art, followed
15 by chlorination or bromination of the ester with N-
chlorosuccinimide or N-bromosuccinimide, for example in
carbon tetrachloride or dibromoethane, to give the com-
pounds of formula (IV) in which W is the bromine atom
or the chlorine atom and V is the group
R90C!C
V can be a group
O
R900C
R9 being as defined above, in which case the corres-
ponding compounds of formula (IV) may be prepared by
reacting the diazotized derivative of p-toluidine with
3-furoic acid to give the compounds of the formula
h L
- 22 -
CH3
/ O
05 HOOC
by the method described in the following literature
reference:
- A. JURASEK et al., Collect. Czech. Chem. Commun.,
1989, 54, 215.
This compound will then be esterified by 'the
conventional methods known to those skilled in the art
to give the compound
C
in which R9 is as defined above, this derivative then
being brominated or chlorinated by reaction with N-
bromosuccinimide or N-chlorosuccinimide, for example in
carbon tetrachloride or 1,2-dichloroethane, to give the
derivative of formula (IV) in which W is the bromine
atom or the chlorine atom and V is the group
O O
/ or /
RsOOC R OOC Br (or C1)
s
V can be a group
C ~ 4 i l'1 l,.' y. n
- 23 - ''
~.r _~ i;. :J ~,', ~ t~
O
NC
05 in which case the acid
CH3
r
HOOC
prepared above will be converted to the acid chloride
by reaction with thionyl chloride and then to the amide
by reaction with ammonia. The amide obtained will be
converted to the nitrile by reaction with phosphorus
oxychloride in dimethylformamide to give the compounds
of the formula
2o CH3 \
O
NC
This derivative will then be brominated or
chlorinated by reaction with N-bromosuccinimide or N-
chlorosuccinimide, for example in carbon tetrachloride
or 1,2-dichloroethane, to give the compounds of formula
(IV) in which W is the bromine atom or the chlorine
atom and V is the group
O O
or
NC ~ NC ~ Br (or C1)
r .1
4~ i) ;) ~'~ ~'
_~" i;n U L) a ti
- 24 -
05
In formula (V), R'1 and R8 are as defined above
and V is as defined in formula (IV).
However, the compounds of formula (V) in which
v is a group
,N ~ , / S . / o /
NC ~ I NC a NC ~ NC / orNC r Br
will react with one equivalent of sodium azide in a
solvent such as dimethylformamide, in the presence of
an ammonium salt such as ammonium chloride, or by
heating in toluene or xylene with a trialkyltin azide,
to give the compounds of formula (V) in which V is the
group
N
Br
N- N_ ~ N_ N_ 'N_
2o N~N~NH N~~ ~NH~ N~~ ,NH N~~ ,NH N~~ ,NH
N N N N
In formula (VI), V is as defined in formula
(IV), but this condensation method will only be used
when V possesses a group unaffected by hydrogenation.
Thus reaction of a 3-amino-1,2,4-triazole of
formula (II):
~NHZ
N
1 N
R~Ni
H
Formula (TI)
.\
C? ~ ~)r~7r~~!'
/ i
s..~ ~ ;M ~~ ) E 3
- 25 -
in which R~ is as defined above (these products being
commercially available or capable of being prepared by
the methods described in the following literature
references:
OS - HUFFMANN and SCHAEFER, J. of Org. Chem., 1963, 28, p.
1812-1816 and p. 1816-1821,
- ALLEN.et al., J. of Org. Chem., 1959, 24, p. 793-796,
and
- Organic Synthesis Collective, volume 3, p. 95),
with the compounds of formula (V), in which R'1 and R8
are as defined above and V is one of the following
groups:
1s N-N
R900C / NC , N~N ~
\ ~ , \ ( , H \ ~ ,
2o N-N
R900C / N NC / N N~N \ / N
I ~ \ I , H \ I '
N-N
2 s R9ppC N C
~ \S ~ ~ \S ' H ~S ,
~/~
N-N
3o R9~C NC
i\ .~\ N i
O ~ O ' H ~O
~) j n ~~ ,n r' n
- ~ ~ ~, C) .) f ; )
N-N
R90OC N C N
w w , N w
O ~ O O
H
05 Br ~r Sr
where R9 is as defined above, will give the compounds
of formulae (VIIa) and/or (VIIb):
R',
R,o
X N
W ~ ~ ~ ~ ~ Y~N~~/ R~~ \ V
N N R, V
Formula (VIIa) Formula (VIIb)
and their tautomeric forms, in which R'1, X, Y and V
are as defined above and R1o is the hydroxyl group when
the compounds of .formula (V) are p-ketoesters and a
lower alkyl radical having 1 to 6 carbon atoms in the
case where these same compounds of formula (V) are ,e-
diketones, by condensation in an aprotic solvent such
as dichloro- or trichloro-benzene, or in an acid
solvent such as acetic acid, or else in an alcohol in
the presence of the corresponding sodium or potassium
alcoholate, or else in pyridine or 2-methyl-5-ethyl-
pyridine in the presence or absence of 4-dimethylamino-
pyridine, at a temperature of between 50° and 200°C.
In the case where V possesses a tetrazole
group, the reaction temperatures should not exceed
140°C so as not to decompose the tetrazole.
The reactions of aminotriazoles or similar
heteroaromatic amines with ,B-ketoesters and ,B-diketone
derivatives are described well in the literature and,
according to the operating conditions, the forms
;~ .ys n C~ r~ :'°
i.'., ::. ~w ~ ~~ 3
- 27 -
obtained are identified. Examples which may be cited
are the studies by
- J.A. VAN ALLAN et al., J. Org. Chem., p. 779 to p.
801 (1959),
05 and by
- L.A. WILLIAMS, J. Chem. Soc., p. 1829 (1960), and
- L.A. WILLIAMS, J. Chem. Soc., p. 3046 (1961).
Thus the compounds VIIa and VIIb will be iden-
tified for separate treatment.
The Applicant has discovered, however, that 2-
methyl-5-ethylpyridine, in the presence or absence of
4-dimethylaminopyridine, is a preferred solvent for
orientating the reaction almost exclusively towards the
formation of the derivatives of formula (VIIb); in
fact, the temperature (170-180°C) and the pH which are
necessary for this orientation can be achieved using
this solvent.
If the derivatives of formulae (VIIa) and
(VIIb) in which Rlo is a hydroxyl group are treated
with a reagent such as P2S5 or Lawesson's reagent, the
derivatives of formulae (VIIa) and (VIIb) in which the
group R1o is a thiol will be obtained.
For example, if the derivatives of formulae
(VIIa) and (VIIb) in which R1a is a hydroxyl group are
heated in POC13, the derivatives of formulae (VIIIa)
and (VIIIb):
Ci
X N \ / X N \ /
Yw ~ ~ \
!~ , \ N N CI V
N N R, V
Formula (VIIIa) Formula (VIIIb)
will be obtained, in which R'z, X, Y and V are as
c~~ y;yr/;,
~..~...U'u' i ~_)
_ 28
defined above.
Hydrogenation of the derivatives of formulae
(VIIIa) and (VIIIb), in the presence of a catalyst such
as palladium-on-charcoal, will make it possible to
05 replace the chlorine with a hydrogen atom, and the
derivatives of formula (IX):
R,
X N ~
N N R ! V
z
Formula (IX)
in which R1, R2, X, Y and V are as defined above, will
be obtained by heating the derivatives of formulae
(VIIIa) and (VIIIb), in the presence of a nucleophile
containing nitrogen, oxygen or sulfur, under reflux in
an alcohol , in the presence or absence of a base such
as Na2C03, or in an autoclave at 100°C.
The derivatives of formulae (VIIa) and (VIIb)
in which R'1 is the group (CHa)a0-benzyl, p being as
defined above, may be hydrogenated in the presence of
palladium-on-charcoal, in acetic acid, to give the
compounds of formulae (VIIa) and (VIIb) in which R'1 is
an alcohol group.
These triazolopyrimidines of formulae (VIIa)
and (VIIb) may also be obtained by reacting the deriva-
tives of formula (X):
35
iT~ '~ ~) () ~ >'~ n
~ ~,. r,. J ~? ~ ~:i
- 29
R,
N ~
NH,---NH N Fi2 'V
05
Formula (X)
in which R1, RZ and V are as defined above, with:
- acids, acid chlorides or carboxylic acid esters,
- isocyanates or isothiocyanates,
- orthoesters,
- carbonyldiimidazole or
urea, potassium xanthogenate, carbon disulfide or an
analogous reagent,
by heating without a solvent or in a solvent such as N-
methylpyrrolidone or an alcohol like ethanol or meth-
oxyethanol, in the presence or absence of a base such
as triethylamine, pyridine or 2-methyl-5-ethylpyridine.
Depending .on the operating conditions, especi
ally the temperature and pH of the reaction, 1,2,4
triazolo[4,3-a]pyrimidine derivatives or their 3,2,4
triazolo[1,5-a]pyrimidine rearrangement products will
be obtained.
The compounds of formula (X) can be obtained by
any one of the known methods of synthesizing 2-hydra
zinopyrimidines (cf.:~The Pyrimidines: The Chemistry of
Heterocyclic Compounds: D.J. BROWN: Wiley Interscience
1970), especially by substituting the derivatives of
formula (XI):
f
~\
.S :1 ~ r ~ n
~i ~ i;,r ~ l~ 1_7
- 30 -
R,
CH,S \ N R2 V
OS
Formula ( 3tI )
in which Rl, R2 and V are as defined above, with
hydrazine hydrate, for example.
The compounds of formula (XI) are obtained by
condensing S-methylthiourea with the derivatives of
formula (V), for example, or by any one of the methods
of synthesizing 2-thiomethylpyrimidines which are known
in the literature (cf.: The Pyrimidines, op. cit.).
The compounds of formula (IX) in which V
possesses an ester group COORS may be hydrolyzed in an
acid or basic medium, or hydrogenated in the case where
R9 is a benzyl, to give the compounds of formula (I) in
which R3 possesses. an acid group.
The compounds of foxmula (IX} in which V
possesses a nitrite group will react with one equiva-
lent of sodium azide in a solvent such as dimethyl-
formamide, in the presence of an ammonium salt such as
ammonium chloride, or by heating in toluene or xylene
with a trialkyltin azide, for example trimethyltin or
tributyltin azide, followed by an acid treatment, for
example with gaseous hydrochloric acid in tetrahydro-
furan, to give the compounds of general formula (I) in
which R~ possesses a tetrazol-5-yl group.
These same compounds in which V possesses a
nitrite group may be converted by reaction with sul
furic acid, or by reaction with hydrogen peroxide, or
else by reaction with polyphosphoric acid, to deriva
tives of general formula (I) in which R3 possesses an
amide group.
f7 ~ n
;
l~ ~ ~ J ~ ~ :_1
- 31 -
The derivatives in which V possesses a nitrile
group or an amide group may also be converted by basic
or acid hydrolysis to derivatives of general formula
(I) in which R3 possesses an acid group.
05 It is possible to obtain addition salts of some
of the compounds of formula (I), especially pharma-
ceutically acceptable addition salts. In particular,
when the compounds of formula (I) contain an acid or
tetrazole group, there may be mentioned the salts of
sodium, potassium, calcium, an amine such as dicyclo-
hexylamine or an amino acid such as lysine. When they
contain an amine group, there may be mentioned the
salts of an inorganic or organic acid, such as, for
example, the hydrochloride, methanesulfonate, acetate,
maleate, succinate, fumarate, sulfate, lactate or
citrate.
The novel compounds according to the invention
possess remarkable pharmacological properties as angio-
tensin II receptor antagonists and antiproliferatives
and can be used in therapeutics for the treatment and
prevention of cardiovascular diseases and in particular
for the treatment of hypertension, cardiac insuffi-
ciency and diseases of the arterial wall, especially
atherosclerosis.
Thus the invention covers the pharmaceutical
compositions which contain as the active principle the
drugs consisting of a pharmaceutically effective amount
of at least one compound of formula (I) as defined
above, as well as one of its pharmaceutically accep
table addition salts where appropriate.
These compositions can be administered by the
buccal, rectal, parenteral, transdermal or ocular
route.
These compositions can be solid or liquid and
can take the pharmaceutical forms commonly used in
:~ ' n n ;o ri~
a , ..
t~w~ aL (~ i) ~_.~ S ~_~
- 32 -
human medicine, such as, for example, simple or coated
tablets, gelatin capsules, granules, suppositories,
injectable preparations, transdermal systems and eye
lotions. They are prepared by the customary methods.
05 In said compositions, the active principle, consisting
of a pharmaceutically effective amount of at least one
compound of formula (I) as defined above, or one of its
pharmaceutically acceptable addition salts, can be
incorporated with excipients normally employed in these
pharmaceutical compositions, such as talc, gum arabic,
lactose, starch, magnesium stearate, polyvidone, cellu-
lose derivatives, cacao butter, semisynthetic glyce-
rides, aqueous or non-aqueous vehicles, fats of animal
or vegetable origin, glycols, various wetting agents,
dispersants or emulsifiers, silicone gels, certain
polymers or copolymers, preservatives, flavorings and
colors.
The invention also covers a pharmaceutical com
position with antagonistic activity towards angiotensin
II receptors, and/or antiproliferative activity, which
permits especially a favorable treatment or prevention
of cardiovascular diseases, in particular hypertension,
cardiac insufficiency and diseases of the arterial
wall, especially atherosclerosis, said composition com-
prising a pharmaceutically effective amount of at least
one compound of formula (I) given above, or one of its
pharmaceutically acceptable addition salts, which may
be incorporated in a pharmaceutically acceptable
excipient, vehicle or carrier.
The dosage varies especially according to the
route of administration, the complaint treated and the
subject in question.
For example, for an adult with an average
weight of 60 to 70 kg, it can vary between 1 and 400 mg
of active principle, administered orally in one or more
r
iv! l.W ,) ~ Lr
- 33 -
daily doses, or from 0.01 to 50 mg, administered paren-
terally in one or more daily doses.
The invention also covers a method of preparing
a pharmaceutical composition, which comprises incor
05 porating a pharmaceutically effective amount of at
least one compound of formula (I) as defined above, or
one of its pharmaceutically acceptable addition salts,
into a pharmaceutically acceptable excipient, vehicle
or carrier. This pharmaceutical composition can be
formulated as gelatin capsules or tablets containing
from 1 to 400 mg of active principle, or as injectable
preparations containing from 0.01 to 50 mg of active
principle.
The invention also covers a method of therapeu
tic treatment for mammals, which comprises adminis
tering to this mammal a therapeutically effective
amount of at least one compound of formula (I) as
defined above, or one of its pharmaceutically accep
table addition salts.
In animal therapeutics, the daily dose which
can be used is normally between 1 and 100 mg per kg.
Further characteristics and advantages of the
invention will be understood more clearly from the
following description of some Preparatory Examples,
which in no way imply a limitation but are given by way
of illustration.
Example 1: Ethyl 3-oxohexanoate
Formula (III): R'1 = n-propyl, RS = 0-ethyl
176 g of 2,2-dimethyl-4,6-dioxo-1,3-dioxane
(Meldrum's acid) are dissolved in 550 ml of dichloro-
methane and 188 ml of pyridine; the mixture is cooled
to 5°C with a bath of water and ice arid 133 ml of
.. ;) c/ fl ;l ~~ !~°
~a ~_ i,~ l) l~ s ~ !
- 34 -
butyryl chloride are added dro;pwise. When the addition
is complete, the mixture is stirred for three hours at
room temperature. The solution is washed with a dilute
solution of hydrochloric acid, dried over magnesium
05 sulfate and evaporated under vacuum to give an oil.
This oil is dissolved in 700 ml of ethanol and the
mixture is refluxed for six hours. The ethanol is
evaporated off under vacuum and the residue obtained is
distilled to give 145.4 g of ethyl 3-oxohexanoate in
the form of an oil.
Boiling point (20 mm of mercury): 98°-100°C.
The compound of Example 2 was prepared by the
procedure of Example 1.
Example 2: Ethyl 3-oxoheptanoate
Formula (III): R'1 = n-butyl, RS = O-ethyl
Boiling point (20 mm of mercury): 115°-120°C.
Example 3: Ethyl 4-benzyloxy-3-oxobutanoate
Formula (III) : R'1 = CH2-O-CH2 l \ ,
R8 = O-ethyl
80 g of 60% NaH are added in portions to 800 ml
of anhydrous THF. The medium is cooled to 10°C and
maintained at this temperature. 500 ml of benzyl
alcohol are then introduced dropwise. A solution of
65.8 g of ethyl 4-chloroacetoacetate in 200 ml of
benzyl alcohol is then added. The mixture is stirred
at room temperature for 20 h. It is neutralized by the
w .~ r) n ;? r~
_R. ~H ~ l? s ,3
_ 35 _
slow addition of acetic acid (120 ml) while being
cooled with an ice bath. The whole is then poured into
a mixture of water and ice and extracted with ether.
The organic phase is washed with a solution of sodium
05 bicarbonate, dried over MgS04 and then concentrated to
give an orange oil. The product is purified by two
successive distillations to give a yellow oil.
Boiling point (under 0.05 mm of mercury): 126-
132°C.
Exa~le 4: 4'-Bromomethyl-2-cyanobiphenyl
CN
Formula (IV): W = Br, V =
a) Preparation of 2-cyano-4'-methylbiphenyl
563.8 g of (4'-methylbiphenyl-2-yl)carboxylic
acid, prepared according to MEYERS A.I.p MIHELICH E.D.;
J. Am. Chem. Soc., 1975, 97(25), 7383, are added in
small portions to 800 ml of thionyl chloride. The mix
ture is refluxed for two hours. The thionyl chloride
is concentrated under vacuum and the residue is poured
into a 28% solution of ammonium hydroxide cooled
beforehand with a bath of water and ice. The mixture
is stirred for 30 minutes and the crystals obtained are
filtered off, washed with water followed by ether and
then dried to give 554.8 g of (4'-methylbiphenyl-2-yl)-
carboxamide in the form of crystals melting at 128°-
132°C. These crystals are taken up in 1300 ml of
thionyl chloride and the mixture is refluxed for 3
hours and then concentrated under vacuum to give an
orange oil. This is taken up in two liters of chloro-
form and washed with water and the organic phase is
~ ,3 c? f> ;1 r~
i
F.~~_I~UC~ i..3
- 36
then dried and concentrated to give 509.8 g of an oil,
which, crystallizes from pentane to give 467.3 g of 2-
cyano-4'-methylbiphenyl.
Melting point: 46°-48°C.
05
b) 4'-Bromomethyl-2-cyanobipheny~l
467.3 g of 2-cyano-4'-methylbiphenyl, prepared
above, are dissolved in 4.7 1 of 1,2-dichloroethane in
the presence of 467.3 g of N-bromosuccinimide and 9.3 g
of benzoyl peroxide. The mixture is heated very
gradually so as to have good control over the exo
thermic effect. It is subsequently refluxed for 4 h,
cooled to 50 ° C and then washed 3 times with hot water
and dried and the organic phase is concentrated to give
cream-colored crystals.
Recrystallization from isopropanol gives 451 g
of white crystals of 4'-bromomethyl-2-cyanobiphenyl.
Melting point: 128°C.
xa ple 5: Ethyl 2-[(2'-cyanobiphenyl-4-yljmethyl]-3-
oxohexanoate
Formula (A): R'1 = n-propyl, RS = O-ethyl,
CN
V =
23 g of ethyl 3-oxohexanoate, prepared in
Example 1, are dissolved in 120 ml of tetrahydrofuran.
30.3 g of 4'-bromomethyl-2-cyanobiphenyl, prepared in
Example 4, and 4.7 g of lithium chloride are added and
the mixture is stirred at room temperature. 39 ml of
diisopropylethylamine are than introduced dropwise,
~ n ;7 ~a ,
- 37 -
causing a slight exothermic effect. The mixture is
subsequently stirred for three hours at room tempera-
ture and 'then for ten hours under reflex. The solvents
are evaporated off under vacuum and the residue is
05 taken up in water and then extracted with chloroform.
__ The organic phase is decanted and then washed with a
dilute solution of hydrochloric acid, dried over mag
nesium sulfate and evaporated under vacuum to give 38 g
of an orange oil.
Purification by chromatography on silica gel
(eluent: CHC1~) gives 32.3 g of ethyl 2-[(2'-cyano-
biphenyl-4-yl)methyl]-3-oxohexanoate.
The compounds of Examples 6 to 10 are obtained
by the procedure of Example 5 using the appropriate ,B
ketoester.
Example 6: Ethyl 2-[(2'-cyanobiphenyl-4-yl)methyl]-3-
oxoheptanoate
Formula (V): R'1 = n-butyl, Rg = O-ethyl,
GN
V =
0
Oil used as such in the next step.
Example 7: Ethyl 2-[(2'-cyanobiphenyi-4-yl)methyl]-3-
oxobutanoate
Formula (V): R'~ = methyl, Ra = O-ethyl,
c .~ ,~ n p r
~ 1 ~.! U ~_, ~ : i
- 38 -
V =
05
Yellow oil purified by chromatography on silica
gel (eluent: chloroform 95%/ether 50).
Example 8: Ethyl 2-[(2'-cyanobiphenyl-~.-yl)methyl]-3-
l0 oxopentanoate
Formula (V): R'1 = ethyl, Rs = O-ethyl,
15 / ~N
V=
Oil purified by chromatography on silica geI
20 (eluent: CHC13 95o/ether 5%).
Example 9: Ethyl 2-[(2'-cyanobiphenyl-4-yl)methyl]-4-
methoxy-3-oxobutanoate
25 Formula (V): R'1 = methoxymethyl, R8 =
O-ethyl,
CN
3G V =
Yellow oil purified by chromatography on silica
gel (eluent: CHC13 95%/ether 5%).
~_~h Jt)~i
- 39 -
Example 10: Ethyl 4-benzyloxy-2-[(2'-cyanobiphenyl-4-
yl)methyl]-3-oxobutanoate
05 Formula (V) : R'1 = GH2-C~-CH2 ,
V = ~ , Ra = O-ethyl
to s
Oil purified by chromatography twice in succes-
sion (eluents: chloroform, then cyclohexane 80%/ethyl
acetate 20%).
Example 11: Ethyl 2-[4-(3-cyano-2-pyridyl)benzyl]-3-
oxohexanoate
2o N , CN
Formula (V): R'1 = CHZ-CH'-CH3, V = I ,
R8 = O-ethyl
a) Preparation of 4-bromobenzyl methyl ether
A solution of sodium methylate, prepared from
11.8 g of sodium and 350 ml of methanol, is introduced
dropwise into a suspension of 117.7 g of 4-bromobenzyl
bromide in 350 ml of methanol. The mixture is stirred
for 2 h at room temperature arid left to stand over
night. The methanol is evaporated off, the residue is
taken up in ether and the organic phase is washed with
water and then dried and concentrated to give a yellow
oil, which ~,s purified by distillation to give 102 g of
bromobenzyl methyl ether as a colorless liquid.
i .) t.) ~ T~~ n
i,r ~,) l ~ i l
- 40
Boiling point under 17 mm of mercury: 112-
114°C.
b) Synthesis of 3-cyano-2-(4-methoxymethylphenyllpyri-
05 dine
2 g of the compound 4-bromobenzyl methyl ether,
prepared above, are added to a suspension of 18 g of
magnesium in 50 ml of anhydrous THF. The formation of
the magnesium compound is initiated with a few crystals
of iodine and, if necessary, by heating with a bath of
warm water. A solution of 121.8 g of 4-bromobenzyl
methyl ether in 200 ml of anhydrous THF is introduced
dropwise so that the temperature does not exceed 40°C.
The components are reacted for 1 h at room temperature
and 800 ml of a solution of zinc chloride in ether are
then introduced under excess nitrogen pressure. A
white precipitate forms. The components are reacted
for 1 h 30 min at room temperature. 800 mg of the
coupling catalyst bis(triphenylphosphine)nickel(II)
chloride, [NiP(phenyl)3]2C12, axe added and a solution
of 76.9 g of 2-chloronicotinonitrile in 300 ml of THF
is then introduced. The mixture is stirred overnight
at room temperature and concentrated under vacuum. The
concentrate is taken up in a mixture of 1 1 of di-
chloromethane, 1 1 of water and 1 1 of the disodium
salt of EDTA. The emulsion is filtered on C~lite 545.
The organic phase is decanted, washed with water, dried
and concentrated to give 133.6 g of an orange oil,
which is purified by chromatography twice in succession
(eluent: chloroform 95%/ether 5%). 69.4 g of 3-cyano-
2-(4-methoxymethylphenyl)pyridine are thus isolated in
the form of an orange oil, which crystallizes.
Melting point: 74°C.
- 41 -
c) Preparation of 3-cyano-2-(4-bromomethylphenyl~pyri-
dine
05 N ,,,, C N
Formula (IV): W = Br, V =
s
69.4 g of 3-cyano-2-{4-methoxymethylphenyl)-
pyridine, prepared in the previous step, are dissolved
in 700 ml of chloroform stabilized with amylene. The
solution is cooled to -10°C. A solution of 66 ml of
BBr3 in 200 ml of chloroform stabilized with amylene is
introduced dropwise so that the temperature does not
exceed 5°C. The mixture is left for 1 h 30 min in an
ice bath. It is hydrolyzed with ice and then with
water. It is filtered and the suspension is taken up
in a mixture of water and chloroform. After decanta-
tion, the organic. phases are combined, dried and then
concentrated to give 78.2 g of cream-colored crystals
of 3-cyano-2-(4-bromomethylphenyl)pyridine.
Melting point: 118°C.
d) Preparation of ethyl 2-[4-(3-cyano-2-pyridvl)-
benzylZ-3-oxohexanoate
N , CN
Formula (V): R'1 = CH2-CH2-CH3, V =
~ s
R8 = O-ethyl
Following the procedure of Example 5, the
expected derivative is obtained in the form of an
orange oil, which is used as such in the next step.
'' -' c) ~) :l y~ n
(; ~ ;,, J a ~ ~;
- 42 -
Example 12: Ethyl 2-(4-(3-cyano-2-thienyl)benzyl]-3-
oxohexanoate
o5 a C1V
Formula (V): R'1 = CHZ-CH2-CH3, V = S~ a
R8 = O-ethyl
a) Preparation of 4-chloro-1-(4-methylphenyl)butanone
A mixture of 560 ml of 4-chlorobutyryl chloride
and 550 ml of toluene is added dropwise to a suspension
of 740 g of AlCl3 in 2 1 of dichloromethane, the tem-
perature being maintained at between 10° and 15°C. The
reaction mixture is stirred for 30 min at room tempera-
ture and poured on to ice. After decantation, the
organic phase is separated off and the aqueous phase is
extracted with dichloromethane. The organic phases are
combined, washed with water and then dried and concen-
traced under vacuum to give 994.5 g of 4-chloro-1-(4-
methylphenyl)butanone in the form of an oil, which is
used in the next step without further purification.
b) Preparation of 3-chloro-2-_(2-chloroethyl~-3-(4-
methylphenyl~,prop-2-en-1-al
390 ml of POC13 are introduced dropwise, at a
temperature of between 7° and 12°C, into a solution of
352.5 g of 4-chloro-1-(4-methylphenyl)butanone, pre-
pared above according to Example 12 a), in 450 ml of
DMF. The temperature is raised gradually, in the first
instance to 50°C over 2 h and then to 75°C over 45 min.
The mixture is poured on to ice and extracted three
times with ether and the organic phases are combined,
washed with water and then dried and evaporated to
give 387.8 g of 3-chloro-2-(2-chloroethyl)-3-(4-methyl-
;1 rs r
y n
/~J _~ Sw L) l1 , ~ 1.1
- 43 -
phenyl)prop-2-en-1-al in the form of an oil, which is
used as such in the next step.
c) Preparation of 4~5-dihydro-3-formyl-2- 4-methyl-
05 phenyl)thiophene
A mixture of 200 g of 3-chloro-2-(2-chloro-
ethyl)-3-(4-methylphenyl)prop-2-en-1-al, prepared in
Example 12 b) , 2 . 2 1 of THF, 276. 5 g of Na2S ~ 9H20 and
373 ml of water is refluxed for 6 h. :It is concentra-
ted under vacuum and the concentrate is taken up in
water and extracted 3 times with ether. The organic
phases are combined, washed with water, dried and
concentrated to give 170.3 g of an oil, which crys-
tallizes.
Melting point: below 50°C.
d) Preparation of 4,5-dihydro-3-formyl-2-(4-met ~~
phenyl)thiophene oxime
132.1 g of hydroxylamine hydrochloride are
added in portions to a solution of 323.5 g of the
aldehyde prepared according to 12 c) in 800 ml of
ethanol. A solution of sodium carbonate, prepared from
100.5 g of NazC03 and 700 ml of water, is than added
dropwise. The mixture is heated at 40°C for 5 min and
the reaction is then left to proceed at room tempera-
ture for 1 h. The mixture is cooled to 15°C and the
solid is filtered off and washed with water and then
with a mixture of isopropyl ether 50%/petroleum ether
50% to give 252 g of oxime. Extraction of the filtrate
with dichloromethane gives a 2nd crop of 99 g of the
expected oxime.
e) Preparation of 3-cyano-4~5-dihydro-2-(4-methyl-
phenyllthiophene
A salution of 171.8 g of the oxime prepared in
~~i , UU~ i
- 44 -
Example 12 d) in 680 ml of acetic anhydride is refluxed
for 3 h. It is concentrated to remove the excess
anhydride and then distilled to give 115.3 g of nitrile
derivative.
05 Boiling point under 0.05 mm of mercury: 140-
150~C.
f) Preparation of 3-cyano-2-f4-methylphenyllthiophene
62 ml of bromine are introduced dropwise into a
solution, preheated to 50~C, of 191.3 g of the nitrile
prepared according to Example 12 e) in 1.85 1 of CC14.
The whole is refluxed until the evolution of HBr
ceases. The CC14 is evaporated off and the residue is
distilled to give 115.3 g of 3-cyano-2-(4-methylphe
nyl)thiophene.
Boiling point under 0.05-0.1 mm of mercury:
130-150'C.
g) 2-~(4-Bromomethyl~~enyl~-cyanothiophene
~ CN
Formula (IV): W = Br, V = S
182.2 g of the compound obtained in Example 12
f) are bromine°ted according to Example 4 to give 133.7
g of 2-(4-bromomethylphenyl)-3-cyanothiophene.
Melting point: 80-84~C.
h) Eth~l 2-f4-(3-cyano-2-thien~rl)benzyl]-3-oxohexanoate
cN
Formula (V): R'1 = CHI-CH2-CH3, V = ,
GS c~ (1 Y~ n
'r~.r ~ ~ E t3
- 45
R8 = U-ethyl
A mixture of 50 g of 2-(4-bromomethylphenyl)-3-
cyanothiophene, prepared above, 40 g of ethyl 3-oxo-
05 hexanoate, prepared in Example 1, 300 ml of THF, 62 ml
of diisopropylethylamine and 15.6 g of Liar is refluxed
for 15 h. It is concentrated under vacuum, a dilute
solution of hydrochloric acid is added and the mixture
is extracted with ethyl acetate. The organic phases
are combined, washed with water, dried and evaporated
to give 62.4 g of ethyl 2-[4-(3-cyano-2-thienyl)-
benzyl]-3-oxohexanoate in the form of an oil, which is
used without further purification.
Example 13: Ethyl 2-[4-(3-cyano-2-furyl)benzyl~-3-oxo-
hexanoate
, Formula (V) : R'1 = CHZ-CHZ-CH3, V - O ~ ~N
R8 = O-ethyl
a ) Preparation of 2 =( 4-methylx~henyl L 3-furanoic acid
70.7 g of p-toluidine, cooled with a bath of
water and ice, are treated with 205 ml of 36% HC1. The
mixture is then stirred at 55°-60°C for 30 min before
being cooled to 0°C again. A solution of 45 g of NaN02
in 220 ml of water is then introduced. The mixture is
stirred for 1 h at 0°C. This cold solution is intro-
duced into a mixture of 49.3 g of 3-furanoic acid, 220
ml of acetone, 23.4 g of CuCl2 and 6.3 g of water,
cooled to -5°C. The whole is stirred at 0°C for 2 h
and then at room temperature for 48 h. Tt is extracted
twice with ether and the organic phase is decanted,
.\
- 46 -
dried and concentrated to give an oil, which gives
crystals when treated with water.. The crystals are
filtered off and washed with 50 ml of a 50% methanol/
water mixture to give 13.4 g of 2-(4-methylphenyl)-3
05 furanoic acid.
Melting point: 166°C.
b) Preparation of 2-(4-methylphenyl)furan-3-carbox-
amide
20 ml of SOC12 are added to a solution of
13.4 g of the furanoic acid prepared above in 70 ml of
toluene. The mixture is refluxed for 3 h and the
excess SOC12 and the toluene are then distilled to give
an oil, which is reacted at 5°C with a solution of 100
ml of 1,2-dimethoxyethane saturated with ammonia. The
precipitate is filtered off and washed with water and
then with isopropyl alcohol to give 7 g of white
crystals of amide.
Melting point: 174°C.
c) Preparation of 3-cyano-2-(4-methylphenyl furan
A mixture of 12.2 g of the amide prepared above
and 65 ml of SOC12 is refluxed for 3 h and concentrated
under vacuum. The oil obtained is taken up in chloro-
form, and water and ice are then added. After decanta
tion, the aqueous phase is extracted with chloroform
and the organic phases are combined, dried and evapora
ted to give an oil. Purification by chromatography on
silica gel (eluent: toluene) gives 7.5 g of an oil,
which crystallizes.
Melting point: 66°C.
c i cz C) n
~.~.,',~Ji.l~l3
47 _
d) 2- ~4-Bromomethylphen~l)-3-cyanofuran
CN
05 Formula (IV): W = Br, V
7.5 g of the compound obtained in Example 13 c)
are brominated according to Example 4 to give, after
purification by chromatography on silica gel (eluent:
pentane 50%/toluene 500), 4.6 g of 5-bromo-3-cyano-2
(4-methylphenyl)furan (melting point: 88°C), 2.2 g of
5-bromo-3-cyano-2-(4-bromomethylphenyl)furan (melting
point: 114°C) and 2 g of 2-(4-bromomethylphenyl)-3
cyanofuran.
Melting point: 108°C.
The compound 5-bromo-3-cyano-2-(4-methyl-
phenyl)furan is subjected to a further bromination
reaction according to Example 4 to give 5-bromo-2-(4-
bromomethylphenyl)-3-cyanofuran, which constitutes the
compound of Example 13 d) bis.
e) Ethvl 2-[4-~(3-cyano-2-furyllbenzyll-3-oxohexanoate
~ ~ CN
Formula (V): R'1 = CH2-CHZ-CH3, V =
Rg = O-ethyl
The resulting derivative 2-(4-bromomethyl-
phenyl)-3-cyanofuran is treated according to Example 5
to give ethyl 2-[4-(3-cyano-2-furyl)benzyl]-3-oxo-
hexanoate in the form of an oil, which is used in the
crude state in the next step.
cz a c~ r~ n ~~~ ;~
-4$- rW<.JV )
Likewise, the derivative 5-bromo-2-(4-bromo-
methylphenyl)-3-cyanofuran of Example 13 d) bis is
treated according to Example 5 to give ethyl 2-[4-(5-
bromo-3-cyano-2-furyl)benzyl]-3-oxohexanoate in the
OS form of an oil, which constitutes the derivative of
Example 13 bis.
Example 14: 3-[(2'-Cyanobiphenyl-4-yl)methyl]-2,4-
dioxopentane
15
Formula (V): R'1 = CH3, Re = CH3, V =
32.8 g of 2,4-dioxopentane, 68 g of 4'-bromo-
methyl-2-cyanobiphenyl, prepared in Example 4, 88 ml of
diisopropylamine and 10.6 g of anhydrous lithium chlo-
ride in 300 ml of tetrahydrofuran are refluxed fox
27 h. The mixture is cooled and the precipitate is
filtered off. The organic phase is concentrated to
dryness to give 88.5 g of crystals. These are taken up
in isopropanol and the mixture is filtered to isolate
38.8 g of unreacted 4'-bromomethyl-2-cyanobiphenyl.
The concentrated mother liquors yield 26.5 g of an oil
which, when purified on silica gel (eluent: chloro
form), gives a further 5.3 g of 4'-bromomethyl-2-cyano
biphenyl and 12.2 g of the expected 3-[(2'-cyanobi
phenyl-4-yl)methyl]-2,4-dioxopentane in the form of a
yellow oil.
4 9 _ ~~ ~~ s~ J a G, L)
Example 15: 5-[(2'-Cyanobiphenyl-4-yl)methyl]-4,6-
dioxononane
05 / CN
Formula (V): R'1 = CH2-CHZ-CHI, V = ~ .
RS = CH2_CHZ-CH3
15.6 g of 4,6-dioxononane, prepared from methyl
propyl ketone and ethyl butyrate in the presence of
lithium amide (according to CA 42 . 4129 f), are dis-
solved in 160 ml of anhydrous DMF. 4 g of 60o NaH are
added in portions. When the exothermic effect has sub-
sided, the mixture is cooled to room temperature and a
solution of 27.2 g of 4'-bromomethyl-2-cyanobiphenyl,
prepared in Example 4, in 90 ml of DMF is introduced
dropwise. The mixture is stirred for 30 min at room
temperature and then heated at 60°C for 2 h. It is
concentrated under vacuum and the concentrate is taken
up in a water/dichloromethane mixture and acidified
with a dilute solution of HC1. After decantation, the
aqueous phase is extracted twice with dichloromethane.
The organic phases are washed with water, dried and
then concentrated to give 36.3 g of an oil, which is
purified by chromatography 'twice in succession (eluent:
chloroform, then cyclohexane 90%/ethyl acetate 10% res
pectively) to give a solid identified by NMR as being
the enol tautomer melting at 105°C, and an oil corres
ponding to the diketone tautomeric form.
Example 15: 2,4-Dioxo-3-[(2'-(1H-tetruzol-5-yl)bi
phenyl-4-yl)methyl]pentane
Formula (V)v R'1 = CH3, R$ = CH3,
s ~ !~ ;') J ~ n
x : ;
_~ iv t_~ l)
- 50 -
N-N
I
/ ~N~
C
05
A mixture of 11.8 g of 3-((2'-cyanobiphenyl-4-
yl)methyl]-2,4-dioxopentane, prepared in Example 14,
200 ml of xylene and 9.3 g of trimethyltin azide is
refluxed for 50 h. After 24 h, a second equivalent of
trimethyltin azide is added.
mhe mixture is cooled and concentrated to give
a viscous oil which, when chromatographed on silica gel
(eluent: chloroform 90%/methanol 10a), gives 9.3 g of
crystals.
An additional treatment with acetonitrile gives
6.2 g of analytically pure 2,4-dioxo-3-[(2'-(1H-tetra-
zol-5-yl)biphenyl-4-yl)methyl]pentane.
Empirical formula: C19H1eN4o2.
Melting point: 166°C.
Example 17: Ethyl 2-[[2'-(1H-tetrazol-5-yl)biphenyl-4-
yl]methyl]-3-oxohexanoate
Formula (V): R'1 = n-propyl, R8 = O-ethyl,
N-N
I ~N
_ /
v - ( H
A mixture of 69.9 g of ethyl 2-[(2'-cyanobi-
phenyl-4-yl)methyl]-3-oxohexanoate, prepared according
to Example 5, 700 ml of anhydrous toluene and 47.5 g of
trimethyltin azide, prepared from sodium azide and tri-
~N ~ ~! z ~..~
- 51 -
methyltin chloride, is refluxed for 24 h. A further
47.5 g of trimethyltin azide are added and reflux is
continued for 16 h. The mixture is concentrated to
500. The orange solution obtained is purified by
05 chromatography twice in succession (eluent: chloroform
90o/methanol 10%, then chloroform 95%/methanol 5%) to
give 58 g of an orange oil, which crystallizes.
Melting point: 65~C.
°Example 18 (method A):
6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-
hydroxy-5-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIa): R'1 = n-propyl, X = N,
NC
Y = CH, R1a = OH, V =
1. 7 g of 3-amino-1, 2 , 4-triazole, 7 g of the ,8-
ketoester prepared in Example 5 and 30 ml of acetic
acid are refluxed for 6 h. The acetic acid is evapo-
rated off. The oil obtained is purified by chromato-
graphy on silica gel~(eluent: CHC13 90%/MeOH 100) to
give 5.2 g of the starting ~-ketoester and 1.2 g of
6-[(2'-cyanobiphenyl-4-yl)methyl]-7-hydroxy-5-propyl-
1,2,4-triazolo[1,5-a]pyrimidine.
Melting point: 200°205'C.
1H NMR (200 MHz; DMSO-d6): 2.65 (t, 2H, propyl
CHZ); 8.2 (s, 1H,
H2).
UV ( 10 ~cg/ml , MeOH ) : as = 209 .1 nm
~b = 257.7 nm
;.. _~ ~) y (~ ;~ n
52 _ I. ,~v3;1
a~ = 286.8 nm.
Example 19 (method B):
6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-
05 hydroxy-7-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
NC
Y = CH, R1o = OH, V =
7.1 g of the ,e-ketoester prepared in Example 5,
1.7 g of 3-amino-1,2,4-triazole and 70 ml of 1,2,4-tri-
chlorobenzene are refluxed for 7 h. The mixture is
concentrated under vacuum. The thick oil obtained is
chromatographed orb silica gel (eluent: CHC13 95a/MeOH
5%) to give 0.8 g of the isomer obtained in Example 18
(melting point: 200°C) and 2.2 g of 6-[(2'-cyanobi-
phenyl-4-y1)methyl]-5-hydroxy-7-propyl-1,2,4-triazolo-
[1,5-a]pyrimidine.
Melting point: 212°C.
1H NMR (DMSO-d6): 2.98 (t, 2H, propyl CH2):
8.1 (s, 1H, H2).
t3V (10 ~sg/ml, MeOH) : as = 207.5 nm
ab = 258.2 nm.
Example 20 (method C):
6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-
hydroxy-7-propyl-1,2,4-triazolo[4,3-a]-
pyrimidine
Formula (VIIa): R'1 = n-propyl, X = CH,
~'\
.. ( y ,.~ j ~ ! ? ;'~ r' f7
~~ ~ lel ~~ I,t j/ ~..7
- 53 -
NC
Y = N, R1o = OH, V =
05
a) 5-[(2'-Cyanobiphenyl-4-yl)methyl]-4-
hydroxy-6-propyl-2-mercaptopyrimidine
11 g of thiourea are added with a spatula to a
solution of sodium methylate prepared from 4.6 g of
sodium and 150 ml of methanol. 34.9 g of the ,e-keto-
ester prepared in Example 5, dissolved in 50 ml of
methanol, are then introduced dropwise. The mixture is
left to stand overnight and then refluxed for 7 h. It
is concentrated under vacuum and the concentrate is
taken up in 500 ml of water and then acidified with
concentrated HC1 to bring the pH to 1. The gummy
precipitate is isolated and taken up in methanol to
give 17.3 g of white crystals of 5-[(2'-cyanobiphenyl-
4-yl)methyl]-4-hydroxy-6-propyl-2-mercaptopyrimidine.
Melting point: 196°C.
b) 5-[(2'-Cyanobiphenyl-4-yl)methyl]-4-
hydroxy-2-methylmercapto-6-propylpyrimidine
NC o
3~ormula (XI): R1 = n-propyl, V =
RZ = OH
17.3 g of the compound obtained above are
introduced in portions into a mixture of 340 ml of
methanol and 2.9 g of KOH. After a clear solution has
c;-a ~~r~,~.,>j;~
r~ n~ ;,. O ~) i .,l
- 54 -
formed, it is cooled and 3.4 ml of ICH3 are then
introduced dropwise.
The mixture is left to react for 2 h at room
temperature.
05 The precipitate is filtered off to give 17.2 g
of 5-[(2'-cyanobiphenyl-4-yl)methyl]-4-hydroxy-2-meth-
ylmercapto-6-propylpyrimidine.
Melting point: 220°C.
a) 5-[(2'-Cyanobiphenyl-4-yl)methyl]-2-hydra-
zino-4-hydroxy-6-propylpyrimidine
NC
Formula (X): R1 = n-propyl, V =
R2 = OH
12.4 g of 5-[(2'-cyanobiphenyl-4-yl)methyl]-4-
hydroxy-2-methylmercapto-6-propylpyrimidine, prepared
above, are dissolved in 370 ml of 2-methoxyethanol. 33
ml of hydrazine hydrate are introduced and the mixture
is then refluxed for 3 h. It is concentrated under
vacuum and the concentrate is taken up in acetonitrile
and triturated. The solid obtained is filtered off
and washed with ether arid isopropyl ether to give
9.9 g of 5-[(2'-cyanobiphenyl-4-yl)methyl]-2-hydrazino-
4-hydroxy-6-propylpyrimidine.
Melting point: 191°C.
d) 6-[(2'-Cyanobiphenyi-4-yl)methyl]-5-
hydroxy-7-propyl-1,2,4-triazolo[4,3-a]-
pyrimidine
Formula (VIIa): R'1 = n-propyl, X = CH,
_:,
:~; f c~ r7 ;~ a) P
lVr ~_ i-.~ U :,? E °~1
- 55 -
05
NC
Y = N, R1o = OH, V =
g of 5-[(2'-cyanobiphenyl-4-yl)methyl]-2-
hydrazino-4-hydroxy-6-propylpyrimidine, prepared as
above, are placed in 200 ml of formic acid. The
10 mixture is refluxed for 4 h. It is concentrated under
reduced pressure and the thick oil obtained is taken up
in water and triturated until it crystallizes.
The compound is purified by chromatography on
silica gel (eluent: CHC13 95%/methanol 50).
This gives 8.3 g of 6-[(2'-cyanobiphenyl-
4-yl)methyl]-5-hydroxy-7-propyl-1,2,4-triazolo[4,3-a]-
pyrimidine.
Melting point: 217~C.
1H NMR (DMSO-ds): 2.6 (t, 2H, propyl CHI): 9
(s, 1H, H3).
UV ( 10 ~ag/ml , MeOH ) : a~ = 210 . 2 nm
ab = 257.5 nm
a~ = 303.4 nm.
Hxample 21: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-
hydroxy-5-propyl-1,2,4-triazolo[4,3-a]-
pyrimidine
Formul;i (VIIb): R'1 = n-propyl, X = CH,
NC s
Y = N, R1o = OH, V = ~
j .a c1 ~ r n
~~ ~ v,~ ~ l' ~ l1
- 56 -
Following the procedure of Example 20, step d),
1.1 g of 6-[(2'-cyanobiphenyl-4-yl)methyl]-7-hydroxy-5-
propyl-1,2,4-triazolo[4,3-a]pyrimidine are obtained at
the same time as the compound described above.
05 Melting point: 204-206°C.
1H NMR (DMSO-d6): 2.9 (t, 2H, propyl CH2); 9
(s, 1H, H3).
UV ( 10 ~cg/ml , MeOH ) : as - 211 . 5 nm
ab = 260 nm.
The compounds of Examples 20 and 21 can also be
obtained by reacting compound 20 c) with triethyl
orthoformate under reflux for 5 h. Tn this case, the
proportion of the compound of Example 21 is found to be
slightly improved.
Example 22 (method D):
6-[(2'-Cyanobighenyl-4-yl)methyl]-7-
hydroxy-5-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIa): R'1 = n-propyl, X = N,
IVC ~.
Y = CH, R1o = OH, V =
500 mg of 6-[(2'-cyanobiphenyl-4-yl)methyl]-5-
hydroxy-7-propyl-1,2,4-triazolo[4,3-a]pyrimidine, pre
pared in Example 20 d) , are heated in a metal bath at
225°C for 2 h 30 min. It is left to cool and taken up
in methanol and then in isopropyl acetate to give 300
mg of cream-colored crystals identical to the compound
of Example 18.
>' ,' :? ry ;1 r~ n
5'J - it ~_ i.n lJ U c 't1
Melting point: 200°C.
Example 23 (method E):
6-[ ( 2'-Cyanobiphenyl-4-yl )methyl ]-5-
05 hydroxy-7-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
CN
Y = CH, R1o = OH, V =
A mixture of 24 g of 3-amino-1,2,4-triazole and
200 g of 5-ethyl-2-methylpyridine is heated to 175°C.
100 g of the ,B-ketoester prepared in Example 5; dis-
solved in 100 ml of 5-ethyl-2-methylpyridine, are
introduced dropwise. The reaction is left to proceed
for 6 h at 175°C. . The ethylmethylpyridine is distilled
off under vacuum and the residue is taken up in a
mixture of water and chloroform. After decantation,
the aqueous phase is extracted with chloroform. The
combined organic phases are washed with a dilute
solution of HCl and then with water, dried and con-
centrated to give an oil, which crystallizes when
triturated in methanol. Recrystallization from n-
butanol gives 35.2 g of cream-colored crystals of
6-[(2~-cyanobiphenyl-4-yl)methyl]-5-hydroxy-7-prapyl-
1,2,4-triazolo[1,5-a]pyrimidine.
Melting point: 210°C.
Purification of the mother liquors by chromato-
graphy on silica gel gives a second crop of 6.9 g of
the expected compound, together with 13.9 g of 'the
derivative 6-[(2'-cyanobiphenyl-4-yl)methyl]-7-hydroxy-
;-
r~_~",ai' c ._'
- 58 -
5-propyl-1,2,4-triazolo[1,5-a]pyrimidine obtained in
Example 18.
Melting point: 196°C.
The yield of the reaction can be improved by
05 about 10a toy adding 10.5 g of 4-dimethylaminopyridine
to the initial mixture.
Example 24: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-
hydroxy-2-methyl-5-propyl-1,2,4-triazolo-
[I,5-a]pyrimidine hydrochloride hemihydrate
Formula (VIIa): R'1 = n-propyl, X = N,
Y = C-CH3, Rio = OH,
CN
p= ~
A suspension of 10 g of compound 20 c) in 100
m1 of phenyl acetate is refluxed for 4 h. It is con-
centrated under reduced pressure. The concentrate is
taken up in water and extracted with chloroform and the
extract is dried and evaporated to give 9.8 g of white
crystals melting at 205°C. These crystals are taken up
in 50 ml of acetonitrile and 40 m1 of a 10% solution of
hydrochloric acid in ether to give 7.5 g of 6-[(2'-
cyanobiphenyl-4-yl)methyl]-7-hydroxy-2-methyl-5-propyl-
1,2,4-triazolo[1,5-a]pyrimidine hydrochloride.
Empirical formula: CZ3H21N50~HC1~~H20.
Melting point: 190°C.
1H NMR (DM50-ds): 2.65 (t, 2H, propyl CH2).
UV (MeOH): as = 213.7 nm
ab = 257.7 nm
a~ = 285 nm.
.r :~ n y r~
- 59 - ~.,<.)~J!';'
Example 25: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-
hydroxy-7-propyl-3-mercapto-1,2,4-triazolo-
[4,3-a]pyrimidine
05 Formula (VIIa): R'1 = n-propyl, X = C-SH,
Y = N, R1a = OH,
CN
1o v =
5.3 ml of carbon disulfide are added to a sus-
pension of 10 g of the compound prepared in Example 20
15 c) in 300 ml of butanol. The mixture is refluxed for
2 h. A further 5.3 ml of CS2 are added and reflex is
then continued for 5 h. The mixture is concentrated
under vacuum. The concentrate is taken up in water and
extracted 3 times with chloroform. The solvent is eva-
20 porated off to give 10.8 g of amorphous crystals, which
are purified by chromatography on silica gel (eluent:
CHC13 90%/MeOH 10%).
A first compound weighing 1.9 g is isolated
and identified as 6-[(2'-cyanobiphenyl-4-yl)methyl]-7
25 hydroxy-5-propyl-3-mercapto-1,2,4-triazolo[4,3-a]pyri
midine. This constitutes the product of Example 25
bis.
Melting point: 240°C.
1H NMR (DMSO-d6): 3.5 (t, 2H, propyl CHa).
30 A second compound weighing 1 g is the expected
product, namely 6-[(2'-cyanobiphenyl-4-yl)methyl]-5-
hydroxy-7-propyl-3-mercapto-1,2,4-triazolo[4,3-a]pyri-
midine.
Melting point: 180°C.
35 1H NMR (DMSO-d6): 2.5 (m, propyl CH2 + DMSO-
~) ;7 n
~.. ~ ;W J ~? ~ '_)
- 60 -
d6).
The third product weicJhing 6.2 g is the star-
ting hydrazino compound 20 c).
05 Example 26: 6-[{2'-Cyanobiphenyl-4-yl)methyl]-3,5-
dihydroxy-7-propyl--1,2,4-triazolo[4,3-a]-
pyrimidine
Formula {V3Ia): R'1 = n-propyl, X = C-OH,
Y = N, Ryo = OH,
C (V
V = \
4.6 g of carbonyldiimidazole are added to a
mixture of 10 g of the compound prepared in Example 20
c) and 500 ml of.THF, heated to 50°C. The whole is
refluxed for 7 h and concentrated under vacuum. The
concentrate is taken up in water and extracted three
times with chloroform. Evaporation of the solvent
gives 12.4 g of amorphous crystals, which are purified
by chromatography on silica gel (eluent: CHC13 95o/MeOH
5%) .
A first compound weighing 3.1 g is isolated and
identified as 6-[(2'-cyanobiphenyl-4-yl)methyl]-3,7-
dihydroxy-5-propyl-1,2,4-triazolo[4,3-a]pyrimidine:
This constitutes the product of Example 26 bis.
Melting point: 228°C.
'-H NMR (DMSO-ds): 3 (t, 2H, propyl CHa).
The second compound weighing 3.8 g is the
expected product, namely 6-[(2'-cyanobiphenyl-4-yl)-
methyl]-3,5-dihydroxy-7-propyl-1,2,4-triazolo[4,3-a]-
pyrimidine.
c~ ~~
- 61 - ~r~~.~i3~' i ~.)
Melting point: 210°C.
1H NMR (DMSO-d6): 2.4 (t, 2H, propyl CHZ).
Using one of the methods described in Examples
05 19 or 23 (method B or method E.), the appropriate amino-
triazoles are reacted with the ~-ketoesters prepared in
Examples 5 to 15 to give the following compounds of
Examples 27 to 43.
Example 27: 6°[(2'-Cyanobiphenyl°4-yl)methyl]-5-
hydroxy-2-methyl-7-propyl-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
Y = C-CHI, R1o = OH,
CN
V- \
Crystallization from methanol. The mother
liquors are purified by chromatography on silica gel
(eluent: CHC13 95%/MeOH 5%).
Melting point: 218-220°C.
A second compound is isolated and identified as
6-[(2'-cyanobiphenyl-4-yl)methyl]-7-hydroxy-2-methyl-5-
propyl-1,2,4-triazolo[1,5-a]pyrimidine. This consti-
tutes the product of Example 27 bis.
Formula (VIIa): R'1 = n-propyl, X = N,
Y = C-CH3, R1o = OH,
NC
V =
-, _>,-,"~n~
' ~ ~ .',, ~ O ~ ~3
- 62 -
Melting point: 204-206pC.
Example 28: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-2-ethyl
5-hydroxy-7-propy:L-1,2,4-triazolo[1,5-a]
05 pyrimidine
Formula (VIIb): R'1 = n-propyl, X = I3,
Y = C-CH2CH3, R1o = OH,
v =
Crystallization from methanol. The mother
liquors are purified by chromatography on silica gel
(eluent: CHC13 95%/MeOH 5%).
Melting point: 216~C.
A second compound is isolated and identified as
6-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethyl-7-hydroxy-5-
propyl-1,2,4-triazolo[1,5-a]pyrimidine. This consti-
tutes the product of Example 28 bis.
Formula (vIIa): R'1 = n-propyl, X = N,
Y = C-CH2CH3, R1o = OH,
NC /
v=
Melting point: 186'C.
F., ~" ;;, i~ .: ~ s )
- 63 -
Example 29: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-n-
butyl-5-hydroxy-1,2,4-triazolo[1,5-a]pyri-
midine
05 Formula (VIIb): R'1 = n-butyl, X = N,
Y = CH, R1o = OH,
NC
V
Purified by recrystallization from n-butanol.
Melting point: 210°C.
Example 30: 2-Amino-6-[(2'-cyanobiphenyl-4-yl)methyl]-
5-hydroxy-7-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
Y = C-NHm Ra.o = OH,
NC
V =
Crystallization from a methanol/chloroform
mixture.
Purification of the mother liquors by chromato
graphy on silica gel (eluent: CHC13 90%/MeOH 200).
Melting point: 260~C.
A second compound is isolated and identified as
2-amino-6-[(2'-cyanobiphenyl-4-yl)methyl]-7-hydroxy-5-
propyl-1,2,4-triazolo[1,5-a]pyrimidine. This consti-
:~ c~ ~ r~ r~
~~ ~ Fn U a a
- 64 -
tutes the product of Example 30 bis.
Formula (VIIa): R'1 = n-propyl, X = N,
Y = C-NH2, R1o = OH,
05
NC
V =
Melting point: 325-330~C.
Example 31: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5
hydroxy-2-methylthio-7-propyl-1,2,4
triazolo[1,5-a]pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
Y = C-SCH3, Rio = OH,
V = \
Purification by chromatography on silica gel
(eluent: CHCl~ 95%/MeOH 5%):
Crystallization from isopropyl acetate,
Melting point: 182°C.
Example 32: 6-[4-(3-Cyano-2-thienyl)benzyl]-5-hydroxy-
7-propyl-1,2,4-triazoio[1,5-a]pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
Y = CH, R1o = OH,
c1 a ~ n ry
~~ ~ ~.,r v) i..) k ~_i
- 65 -
NC
V =
05
Crystallization from chloroform/water. Puri-
fication by recrystallization from 2-methoxyethanol.
Melting point: 246~C.
Example 33: 6-[4-(3-Cyano-2-pyridyi)benzyl]-5-hydroxy-
7-propyl-1,2,4-triazolo(1,5-a]pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
Y = CH, R1o = OH,
N
V =
NC
Crystallization from methanol. The mother
liquors are purified by chromatography on silica gel
(eluent: CHzGl2 97.5%/MeOH 2.5%). The whole is puri-
fied by recrystallization from methanol.
Melting point: 212~C.
Example 34: 6-[4-(3-Cyano-2-thienyl)benzyl]-5-hydroxy-
2-methyl-7-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
Y = C-CH3, Rio = OH,
NC / _
S
V =
~
; .~ :, ~ C1 r) P
f~ z r,~ trl i;S ( :,.
- 66 -
Crystallization from a water/chloroform mix-
ture. Purification by recrystallization from 2-meth-
oxyethanol.
Melting point: 277°C.
05
Example 35: 6-(4-(3-Cyano-2-furyl)benzyl]-5-hydroxy-7-
propyl-1,2,4-triazolo(1,5-a]pyrimidine
Formula (VIIb): R'~ = n-propyl, X = N,
Y = CH, R1o = OH,
N C.
V =
Melting point: 256°C.
Example 36: 7-Butyl-6-[(2'-cyanobiphenyl-4-yl)methyl]
5-hydroxy-2-methyl-1,2,4-triazolo[1,5-a]
pyrimidine
Formula (VIIb): R'1 = n-butyl, X = N,
Y = C-CH3, Rzo = OH,
V =
Purified by recrystallization from n-butanal.
Melting point: 230°C.
' c i n ~ ri :,
F.e _~. fen s.i s. ~ 1 ~_."
- 57
Example 37 : 6-[ ( 2'-Cyanobiphenyl-4--yl )methyl ]-5-
hydroxy-2-hydroxymethyl-1,2,4-
triazolo[1,5-a]pyrimidine
05 Formula (VIIb): R'1 = n-propyl, X = N,
Y = C-CH20H, R1o = OH,
NC
V =
Purification by chromatography on silica gel
(eluent: CHC13 95%/MeOH 5%).
Melting point: 214°C.
Example 38: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-
hydroxy-7-methoxymethyl-1,2,4-
triazolo[1,5-a]pyrimidine
Formula (VIIb): R'~ _ -CH2-OCH3, X = N,
Y = CH, R1o = OH,
NC
= w
Purified by chromatography on silica gel
(eluent: chloroform 95%/methanol 5%).
Melting point: 188°C.
A second compound is isolated and identified as
6-[(2'-cyanobiphenyl-4-yl)methyl]-7-hydroxy-5-methoxy-
methyl-1,2,4-triazolo[1,5-a]pyrimidine. This consti-
Lutes the product of Example 38 bis.
~d~Yd'V~u3L
- 68 -
formula (VIIa): R'.~ _ -CHI-OCH3, X = N,
Y = CH, R1o = OH,
PEI C
05
V =
Melting point: 240'C.
Example 39: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-
hydroxy-7-methyl-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (VIIb): R'1 = CH3, X = N, Y = CH,
NC
R1o = OH, V =
The product is purified by chromatography on
silica gel (eluent: CHC13 95%/MeOH 50) and crystallized
from methanol.
Melting point: 212'C.
second compound is isolated and identified as
6-[(2'-cyanobiphenyl-4-yl)methyl]-7-hydroxy-5-methyl-
1,2,4-triazolo[1,5-a]pyrimidine: This constitutes the
product of Example 39 bis.
Formula (VIIa): R'1 = CH3, X = N, Y = CH,
N~
R1o = OH, V =
-
1..~ ~. f~r iJ l~ i '_S
Melting point: 252'C.
Example 40: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-ethyl-
5-hydroxy-1,2,4-triazolo[1,5-a]pyrimidine
05
Formula (VIIb): R'1 = ethyl, X = N, Y = CH,
NC /
R1o = OH, V =
Isolated by chromatography on silica gel
(eluent: CHC13 95%/MeOH 5%) and purified by recrystal-
lization from n-butanol.
Melting point: 224~C.
A second compound is isolated and identified
as 6-[(2'-cyanobiphenyl-4-yl)methyl]-5-ethyl-7-hydroxy
1,2,4-triazolo[1,5-a]pyrimidine. This constitutes the
product of Example 40 bis.
Formula (VIIa): R'y = -CHZ-CH3, X = N,
Y = CH, R1o = OH,
, IVC
V = ~
Melting point: 234'C.
ff ~ ~ i~ ~) t
.. .. . ~., r.n 'L.~ l f ~ i f
- 70 -
Example 41: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-2-N,N-
diethylamino-5-hydroxy-7-propyl-1,2,4-tri-
azolo[1,5-a]pyrimid:ine
05 Formula (VIIb): R'1 = n-propyl, X = N,
Y = C-N~ . R1o = OH,
NC
V =
The product is crystallized from methanol.
Melting point: 220°C.
A second compound is isolated in the form of
amorphous crystals after chromatography of the mother
liquors on silica gel (eluent: CHC13 80%/isopropylamine
20~). It is identified as 6-[(2'-cyanobiphenyl-4-
yl)methyl]-2-N,N-diethylamino-7-hydroxy-5-propyl-1,2,4-
triazolo[1,5-a]pyrimidine. This constitutes the com-
pound of Example 41 bis.
Formula (VIIa): R'1 = n-propyl, X = N,
Y = C-N , R1o = OH,
NC
v=
c~ ~ -; ~ r1
i~.,I ~ Fd t~ SJ A ~~J
- 71 -
Example 42: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5,7-
dipropyl-i,2,4-triazolo[1,5-a]pyrimidine
Formula (VIIa): R'1 = n-propyl, X = N,
05 Y = CH, R1o = n-propyl,
/
V =
Product purified by chromatography on silica
gel (eluent: chloroform 95%/methanol 5%).
Melting point: 160'C.
Example 43: 7-Benzyloxymethyl-6-[(2'-cyanobiphenyl-4-
yl)methyl]-5-hydroxy-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIb) : R', _ '~/-(2 Q-C
X = N, Y = CH, R1o = OH,
N(i
V= ~ I
Purified by recrystallization from butanol
followed by chromatography on silica gel (eluent:
chloroform 95o/methanol 50).
Melting point: 218'C.
A second compound is isolated and identified as
5-benzyloxymethyl-6-[(2'-cyanobiphenyl-4-yl)methyl]-7-
i a c1 ~~'~ :? t~) "
- ~2 - ~~.s~J~~ . ?7
hydroxy-1,2,4-triazolo[1,5-a]pyrimidine. This consti-
tutes the product of Example 43 bis.
05 Formula (VIIa) : R'1 = -~~2-Q-C1-!2 ~ ~ ,
X = N, Y = CH, R1o = OH,
NC
V =
Melting point: 260°C.
Example 44: 5-Chloro-6-[(2'-cyanobiphenyl-4-yl)methyl]-
7-propyl-1,2,4-t.riazolo[1,5-a~pyrimidine
Formula (VIIIb): R'1 = n-propyl, X = N,
25
NC
Y = CH, V
25.9 g of the compound prepared in Example 19
or 23 are added in portions to 260 ml of POC13. The
mixture is refluxed for 4 h. It is concentrated under
vacuum, the concentrate is taken up in 200 ml of chlo-
roform stabilized with amylene, and a solution of water
and ice is then added. After decantation, the aqueous
phase is extracted with chloroform and the organic
phases are combined. After washing with water and
drying, they are concentrated under vacuum to give a
thick oil. The product is crystallized from isopropyl
cl ~) >-) rl n
l.~ (,~ ;j ~! ~ 1 i
- 73 -
acetate to give 21 g of 5-chloro-6-[~2'-cyanobiphenyl-
4-yl)methyl]-7-propyl-1,2,4-triazolo[1,5-a]pyrimidine.
Melting point: 138°C.
05 The derivative of Example 45 is obtained by the
procedure of Example 44 using the derivative prepared
in Example 18.
Example 45: 7-Chloro-6-[(2'-cyanobiphenyl-4-yl)methyl]-
5-propyl-1,2,4-triazolo[1,5-a]pyrimidine
Formula (VIIIa): R'1 = n-propyl, X = N,
NC
Y = CH, V = \
Melting point: 132°C.
Example 46: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-
mercapto-5-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (IX): RZ = n-propyl, X = N, Y = CH,
NC
R 1 = SH I V = \
V
A mixture of 5 g of the chlorinated compound
obtained in Example 45, 2 g of thiourea and 150 ml of
ethanol is refluxed for 7 h and concentrated under
vacuum. The yellow solid is taken up in 60 ml of a 0.5
E f 43 :7 n n
<~~;~JCJ~'t3
_ 74 _
N solution of NaOH. The small amount of insoluble
material is filtered off. The filtrate is acidified
with acetic acid. The yellow precipitate is filtered
off and purified by chromatography on silica gel
05 (eluent: chloroform 90%/metha:nol 10%) to give 3.4 g of
6-[(2'-cyanobiphenyl-4-yl)methyl]-7-mercapto-5-propyl-
1,2,4-triazolo[1,5-a]pyrimidine.
Melting point: 200-205°C.
Example 47: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-mer-
capto-7-propyl-1,2,4-triazolo[1,5-a]pyri-
midine
Formula (IX): R1 = n-propyl, X = N, Y = CH,
20
NC
R2 = SH, V = ~
A mixture of 11.1 g of the derivative prepared
in Example 19 or Example 23, 350 ml of toluene and
13.4 g of Lawesson's reagent is refluxed for 2 h. The
yellow solid obtained is filtered off. Purification by
chromatography on silica gel (eluent: CH2C12 90%/
acetone 10%) gives 10 g of 6-[(2'-cyanobiphenyl-4-yl)-
methyl]-5-mercapto-7-propyl-1,2,4-triazolo[1,5-a]pyri-
midine.
Melting point: 226°C.
Example 48: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-propyl
1,2,4-triazolo[1,5-a]pyrimidine
Formula (IX): R1 = n-propyl, X = N, Y = CH,
:~ rn r-~ .
s~ ' i 7
- ,~ 5 - l.~ .f~ i,~ i1 U i :~
NC
RZ = H~ V = \
05
A solution of 5.4 g of the compound prepared in
Example 44 in 110 ml of 2-methoxyethanol containing
1.2 g of anhydrous sodium acetate is hydrogenated at
atmospheric pressure and room temperature in the pre-
sence of 1.4 g of 5% Pd-on-charcoal. The system is
purged with nitrogen. The catalyst is filtered off on
Celite 545 and washed with hot 2-methoxyethanol. The
filtrate is concentrated and the crystals obtained are
taken up in ether to give 3.7 g of crude product.
Purification by chromatography on silica gel (eluent:
dichloromethane 90%/acetone 10%) gives 2.5 g of white
crystals of 6-[(2'-cyanobiphenyl-4-yl)methyl]-7-propyl-
1,2,4-triazolo[1,5-a]pyrimidine.
Melting point: 180'C.
Example 49: 6-j(2'-Cyanobiphenyl-4-yl)methyl]-5-meth-
oxy-7-propyl-1,2,x-triazolojl,5-a]pyrimi-
dine
Formula (IX): R1 = n-propyl, X = N, Y = CH,
NC
R2 = OCH3, V =
A solution of sodium methylate, prepared from
0.8 g of sodium and 25 ml of methanol, is added to a
solution of 11.6 g of the compound of Example 44 in 120
ml of 1,2-dimethoxyethane. The mixture is stirred at
- 6~~.:J~.j~ iv
room temperature for 3 h. The insoluble material is
filtered off and the filtrate is concentrated. The
crystals obtained are taken up in water, filtered off
and washed firstly with water and then with isopropyl
05 alcohol and ether to give 9.5 g of 6-[(2'-cyanobi-
phenyl-4-yl~methyl]-5-methoxy-7-propyl-1,2,4-triazolo-
[1,5-a]pyrimidine.
Melting point: 166°C.
Example 50: Ethyl [6-[(2'-cyanobiphenyl-4-yl)methyl]-7-
propyl-1,2,4-triazolo[1,5-a]pyrimidin-5-
yl]mercaptoacetate
Formula (TX): R1 = n-propyl, X = N, Y = CH,
I5 R~ _ -S-CH2-COOCH2-CH3,
NC '
0.6 g of 60% NaH is added in portions to a
solution of 1.8 g of ethyl mercaptoacetate in 50 ml of
toluene. The mixture is maintained at a temperature of
40° for ~ h and then cooled to room temperature. A
solution of 5 g of the compound prepared in Example 44
in 50 ml of anhydrous toluene is then introduced. The
reaction is left to proceed at room temperature for 3 h
and then at 50 ° C for 4 h. A second equivalent of the
sodium salt of ethyl mercaptoacetate, prepared as
above, is added to complete the reaction. After hydro-
lysis and decantation, the organic phase is washed with
water and then with a dilute solution of acetic acid,
dried and concentrated. The oil obtained is purified
by chromatography on silica gel (eluent: dichloro-
~~ W ,~ t) i 1 ~ )
- 77 -
methane 90%/acetone 10%) to give 5.4 g of ethyl [6-
[(2'-cyanobiphenyl-4-yl)methyl]-7-propyl-1,2,4-triazo-
l0[1,5-a]pyrimidin-5-yl]mercaptoacetate.
Melting point: 76°C.
05
The compound of Example 51 is obtained by the
procedure of Example 50 using 2-methoxyethanol instead
of ethyl mercaptoacetate.
Example 51: [6-((2'-Cyanobiphenyl-4-yl)methyl~-7-
propyl-1,2,4-triazolo(1,5-a~pyrima.din-5-
yl] 2-methoxyethyl ether
Formula (IX): Ry = n-propyl, X = N, Y = CH,
R2 = -0-CH2-CH2-OCH3,
NC
v=
Product crystallized from isopropyl ether.
Melting point: 102°C.
Example 52: 5-Amino-6-[(2'-cyanobiphenyl-4-yl)methyl]-
7-propyl-1,2,4-triazolo[1,5-a~pyrimidine
Formula (IX): R1 = n-propyl, X = N, Y = CH,
N C
R2 = NH2~ V =
A mixture of 10 g of the derivative prepared in
S~ , c'~ ; ~ ( 1 Y~~ ~,
- ~~ ~ f. i' l5 E ~
Example 44 and 200 ml of a soluton of 1,2-dimethoxy-
ethane saturated with ammonia is placed in an auto-
clave. It is heated at 125°C for 24 h and taken up in
a chloroform/water mixture. After decantation, the
05 aqueous phase is extracted. The organic phases are
combined, dried and concentrated to give 8.1 g of 5-
amino-6-[(2'-cyanobiphenyl-4-yl)methyl]-7-propyl-1,2,4-
triazolo[1,5-a]pyrimidine.
Melting point: 206°C.
Example 53: 6-[(2'-Cyanobiphenyl-4-yl)anethyl]-7-N,N-
diethylamino-5-propyl-1,2,4-triazolo-
[ ~. , 5-a ] pyrimidine
Formula (IX): R2 = n-propyl, X = N, Y = CH,
NC
CH2-CH3
~ ,
R1 = N ~ = W
2 0 C H2-C H3
A mixture of 5 g of the chlorinated derivative
of Example 45, 100 ml of ethanol, 16 ml of diethylamine
and 2.5 g of sodium carbonate is refluxed far 4 h. It
is concentrated under vacuum and the thick oil is taken
up in water. It is extracted three times with dichlo-
romethane and the extracts are dried and concentrated.
The compound obtained is purified by chromatography on
silica gel (eluent: chloroform 95%/methanol 5%) to give
5 g of 6-[(2~-cyanobiphenyl-4-yl)methyl]-7-N,N-diethyl-
amino-5-propyl-1,2,4-triazolo[1,5-a]pyrimidine in the
form of an orange oil.
The following compounds of Examples 54 to 58
are obtained by reacting one of the derivatives des-
-.,
a. ~; F, :, ~~~ r~
~~r;~U~~
cribed in Examples 44 or 45 with appropriate amines by
either one of the two methods described in Examples 52
and 53.
05 Example 54: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-N,N-
diethylamino-7-propyl-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (IX): R1 = n-propyl, X = N, Y = CH,
1s
CH2-CH3 NC ,'
R2 = N V = \
CH2-CH3
Product crystallized from hot isopropyl ether.
Melting point: 133~C.
Example 55: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-7-propyl
5-(pyrrolidin-1-yl)-1,2,4-triazolo[1,5-a]
pyrimidine
Formula (IX): R1 = n-propyl, X = N, Y = CH,
NC
R2 = -N~ , V =
Product crystallized from hot isopropyl ether.
Melting point: 166'C.
_'
c, ~ ;f ;~ :, :~ .~
- 8 O - ~ ~ 1'd w LS ~ '_~
Example 56: 6-[(2~-Cyanobiphenyl-4-yl)methyl]-7-propyl-
5-(morpholin-4-ylethylamino)-1,2,4-tri-
azolo[1,5-a]pyrimidine
OS Formula (IX): R1 = n-propyl, X = N, Y = CH,
RZ = NH--CH2-CH2- p ,
NC
V= \
Oily product purified by chromatography on
silica gel (eluent: chloroform 95o/methanol 5%).
Example 57: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-(pipe-
ridin-1~yl)-7-propyl-1,2,4-~triazolo[1,5-a]-
pyrimidine
Formula (IX): R1 = n-propyl, X = I3, Y = CH,
NC
RZ=_N~, v= ~ I
Compound purified by recrystallization from 2-
methaxyethanol.
Melting point: 266°C.
y i ", (~' ~'1 7~ n
- 81 y ~l ~ ~.'.~ <) ;'~ ~ '_)
Example 58: 6-[(2"-Cyanobiphenyl-4-yl)methyl]-5-hydra-
zino-7-propyl-1,2,4-triazolo[1,5-a]pyrimi-
dine
05 Formula (IX): R1 = n-propyl, X = N, Y = CH,
NG o
R2 = NH-NH2, V =
Product crystallized from ether.
Melting point: 161'C.
Example 59: 5-Azido-6-[(2"-cyanobiphenyl-4-yl)methyl]-
7-propyl-1,2,4-triazolo(1,5-a]pyrimidine
Formula (IX): R1 = n-propyl, X = N, Y = CH,
2~ NG
0
R2 = N3. V = \
10.3 g of the compound prepared in Example 58,
2.3 ml of concentrated HCl and 300 ml of acetic acid
are mixed. A solution of 1.9 g of NaN02 in 20 ml of
water is added. The mixture is left overnight at room
temperature. Water is added and the mixture is decan-
ted and extracted with ethyl acetate. The organic
phases are combined, washed with water, dried and
evaporated. Purification by chromatography twice in
succession (eluent: dichloromethane 95o/methanol 5% and
dichloromethane 90o/methanol 10%) gives 4.3 g of 5-
azido-6-[(2"-cyanobiphenyl-4-yl)methyl]-7-propyl-1,2,4-
it .j :~ r1 W ~ n
- 8 2 - i'~ .~. ~ .r t1 a i I_D
triazolo[1,5-a]pyrimidine.
Melting point: 134°C.
This same compound can be considered as a tri
cyclic derivative according to the known equilibrium of
05 azides in the 2-position of nitrogen-containing rings
(cf. Temple and Montgomery, J. Org. Chem., 30, 826
(1965)).
Example 60: 3-Amino-5-hydroxymethyl-1,2,4-triazole
Formula (II): R~ _ -CH20H
A mixture of 136 g of aminoguanidine bicarbo-
nate and 80 g of glycolic acid is heated gradually to
120°C. The reaction is continued for 5 h at this tem-
perature. The mixture is taken up in 100 ml of ethanol
and the solid is filtered off to give 45.7 g of 3-
amino-5-hydroxymethyl-1,2,4-triazole.
Melting point: 192-194°C.
Example 61: 3-Amino-5-N,N-diethylamino-1,2,4-triazole
Formula (II): R~ = N
10.3 ml of diethylamine are added to a solution
of 16.1 g of dimethyl N-cyanodithioiminocarbonate in
160 ml of acetonitrile. The mixture is stirred for one
hour at room temperature and then refluxed until no
more methylmercaptan is evolved. The solution is
cooled with an ice bath and 5 m1 of hydrazine hydrate
are introduced. The mixture is refluxed for 4 h.
After distillation of the solvent, the product is taken
up in acetonitrile to give 8.9 g of white crystals of
- 8 3 - ~ ~ i7 L' s ~.)
3-amino-5-N,N-diethylamino-1,2,4-triazole.
Melting point: 134°C.
Example 62: 7-Hydroxy-5-propyl-6-[(2'-(1H-tetrazol-5
05 y1)biphenyl-4-yl)methyl)-1,2,4-triazolo
[1,5-a]pyrimidine
Formula (I): R1 = OH, R2 = n-propyl, X = N,
N-NH
!J
N~ i
Y = CH, R3 = N
A mixture of 7.8 g of the ,e-ketoester of
Example 17, 1.7 g of 3-amino-1,2,4-triazole and 70 ml
of 1,2,4-trichlorobenzene is heated at 120°C for 7 h.
The precipitate obtained is purified by chromatography
on silica gel (eluent: CHaCl2 80%/ methanol 20%). The
compound obtained is dissolved in a 1 N solution of
NaOH, the insoluble material is filtered off and the
clear solution is acidified by bubbling SOz to give
2.4 g of a white precipitate of 7-hydroxy-5-propyl-6
[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-tri
azolo[1,5-a]pyrimidine.
Empirical formula: CZZHZON8OØ5H20.
Melting point: 260-265°C with decomposition.
1H NMR (DMSO-d6): 2.6 (t, 2H, propyl CH2)~ 8.2
(s, 1H, H2).
UV (MeOH): as = 210 nm
a6 = 250 nm.
i 7 ~~ :)
/-.' J. Vd 'v L
_ 84
Example 63: 5-Hydroxy-7-propyl-6-[(2'-(1H-tetrazol-5-
yl)biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
OS Formula (I): R1 = n-propyl, RZ = OH, X = N,
N-NH
N~
~N
Y = CH, R3 = \ I
~ mixture of 25 g of the compound obtained
according to Example 19 or 23, 750 ml of xylene and
34.5 g of trimethyltin azide is refluxed for 50 h. The
white precipitate obtained is filtered off. It melts
at 290°C with decomposition. This compound is suspen-
ded in 500 ml of THF. Gaseous hydrochloric acid is
bubbled in for 30 min to give a total solution, which.
is then concentrated under vacuum. The concentrate is
taken up in water and triturated. The gum obtained is
crystallized from acetonitrile. Recrystallization from
isopropanol gives 15.2 g of the expected derivative.
Melting point: 242°C.
The mother liquors are concentrated, the
concentrate is rendered basic with a 1 N solution of
KOH and extraction is carried out with chloroform,
followed by neutralization with acetic acid. The
precipitate obtained is recrystallized twice from
isopropanol to give 4.5 g of a second crop of the
compound 5-hydroxy-7-propyl-6-[(2'-(1H-tetrazol-5-yl)-
biphenyl-4-yl)methyl]-1,2,4-triazolo[1,5-a]pyrimidine.
Empirical formula: C22H2oN80.
Melting point: 242-244°C.
1H NMR (DMSO-d6): 2.91 (t, 2H, propyl CH2)1
8.11 (s, 1H, H2).
~. . .1. . ' , ... .,.. . ~~~, . ~ . ~~ .. , .. .
' . . :,. .. , ..: . . ~ " .. , :'. .~, : , .'
:~n.;,~;~
8 5 _ o-s .A~. i,~ v) t! a :.)
The following compounds of Examples 64 to 95
were prepared by one or other of the procedures des-
cribed in Examples 62 and 63.
05 Example 64: 7-Hydroxy-2-methyl-5-propyl-6-[(2'-(iH-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[1,5-a]pyrimidine hemisulfate
Formula (I): R1 = OH, R2 = n-propyl, X = N,
N-N H
//
i
~N
Y = C_CH3. R3 =
Empirical formula: C23H22N8O~0.5HZS04.
Melting point: 236'-238'C.
1H NMR (DMSO-ds): 2.6 (t, 2H, propyl CHZ).
UV (MeOH): as = 212.1 nm
ab = 250 nm.
Example 65: 5-Hydroxy-7-propyl-6-[(2'-(1H-tetrazol-5-
yl)biphenyl-4-yl)methyl]-1,2,4-triazolo-
[4,3-a]pyrimidine
Formula (I): R1 = OH, R2 = n-propyl,
X = CH, Y = N,
N-NH
//
~~N' /
R3 =
Empirical formula: CZZH2oN80.
Melting point: 251'C.
!-
FW. i~a V ~f f t~
- 86 -
1H NMR (DMSO-d6): 2.55 (t, 2H, propyl CH2);
9 (s, 1H, H3).
Example 66: 5-Propyl-7-mercapto-6-[(2'-(1H-tetrazol-5-
05 yl)biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = SH, R2 = n-propyl, X = N,
N-~N f-4
N
~N /
Y = CH, R~ _
Empirical formula: C22H2oN$S.
Melting point: 288°C.
1H NMR (DMSO-d6): 2.59 (t, 2H, n-propyl CH2);
8.6 (s, 1H, H2).
Example 67: 5,7-Dimethyl-6-[(2'-(1H-tetrazol-5-yl)-
biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = CH3, R2 = CH3, X = N,
N-NH
II
N~Ni /
Y = CH, R3 =
This compound was obtained by the procedure of
Example 62 using the 2,4-dioxo-3-[(2'-(1H-tetrazol-5
yl)biphenyl-4-yl)methyl]pentane prepared in Example 16.
Empirical formula: C21H18N8.
Melting point: 264°C.
:\
y .t :~ ;
~~ ~ 'r.r U i1 '~
_ 87
1H NMR (BMSO-d6): 2.48 (s, 3H, CH3); 2.81 (s,
3H, CH3); 8.56 (s, 1H, H2).
Example 68: 2-Ethyl-7-hydroxy-5-propyl-6-[(2'-(1H-
05 a tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4
triazolo[1,5-a]pyrimidine
Formula (I): R1 = OH, R2 = n-propyl, X = N,
Y = C-CH2-CHI,
15
N-NH
N~
~N
R3 =
Empirical formula: C24H2,~N8O.
Melting point: 246°C.
1H NMR (DMSO-d6): 2.57 (m, 2H, propyl CH2 +
DMSO-ds).
Example 69: 7-N,N-Diethylamino-5-propyl-6-((2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo(1,5-a]pyrimidine
'CH2-CH3
Formula (I): R1 = N ,
~CH2-CH3
R2 - n_proPYl. X = N. Y = CH.
N-NH
N//
aN
R3 = ~ I
Empirical formula: C25N29Ns~
:-~\
- ss - ~.~~L"'r~~ ' ,o
Melting point: 192~C.
1H NMR (DMSO-d6): 2.65 (t, 2H, n-propyl CH2);
8.5 (s, 1H, H2).
05 Example 70: 5-Azido-7-propyl-6-((2'-(1H-tetrazol-5-yl)-
biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, Rte= N3, X = N,
N-NH
n
N~ ~ /
N
Y = CH, R3 =
Empirical formula: C22H19Na1~
Melting point: 212-213~C.
1H NMR (DMSO-ds): 3.17 (t, 2H, n-propyl CH2);
4.06 (s, 2H, benzyl CH2,
azido/tetrazole equilibrium
10%); 4.47 (s, 2H, benzyl
CHZ); 8.56 (s, 1H, H2, azido/
tetrazole equilibrium ~ 10%);
8.7 (s, 1H, Hue).
Example 71: 3,5-Dihydroxy-5-propyl-6-[(2'-(1H-tetrazol-
5-yl)biphenyl-4-yi)methyl]-1,2,4-triazolo-
[4,3-a]pyrimidine
Formula (I): R1 = n-propyl, R2 = OH,
X = COH, Y = N,
s a .t cy ;
F.r r.r U y~ , ~,a
_ 89 _
N-N1~
N'~ ~
R3 = N
05
Empirical formula: C22H2oN802.
Melting point: 252'C.
1H NMR (DMSO-ds): 2.93 (t, 2H, n-propyl CHZ);
3.7 (s, 2H, benzyl CH2).
Example 72: 5-Hydroxy-2-methyl-7-propyl-6-[(2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[4,3-a]pyrimidine
Formula (I): R, = n-propyl, R2 = OH, X = N,
N-NH
N
'N /
Y = C-CHsr R3 = ~
Empirical formula: C23H22N8O.
Melting point: 286°C.
~H NMR (DMSO-d6): 2.85 (t, 2H, n-propyl CH2);
3.84 (s, 2H, benzyl CH2).
- Example 73: 2-Ethyl-5-hydroxy-7-propyl-6-[(2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[4,3-a]pyrimidine
Formula (I): R1 = n-propyl, R2 = OH, X = N,
Y = C-CH2CH3,
c~ ~i ,.~ ~ .,
... o~ a. :~~ J ,J ~ :l
90 -
Nr-NH
~N
R3 =
05
Empirical formula: C24H24N80.
Melting point: 260°C.
'-H NMR (DMSO-d6): 2.86 (t, 2H, n-propyl CH2);
3.85 (s, 2H, benzyl CHZ).
Example 74: 7-Butyl-5-hydroxy-6-[(2'-(1H-tetrazol-5-
yl)biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = n-butyl, RZ = OH, X = N,
N-NH
~N
Y = CHI R~ _ ~ I
ZO
Empirical formula: Cz3H22Na0.
Melting point: 255°C.
1H NMR (DMSO-dg): 2.92 (t, 2H, n-propyl CHZ);
3.86 (s, 2H, benzyl CH2);
8.11 (s, 1H, H2).
Example 75: 2-,amino-5-hydroxy-7-propyl-6-[(2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, Rz = OH, X = N,
;: :~ :, ;;~ r- ~
G.~ .~ i;. ~ .,~5
- 91 -
N-NH
//
N\ i
N
Y = C-NH2, R3 =
05
Empirical formula: C22H21N90.
Melting point: 282°C.
1H NMR (DMSO-d6): 2.76 (t, 2H, n-propyl CH2):
3.8 (s, 2H, ben2yl CHz).
Example 76: 5-N,N-Diethylamino-7-propyl-6-[(2~-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo(1,5-a]pyrimidine
Formula (I): R1 = n-propyl,
~CNZ-CH3
R? = N~ , x = N,
Cw2-CH3
2p N-NH
N~ ~
Y CH, R3 N
Empirical formula: C26H29N9~
Melting point: 140°C, then 205°C.
1H NMR (DMSO-d6): 2.91 (t, ZH, propyl CH2);
4.07 (s, 2H, benzyl CH2);
8.32 (s, 1H, H2).
Example 77: 5-Amino-7-propyl-6-[(2'-{1H-tetrazol-5-yl)-
biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, R2 = -NH2,
'~ .l :.. !1 ;~) P
~; ~ ., .~a ;y, '~ ~
_ 92 _
X = N, Y = CH,
N-NH
It
N~ ~
N
05 R _
3
Empirical formula: C22H21N~.
Melting point: 276~C.
1H NMR (DMSO-d6): 2.98 (t, 2H, propyl CH2)
4.03 (s, 2H, benzyl CH2)p 8.1
(s, 1H, HZ).
Example 78: 5-Hydroxy-2-mercaptomethyl-7-propyl-6-[(2°-
(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-
1,2,4-triazolo[1,5-a)pyrimidine
Formula (I): R1 = n-propyl, R2 = OH, X = N,
N-NH
~N
Y = C-SCH3, R3 =
Empirical formula: C23Hz2N80S.
Melting point: 260°C.
xH NMR (DMSO-d6): 2.85 (t, 2H, n-propyl CH2);
3.84 (s, 2H, benzyl CH2).
Example 79: 5-Hydroxy-7-propyl-6-[4-[3-(1H-tetrazol-
5-yl)-2-thienyl]benzyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, R2 = OH, X = N,
r,'.. .~ .sj ~ ~ ~ 7
- 93 -
Y = CH, R3 = N
05 N~~N~N H
Empirical formula: C2oH18N80S.
Melting point: 275~C.
1H NMR (DMSO-d6): 2.95 (t, 2H, n-propyl CH2);
3.91 (s, 2H, benzyl CHz);
8.12 (s, 1H, H2).
Example 80: 5-Hydroxy-7-propyl-6-[4-[3-(1H-tetrazol-5-
yl)-2-pyridyl]benzyl]-1,2,x-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, Ra = OH, X = N,
,N
N.,N
Y = CH, R3 = ~ N
N~
H
Empirical formula: C21H19N90.
Melting point: 244'C.
1H NMR (DMSO-ds): 2.91 (t, 2H, n-propyl CHa);
3.89 (s, 2H, benzyl CH2);
8.11 (s, 1H, Ha with pyridine
Ha).
- \s/
- 94 -
Example 81: 5-Hydroxy-2-methyl-7-propyl-6-[4-[3-(1H-
tetrazol-5-yl)-2-thienyl]benzyl]-1,2,4-
triazolo[1,5-a]pyrimidine
05 Formula (I): R1 = n-propyl, R2 = OH, X = N,
S
Y = C-CH3, R3 =
N-
NwN~NN
Empirical formula: CZiH2oN80S.
Melting point: 287°C.
1H NMR (DMSO-d6): 2.9 (t, 2H, n-propyl CH2);
3.89 (s, 2H, benzyl CH2).
Example 82: 7-Butyl-5-hydroxy-2-methyl-6-[(2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[1,5-a]pyrimidine
Formula (I): R1 = n-butyl, R2 = OH, X = N,
N-NH
i~
NON ~ /
Y = C_CHsi Rs = .\
Empirical formula: CZQHZQN80.
Melting point: 275aC.
1H NMR (DMSO-ds): 2.87 (t, 2H, n-butyl CH2);
3.84 (s, 2H, benzyl CH2).
ii ~~ :~ fl ~J "'~ :~
- 95 ° ~~.'."~J~a )
Example 83: 5-Hydroxy-2-hydroxymethyl-7-propyl-6-[(2'-
(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-
1,2,4-triazolo[1,5-a]pyrimidine
05 Formula (I): R1 = n-propyl, RZ = OH, X = N,
N-NH
II
N~ ~
N
Y = C-CHZOH, R3 =
Empirical formula: C23HZZN802.
Melting point: 274°C.
1H NMR (DMSO-d6): 2.88 (t, 2H, n-propyl CH2):
3.86 (s, 2H, benzyl CH2).
Example 84: 5-Mercapto-7-propyl-6-[(2'-(1H-tetrazol-5-
yl)biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, R2 = SH, X = N,
N-NH
NI
~N
Y = CH, R3 =
s
Empirical formula: C22H2oN8S.
Melting point: 278°C.
1H NMR (DMSO-ds): 2.87 (t, 2H, n-propyl CH2);
3.37 (s, 2H, benzyl CH2)p
8.29 (s, 1H, H2).
Example 85: 5-Hydroxy-7-methoxymethyl-6-[(2'-(1H-
tetrazol-5-yl)bip:henyl-4-yl)methyl]-1,2,4-
triazolo[1,5-a]pyrimidine
05 Formula (I): R1 = -CH2-O-CH3; R2 = OH,
X = N, Y = CH,
N--N H
N~
'N
R _
3
Empirical formula: C21Hs8N802~
Melting point: 264~C.
1H NMR (DMSO-ds): 3.91 (t, 2H, benzyl CH2);
4.79 (S, 2H, O-CHa); 8.12 (S,
1H, Ha).
Example 86: 7-Propyl-5-(pyrrolidin-1-yl)-6-[(2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, R2 = °N~ ,
X = N, Y = CH,
N-NH
Nl
'N
R3 =
Empirical formula: C26H2~N9.
Melting point: 280~C.
1H NMR (DMSO-ds): 2.94 (t, 2H, propyl CH2);
:t ,~ ;~ r~ p r) '~
r.~ ~;, ~ ;~, f ;o
- 97
4.22 (s, 2H, benzyl CH2);
8.18 (s, 1H, HZ).
Example 87: 5-Hydroxy-7-methyl-6-[(2'-(1H-tetrazol-5
05 yl)biphenyl-4-yl):~ethyl]-1,2,4-triazolo
[1,5-a]pyrimidine
Formula (I): R1 = -CHI, Ra = OH, X = N,
to ~N-NH
N~ ~
N
Y = CH, R3 =
15 Empirical formula: C~oH16N80.
Melting point: 248°C.
1H NMR (DMSO-d6): 2.56 (s, 3H, CH3); 3.86 (s,
2H, benzyl CHI); 8.11 (s, 1H,
Hz).
Example 88: 7-Ethyl-5-hydroxy-6-[(2'-(1H-tetrazol-5-
yl)biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = -CH2-CH3, RZ = OH, X = N,
N-NH
~N
Y = CH, R3 = ~
Empirical formula: C21H~SNsO.
Melting point: 245°C.
1H NMR (DMSO-d6): 2.94 (q, 2H, ethyl CH2); 3.87
(s, 2H, benzyl CH2); 8.12 (s,
f ~ W
gg _
1H, H2).
Example 89: 2-N,N-Diethylamino-5-hydroxy-6-[(2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-~.,2,4-
05 triazolo[1,5-a]pyrimidine
Formula (I): R1 = n°propyl, RZ = OH, X = N,
~CH2-CH3
Y - C~N~
1o CH2-CH3
N-NH
N
'N /
R3 =
Empirical formula: C26H29NgO.
Melting point: 207°C.
1H NMR (DMSO-ds): 2.79 (t, 2H, n-propyl CH2);
3.80 (s, 2H, benzyl CH2).
Example 90: 5-(Morpholin-4-ylethylamino)-7-propyl-6-
[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)-
methyl]-1,2,4-triazolo[1,5-a]pyrimidine
Formula (I): R1 = n-propyl,
R2 = NH-CH2-CH2-N O ,
X = N, Y = CH,
N-NH
ii
N~ ~
R3 = N \
-'. , :.~:~~-~«
- is .~. i~ :j ;.' ! v.)
Empirical formula: CleH32N1~0.
Melting point: 236°C.
'-H NMR (DMSO-d6): 2.99 (t, 2H, n°propyl CH2);
4.02 (s, 2H, benzyl CH2);
05 8.13 (s, 1H, Hs).
Example 91: 5,7-Dipropyl-6-[(2'-(1H-tetrazol-5-yl)bi-
phenyl-4-yl)methyl]-1,2,4-triazolo[1,5-a]-
pyrimidine
Formula (I): R1 = n-propyl, RZ = n-propyl,
X = N, Y = CH,
N-NH
N~[~s
R3
Empirical formula: C?SHZ~Ne.
Melting point: 226°C.
1H NMR (DMSO-ds): 2.66 (t, 2H, n-propyl CHZ);
3.15 (t, 2H, n-propyl CH2);
4.14 (s, 2H, benzyl CHZ);
8.26 (s, 1H, HZ).
Example 92: 7-Propyl-6-[(2'-(1H-tetrazol-5-yl)biphenyl-
4-yl)methyl]-1,2,4-triazolo[1,5-a]pyrimi-
dine
Formula (I): R1 = n-propyl, R2 = H, X = N,
N-NH
N~
~N
Y = CH, R3 =
sz.; :?n~~!~
~,r ~. ~,~ C) _~ t '_a
- 100
Empirical formula: C2.~H2oN8.
Melting point: 238~C.
1H NMR (DMSO-d6): 3.16 (t, 2H, n-propyl CH2);
4.21 (s, 2H, benzyl CHZ);
05 8.65 (s, 1H); 8.82 (s, 1H).
Example 93: 7-Benzyloxymethyl~-5-hydroxy-6-[(2'-(1H-
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[1,5-a]pyrimidine
Formula ( z ) . R1 = CH2-O-CH2 ~ ~ ,
Ra = OH, X = N, Y = CH,
1s N-NH
~N
R3 =
Empirical formula: C2~H22N802°
Melting point: 270-5°C (decomposition).
1H NMR (DMSO-d6): 3.86 (s, 2H, benzyl CHZ};
4.62 (s, 2H, O-CHZ); 4.88 (s,
2H, O-CH2); 8.11 (s, 1H, H2).
Example 94: 5-(Pigeridin-1-yl)-7-propyl-6-[(2'-(1H°
tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4-
triazolo[1,5-a]pyrimidine
Formula (I): R~ = n-propyl, R2 = N
X = N, Y = CH,
~r i "~ U ;'? Y,1 1
- 101
N-NH
//
N..N i
R3 =
05
Empirical formula: CZ,H2~N9.
Melting point: 266°C.
1H NMR (DMSO-d6): 2.88 (t, 2H, n-propyl CHZ);
4.09 (s, 2H, benzyl CH2);
8.35 (s, 1H, H2).
Example 95: [a-Propyl-6-[(2'-(1H-tetrazol-5-yl)bi-
phenyl-4-yl)methyl]-1,2,4-triazolo[1,5-a]-
pyrimidin-5-yl] 2-methoxyethyl ether
Formula (I): R1 = n-propyl,
RZ = O-CHa-CHZ-O-CH3~ X = Nr
N-NH
//
2o N~N~ /
Y = CI-I, R3 =
Empirical formula: C25HZSI'IsOa~
Melting point: 224°C.
1H NMR (DMSO-ds): 3.14 (t, 2H, n-propyl CH2);
4.04 (s, 2H, benzyl CHa);
8.41 (s, 1H, H2).
Example 96: 5-Chloro-7-propyl-6-[(2'-(1H-tetrazol-5-
yl)biphenyl-4-yl)methyl]-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (I): R1 = n-propyl, R2 = C1, X = N,
- ~ ;1 ;, ,) rl ,~
- 102 -
[~i ° PJ H
a~
Y = CH, R3 = \
05
Obtained by diazotization of the derivative of
Example 77 and treatment of the diazonium salt with
cuprous chloride according to the classical Sandmeyer
reaction.
Example 97: 6-[(2'-Aminocarbonylbiphenyl-4-yl)methyl]-
5-hydroxy-7-propyl-1,2,4-triazolo[4,3-a]-
pyrimidine
Formula (I): R1 = n-propyl, R2 = OH,
X = CH, Y = N,
O
I I
R3 = H2N~C
2 g of the compound of Example 20 d) in 200 ml
of 1 N NaOH are refluxed for 4 h. The mixture is con-
centrated under vacuum and the concentrate is acidified
with 200 ml of 1 N ~HC1. The crystals obtained are
purified by recrystallization from 2-methoxyethanol to
give 1.6 g of 6-[(2'-aminocarbonylbiphenyl-4-yl)
methyl]-5-hydroxy-7-propyl-1,2,4-triazolo[4,3-a]pyrimi
dine .
Empirical formula: C2ZH21N5O2'
Melting point: 258°C.
1H NMR (DMSO-d6): 2.61 (t, 2H, n-propyl CH2):
3.9 (s, 2H, benzyl CHZ)p 9
(s. 1H. H3).
.~ i1 ~~, ~ r~ n
F.: X ,,~ ~J :.~ t ~_~
- 103
Example 98: 6-[(2'-Carboxybiphe:nyl-4-yl)methyl]-5-
hydroxy-7-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine
05 Formula (I): R~ = n-propyl, R2 = OH,
X = N, Y = CH,
COON
R3 =
A mixture of 9.4 g of the product obtained in
Example 97, 200 ml of ethylene glycol and 20 ml of con-
centrated NaOH is refluxed for 10 h. The ethylene
glycol is distilled, 200 ml of water are added and the
mixture is acidified with a solution of HC1. The crys-
tals obtained are purified by recrystallization from 2--
methoxyethanol to give 5.8 g of 6-[(2'-carboxybiphenyl-
4-yl)methyl]-5-hydroxy-7-propyl-1,2,4-triazolo[1,5-a]-
pyrimidine.
Empirical formula: C22HaoNa~a~
Melting point: 265°C.
1H NMR (DMSO-d6): 2.96 (t, 2H, n-propyl CH2);
3.92 (s, 2H, benzyl CH2);
8.12 (s, 1H, H2).
The compound of Example 99 was prepared by the
procedure of Example 23.
Example 99: 6-[4-(5-Bromo-3-cyano-2-furyl)benzyl]-5-
hydroxy-7-propyl-1.,2,4-triazolo[1,5-a]-
pyrimidine
Formula (VIIb): R'1 = n-propyl, X = N,
~~ ..3. i.~ vi 9 6 ~I
- 104 -
Y = CH, R1o = OH,
NC
r\
05
Br
Melting point: 262°C.
Example 100: 6-[(2'-Cyanobiphenyl-4-yl)methyl]-5-
hydroxy-7-hydroxymethyl-1,2,4-triazolo-
[1,5-a]pyrimidine
Formula (VIIb): R'1 = CH20H, X = N,
Y = CH, R1o = OH,
NC
V= ~
A solution of 9 g of the compounds prepared in
Example 43 in 360 ml of acetic acid is reduced by cata-
lytic hydrogenation in the presence of 1.8 g of 5%
palladium-on-charcoal. The reaction is carried out at
atmospheric pressure and at 50°C. The catalyst is fil-
tered off on Celite 545 and washed with acetic acid and
the filtrate is concentrated and then purified by
chromatography on silica gel (eluent: chloroform 95%J
methanol 5a) to give 4.5 g of the starting material arid
2.2 g of 6~-[(2'-cyanobiphenyl-4-yl)methyl]-5-hydroxy-7-
hydroxymethyl-1,2,4-triazolo[1,5-a]pyrimidine.
Melting point: 262°C.
This same compound can also be obtained by
reaction with BBr3 in chloroform.
isr I ;
- 105 -
The following derivatives of Examples 101 and
102 were prepared by the procedure of Example 63.
Example 101: 5-Hydroxy--7-hydroxymethyl-6-((2'-(1H-
os tetrazol-5-yl)biphenyl-4-yl)methyl]-1,2,4
triazolo(1,5-a]pyrimidine
Formula (I): R1 = CH20H, R2 = OH, X = N,
l0 N-N H
Il
N
Y = CH, R3 = ~
15 Empirical formula: C2oH15N5O2.
Melting point: >360°C (decomposition).
1H NMR (DMSO-ds): 3.93 (s, 2H, benzyl CHZ);
4.82 (s, 2H, CH2-O); 8.06 (s,
1H, H2).
Example 102: 6-[4-[5-Bromo-3-(1H-tetrazol-5-yl)-2-
furyl]benzyl]-5-hydroxy-7-propyl-1,2,4-
triazolo[1,5-a]pyrimidine
Formula (I): R~ = n-propyl, R2 = OH,
X = N, Y = CH,
N-N
N! t
R3 =
Br
' Empirical formula: C2oHI~BrNg02.
.~ .t cp ~y r~ '
' ' (.v ~ .a
~d A ta! V...'
- 106 -
Melting point: >360~C.
~H NMR (DMSO-d6): 2.93 (t, 2H, n-propyl CH2);
3.92 (s, 2H, benzyl CH2); 6.9
(s, 1H, furan proton); 8.03
05 (s, 1H, H2).
PI3ARMACO'.LOGY
A ~ Study of the adrenal angiotensin II receptors
I. Principle
The affinity of the products of the Examples
for the angiotensin II receptors is evaluated by the
technique of displacing a radioligand specifically
bound to rat adrenal angiotensin II receptors.
II. Procedure
An aliquot of a rat adrenal gland homogenate
incubates in the presence of a single concentration of
~125I]_SIAII (Sarl,Tyr4,Ilee-angiotensin II), which is
an angiotensin II receptor antagonist, and two concen
trations of competing agents (10-5 M, 10-~ M) for 60
min at 25~C.
The reaction is completed by the addition of a
buffer, followed by rapid filtration through glasspaper
filters. The non-specific binding is determined in the
presence of angiotensin II.
III. Expression of the results
The results are expressed, for the concentra-
tions tested, as the percentage displacement of the
radioligand specifically bound to the adrenal angio-
- 107 -
tensin II receptors.
IV. Results
..~ v,~ l) (~,j 7~! '~ 1
05
Product of % displacement
of the labeled
ligand
1E-5M 1E-7M
Example 62 65 52
Example 63 61 52
Example 65 63 47
Example 68 69 59
Example 69 69 19
Example 75 61 60
Example 76 63 28
Example 77 63 31
Example 79 58 26
Example 82 58 11
B ~ Measurement of the inhibition of the cell prolife
ration induced b~ growth factors (example: Platelet
Derived Growth Factor, or PDGF) in rat aorta smooth
muscle cells
I. Principle
The inhibition of the cell proliferation
induced by a growth factor (example: PDGF) is evaluated
by measuring the incorporation of 3H-thymidine in rat
aorta smooth muscle cells (VSMC).
II. Procedure
The VSMC are cultivated at 37'C in 5% C02 until
subconfluence is reached, and are then placed for 24
hours at rest in a serum-poor medium. They are subse-
quently pretreated for one hour with the test molecule
(10-4 bI) and then stimulated for 22 hours with a growth
G.~ .~ :,. ,j ~?
- 108 -
factor (example: PDGF). 3H-Thymidine is incorporated
during the last 4 hours. All these steps are performed
at 37~C in 5% C02.
The reaction is terminated by sucking off the
05 reaction medium, detaching the cells and then filtering
the lyzed cells through glassfiber filters.
III. Expression of the results
The results are expressed as the percentage
inhibition of the stimulation of incorporation of 3H-
thymidine due to the action of the growth factor.
IV. Results
Product % inhibition of the incorporation
of
of 3H-thymidine induced by PDGF
1E-4M
Example 100
69
TOXTCOLOGY
The products of the Examples described have an
excellent tolerance after oral administration.
Their 50% lethal dose in rats was found to be
greater than 300 mg/kg.
CONCLUSION
The products of the Examples described have a
good affinity for the angiotensin II receptors. In
this respect they may be used beneficially for the
various pathological conditions in which angiotensin II
is involved, in particular for the treatment of arte-
~\
- 109 - ! ~)
~ ~ .~ i,W~ :j
rial hypertension and cardiac insufficiency, in dosages
of 1 to 400 mg by oral administration and of 0.01 to 50
mg by intravenous administration, in one or more dosage
units per day. Furthermore, some of the compounds also
05 have an antiproliferative activity and in this respect
are of potential value in the treatment of prolifera-
tive diseases such as atherosclerosis.
15