Note: Descriptions are shown in the official language in which they were submitted.
21307fi7
C~SE 5371
SO~NOJ~g nEMOVAL ~nOM ~LUE GAS BY AMMOlnA
WET SCRUBBlI~G USlI~G IBON ~,'H~;-.~TE CATALYSTS
BACKGnOUl~D 0~ T~E INVE~TIO~
1. ~leld of * P l~ver~on
The present invention relates in general to the
~contAm;nAtion of flue gas and in particular to a new and
5 useful method and system for removing various decontAm;nA~ts
from flue gas through the use of ~mnnl ~ and iron chelate
catalyst sc~lhh;n~. -
2 . ~)esc~io~ of ~ P,~Ls~ed ~.t
In the pcwer plant field, it is ~,.,~"l to use A~n~;A in
order to scruh or clean the cQnt~;nAnt.~ from flue gas. Someof the cont~m;nAnt~ contA;n~A in flue gas ~,~Lise NOx, SO2,
Hg, etc. Past laboratory studies have indicated that iron
chelates such as Fe2+ EDr~ ~Ethyl~e~; A~l ~tetraacetate)
catalysts can assist in ~mmonl ~ scrubbing of flue gas for
removing NOx. In turn, several systems and methods were
developed using iron chelates as catalysts for assisting in
NOX removal.
U.S. Patent 4,079,118 discloses a method for removing
nitrogen oxides using Ferric ion ~l~ ccmplex solutions. This
- 21~0767
C~SE 5371
reference focuses on using Ferric ion for ~n~;,3 scrubbing
for the removal of NOX only.
U.S. Patent 4,167,578 t~A~h~ a method of calcium
scrubbing using an iron EDI~ chelate for removing NOX only.
The process disclosed, however, utilizes a ccmplex hydrolysis
of Ca(NH2SO3) 2 in which ~mmnn;um sulfate is pro~-3c~. This
~nn;~ recovery through stripping can be rather expensive.
U.S. Patents 4,288,421 and 4,255,401 teach a scrubbing
process using potassium sulfite and iron chelate salt for NOX
and SO2 scrubbing involving a complex recovery process which
produces sulfur, potassium ~r3rhnn~te a3~d ~mnn;,3 -U.S. Patent
5,106,601 provides for a NOX and SO2 removal process using
phosphates emulsicn, pr~ferably in a wet scrubber, without a
chelating agent. The by products of this process are calcium
and ammonia phosphate.
Presently, there are no known processes or systems for
efficiently removing SOx, NOX and Hg from the flue gas in an
~mmnn;~ wet scru~ber through the use of an iron chelate
catalyst.
The present invention pertains to method of removing
cont~;n~nt~, such as NOX, SOx, Hg from a flue gas. The
~130767
CASE 5371
present invention o~l~ises providing the flue gas to an
absorber tank or serubber reaetor. A metal ehelate eatalyst,
sueh as iron ethyl~n~ mi n~tetraacetatel Fe2+ ~lA, is
provided to an ~mnn;~ solution, whieh is in turn provided to
the absorber tank for mixing with the flue gas. After mlxing
the ~mnn;~ and eatalyst solution with the flue gas, the
solution is oxidized and the metal ~h~l~te eatalyst is
separated from the solution.
me present invention also pertains to a system for
removing eont~m~n~nt~ sueh as SOx, NOX and Hg from a flue gas
whieh e~l~rises an absorber tank having a flue gas inlet for
reeeiving the eont~minated flue gas and a flue gas outlet for
passing a elean flue gas from the tank. me absorber tank
also has an exit for passing oxidized solution from the
absorber tank. A reeyeler is used to separate the metal
ehelate eatalyst from the used solution baek into the system.
The various features of novelty whieh eharaeterize the
invention are pointed out with partieularity in the elaims
annexed to and forming a part of this diselosure. For a
better underst~n~;ng of the invention, its operating
advantages and specific objects att~ne~ by its uses,
referenee is made to the ae~ n~ing drawings and deseriptive
matter in which a preferred embo~; m~nt of the invention is
illustrated.
`- 21~076~
C~SE 5371
RRn~F nF~ llCX~ OF ll~FJ n~vvn~(~s
In the drawings:
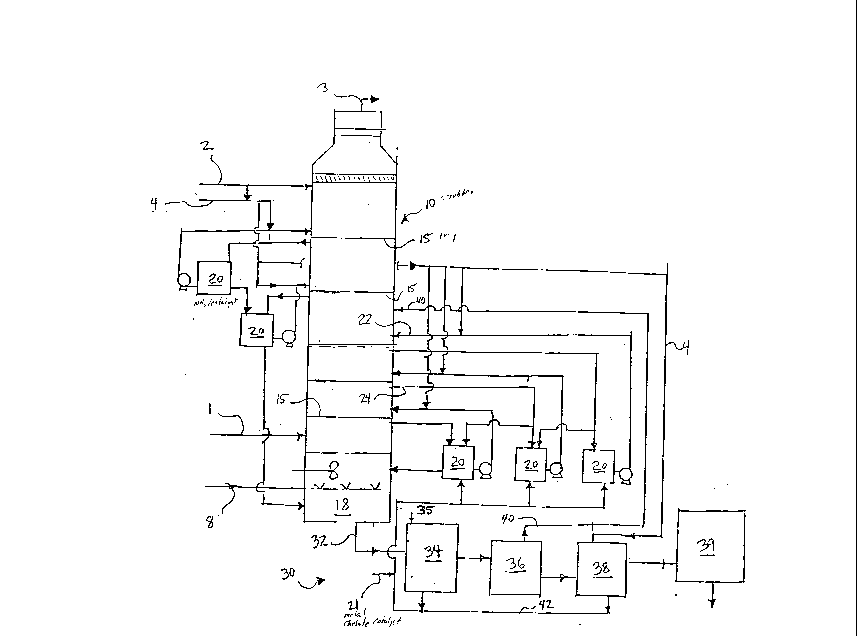
Fig. 1 is a schematic view illustrating a system according
to the present invention.
nE~ h~ CX~ o~ F ~ hh~RI~ F~ODrVn~TS
The present invention, as illustrated in Fig. 1,
~ ises a method and system for removing SOx, NOX and Hg from
a flue gas by amm~n;~ wet scrubbing using an iron chelate
catalyst. The system ~ ises an absorber or scrubber 10
having ~ flue gas inlet 1 which provides flue gas eont~;n;ng
SOx~ NC~ and Hg to the absorber 10. The absorber 10 has a
plurality of trays 15 located within the ~h~orher 10. An
ammonia and catalyst solution 20 is provided to the absorber
10 near the trays 15 through an inlet 22 located near each
tray 15. A metal chelate catalyst, such as iron E~lA 21 is
provided along with an ~n~i~ solution 4 which makes up the
ammonia brine s3lution 20 which eQ~t~;n.~ both ammonia solution
and the iron ~Ll~ catalyst. The ammonia brine solution 20 is
regulated at the ~h~orh~r 10 by both the inlets 22 and outlets
~os~
24 which are also located near the trays 15. ~mn~;~
brine solution 20 is penmitted to flow through outlets 24 back
to their original source.
2130767
C~SE 5371
The trays 15 are .~r~c~ at least four feet apart from
each other in order to m;n;m;~ entr~;nm~nt and optimize mass
transfer effectiveness within the absorber 10. The present
invention does not require any spray nozzles and thus the
trays 15 are sufficient for providing absorption of the flue
gas by the ~m~n;~ brine solution 20. Am~n~;~ 4 and water 2
are also provided to absorber 10 for m~;nt~;n;ng sufficient
ammonia conc~ntration control at the different stages 15 for
obtaining the required SO2/NH3 vapor efficiency which is
dep~n~t upon the liquid conc~ntration of bisulfite/sulfite
and ~mm~n;~ 4.
Onee the flue gas is mixed at each tray 15 with the
~m~ia brine catalyst solution 20 for cl~n;~ the flue gas,
the solution 20 collects at the bottom of the absorber 10 in
an oxidation zone 18. At this point, the solution 20 in
oxidation zone 18 cont~;nR ~hsorhed cont~m;n~t~ such as NOX,
Sx and Hg and is exposed to oxidation air 8 for oxidizing the
absorbed solution.
m e catalys~ c~nc~ntration 21 ranges from 0.05 to 0.30
moles/liter (Fe~+ EDTA) in the liquor 20 dep~ upon the gas
phase SO2 and NOX co~c~ntration range (SO2 500-3000 ppm, NOX,
100 - 600 ppm typical range). me oxidation step is easily
~c~mpl;.~he~ at the bottom of the tower 10 and is controlled
for the optimum operation. me basic ~h~mi.~try will not
2130767
C~SE 5371
change significantly.
Nitric oxide (NO), normally insoluble in water, is
absorbed in a bisulfite solution at a moderately low pH, where
the NO is catalytically r~ by bisulfite to ammonium ions.
Bisulfite }s oxidized in this reaction to dithionate ion and
imido ~ nd as shown by equations (1) and (2) listed below.
catalyst
NO(g) + H20 ~~~O(abs) (1)
~ catalyst ~ ,t
NO~s) + 3NH4 HS03 - (NH4) 2 NH(S03) 2 + 1/2 (NH4) 2 S26 + H20
(lmldo) (dlth1onate) (2)
catalyst
2NH4 HS03 + 1/2 2 - (NH4)2 S26 + H20 (3)
(NH4) 2 S03 + 1/2 2 - (NH4) 2 SO4 (4)
Mercury (Hg) is also absorbed in the solution in the
presence of chelate catalyst.
These side reactions in equations (3) and (4) reduce the
availability of bisulfite for the desired reaction. In the
presence of the catalyst 21, dithionate formation is
30 pr~mlnAnt over the sulfate formation. Ammonia 4 is supplied
to the absorbent solution 20 in order to m~;~t~in pH values
between 5.5 to 6.5 in the ab~lL~r 10. The clean flue gas
leaves the absorber at outlet 3 and may be acid w~.~h~ and
reheated to reduce plume fonmation.
2130767
C~SE 5371
The spent ~mm~n~ A brine solution 20 eolleeted at the
oxidation zone 18 exits the ~h~orh~er 10 at outlet 32 loeated
near the oxidation zone 18 at the bottom of absorber 10 for
providing the spent brine solution to a eatalyst reeovery
system 30. The spent brine solution from the absorber bottom
is oxidized and ~ne~n~rated prior to it being sent to the
eatalyst reeovery seetion 30. A eatalyst reeoverer 34, auch~
a e~ntrifugc, reeeives the spent brine solution 20, whieh
has beenboxHidized~ and subjeets the spent solution to
aeidifying~an~ eooling in order to erystalize the ehelating ~,~n ~/61
agent 21 prior to sephara ~ n by the~ceentrifuge 3~. The
separated solution is tQa to ~ C in autoelave where
dithionate and imidosulfonate are hydrolysed to deecmpose into
sulfate and bisulfate at a ~ro~oser 36 as shown ~y equations
15 (5) and (6) below. f~R
(N~)~ S
~3Nl~ + 2H2O - (NH4) 2 SOg + NH4 HSO4 (5)
(N~ ) 2 S26 - (N~) 2 SO4 + SO2t (6)
Liberated SO2, repres~nt~ at 40, is separated at
decomposer 36 and is reeyeled to absorber tower 10. The
acidic solution is then neutralized by ~mmn~;~ in a
neutralizer 38 so as to convert ~m~nn;um bisulfate to ammonium
sulfate. Hy~roxide 42 is separate from the solution and is
recycled to the absorber 10 along with eatalyst 21 as
recovered.
A~mn~; um sulfate in solution is e~d~o~ated by steam
heating and (NH4)2 S04 iS crystallized out and filtered by an
ammonium sulfate recoverer 39 such as a filter.
Clear scrubbing solution is used for both SO2 and NOX
2 13 0 7 6 7 CASE 5371
removal in the tray tower system 10. The number of stages or
trays 15 can be r~ r~ by using ~ ,ietary type trays. Air
toxic metals such as mercury (Hg), are also removed
effectively. Total liquid to gas ratio (L/G) will not exceed
40-45 for 95~ SO2 removal and 80-90~ NOX removal ~r~n~;ng upon
the inlet conc~ntrations. me byproduct brine can be disposed
of as fer~ r.
Am~nniA fumes can be re~nc~ to eomply with opacity by
acid w~hing of the mist el;m;nAtor, or reheating before the
stack emission. Also, a proven wet electrostatic precipitator
can be used to reduce fine particulates from the scrubber
system. me nitric oxide (NO) is converted to ammonia 4 by
the chelating agent 21 and this r~nce~ make-up reagent
~mo~l~. me absorption efficiency for NOX increases with
lower oxygen eoncentration and increases with higher S02
conc~ntration. S03 and HCl gases arse also reduce~
subst~nt'~lly by ~ serubbing at the~inlet sectionq~ y
In a normal dry seleetive eatalytic r~llc~r SCR, the
limitation for higher SO3/SO2 due to bisulfate formation and
perlodic repl~"~"~ of catalyst is required. Some trace
metals are likely to be poisoned and plug up the pores.
SOx/NOx/mercury removal (air toxic) using the present invention
with m;n;ml~ field modification is also possible. Also,
partieulate removal efficiency is high due to more numbers of
stages and m;ni~ ~ ~ solids (~1~3 in slurry in scrubbing
solution.
While a ~r~c;fic ~ho~;m~nt of the invention has been
shown and deseribed in detail to illustrate the application of
the principles of the invention, it will be understood that
the inv~nt;~n may be ~mho~;ed otherwise without departing from
sueh principles.