Note: Descriptions are shown in the official language in which they were submitted.
-- ~ILE P~N 1
~ TR A N~ LAT I O N f~ r~
DESCRIPTION
ELECTRODE AND SECONDARY BATTERY USING THE SAME
Technlcal Fleld
The present lnventlon relates to an electrode uslng
carbon f-bers and a chargeable/dlschargeable secondary battery
uslng the same.
Background Art
In recent years, small secondary batteries havlng high
capacltance have been remarkably demanded wlth the spread of
portable devlces such as vldeo cameras and notebook-type personal
computers. Most of the secondary batterles curren~iy used are
nlckeL-cadmlum batterles whlch use alkallne electrolytic
solutions. Such secondary batterles, however, show low ~attery
voltages of about 1.2 V, and therefore are difflcult to be
lmproved ln energy denslty. Under these clrcumstances, it has
been :Lnvestlgated the hlgh energy-type secondary batterles uslng
llthlum metal, whlch ls the basest metal, for negatlve electrode.
However, the secondary batterles ln whlch llthlum metal ~ -~
ls used for negatlve electrode have disadvantages such that the
llthlum develops to dendrltes by the (re)charglng/discharglng
cycle~ whlch may cause a short clrcult and further cause the
danger of lgnltlon of the batterles. In addltlon, as llthlum
me~al used ln a secondary battery ls very actlve, such a battery
ltself lnvolves hlghly dangerous factors. Therefore, they are
quest:Lonable ln domestlc appllcablllty. In order to solve the
problems relatlng to safety descrlbed above, llthlum lon
secondary batterles uslng varlous carbonaceous materials have
been proposed recently, by whlch hlgh energy lnherent to the
- 2 - ~ 8~7
llthlum electrode can be ylven. The secondary batterles of thls
type are devlsed by utlllzlng the phenomenon that, slnce the
carbonaceous materlal doped wlth llthlum lons at charglng comes
to have the same electrlc potentlal as metal llthlum, the
carbonaceous materlal doped wlth llthlum lons can be used for
negatlve electrode in place of metal lithlum. In thls type of
secondary battery, when dlscharged, the llthlum lons whlch have
been doped to the carbonaceous material are dedoped from the
negative electrode and go back to the carbonaceous materlal to
whlch the llthlum lons have been doped orlginally. Therefore,
the use of carbonaceous materlal doped wlth llthlum lons for
negatlve electrode never causes the problem of dendrlte
productlon, and furthermore glves excellent safety slnce metal
llthlum ls not present; therefore has now been investlgated
extenslvely.
As the secondary batterles utlllzlng the doplng of
llthium lons to carbonaceous materlal, those have been known, for
example, dlsclosed in Japanese Patent Appllcatlor. ~ald-open Nos.
90861/1987 and 122066/1987. The carbonaceous materlals used ln
the references above are generally ln a form of powder, and
therefore ls requlred to be lncorporated wlth a polymer as a
blnder such as Teflon and poly(vlnylldene fluorlde) for moldlng
lnto an electrode. That ls, an electrode can be prepared ln the
manne!r that a powdery carbonaceous material ls mlxsd wlth a
blnder and then adhered to a metal mesh, or applled on a metal
foll as a slurry. On the contrary, as for carbon flbers, there
has been no precedent ln whlch carbon flbers are practlcally used
for electrodes of secondary batterles lndustrlally. Therefore,
the form or structure of electrode to be preferably employed or
the preparatlon technlque of such electrode has been quite
- 3 _ ~13 08 Q7
unkn~wn. In particular, the most serious technical problems are
how to shape carbon fibers into an electrode, how to take the
electrica:L contact of the carbon fiber with a current collector,
how to so.Lve a problem of electrlcal short circuit between a
posltlve electrode and a negatlve electrode caused by the
penetration of fluffs of the carbon fibers through a separator,
and so on
However, when carbon fibers are used in a form of non-
woven fabric or woven fabric, the electrode can be prepared
wlthout or, lf any, a trace amount of a binder. In addition, it
is recognlzed that the use of carbon fibers for electrode is
excellent with respect to chemical stability against
electrolytes, structural stability against volume expansion
caused by doping, cyclicity of (re)charging and discharging, and
so on. As the secondary batteries using such electrodes, those
have been known, for example, disclosed in Japanese Patent
Application Lald-open Nos. 54181/1985 and 103991/1987. The
electrode~; using carbon flbers as descrlbed above, however, have
a defect that the electrlcal connectlon with a metal, l.e. a
taklng-out. electrode~ becomes difficult. In case of a carbon
powder electrode, since the carbon powder is mlxed with a binder
and then adhered to a metal mesh or applied on a metal foil as a
slurry as descrlbed above, the metal mesh or metal foll can be
used as a collectlng electrode for the connection wlth a
termlnal. On the contrary, in case of a carbon flber electrode,
lt has been trled to lnsert the ends of the carbon flbers lnto a
mesh-~haped or foll-shaped current collectlng metal electrode to
be fixed. However, the carbon flber tend to become lnto pleces
and to be broken easily, and consequently the use of the carbon
flbers ls stlll dlsadvantageous ln workablllty in preparatlon of
~ 4 ~ 21~8~7
electrode, as well as mechanical strength and durablllty of the
resul~ing electrode. Further, such problem also occurs that the
fluffs, i.e. broken flbers, penetrate through a separator to
cause the electrlcal contact between a positlve electrode and a
negatlve electrode, whlch resultlng ln an lnternal short clrcult.
Furthermc,re, such problem occurs that, slnce the carbon flbers
are merely~ lnserted lnto the current collecting metal electrode,
the voltaqe to be applled to the carbon flbers dlffers from that
applled to the carrent collecting metal electrode due ~o the
contact reslstance of the carbon flbers. Thls can be detected by
the phenomenon that the voltage returns lmmedlately to the
lnltlal state when the appllcatlon of voltage ls stepped, ln
other worcls, the lncrease in so-called overvoltage, and so on.
Stlll furt.her, such problem occurs that, when the surface area of
the electrode ls lncreased, the dlfference ln potentlal at the
polnts far from the current collectlng metal electrode becomes
large due to the reslstance of the carbon flbers and, as the
result, unlform doping and dedoping hardly occurs.
Brlef Descrlp_ on of Drawings
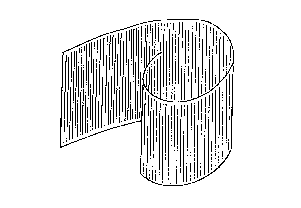
FiLg. l ls a schematlc lllustratlon of an embodlment of
the electrode according to the present lnvention, ln which an
electroconductlng foll is applled to a carbon flber sheet.
FiLg. 2 ls a schematlc lllustratlon of another embodlment
of the electrode accordlng to the present lnventlon, ln which an
electroconductlng foil ls applled to a carbon flber sheet.
F:Lg. 3 ls a schematlc lllustratlon of another embodlment
of the electrode accordlng to the present lnventlon, ln which
electroconductlng wlres are arxanged ln carbon flbers ln the
parallel dlrectlon ~o the flbers.
. .
- 5 - ~13~8~7
Flg. 4 is a schematlc illustration of another embodimen~
of the el,ectrode according to the present invention, in which the
carbon fllbers are woven in networks of electroconductlng wlres.
In Flgs. 1 to 4, 1 stands for a carbon flber, 2 for a
electroconductlng wlre, 3 for the dlrection of the taklng-out
elect.rode, 4 for a carbon flber sheet, and 5 for an
electroconductlng wlre, respectlvely.
Best Mode for Carrylng Out of the Invention
In order to solve the problems descrlbed above, the
present inventlon ls constructed as the followlngs.
That ls, the first lnventlon of the present appllcatlon
ls to provlde an electrode comprlslng an unl-dlrectionally
arranged body of carbon flbers and a secondary battery uslng the
same. In the present inventlon, the form ln which the carbon
flbers are arranged ln an unl-axlal dlrectlon can glve excellent
packed density and handllng property of the carbon flbers. In
thls case, lt ls preferable that the carbon fibers are placed ~
unlformly. If there exlst some uneven areas in the carbon flber
arrangement, unlform doplng can sometlmes not be glven.
The electrode accordlng to the second lnventlon of the
present appllcatlon ls characterlzed by comprlsing a carbon flber
sheet and a foll or wlres havlng electrlcal conductlvlty.
The practlcal modes prefera~ly employed in the present
lnventlon are lllustrated concretely ln the followings wlth
reference to the drawlngs attached.
Flg. 1 lllustrates an embodlment of the electrode of the
present lnventlon, ln whlch carbon flbers are arranged ln an unl-
axlal dlrectlon and an electroconductlng foll ls applled thereto.
Flg 3 lllustrates another embodlment of the elec.rode of the
- 6 - ~13 ~8a7
present invention, in whlch carbon flbers are arranged ln an uni-
axlal dlrectlon and electroconducting wlres are also arranged ln
the same dlrectlon as the carbon fibers. In Flg. 3, 1 stands for
carbon flbers, 2 for electroconductlng wlres represented by metal
flbers, and 3 for the dlrection connectlng wlth the taklng-out
electrode, respectlvely. In the case where the carbon flbers are
arranged iLn an unl-axlal direction as shown ln these drawlngs, by
arranglng the electroconducting wlres ln the dlrec~lon vertlcal
to the dlrectlon of the carbon flbers so that the carbon flbers
are bound wlth the electroconductlng wlres, the carbon flbers
come to be flxed to some extent; whlch ls the more preferable
mode for practlce. In additlon, as shown ln Fig. ~, by weavlng
the carbon flbers arranged ln an unl-axlal dlrectlon lnto the
networks of electroconductlng wlre, not only the carbon flbers
can be prevented from becomlng lnto pleces, but also the
e~ectrlcal collectlon efflclency becomes well.
In order to lmprove the electrlcal conductlvlty wlth the
carbon flbers, a method ln whlch a sheet-shaped carbon fibers
arranged in an unl-axlal dlrectlon are closely adhered on an
electroconductlng foll represented by a metal foll ls preferably
employed. Thls method can be carrled out, for example, by
adherlng a part or all of the carbon flbers on a metal foll under
compression by means of roll press and the llke, or adherlng the
carbon flbers on a metal foll uslng a small amount of a resln
such as Teflon and poly(vlnylldene fluorlde) as a blnder.
In case where the electrode ls rolled up, The directlon
of the carbon flbers to be arranged ls preferably approxlmately
vertlcal agalnst the rolled dlrectlon. Thls ls because that,
this arrangement can prevent the loosenlng of the carbon flbers
placed lnslde of the metal foll and can make the carbon flbers to
- 7 - ~ 8 07
be hardly broken when the electrode ls rolled up. Furthermore,
by such arrangement, there can also be prevented the penetration
of the broken carbon fiber edges through a separator or the
sticking of the broken carbon fiber edges out of the boLh ends of
the electrode by moving ln zlgzag directlons. The penetratlon
through a separator and stlcklng out of the both ends of the
electrode of the broken carbon flber edges are undesirable slnce
they may cause the electrlcal short clrcuit with the positive
electrode.
As described above, the electrode in which the carbon
fibers are integrated with the metal foil enables to lower the
contact resistance of the carbon fibers and to make the distance
between the metal collector and the carbon fibers 4horter and, as
the result, more uniform potential in the carbon flbers can be
giverl. Therefore, the decrease in capacitance caused by
overvoltage resulting from the contact reslstance and the non-
unifc)rm doping caused by the non-uniform potential in the carbon
fibers ca:n also be prevented.
T:he weight of the carbon fibers to be arranged ln an uni-
axia]. direction is.preferably not smaller than 30 g/m2 and not
larger than 200 g/m2, and more preferably not smaller than 50
g/m2 and not more larger than 150 g/m2. When the weight is too
large, thle carbon fiber sheet itself becomes thick and the
reslEItanc,e of the thickness dlrection becomes hlgk whlch results
ln oc:caslonal non-unlform doping and dlfficulty in use of the
resu].ting electrode at hlgh output current. On the other hand,
when the weight is too small, the amount ratio of the carbon
fibers, l.e. actlve material, based on the whole amount of the
resul.ting negative electrode becomes small, which results in
decre!ase .in the amount of the carbon fibers to be packed ln a ~:
- 8 - 2130807
battery and reductlon in energy density of the battery.
The carbon fiber ~o be used in the present lnventlon is
not partlcularly llmited, but a fllament prepared by flrlng an
organlc substance is generally used. Speclflc examples of such
carbon fiber lnclude a PAN-based carbon flber prepared from
polyacrylonltrlle (PAN), a pltch-based carbon flber prepared from
pltch of coal, petroleum or ~he like, a cellulose-based carbon
flber preparea from cellulose and a vapor phafie grown carbon
flber prepared from gas of a low molecular organlc material. In
addltlon, other carbon flbers prepared by flring poly(vinyl
alcohol), lignin, poly(vinyl chloride), a polyamlde, a polyimide,
a phenol resin, furfuryl alcohol and so on can also be employed.
The carbon flber to be used ls suitably selected f-om those
llsted above dependlng on the lntended propertles vf the
resultlng electrode or battery.
Among the carbon flbers llsted above, when used for a
negatlve electrode of a secondary battery ln whlch a nonaqueous
electrolyt.lc solutlon contalnlng an alkall metal salt ls used,
preferably employed are a PAN-based carbon flber, a pltch-based
carbon flber and a vapor phase grown carbon fiber. In
partlcular, from the vlewpolnt of a good doplng property wlth
llthium lons, a PAN-based carbon flber ls most preferable.
In the present lnventlon, the carbon flbers obtained by
firlng may~ be sub~ected to any subsequent treatment and any type
of carbon flber may be employed, so long as lt retalns a form of
carbon flber. In partlcular, the carbon flber which ls sub~ected
to the chalrglng/dlscharglng treatment ln an electrolytlc solutlon
prlor to incorporatlng lnto a battery are effectlvely used slnce
lt can recluce the lnltlal capaclty loss (l.e. retentlon) lnherent
to a carbonaceous materlal. The lnltlal capaclty loss results
~13080'7
from the phenomenon that a part of dopants (e.g. llthlum ion)
whlch are doped durlng the lnitial charging step remalns in the
carbonaceous material and the residue i5 not dedoped in the
subsequent. discharging step. In order to lmprove the capacity of
a secondary battery, it is effective to reduce the initlal
capaclty ].oss. The carbon fiber ltself has an electrlcal
conductivity and is a continuous material, and therefore suitable
for the prevlous charging/discharging treatment. Speclflc
example of such prevlous treatment method ls that ln whlch the
carbon flber ls doped or dedoped in an electrolytlc solu~lon
contalning lithlum ions.
In the second invention of the present application, the
form or shape of the carbon flber sheet ls not partlcularly
llmlted, but is preferably a sheet-shaped structural in which the
carbon fibers are arranged in an uni-axial direction. In the
cloth-type or felt-type carbon fiber sheet, any form may be
employed such as woven fabric, knit fabric, plalted fabric, lace,
mesh, felt., paper, non-woven fabrlc and mat.
The dlameter of the carbon flber to be used ln the
present invention should be determined so that the carbon flbers
can be prepared ln the form as descrlbed above, and preferably l
to 500 am. more preferably 3 to lO am. It is also preferable to
use several klnds of carbon flbers havlng different diameters
indlvldua]Lly.
A~; the metal to be used as an electrically conductive
foil and wire, there can be employed gold, silver, copper,
platinum, rhodium, aluminum, iron, nickel, chromium, manganese,
lead, zinc, tungsten, titanium, and so on. In addition, alloys
of the me1als listed above can also be employed, such as
stainless steel. These metals may be coated of their surfaces
-- 10 --
21308Q~I
wlth varlous substances so long as they are impalred of thelr
electrica:L conductlvlty. The metal or coated one thereof ls made
lnto a fo:Ll or a wlre, and then arranged wlth the carbon flbers
ln the fo:rms shown ln the drawlngs. In case of metal foll, a
thin foll ls preferably used, slnce the thlck metal foll causes
to decrease ln the amount of the actlve material to be stored ln
a battery. The thlckness of the metal foll ls preferably about 5
to 100 ~m. In partlcular, from the viewpolnts of electrlc
reslstanc,s and thlckness and cost of the metal foll to be used,
copper foil ls preferably employed. On the other hand, ln case
of metal wlre, the dlameter should be determlned depending on
propertles, dlameter and shape of the carbon flber used, so that
the c:urrent collectlng effect ls enhanced or the carbon flbers
are bundlled easlly, but ls preferably about 1 to 200 ~m, more
preferably 5 to 100 ~m. In order to lncrease the lntenslty of
bundllng of carbon flbers, lt ls preferable to bundle several
flne metal wires ln a twlsted form.
The ratlo between the carbon flbers and the
electroconductlng wlres ln an electrode of the present lnvention
should be determlned sultably taklng into conslderatlon of the
propertles and current collecting efficlency of the resulting
electrode and so on. However, the ratlo of the electroconducting
wlre~3 to the carbon fibers of the resulting electrode is
preferably 1 to 10~ by weight and 0.2 to 2~ by volume, and more
preferably 2 to 8~ by weight and 0.4 to 2~ by volume.
In the flbrous and cloth-shaped carbon flbers, the
part:Lal breaking of the single flber ln the carbon flber bundle,
l.e. fuzzlng, tends to occur. The fluffs sometlmes penetrate
through a separator to contact wlth a posltlve electrode, whlch
causes the internal short clrcult. In order to prevent thls
~130g~
defect, 11 is effectively carried out to paste and coat a part or
all of carbon fibers with a resin. The resin to be used is not
particularly limited, and a conventlonal thermoplastlc or
thermoset1ing resin can be employed. In particular, preferably
used are il fluororesln, an olefln resin, an epoxy resin, an
urethane :resln, an acryl resln and a polyester resln slngly or in
comblnatlon thereof, and a modified one thereof.
The method for pasting and coating carbon *ibers with a
resin is ~lOt particularly limited. However, it is preferable to
paste and coat carbon fibers by passing the carbon fibers through
a polymer solution or emulsion vessel, or by spraying the
solution or emulsion thereon. When the amount of the polymer to
be cc,ated on the carbon fibers is too small, the fuzzing of the
carbon flbers can not be depressed sufficiently. On the other
hand, when the amount of the polymer is too large, the function
of the carbon fibers themselves as active material tends to be
reduced.
From these reasons, the amount of the polymer used for
coating oE carbon fibers is preferably not less than 0.1 part by
welght and not more than 15 parts by weight based on 100 parts by
welght of the carbon flbers. When the amount is less than O.l
part by w,elght, the fuzzing can not be prevented sufficiently.
On the ot~her hand, when the amount is over 15 parts by welght,
the electrlcal propertles of the carbon flbers as negatlve
electrode actlve materlal ls affected. In partlcuiar, when the
dlscharglmg current becomes over 500 mA per l g of the negative
elect.rode actlve materlal, the lnitial discharge capacity tends
to be resuced to 70~ of that given when the carbon fibers are
uncoclted.
From these reasons, the coating amount of a polymer is
213080 ~
most preferably 0.5 to 10 parts by welght, and partlcularly 0.S
to 8 parts by welght. In the method for coatlng a polymer on
carbon flbers, ln the case where the polymer ls solved ln an
water soluble organlc solvent such as N-methylpyrrolldone, lt ls
more effectlve to preclpltate the polymer by wet solidlflcatlon
ln wa~er cr a mlxed solutlon of an organlc solvent and water.
The separator to be used ln the present lnvention ls not
partlcularly limlted, and a commerclally avallable product can
also be emlployed, so long as lt ls a porous fllm, a woven fabrlc,
a non-woven fabrlc and so on havlng lnsulatlng property, such as
that made of polyolefln, polypropylene, polytetrafluoroethylene,
polyethylene and polyacetal. The fllm thlckness o~ the separator
ls prefera.bly not larger than 200 ~m, and more pre~erably not
larger tha.n 50 ~m, for the purpose of reduclng the lnternal
reslstance of the resultlng battery. More speclflcally, "
Cellguard" (a trade name produced by Dalcel kabushlkl Kaisha) and
"Hlghpore" (a trade name produced by Asahl Kàsel Kogyo Kabushikl
Kalsha) are preferably used.
As. the materlal used for a posltlve electrode as a
const.ltuen.t of a secondary battery, a carbon flber can be used.
In ad~ltlon, there can also be used artlflcial or natural
graph.lte, carbon fluorlde, an lnorganic compound such as a metal
and a meta.l oxlde, and an organic hlgh molecular compound. When
an lnorgan,lc compound such as a metal and a metal oxlde is used
for a posltlve electrode, the charglng/dlscharglng reaction
occuris utl.lizing the phenomenon of doping and dedoplng of
catloms. On the other hand, when an organlc hlgh molecular
compound is used for a posltlve electrode, the
charg.lng/d!lscharglng reactlon occurs utlllzlng the phenomenon of
doplng and~ dedoplng of anlons. Thus, the charglng~dlschargi;ng
- 13 - 2130~ 0 ~
react.lon ~akes various manners according to the kinds of the
subst.ances employed, and is suitable selected accordlng to the
lntended propertles of the posltlve electrode of the resultlng
battery.
Speclflc examples of the positive electrode material
include lnorganlc compounds such as oxides and chalcogenldes of
transltlon metals lnvolving alkall metals; con~ug~ted polymers .
such as polyacetylene, poly(para-phenylene), poly(phenylene :
vinyl.ene), polyaniline, polypyrrole and polythiophene; bridged
polymers havlng dlsulfide bond(s); thionyl chlorlde; and so on;
whlch are compounds used in conventional secondary batterles.
Among thel3e, in case of a secondary battery using a nonaqueous
elect.rolyLic solution containing lithlum ions, an ~xides or
chalc:ogen:lde of a transltion metal such as cobalt, manganese,
molybdenum, vanadlum, chromlum, lron, copper or tltanlu~ is
prefe!rabl~y used. In partlcular, compounds LlCoO2 and LlN102 are
most preferable slnce they exhlblt hlgh voltage and large energy
densi.ty.
The electrolytlc solutlon to be used for the secondary
battery ln whlch the electrode of the present invention is used
18 not pa:rtlcularly limlted, and a conventional one ls employed
such as an acldlc or alkaline aqueous solutlon or non-aqueous
solvont. In partlcular, as an electrolytlc solution for a
secondary battery uslng a non-aqueous electrolytlc solutlon
contalnlng an alkall metal salt llsted above, there are
preferably used propylene carbonate, ethylene carbonate, 1-
buty~olactone, N-methylpyrrolldone, acetonltrlle, N,N-
dlmet.hylformamlde, dlmethylsulfoxlde, tetrahydrofuran, 1,3-
dloxolane, methyl formate, sulfolane, oxazollne, thinyl chlorlde,
1,2-climetlhoxyethane, diethylene carbonate, derlvatlves thereof
- 14 -
2130813'`~
and mixtures of two or more of them. As the electrolyte to be
contained in the electrolytlc solutlon, are preferably employed
halldes of alkall metals, especlally of llthlum, perchlorates,
thlcyanates, borofluorides, phosphofluorldes, arsenofluorldes,
alumlnofluorldes, trlfluoromethylsulfates, and so on.
For the appllcatlon to a secondary battery whlch uses a
non-aqueous electrolytic solutlon containing an alkall metal
salt, the electrode comprlslng carbon fibers of thç present
lnventlon utlllzes a phenomenon of doping of catiors or anlons to
carbon fibers. Therefore, this electrode can be used for both of
negatlve and posltlve electrodes, and preferably for a negatlve
electrode of a secondary battery. In partlcular, when catlons
represented by lithlum lons are doped, the carbon ,ibers show
excellent propertles as a negatlve electrode material for high
energy-type battery whlch exhlblts high capaclty and base
potentlal. In addltlon, slnce the carbon flbers are used in a
flbrous form, they can make thelr contact resistance lower
compared with carbon powder, and as the result hlgh current
dlscharge becomes possible.
The secondary battery using the electrode of the present
lnventlon can be applled to various portable small electronlc
devlces such as video cameras, personal computers, word
processors, radlos wlth cassette, portable telephones and so on,
due to lt~l characterlstlcs of llghtwelght, hlgh capacltance and
hlgh enerqy denslty.
Examples
The present lnventlon wlll be lllustrated ln more detail
wlth reference to the followlng examples; however, these examples
are lntencled to be understood to llmit the scope of the present
- 15 - 2130807
invention.
Example 1
12000 pieces of carbon fibers "TORAYCA T300" (a trade
name produced by Toray Industries, Inc.) were bundled into a tow-
shaped structural body, fixed its ends with an electroconductive
copper paste. Five of the resulting tow-shaped structural bodles
are arranged ln an unl-dlrectlon, and then adhered of their ends
with copper foils, to glve a sheet of 20 mm ln long, 50 mm ln
wide and about 0.3 mm in thlck. The welght of the resultlng
carbon flber sheet was 230 mg.
For the determlnation of the capacitance of the unl-
dlrectlonally arranged body of the carbon flbers for a secondary
battery, a trlode-type cell was prepared uslng llthlum folls as a
counter electrode and a reference electrode and uslng a solutlon
ln whlch 1 M of llthlum perchlorate had been dlssolved ln
propylene carbonate as an electrolytlc solutlon. The resultlng
cell was charged untll the voltage reached to 0 V at a constant
current of 20 mA, and after restlng for 20 minutes, was
dlschargeld untll the voltage reached to 1.5 V at a constant
current of 20 mA to determlne the discharge capaclty. As the
resu].t, the capacltance of the cell per welght was 304 mAh/g, and
lt was proved that thLs method could glve a hlgh dlscharge
capac:lty.
le 2
(1) Preparatlon of electrode
On a copper foll of 15 ~m in thick, commercially
available PAN-based carbon fibers "TORAYCA T-300" (a trade name
produced by Toray Industrles, Inc.) were placed ln an unl-axlal
- 16 -
21308~7
directlon unlformly, to glve an electrode comprlslng a copper
foll and carbon fibers, ln whlch the welght of the carbon flbers
was 100 g,/m2.
(2) Prepara~lon of posltive electrode
Commercially available lithium carbonate (Li2C03) and
basic cobi~lt carbonate (2CoC03~Co(OH)2) were weighed so that the
molar ratlo of these components became Li/Co = 1/1, and then
mixedL wlth each other using a ball mill. The resulting mixture
was treated by hea~ing at 900~ for 20 hours, to give LiCoO2. The
resulting LiCoO2 was crushed using a ball mill. A slurry for a
positive electrode was prepared by mixing the LiCoO2, artificial
graph,lte as an electroconducting material, poly(vinylidene
fluoride) (PVDF) as a binder and N-methylpyrrolidone as a solvent
in a miximg ratlo of LlCoO2/ artiflcial graphite/ PVDF - 80/lS/5
by welght. The resultlng slurry was applled on an aluminum foil,
drled and pressed; whereby a positive electrode was given.
(3) Preparation of battery
Two kinds of electrodes prepared in steps (1) and (2)
above!, respectively, were superposed upon each other wlth
lnterposing a separator of a porous polypropylene fllm
("Cellguard #2500"; a trade name by Daicel Kagaku Kabushiki
Xalsha) therebetween, and then rolled up, to give a cylindrical
electrode body. The resul~ing electrode body was immersed ln~o a
beake~r cell ln which an electrolytlc solution of propylene
carbonate containing 1 M oE lithium perchlorate was put.
Termlnals were taken out from the copper foil and the aluminum
foll, respectlvely; thus a secondary battery was prepared.
~4) Evaluation of battery
The secondary battery thus prepared was evaluated for its
charging property. That is, the secondary battery was charged
- 17 - ~13~8Q7
un~ he voltage reached to 4.3 V at a constant current of 40
mA/g as the current density per weight of the carbon fibers, and
was discharged. The dlscharge capacity of the secondary battery,
whlch was determlned from the charge amount given by the
dlschargln,g, was 320 mAh/g per weight of the carbon flbers used
ln thls battery.
Example 3
As the carbon flbers, commerclally avallable PAN-based
carbon flbers ("TORAYCA T-300"; a trade name produced by Toray
Industrles, Inc.; 3K; 3000 flbers) were used. As the polymer for
pastlng and coatlng of the carbon fibers, a commerclally
avallable poly(vlnylidene fluoride) resln ("Neoflon VP-850"; a
trade name produced by Dalkln Kagaku Kabushlki Kaisha) was used
by dlssolvlng ln N-methyl-2-pyrrolldone.
The carbon flbers were lmmersed in the PVDF solu~ion, and
then further lmmersed ln a solutlon havlng a compositlon of
water: N-rnethyl-2-pyrrolldone = 1:1 (by welght) to solldlfy the
polym,er. The resultant was dried at 150~ for 1 hour, to give
carbon flbers pasted with the polymer. The PVDF polymer-adhered
carbon flbers thus prepared had an adhesion amount of the polymer
of 5~, by welght based on the welght of the carbon flbers and an
avera,ge pore dlameter measured uslng a SEM photograph of about 15
~m.
In order to examine the lnfluence of fluffs of the carbon
flbers pasted and coated with the polymer agalnst a separator,
the c:arbon flbers ls lnserted lnto polypropylene porous fllm
("Cellguard"; a trade name produced by Dalcel Kayaku Kabushlkl
Kalsha) to be flxed and then wound up around a sta~nless steel
- 18 ~ '~i30~'7
rod. The resultant was applied with a llne pressure of 2 kg/cm
for 10 minutes to observe whether the carbon flbers penetrated
through a separator or not. As the result, no penetration of
carbon flbers was not observed.
Accordlng to the same method as Example 2, a trlode-type
beaker cell was prepared uslng these carbon fibers woven into
networks of nlckel fine wires as a working electrode, metal
lithi.um as a counter electrode and a reference elec~rode, and 1 M-
LlClC)4/propylene carbonate as an electrolytic solution. The
resultlng cell was charged (i.e. doped with lithium ions) until
the voltage reached to 0 V (vs Li+/Li) for the reference
electrode at a constant current of 100 mM/g based on the weight
of the carbon fibers, and after resting for 20 minutes,
dlscharged (l.e. dedoped) under the same condltion until the
voltage reached to 1.5 V (vs Lil/Li); thus the cell was charged
and discharged to determine the discharge capacity.
As the result, the discharge capacity was 350 mAh/g,
whlch was the same vale as that given when the carbon flbers were
not pasted or coated with PVDF polymer. Accordingly, the
reduction in discharge capacity caused by the pasting and coating
wlth a polymer was not recognized.
On the other hand, when the carbon fibers were used as
they were wlthout pasting or coating wlth a polymer, the
dlscharge capacity given was 351 mAh/g. However, when the
penetration of the carbon fibers through a separator was
exam:Lned, 10 to 15 of penetrating ponts by the carbon flbers were
obse:rved on the separator.
Example 4
(1) Preparation of electrode
.
2130807
2() mg of commerclally avallable PAN-based carbon flbers "
TGRAYCA T--300" ~a trade name produced by Toray Industries, Inc.)
were arranged ln an unl-axlal directlon, and woven thelr ends
wlth nlckel flne wires ~diameter: l00 am) in the direction
vertlcal 1:o the arranged direction of the carbon flbers, and
bundled as shown in Fig. 3; thus an electrode was prepared. In
the resul1:ing electrode, the welght ratio between ~he carbon
fibers and the metal fine wires was l00:l.
(2) Evaluatlon of charging property
The evaluation of charging property was carried out using
the elect:rode prepared above. In this evaluation, a trlode-type
liquid cell was used in which propylene carbonate containing l M
llthlum perchlorate was used as an electrolytic sclution and
metal llthium foils were used as a counter electrode and a
reference electrode, respectlvely, and the liquld cell was
charged untll the voltage reached to 0 V (vs. Li /Li) at a
constant current of 40 mA/g-as the current density per welght of
the carbon flbers. As the result, the return of the voltage,
l.e. overvoltage, glven after restlng for 20 mlnutes was l0 mV.
Example 5
(l) Preparatlon of electrode
20 mg of commercially avallable PAN-based carbon flbers "
TORAS'CA T-300" (a trade name produced by Toray Ind-1strles, Inc.)
were arranged ln an unl-axlal dlrectlon, and woven thelr ends
with nlckel fine wlres (dlameter: l00 ~m) ln a network form as
shown ln Flg. 4; thus an electrode was prepared. In the
resultlng electrode, the welght ratio between the carbon fibers
and the metal flne wlres was l00:l.
(2) Evaluation of charglng property
- 20 -
2~30807
The evaluatlon of charglng property was carrled out uslng
the electrode prepared above ln the same manner as Example 1. As
the result., the overvoltage given after charglng was 0.5 mV.
Example 6
A coin-shaped secondary battery was prepared uslng the
carbon flbers of Example 3, whlch had been pasted and coated with
a PVDF polymer, as a negatlve electrode, the LiCoO / artificlal
graphlte/ PVDF of Example 2 as a posltlve electrode; ln a manner
that the posltlve electrode and the negative electrode were
superposecl upon each other with lnterposlng a separator. In thls
secondary battery, 1 M-LlClO4/propylene carbonate ~as used as an
electrolyt.lc solutlon.
The charglng/dlscharglng test was carrled out using 100
pleces of the coln-shaped secondary batteries prepared above. As
the result., no defectlve such as short clrcult was not observed,
and all of the secondary batteries tested were operated normally.
Example 7
(1) Preparatlon of carbon fiber electrode and
charglng/cllscharging thereof
A beaker cell was prepared uslng commerclally
avallable PAN-based carbon flbers "TORAYCA M40" (a trade name
produced by Toray Industrles, Inc.) bundled wlth ~1 wlre of a
current collector as a worklng electrode, and uslng metal llthlum
as a count.er electrode and a reference electrode, and uslng 1 M-
LlClO4/propylene carbonate as an electrolytlc solutlon.
In the resultlng cell, llthlum lons were doped untll the
voltage re!ached to 0 V (vs. Ll /Ll) to the reference electrode
and then cledoped under the same condition until the voltage
- 21 - 2130807
reached to 1.5 V (vs. Ll+/Ll); thus the charging and discharglng
of the ce:Ll was completed.
(:2) Preparatlon of secondary battery and evaluatlon
thereof
The carbon fibers which had been charged and discharged
prevlously ln the step (1) above were arranged on a Ni mesh. The
resultant was superposed upon the positlve electrode prepared in
the same manner as Example 4 with interposing a separator; thus a
coln shaped cell was prepa:red. In thls cell propylene carbonate
contalnlng 1 M llthium perchlorate was used as an electrolytic
solution. When this cell was charged and discharged the Coulomb
s efficiency given was 96~. In this cell by the previous
charging and discharging the inltlal volume loss was reduced
from the value of 30 mAh/g glven when no treatment was carrled
out to the value 5 mAh/g.
Industrlal Applicabllity
As descrlbed above the electrode of the present
lnven,tlon comprlses an unl~directlonally arranged body of carbon
flbers or one comprlslng the carbon fibers and electrlcally
conductlve foll or wlres. By using the electrode for a
chargeable/dlschargeable secondary battery ln which a
carbonaceous materlal capable of doplng and dedoplng of llthlum
ions is u~3ed as a negatlve electrode actlve materlal there can
be provlded a secondary battery havlng hlgh capacltance and hlgh
outputtlng property.