Note: Descriptions are shown in the official language in which they were submitted.
2~317 4S
TITLE OF THE INVENTION
LOW GLOSS POWDER COATING COMPOSITION AND
METHOD FOR COATING THEREWITH
FIELD OF THE INVENTION
The present invention relates to a powder
coating composition for forming a low gloss coating
film, in particular to a powder coating composition
based on polyester resins capable of adjusting the
gloss of the resulting coating film at any voluntary
value, as well as to a method for coating various
substrates with it.
BACKGROUND OF THE INVENTION
Low gloss powder coatings have been used for
forming lusterless coating films. Up to date, low
gloss powder coating compositions based on polyester,
in which the gloss value of the coating is reduced by
incorporating an extender pigment, such as silica,
talc or the like, have been known. These powder
coating compositions reduce the gloss value by the
formation of minute surface irregularities on the
coating film due to the incorporation of the extender
pigment.
In such powder coating compositions, however,
the gloss value will not be lowered down to 50 or less,
even when the content of the extender pigment in the
coating composition is increased over 30 % by weight.
In addition, the resulting coating film becomes hard
and brittle together with a quite inferior appearance,
when such a high content of the extender pigment is
employed.
A powder coating composition for glossless
coating based on polyester resins has been known, in
which the above-mentioned defects were obviated by
compounding two polyester resins exhibiting different
curing reaction rates (Japanese Patent Application
Kokai Nos. 154771/1988, 1770/1989, 98671/1989 and
109468/1991). This powder coating composition realizes
the reduction of gloss value by forming -a fine wrinkle
pattern over the coating surface due to a difference in
the rate- of curing reaction of the polyester with the
hardener existing between the two polyester resins
incorporated and permits the coating to reach a gloss value
of below 10.
However, these conventional powder coating
compositions suffer from a large fluctuation in the
gloss value caused even by a small difference in, for
example, the mixing proportion of the components, the
condition of baking of the resulting coating and so on.
In the industrial production of such powder coating
compositions, occurrence of fluctuation in the mixing
proportion cannot be avoided, resulting in considerable
unequalities between the production lots, so that it is
difficult to maintain a definite gloss value requested
by users. Thereto adds further a problem that coating
films of the same gloss value are difficultly
'L ~ 2
2I317~5
obtainable even using the same coating composition, if
different baking conditions are to be employed.
SUMMARY OF THE INVENTION
An object of the present invention is to
obviate the above-mentioned problems and to provide a
powder coating composition capable of forming a low
gloss coating which exhibits a desired definite low
gloss value with only a little fluctuation in the gloss
value even if an unequality in the mixing proportion of
each component and in the baking condition of the
resulting coating occurs and which permits adjustment
of the gloss at any voluntary value over a considerable
range.
Another object of the present invention is to
provide a method for coating a substrate with the
above-mentioned powder coating composition as well as
low gloss coating films obtained thereby.
The powder coating composition for low gloss
coating according to the present invention comprises
three thermosetting polyester resins (A), (B) and (C)
and a hardener (D), the weight ratio of the polyester
resin (A) to the polyester resin (B) being in the range
of 90/10 - 70/30 and the weight ratio of the polyester
resin (A) to the polyester resin (C) being in the range
of 85/15 - 60/40 and the equivalent ratio of the
polyester resin (A, B or C) to the hardener (D) being
each in the range of 0.8 to 1.25, wherein the polyester
resins (A), (B) and (C) are selected from those in
2131 7~5
which the moduli of elasticity Ea~ Eo and Ec for the
products of the curing reactions of the resins (A), (B)
and (C) with the hardener (D), respectively, after 3
minute's curing with the hardener (D) at 200~C hold a
relationship that the differences between the moduli of
elasticity Eb - a = Eo-E~ and Ec a = EC-E~ are maintained
in the following ranges, respectively:
20 dyn/cm2< Eb a < 103 dyn/cmZ and
1 dyn/cm2 < Ec a < 10 dyn/cm2
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 illustrates the coating film according
to the present invention in a schematic sectional view.
Fig. 2 is a graph showing the course of
temporal variation of the moduli of elesticity E~, Eo
and Ec for the products of the curing reactions of the
polyester resins (A), (B) and (C), respectively, with
the hardener (D) at 200 ~C for Example 1.
Fig. 3 is a graph showing the relationship
between the mixing ratio of the polyester resins
(A)/(B) and the coating gloss value from the curing
reaction of polyesters (A), (B) with a hardener (D),
wherein the polyesters exhibit different curing rates
with the hardener (D).
Fig. 4 illustrates the coating film according
to a typical conventional powder coating composition in
a schematic sectional view.
DETAILED DESCRIPTION OF THE INVENTION
Polyester resins exhibit different curing
reaction rates and different gelation times due to the
difference in the reactant group, its content, the
hardener, its amount used and so on. When a mixture of
such polyester resins of different reaction rates and a
hardener is cured, minute irregularities will be
formed over the coating surface due to the difference
in the curing reaction rate between them, whereby a
delustered coating is obtained.
For decreasing the gloss value of the coating
in a binary polyester resin mixture, the lowering of
the gloss value will be higher for a mixture of
polyester resins with greater reaction rate difference.
It is even possible to produce a coating layer having a
gloss value of 10 or less. However, as seen in Fig. 3,
the gloss value will vary considerably, when the mixing
ratio of the two resins deviates only a little from a
definite mixing proportion, at which the resulting
coating has a minirllm gloss value.
Fig. 3 is a graph showing the relationship
between the mixing ratio of the polyester resins in a
binary mixture of polyesters (A) and (B), on the one
hand, and the gloss value of the resulting coating, on
the other hand. As seen, a gloss value of 5, for
example, is attained by a mixture of 75 parts by weight
of the polyester resin (A) and 25 parts by weight of
the polyester resin (B), whereas the gloss value rises
up to a value of 16 - 17 upon deviation of the mixing
ratio to 75/23 or to 75/27.
If a gloss value difference of 10 or higher
213174~
exists between two coatings, they are visually
discriminated as different coatings. Even using the
same coating composition containing 25 parts by weight
of the polyester resin (B), considerably different
gloss values of the resulting coatings, in despite of
consistent other properties, may be obtained, when the
baking condition is altered from 200~C for 10 minutes
to 180~C for 20 minutes.
In contrast thereto, if a binary mixture of the
polyester resin (A) and the polyester resin (B) having
a greater rate of curing reaction with a hardener (D)
for the resin (B) than for the resin (A) is combined
with a further polyester resin (C) exhibiting a rate of
curing reaction with the hardener (D) somewhat higher
than that of the resin (A), the gloss value change
caused by an about 10 % variation in the weight ratio
of the resin (B) to the resin (A) can be restricted
within a gloss value of 5.
According to the present invention, three
polyester resins (A), (B) and tC) and a hardener (D)
are employed. Here, the weight ratio of the polyester
resin (A) to the polyester resin (B) is in the range of
90/10 - 70/30 and that of the polyester resin (A) to
the polyester resin (C) is in the range of 85/15
60/40 and the equivalent ratio of the polyester resin
(A, B or C) to the hardener (D) is in the range of 0.8
to 1.25, wherein the polyester resins (A), (B) and (C)
are selected from those in which the moduli of
elasticity Ea~ Eb and Ec for the products of the curing
reactions of the resins (A), (B) and (C) with the
213~74S
hardener (D), respectively, after 3 minutes' curing
with the hardener (D) at 200~C hold such a relationship
that the differences between the moduli of elasticity
ED - a = E~ ~Ea and Ec - a = Ec-E~ are maintained in the
following ranges, respectively:
20 dyn/cm2< Eb a < 103 dyn/cm2 and
1 dyn/cm2 < Ec a < 10 dyn/cm2
These polyester resins hold such a relationship
therebetween that the differences of the moduli of
elesticity E~ a and Ec a are maintained within the
range as given above. While there is no limitation for
the absolute values of the moduli of elesticity Ea~ Eb
and Ec~ they are held in the relationship:
Eb ~ Ec > EA
Fig. 2 is a graph showing the course of
variation of the moduli of elasticity Ea~ E~ and Ec in
time observed for an embodiment wherein each of the
polyester resins (A), (B) and (C) of Example 1 as given
in Table 1 appearing afterwards is subjected to a
curing reaction with the hardener (D) at 200~C . Fig. 2
indicates the above circumstances. Thus, according to
the present invention, three polyester resins (A), (B)
and (C) are employed, in which the difference of Eb ~Ea =
Eb - a is large and the difference of Ec ~Ea = Ec- a iS
small.
For the parameter of the reaction rate, there
may be employed, for example, the reaction rate
constant, the gelation time, the rate of variation of
the modulus of elasticity and so on. Determination of
the reaction rate constant is difficult to carry out
for such a reaction as the curing of a polymer resin
for coating where a molecular weight distribution is
included. Determination of the gelation time is not
accurate, since the end point (gelation time) is, in
general, determined by visual observation on a hot
plate. In contrast, the determination of variation in
the modulus of elasticity can be effected with a high
accuracy using, for example, Laboplastomill* of Toyo
Seiki K.K. or Soliquidmeter* of Rheology K.K.
The modulus of elasticity is determined using
these apparatuses by heating a curable mixture composed
of the polyester resins and a hardner in a mixing
proportion in terms of equivalent ratio of each
polyester resin (A, B or C) to the hardener (D) of 0.8
- 1,25 at 200 ~C and observing the elasticity at this
temperature after three minutes. The difference of
modulus of elasticity is the difference of such values
for the different polyester resins.
The polyester resins (A), (B) and (C) to be
incorporated in the powder coating composition for low
gloss coating according to the present invention are
each a condensation product of a polycarboxylic acid,
such as terephthalic acid, isophthalic acid, phthalic
anhydride, 2,6-naphthalene dicarboxylic acid, succinic
acid, adipic acid, azelaic acid, trimellitic acid,
pyromellitic acid, and the like, for the acid
component, with a polyol, such as trimethylolethane,
trimethylolpropane, 3-methylpentane-1,3,5-triol,
glycerin, ethylene glycol, diethylene glycol,
2-propanediol, neopentyl glycol, pentaerythritol and
~ .
* Trade-mark
the like, for the alcoholic component.
The polyester resins (A), (B) and (C) to be
incorporated in the powder coating composition
according to the present invention exhibit different
reaction rates for one and the same hardener (curing
agent) so as to bring about a difference between Eb A
and Ec . as mentioned above. Such difference in the
reaction rate can be brought about by, for example,
selecting the number of the functional groups, such as
hydroxyl groups and carboxyl groups, in the resins or
selecting the amount of the catalyst for accelerating
the curing reaction between such functional groups and
the hardener.
For providing such difference in the reaction
rate by selecting the content of the hydroxyl groups in
the resins, it is preferable to use as the polyester
resin (A) those which have hydroxyl values in the range
of 20 - 38 mg KOH/g. As the commercial products for
these resins, there may be employed, for example, Ester
Resins ER-6610, ER-6650 and ER-6800 (all trademarks) of
Nippon Ester Co., Ltd.; Upicacoat GV-150, GV-160,
GV-165 and GV-730 (all trademarks) of Japan Upica Co.,
Ltd.; and Finedic M-8020 (trademark) of Dainippon Ink &
Chemlcals, Inc. as well as products corresponding to
them.
As the polyester resin (B), it is preferable to
use those which have hydroxyl values of 100 mg KOH/g or
more, such as those commercially available products of
30-3011 and 30-3002 ~both trademarks) of McWHORTER
Technologies, Inc.; XI-7009 (trademark) o~ Nippon Ester
.~; . 9
21317~'5
Co., Ltd. and corresponding products.
As the polyester resin (C), it is preferable to
use those which have hydroxyl values in the range from
40 to 80 mg KOH/g, such as those commercially available
products of, for example, Upicacoat GV-740, GV-741
and GV-710 (all trademarks) of Japan Upica Co., Ltd.;
Ester Resin ER-6570 (trademark) of Nippon Ester Co.,
Ltd. and corresponding products.
For the case where the difference in the
reaction rate is brought about by the difference in the
carboxyl group content, it is preferable to use as the
polyester resin (A) those which have acid values in the
range from 20 to 45 mg KOH/g. As the commercial
products for these resins, there may be employed, for
example, Ester Resins ER-8105 and ER-8107 (both trade-
marks) of Nippon Ester Co., Ltd.; Finedic A-229-M,
M-8900 and M-8930 (all trademarks) of Dainippon Ink &
Chemicals Inc.; Uralac P-2400 and P-3500 (both trade-
marks) of DSM Resins BV; Crilcoat 320 (trademark) of
UCB Chemicals, Inc. and other corresponding products.
As the polyester resin (B), it is preferable to
use those which have acid values of 100 mg KOH/g or
more, such as those commercially available products of
Ester Resins XE-3009, XE-93001 and XE-94002 (all trade-
marks) of Nippon Ester Co., Ltd.; Upicacoat PX3064
(trademark) of Japan Upica Co., Ltd.; Finedic M-8540
(trademark) of Dainippon Ink & Chemicals Inc. and
corresponding products.
As the polyester resin (C), it is preferable to
use those which have acid values in the range from 53
1 0
to 90 mg KOH/g, such as those commercially available
products of, for example, Ester Resins ER-8100 and
ER-8101 (both trademarks) of Nippon Ester Co., Ltd.;
Upicacoat GV-230 (trademark) of Japan Upica Co., Ltd.;
Finedic M-8500 and M-8830 (both trademarks) of
Dainippon Ink & Chemicals Inc.; Uralac P-2065
(trademark) of DSM Resins BV; and Crilcoat 340
(trademark) of UCB Chemicals, Inc. and corresponding
products.
While the hydroxyl value and the acid value for
the ester resins mentioned above are important as a
parameter for the selection of the resins, the
selection is not restricted to these values.
Thus, other resins having acid values or hydroxyl
values outside the above-mentioned range may also be
employed, so long as a difference in the modulus of
elasticity as a measure of a difference in the reaction
rate can be brought about.
The mixing ratio of the three polyester resins
may be in the range such that the weight proportion of
the polyester resin (A)/polyester resin (B) is in the
range of from 90/10 to 70/30, preferably from 85/15 to
74/26, with the weight proportion of the polyester
resin (A)/polyester resin (C) in the range of from
85/15 to 60/40, preferably from 82/18 to 62/38. If the
mixing ratio is outside of such limitation, the
contemplated gloss value of the resulting coating is
difficult to attain and the gloss value becomes
unstable.
The coating gloss value to be attained can be
L~
1 1
7 ~ 7 ~ ~,
adjusted at every voluntary value by suitably selecting
the difference (Eb~Ea ) of the modulus of elasticity
values Eb and E~ as defined previously for the curing
reaction products of the polyester resins (A) and (B)
with the hardener (D), respectively, within the
definite range explained previously. For example, the
larger the value Eo a ~ the lower the gloss value will
be and vice versa. This phenomenon can be utilized for
adjusting the gloss value of the resulting coating film
at any voluntary value within the range of 3 - 50.
It is preferable for the powder coating
composition according to the present invention that the
glass transition temperature Tg of -each of the
polyester resins (A), (B) and (C) is in the range of 35
- 100 ~C . If Tg is not higher than 35~C , the powder
coating composition tends to agglomerate to form solid
grains and causes even blockings. If Tg exceeds
100 ~C , the smoothness of the resulting coating film
will tend to be inferior.
While the powder coating composition according
to the present invention comprises the three polyester
resins (A), (B) and (C) as inevitable components, it is
possible that further polyester resins are incorporated
so as to provide each a stepwise curing rate difference
upon curing of the coating film.
For the hardener (D) to be employed according
to the present invention, any compound capable of
causing curing reaction in a pulverous condition with
the polyester resins to be employed according to the
present invention may be used. For example, blocked
.. .
1 2
2131745
isocyanate compounds and amino compounds may be
employed for the polyester resins having hydroxyl
functional groups. The blocked isocyanate compounds
may be those in which aliphatic, aromatic and alicyclic
polyisocyanates, such as tolylene diisocyanate,
isophorone diisocyanate and hexamethylene diisocyanate,
are blocked with a known blocking agent, such as
methanol, isopropanol, butanol, benzyl alcohol, ethyl
lactate, methyl ethyl ketoxime or & -caprolactam. As
the commercial products therefor, for example, Adduct
B-1530, B-1065 and BF-1540 (all trademarks) of Huls AG
and 24-2400 (trademark) of McWHORTER Technologies,
Inc., may be enumerated. The amino compounds may be
those having two or more amino groups, such as melamine
resins and gll~n~rine resins. As the commercial product
therefor, for example, POWDERLINK 1174 (trademark) of
American Cyanamid Co., may be used.
For the polyester resins having carboxyl groups
as the functional group, glycidyl compounds and amide
compounds may be employed as the hardener. The glycidyl
compounds may be those having two or more glycidyl
groups, such as diglycidyl terephthalate, diglycidyl
p-oxybenzoate, triglycidyl isocyanurate, compounds of
hydantoin, cycloaliphatic epoxy resins, aliphatic epoxy
resins, epoxy resins based on bisphenol-A, epoxy resins
based on cresol novolaks, epoxy resins based on phenol
novolaks and the like. The amide compounds may be those
having two or more functional groups of hydroxy etc.,
such as tetra-~ -hydroxyalkyl amide. As the commercial
product therefor, for example, PRIMID XL-552 (trademark)
213174~
of Rohm & Haas Co. may be enumerated.
As the catalyst for the curing reaction, for
example, imidazole compounds and phosphorus-containing
compounds may be used. As the imidazole compounds, for
example, 2-methylimidazole, 2-isopropylimidazole,
2-heptadecylimidazole, 2-undecylimidazole and 2-phenyl-
4,5-dihydroxymethyl-imidazole, may be enumerated. As
the commercial product therefor, for example, CURESOLE
Cl 7 z (trademark) of Shikoku Chemicals Corp. may be
enumerated. As the phosphorus-containing compounds,
for example, triphenylphosphine, tri(nonylphenyl)-
phosphine and triethylphosphine may be enumerated.
The proportion of each of the polyester resins
(A), (B) and (C) relative to the hardener (D) should be
maintained within a range of 0.8 to 1.25, preferably
0.84 to 1.25 equivalents per one equivalent of the
hardener (D). If the equivalent ratio of the polyester
resin to the hardener is less than 0.8, the cross-
linking of the polyester resins in the coating film is
insufficient and, if this exceeds over 1.25, the
material properties of the resulting coating film
become inferior due to the unreacted remainder of the
hardener in the coating film.
The powder coating composition according to the
present invention may contain, on requirement, pigments
up to a content of 70 parts by weight per 100 parts by
weight of the sum of the polyester resins plus the
hardener. As the pigments, inorganic pigments, such as
titanium dioxide, carbon black and iron oxide;
extender pigments, such as talc, precipitated barium
1 4
7 4 ~
.~.,
sulfate and silica; and organic pigments, such as
cyanin blue, azo pigments and so on may be employed.
Other additives and modifièrs, such as leveling agent,
antifoaming agent, antioxidant, W-absorber and so on
may be incorporated within a range not obstructing the
material properties of the coating film.
The powder coating composition for low gloss
coating according to the present invention is prepared
by blending the powder components described above and
preferably melting the blend at a temperature not
reaching the hardening temperature of the composition,
for example, at 80 - 100~ , with kneading and crushing
the cooled solidified mass. The average particle size
of the so-prepared powder coating composition may, in
general, be in the range of 20 - 150~ m, preferably in
the range of 30 - 100 ~ m.
The powder coating composition according to the
present invention prepared as above, can be coated on
various substrates by means of a powder coating
technique, such as electrostatic spray coating, with
subsequent h~ki ng of the coated layer at a temperature
capable of causing curing of the polyester resins and
the hardener to form a lustreless coating film.
Any substrate can be coated by the powder coating
composition according to the present invention,if it is
capable of withst~n~;ng the condition used in baking the
coated layer. The substrate may preferably be a metal plate
with a thickness of 0.2 to 2 mm, such as steel panels,
galvanized steel panels, aluminum panels and stainless
- 15 -
~i
steel panels.
For effecting coating of such a substrate withthe powder coating composition according to the present
invention, a commercial electrostatic coater (with an
application voltage of -50 to -90 kV) or other dry
application apparatus for powder coating may be
employed to build up a uniform coating layer, whereupon
the resulting coated layer is subjected to baking in a
baking furnace, such as hot blast baking furnace,
infrared furnace and induction furnace, at a
temperature of 150 - 300~C , preferably 160 - 250~C for
20 seconds to 60 minutes, preferably for 30 seconds to
minutes, in order to obtain a lustreless coating
film in a thichness of 20 - 200 ~ m, preferably 30 -
100 ~ m. By such coating procedures, lustreless coating
of every gloss value can be obtained.
Such a low gloss value of the coating film is
brought about by scattered light reflection from the
finally cured irregular coating surface with a fine
surface irregularity. In the conventional binary
polyester resin system, a comparatively monotonous
surface irregularity is apt to be re~cheA.
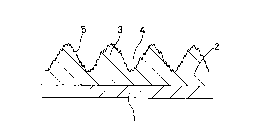
Fig. 4 shows such a surface irregularity of the
coating layer by a conventional binary resin powder
coating composition in a schematic sectional view. The
numeral 1 represents the substrate and 2 is the low
gloss coating layer. On the coating layer 2, a fine
wrinkle pattern with relatively simple-shaped
irregularity composed of protrusions 3 and indentations
4 is produced due to the difference in the curing
- 1 6
reaction rate between the two polyester resins, whereby
a mat appearance is brought about. In this case, the
lowest gloss value is o~tained, when the distance
between the neighboring two protrusions 3 is the
smallest, namely, when the density of the irregularity
is the highest. Here, a relatively high gloss-value by
an enwidening of the protrusion distance is apt to
occure, since a possible deviation of the mixing
proportion of the two polyester resins may exist.
In contrast thereto, by the powder coating
composition according to the present invention, as seen
from the schematic illustration of the coating layer
section shown in Fig. 1, additional -secondary fine
irregularity 5 is formed on the wrinkling surface with
protrusions 3 and indentations 4, due to the employment
of at least three polyester resins for realizing a step
wise difference in the curing reaction of the resins in
the coating layer 2. This secondary fine irregularity
5 provides a rather uniform scattering of the reflected
light, even when some enwidening of the protrusion
distance on the coating layer may occur due to a
possible fluctuation or inconsistency of the resin
mixing proportion or the h~king condition. It is
believed that the primary wrinkle by the protrusions 3
and the indentations 4 is caused by the curing reaction
rate difference between the polyester resins (A) and
(B) and the secondary irregularity 5 is caused by the
curing reaction rate difference between the polyester
resins (A) and (C).
Thus, according to the present invention, an
1 7
. '
.
213171~
intended constant low gloss value below 50 can be
attained even if the mixing proportion of the polyester
resins (A) and (B) deviates by an amount of about 10 %
by weight. When a different baking condition is to be
employed, the gloss value is maintained at a constant
low value due to the formation of the secondary fine
irregularity 5.
As described above, a low gloss coating film
can be obtained using the powder coating composition
according to the present invention, even if some
fluctuations and inconsistencies in the resin blending
condition and coating layer baking condition occur,
since three polyester resins exhibiting definite curing
reaction rate difference between them are incorporated.
Thus, according to the present invention, a powder
coating composition for a low gloss coating film is
provided, which, together with a superior mechanical
strength of the coating film, can maintain a
contemplated gloss value without suffering from an
intolerable deviation and which is capable of adjusting
the gloss value at any voluntary value within a wide
range.
PREFERRED EMBODIMENT OF THE INVENTION
Below, the present invention will further be
described in detail by way of Examples and Comparative
Examples, wherein it is to be noted that the present
invention is by no means restricted by such Examples.
All the numerical values for the blending proportion of
7 4 S ~
. ..~
the components are on the weight basis,
Examples 1 to 27, Comparative Examples 1 to 38
Using a dry blender (Henschel* Mixer of Mitsui
Miike Engineering Corp.), powder blend components for
each of the powder coating compositions of Examples 1
to 27 as well as of Comparative Examples 1 to 38 as
given in Tables 1 - 4 below were blended uniformly for
about 1 minute, whereupon the resulting blend was
subjected to melt kne~ing on an extruding kneader
(Buss-co-kneader PR-46* of BUSS AG). The kneaded mixture
was cooled to solidify and the resulting solid mass was
crushed on a hammer mill, whereupon the crushed mass
was screened on a wire sieve of 150-mesh (mesh size
105 ~ m) to obtain a powder coating composition.
Each of steel test panels with a thickness of
0.5 mm which had been treated by zinc phosphate was
coated by an electrostatic coating with each of the
powder coating compositions prepared as above to a dry
powder coating layer thickness of about 50 - 60 ~ m and
the coating layer was baked at 180~ for 20 minutes or
at 200~ for 10 minutes. The coating films baked at
180 ~ for 20 minutes were tested for their gloss
values, Erichsen values and impact resistances. The
coating films baked at 200~ for 10 minutes were tested
for their gloss values. The test results are recited
also in Tables 1 - 4.
For determining the modulus of elasticity, each
mixture composed of the polyester resin and the
hardener in a mixing proportion in terms of equivalent
ratio as given in Tables 1 - 4 was filled in the
* Trade-mark
, . 1 9
21 3I 7~
sample holder of a rheometer (MR-300 Soliquidmeter of
Rheology K.K.) heated at 200~C to melt it. After 3
minutes, the modulus of elasticity for each sample
mixture was determined. From this, the differences of
the moduli of elasticity between the polyester resins
were calculated. In Fig. 2, the course of temporal
change of the moduli of elasticity during 30 minutes
after filling the sample holder with each sample mixture
of combination of the polyester resins (A), (B) and (C)
and the hardner (D) of Example 1 is graphically shown.
2 0
213174~
Table
Components and ExamPle
Material Property 1 2 3 4 5 6 7
Polyester resin (A)
ESTER RESIN ER-6610 " 82.0 82.0 82.0
UPICACOAT GV-150 2)
FINEDIC M8020 3) 80.0
ESTER RESIN ER-6650 4) 75.0 75.0 75.0
Polyester resin (B)
30-3011 5)16.0 18.0 20.0 22.0 25.0 27.0
XI-7009 3' 18.0
30-3002 7)
Polyester resin (C)
UPICACOAT GV-740 8) 23.0
ESTER RESIN ER-6570 9)18.0 18.0 18.0 46.0 46.0 46.0
Hardener (D)
ADDUCT B-1530 '~' 55.0 55.0 55.0
24-2400 "' 24.0 24.0 24.0 26.0
POWDERLINK 1174 12 )
Titanium dioxide 60.0 60.0 60.0 50.0 50.0 50.0 60.0
MODAFLOW 13) 0.8 0.8 0.8 1.2 1.2 1.2 0.8
OH/NCO equival. ratio 1.1 1.0 0.9 1.1 1.0 0.96 1.25
Resin wt. ratio (A/B)84/16 82/18 80/20 77/23 75/25 74/26 82/18
Resin wt. ratio (A/C)82/18 82/18 82/18 62/38 62/38 62/38 78/22
Difference Eo ~ 107- 8 102. 8 102- 8 102- 5 102- 5 102- 5 102- ~
Difference Ec-. 10~ 5 10~ 5 10~ 5 10~ 5 10~. 5 10~. 5 10~ 9
Gloss (200~,10min) 28) 9 5 9 24 21 23 34
Gloss (180~,20min) 28) 8 6 9 24 20 22 31
Erichsen value (mm)29'>7.0 >7.0 >7.0 >7.0 >7.0 >7.0 >7.0
Impact resistance (cm)50 50 50 50 50 50 50
". lOOOg) 30)
'' 2l3l7~
Table 1 (cont.)
Components and Example
Material Property 8 9 10 11 12 13
Polyester resin (A)
ESTER RESIN ER-6610 " 75.0
UPICACOAT GV-150 2~ 80.0 80.0 80.0
FINEDIC M8020 3) 80.0 80.0
ESTER RESIN ER-6650 4)
Polyester resin (B)
30-3011 5) 25.0
XI-7009 6) 20.0 22.0
30-3002 7) 18.0 20.0 22.0
Polyester resin (C)
UPICACOAT GV-740 8) 23.0 23.0 44.0 44.0 44.0
ESTER RESIN ER-6570 9) 46.0
Hardener (D)
ADDUCT B-1530 '~'
24-2400 "' 26.0 26.0 41.0 41.0 41.0
POWDERLINK 1174 12) 22.0
Titanium dioxide 60.0 60.0 72.0 72.0 72.0 50.0
MODAFLOW I 3) O. 8 0.8 1.0 1.0 1.0 1.2
OH/NCO equival. ratio 1.15 1.07 0.93 0.89 0.84 1.0
Resin wt. ratio (A/B) 80/20 78/22 82/18 80/20 78/22 75/25
Resin wt. ratio (A/C) 78/22 78/22 65/35 65/35 65/35 62/38
Difference Eb a lo2 a lo2 o 10~ 5 10~ 5 10~' 5 102' 5
Difference Ec-. 10~ 9 10~ 9 10~ 8 10~ 5 10~ ~ 10~ 8
Gloss (200~,10min) 28) 32 30 47 45 46 20
Gloss (180~.20min) 28) 33 32 45 46 47 22
Erichsen value (mm)29' >7.0 >7.0 >7.0 >7.0 >7.0 >7.0
Impact resistance (cm) 50 50 50 50 50 40
(~ ~ , lOOOg) 30)
213174S
Table 2
Components and Example
Material Property 14 15 16 17 18 19 20
Polyester resin (A)
FINEDIC A-229-U 15) 82.0 82.0 82.0
FINEDIC M-8620 18)
ESTER RESIN ER-8105'7' 75.0 75.0 75.0
URALAC P-3500 18) 80.0
Polyester resin (B)
ESTER RESIN XE94002l9~ 16.0 18.0 20.0 22.0 25.0 27.0
ESTER RESIN XE930012~~ 18.0
FINEDIC M-8540 21)
Polyester resin (C)
URALAC P-2065 22) 23.0
UPICACOAT GV-230 2a) 24.0 24.0 24.0 46.0 46.0 46.0
Hardener (D)
Triglycidyl i-~y~nula~e 9.3 10.6 12.3 15.2 17.4 22.4 11.5
EHPE-3150 24)
EPIKOTE 1003F 25)
PRIMID XL-552 28)
CURESOLE C~7z 2 7)
Titanium dioxide 60.0 60.0 60.0 50.0 50.0 50.0 60.0
MODAFLOW 13) 0.8 0.8 0.8 1.2 1.2 1.2 0.8
COOH/Glycid. eq. ratio 1.1 1.0 0.9 1.1 1.0 0.8 1.25
Resin wt. ratio (A/B) 84/16 82/18 80/20 77/23 75/25 74/26 82/18
Resin wt. ratio (A/C) 82/18 82/18 82/18 62/38 62/38 62/38 78/22
Difference Eb-~ lo2. 9 102. 9 10Z~ 9 lOZ' 3 102' 3 102' 3 102' 1
Difference Ec-~ 10~ 4 10~ 4 10~ 4 10~ ~ 10~ ~ 10~ ~ 10~ 8
Gloss (200~,10min) 28) 6 7 6 31 30 33 25
Gloss (180~.20min) 28) 7 6 5 30 32 35 25
Erichsen value (mm)29) >7.0 >7.0 >7.0 >7.0 >7.0 >7.0 >7.0
Impact resistance (cm) 50 50 50 50 50 50 50
~ . 1000g) 30)
2131745
Table 2 (cont.)
Components and Example
Material Property 21 22 23 24 25 26 27
Polyester resin (A)
FINEDIC A-229-M l5) 80.0 80.0 80.0 75.0
FINEDIC M-8620 16) 80.0
ESTER RESIN ER-8105l7)
URALAC p-3500 18) 80.0 80.0
Polyester resin (B)
ESTER RESIN XE94002l9~ 18.0 25.0
ESTER RESIN XE930012~~ 20.0 23.0
FINEDIC M-8540 Z~ 18.0 20.0 22.0
Polyester resin (C)
URALAC P-2065 ZZ~ 23.0 23.0
UPICACOAT GV-230 Z3) 44.0 44.0 44.0 24.0 46.0
Hardener (D)
Triglycidyl i-cy~nurate 13.1 14.3
EHPE-3150 Z4~ 27.3 28.8 30.3
EPIKOTE 1003F 25) 75.5
PRIMID XL-552 Z6) 9.3
CURESOLE Cl7Z 27) 0.4 0.4 0.4 0.4
Titanium dioxide 60.0 60.0 72.0 72.0 72.0 72.0 50.0MODAFLOW 13) 0.8 0.8 1.0 1.0 1.0 1.0 1.2
COOH/Glycid. eq. ratio 1.15 1.1 0.95 0.9 0.85 1.0 1.0Resin wt. ratio (A/B) 80/20 78/2282/18 80/20 78/22 82/18 75/25
Resin wt. ratio (A/C) 78/22 78/2265/35 65/35 65/35 77/23 62/38
Difference Eo a lo2. 1 lo2. 1lol~ 4101- 4 101' 4 101' 3 102' Z
Difference Ec-~ 10~ 8 10~ 8 10~ 6 10~ 5 10~ 5 10~ 9 10~ 7
Gloss (200~.10min) 28) 23 24 41 43 42 45 35Gloss (180~,20min) Z8) 24 25 42 41 44 46 37
Erichsen value (mm)29' >7.0 >7.0 >7.0 >7.0 >7.0 >7.0 >7.0
Impact resistance (cm) 50 50 50 50 50 50 50
(~ ¢' ". 1000~) 30)
2 4
21317~
Table 3
Components and Comparative Example
Material Property 1 2 3 4 5 6 7
Polyester resin (A)
ESTER RESIN ER-6610 " 82.0 82.0 82.0
UPICACOAT GV-150 Z~
FINEDIC M8020 3) 80
ESTER RESIN ER-6650 4) 75.0 75.0 75.0
Polyester resin (B)
30-3011 5) 16.0 18.0 20.0 22.0 25.0 27.0
XI-7009 6) 18.0
30-3002 7)
Polyester resin (C)
UPICACOAT GV-740 8)
ESTER RESIN ER-6570 9'
Hardener (D)
ADDUCT B-1530 IO) 48.0 48.0 48.0
24-2400 "' 28.0 28.0 28.0 33.0
Titanium dioxide 51.0 51.0 51.0 59.0 59.0 59.0 53.0
MODAFLOW 13) 1.0 1.0 1.0 1.0 1.0 1.0 1.0
DURCAL 40 14)
OH/NCO equival. ratio 1.29 1.2 1.11 1.07 1.0 0.961.33
Resin wt. ratio (A/B)
Resin wt. ratio (A/C)
Gloss (200~,10min) 28) 15 4 14 30 20 31 45
Gloss (180~,20min) 28) 18 8 19 37 26 38 56
Erichsen value (mm)29' 5.4 >7.0 >7.0 >7.0 >7.0 >7.0 4.2
Impact resistance (cm) 30 50 50 50 50 50 30
(~ ¢' . lOOOg) 30)
2 5
213I 74S
Table 3 (cont.)
Components and Cl ative Example
Material Plop~lty 8 9 10 11 12 13 14
Polyester resin (A)
ESTER RESIN ER-6610 " 88.5 80.0
UPICACOAT GV-150 Z~ 80.0 80.0 80.0
FINEDIC M8020 3) 80 80
ESTER RESIN ER-6650 4)
Polyester resin (B)
30-3011 5)
XI-7009 6) 20.0 22.0 20.0
30-3002 7) 18.0 20.0 22.0
Polyester resin (C)
UPICACOAT GV-740 8) 34.0
ESTER RESIN ER-6570 9'
Hardener (D)
ADDUCT B-1530 '~' 11.5 31.0
24-2400 "' 33.0 33.0 28.0 28.0 28.0
Titanium dioxide 53.0 53.0 51.0 51.0 51.0 66.0
MODAFLOW 13) 1.0 1.0 1.0 1.0 1.0 I.O 1.0
DURCAL 40 14) 40.0
OH/NCO equival. ratio 1.25 1.17 0.89 0.830.75 1.0 1.43
Resin wt. ratio tA/B) 80/20
Resin wt. ratio (A/C) 70/30
Gloss (200~.10min) 28) 34 47 58 43 55 53 23
Gloss (180~.20min) 28) 44 58 62 50 60 62 25
Erichsen value (mm)Z9~ >7.0 >7.0 >7.0 >7.0 5.3 2.5 5.2
Impact resistance (cm) 50 50 50 50 30 10 30
". lOOOg) 30)
2 6 -
21317~
Table 3 (cont.)
Components and Comparative Example
Material Property 15 16 17 18 19
Polyester resin (A)
ESTER RESIN ER-6610 ') 80.0
UPICACOAT GV-150 2) 75.0 60.0 93.0
FINEDIC M8020 3) 72.0
ESTER RESIN ER-6650 4)
Polyester resin (B)
30-3011 5)25.0 20.0
XI-7009 8) 28.0
30-3002 7) 40.0 7.0
Polyester resin (C)
UPICACOAT GV-740 8) 65.0 10.0
ESTER RESIN ER-6570 9~ 35.0 26.0 50.0
Hardener (D)
ADDUCT B-1530 '~' 58.0 37.0
24-2400 ")63.0 36.0 26.0
Titanium dioxide 79.0 65.0 70.0 89.0 59.0
MODAFLOW 13~1.0 1.0 1.0 1.0 1.0
DURCAL 40 14)
OH/NCO equival. ratio 0.77 1.0 1.0 1.0 1.25
Resin wt. ratio (A/B) 75/25 60/40 93/7 80/20 72/28
Resin wt. ratio (A/C) 68/32 70/30 65/35 55/45 88/12
Gloss (200~.10min) 28) 17 72 80 65 73
Gloss (180~,20min) Z8) 22 75 85 67 77
Erichsen value (mm) 29)5.0 6.5 >7.0 >7.0 >7.0
Impact resistance (cm) 30 50 50 40 50
(~ ~, lOOOg) 30)
2 7 ~
21317~5
Table 4
Components and C, . ative Example
Material Property 20 21 22 23 24 25 26
Polyester resin (A)
FINEDIC A-229-M 15) 82.0 82.0 82.0
ESTER RESIN ER-8105'7' 75.0 75.0 75.0
URALAC P-3500 18) 80.0
Polyester resin (B)
ESTER RESIN XE94002'9~16.0 18.0 20.0 22.0 25.0 27.0
ESTER RESIN XE930012~~ 18.0
FINEDIC M-8540 21)
Polyester resin (C)
URALAC P-2065 22)
UPICACOAT GV-230 23)
Hardener (D)
Triglycidyl i-cyanurate5.9 6.8 7.8 10.7 12.4 13.6 8.1
EHPE-3150 24)
CURESOLE C~7z 2 7~
Titanium dioxide 51.0 51.0 51.0 59.0 59.0 59.0 53.0
MODAFLOW 13) 1.0 1.0 1.0 1.0 1.0 1.0 1.0
DURCAL 40 '~'
COOH/Glycid. eq. ratio1.3 1.2 1.1 1.1 1.0 0.951.33
Resin wt. ratio (A/B)
Resin wt. ratio (A/C)
Gloss (200~,10min) 28) 20 6 21 37 21 35 57
Gloss (180~,20min) 28) 25 13 27 42 28 43 69
Erichsen value (mm)25)4.3 >7.0 >7.0 >7.0 >7.0 >7.0 3.5
Impact resistance (cm)30 50 50 50 50 50 20
(~ ~ ". lOOOg) 30)
21317~5
Table 4 (cont.)
Components and Comparative Example
Material Property 27 28 29 30 31 32 33
Polyester resin (A)
FINEDIC A-229-U 15) 80.0 80.0 80.0 95.6 80.0
ESTER RESIN ER-8105l7'
URALAC p-3500 18) 80.0 80.0
Polyester resin (B)
ESTER RESIN XE94002'9'
ESTER RESIN XE930012~~ 20.0 22.0 20.0
FINEDIC U-8540 21) 18.0 20.0 22.0
Polyester resin (C)
URALAC P-2065 22)
UPICACOAT GV-230 23) 34.0
Hardener (D)
Triglycidyl i-cYanurate 9.2 10.4 4.4 9.9
EHPE-3150 24) 23.3 28 31.9
CURESOLE C~7Z 27) 0.4 0.4 0.4
Titanium dioxide 53.0 53.0 51.0 51.0 51.0 66.0
MODAFLOW 13) 1.0 1.0 1.0 1.0 1.0 1.0 1.0
DURCAL 40 14) 40.0
COOH/Glycid. eq. ratio 1.25 1.71 0.9 0.8 0.75 1.0 1.43
Resin wt. ratio (A/B) 80/20
Resin wt. ratio (A/C) 70/30
Gloss (200~.10min) 28) 45 52 63 53 65 68 24
Gloss (180~.20min) 28) 57 66 71 61 73 75 26
Erichsen value (mm)2~ >7.0 >7.0 >7.0 >7.0 3.2 3.5 4.1
Impact resistance (cm) 50 50 50 50 20 30 30
~ . 1000g) 30)
213174S
Table 4 (cont.)
Components and Comparative Example
Material Property 34 35 36 37 38
Polyester resin (A)
FINEDIC A-229-M 15) 80.0
ESTER RESIN ER-810517~ 75.0 60.0 93.0
URALAC P-3500 18) 72.0
Polyester resin (B)
ESTER RESIN XE94002'9~25.0 20.0
ESTER RESIN XE93001Z~~ 28.0
FINEDIC M-8540 Z" 40.0 7.0
Polyester resin (C)
URALAC P-2065 ZZ~ 35.0 65.0 10.0
UPICACOAT GV-230 Z3) 26.0 50.0
Hardener (D)
Triglycidyl i-~y~nu~ a~e23.2 24.9 16.4 18.5 15.6
EHPE-3150 Z4'
CURESOLE C, 7z Z7)
Titanium dioxide 79.0 65.0 70.0 89.0 59.0
~ODAFLOW I 3 ) 1 . O 1 . O 1 . O 1 . O 1 . O
DURCAL 40 14'
COOH/Glycid. eq. ratio0.77 1.0 1.0 1.0 1.25
Resin wt. ratio (A/B) 75/25 60/40 93/7 80/20 72/28
Resin wt. ratio (A/C) 68/32 70/30 65/35 55/45 88/12
Gloss (200~,10min) Z8) 16 75 85 68 79
Gloss (180~,20min) 28) 21 81 89 72 83
Erichsen value (mm)29) 4.5 3.1 >7.0 >7.0 >7.0
Impact resistance (cm) 30 20 50 40 50
~ . 1000g) 30)
Notes in Tables 1 - 4:
1) Trademark of the product of Nippon Ester Co., Ltd.: a hydroxyl group-
containing polyester resin having a hydroxyl value of 31 mgKOH/g and a
Tg of 66~C
3 O
2131745
2) Trademark of the product of Japan Upica Co., Ltd.: a hydroxyl group-
containing polyester resin having a hydroxyl value of 34 mgKOH/g and a
Tg of 59~C
3) Trademark of the product of Dainippon Ink & Chemicals, Inc.: a hydroxyl
group-containing polyester resin having a hydroxyl value of 30 mgKOH/g
and a Tg of 53~C
4) Trademark of the product of Nippon Ester Co., Ltd.: a hydroxyl group-
containing polyester resin having a hydroxyl value of 31 mgKOH/g and a
Tg of 61~C
5) Trademark of the product of McWHORTER Technologies, Inc.: a hydroxyl
group-containing polyester resin having a hydroxyl value of 295 mgKOH/g
and a Tg of 44~C
6) Trademark of the product of Nippon Ester Co., Ltd.: a hydroxyl group-
containing polyester resin having a hydroxyl value of 234 mgKOH/g and a
Tg of 48~C
7) Trademark of the product of McWHORTER Technologies, Inc.: a hydroxyl
group-containing polyester resin having a hydroxyl value of 136 mgKOH/g
and a Tg of 49~C
8) Trademark of the product of Japan Upica Co., Ltd.: a hydroxyl group-
containing polyester resin having a hydroxyl value of 51 mgKOH/g and a
Tg of 57~C
9) Trademark of the product of NiPpon Ester Co., Ltd.: a hydroxyl group-
containing polyester resin having a hydroxyl value of 40 mgKOH/g and a
Tg of 65~C
10) Trademark of the product of Huls AG: a blocked isocyanate compound: an
& -caprolactam-blocked isophorone diisocyanate: equivalent weight = 280
(g/eq.), Tg = 50~C
11) Trademark of the product of McWHORTER Technologies, Inc.: a blocked
isocyanate compound: an ~ -caprolactam-blocked isophorone diisocyanate:
equivalent weight = 240 (g/eq.), Tg = 52~C
12) Trademark of the product of American Cyanamid Co.,: glycolyl
tetramethoxymethyl: equivalent weight = 107 (g/eq.), M.P. = 100 ~C
13) Trad kof the product of Monsanto Co., a leveling agent
14) Trademark of the product of Hoechst Gosei K.K.: an extender pigment:
average particle size = 30~m
15) Trademark of the product of Dainippon Ink & Chemicals, Inc.: a carboxyl
group-containing polyester resin having an acid value of 24 mgKOH/g and
a Tg of 77~C
16) Trademark of the product of Dainippon Ink & Chemicals, Inc.: a carboxyl
21~174~
group-containing polyester resin having an acid value of 26 mgKOH/g and
a Tg of 69~C
17) Trademark of the product of Nippon Ester Co., Ltd.: a carboxyl group-
containing polyester resin having an acid value of 45 mgKOH/g and a Tg
of 63 ~C
18) Trademark of the product of DSM Resins BV: a carboxyl group-containing
polyester resin having an acid value of 30 mgKOH/g and a Tg of 65 ~C
19) Trademark of the product of Nippon Ester Co.. Ltd.: a carboxyl group-
containing polyester resin having an acid value of 125 mgKOH/g and a Tg
of 56 ~C
20) Trademark of the product of Nippon Ester Co., Ltd.: a carboxyl group-
containing polyester resin having an acid value of 180 mgKOH/g and a Tg
of 55 ~C
21) Trademark of the product of Dainippon Ink & Chemicals, Inc.: a carboxyl
group-containing polyester resin having an acid value of 220 mgKOH/g
and a Tg of 57~C
22) Trademark of the product of DSU Resins BV: a carboxyl group-containing
polyester resin having an acid value of 80 mgKOH/g and a Tg of 60 ~C
23) Trademark of the product of Japan Upica Co., Ltd.: a carboxyl group-
containing polyester resin having an acid value of 55 mgKOH/g and a Tg
of 69 ~C
24) Trademark of the product of Daicel Chemical Ind., Ltd.: an alicyclic
epoxy resin with an equivalent weight of 185 (g/eq.) and a softening
point of 74 ~C
25) Trademark of the product of Yuka Shell Epoxy K.K.: an epi-bis type
epoxy resin with an equivalent weight of 750 (g/eq.) and an softening
point of 96 ~C
26) Trademark of the product of Rohm & Haas Co.: a ~ -hydroxy alkylamide
(HAA) with an equivalent weight of 84 (g/eq.) and a M.P. of 123 ~C
27) Trademark of the product of Shikoku Chemicals Corp.: 2-heptadecyl
imidazole. a curing catalyst; M.W. = 222, M.P. = 88 ~C
28) Gloss value: according to JIS K-5400 (1990) 7.6. "Specular Gloss (60~)"
29) Erichsen value: according to JIS K-5400 (1990) 8.2.2. "Break Distance
Method"
30) Impact resistance: according to JIS K-5400 (1990) 8.3.2. on DuPont-
Method
3 2
213174~
As seen in Tables 1 and 2, the experimental
conditions of Examples 1, 2 and 3 as well as those of
Examples 14, 15 and 16 were selected in such a manner
that the weight proportion of the polyester resin (B)
differs by about 10 % successively. The results of
these Examples showed that the increase in the gloss
value by such difference in the resin content amounts
to at the most only 4 (i.e. 9 minus 5) which is well
within a visually indiscrimiable range. Also, the
experimental conditions in each ternary experimental
group of Examples 4, 5 and 6, of Examples 7, 8 and 9,
of Examples 10, 11 and 12, of Examples 17, 18 amd 19,
of Examples 20, 21 and 22 or of Examples 23, 24 and 25
were selected so that the weight proportion of the
polyester resin (B) differs by about 10 % successively.
They also indicate that the difference in the gloss
value among each three experiments falls under a
visually indiscriminable range. The hardeners used in
Examples 13, 26 and 27 were an amino compound, an
epi-bis type epoxy resin and an amide compound,
respectively.
Comparayive Examples 1 to 12 and Comparative
Examples 20 td 31 as recited in Tables 3 and 4,
respectively, represent the cases of using a binary
polyester resin mixture, wherein each set of three
Comparative Examples, namely, Comparative Examples 1 to
3, Comparative Examples 4 to 6, Comparative Examples 7
to 9, Comparative Examples 10 to 12, Comparative
Examples 20 to 22, Comparative Examples 23 to 25,
Comparative Examples 26 to 28 and Comparative Examples
21~174S
29 to 31, represents the case in which the weight
proportion of the polyester resin (B) differs by about
% successively. The results of these experiments
showed that the resulting gloss values of the coatings
were visually discriminable from each other. It was
also shown that the gloss value differed when the
baking condition was different. Comparative Examples 13
and 32 represent the case where the delustreing was
attained by the employment of an extender pigment,
which both gave gloss values over 50, with quite
inferior coating film properties, e.g. Erichsen value
and impact resistance.
In Comparative Examples 14, 15, 33 and 34 as
given in Tables 3 and 4, the equivalent ratio of the
constituent polyester resins relative to the hardener
was outside the range defined according to the present
invention. The results of these Comparative Examples
show that the coating film properties, e.g. Erichsen
value and impact resistance, are inferior.
In Comparative Examples 16 to 19 and
Comparative Examples 35 to 38, the blend proportion of
the polyester resins was chosen to be outside the range
defined according to the present invention, wherein
Comparative Examples 16, 17, 35 and 36 represent the
case where the weight ratio of the polyester resin (A)
to the polyester resin (B) was outside the inventive
range, whereas Comparative Examples 18, 19, 37 and 38
represent the case where the weight ratio of the
polyester resin (A) to the polyester resin (C) was
outside the inventive range. The gloss values of the
3 4
213174~
resulting coatings in these Comparative Examples were
over 60, so that they do not fall under the scope of
the present invention.
3 5