Note: Descriptions are shown in the official language in which they were submitted.
~ CA 021320~6 1998-02-25
A METHOD OF BLEACHING PULP
The present invention relates to a method of bleaching
pulp to a brightness of at least ISO 80 by utilizing at
least two of the following bleaching agents: oxygen,
ozone, and hydrogen peroxide.
Efforts have been made, e.g., for environmental reasons
to lessen the use of elementary chlorine in bleaching of
chemical pulp. The first efforts led to use of chlorine
dioxide, which as such is a better alternative than ele-
mentary chlorine in terms of environmental protection.
Also marketing factors are turning the trend in the di-
rection of total abandonment of chlorine, both elementary
chlorine and compounds of chlorine. Very many customers
attach great importance to the totally chlorine free
(TCF) manufacture of the product they are using. Manufac-
ture free of gaseous elementary chlorine (ECF) is
regarded as the minimum requirement.
A few years ago, when ozone bleaching of medium consist-
ency pulps had developed to a form applicable in an in-
dustrial scale (cf. FI 89516), most companies who de-
veloped processes and equipment for the pulp industry
concentrated almost exclusively on ozone bleaching to
develop it to a form that could be utilized in their own
processes.
However, ozone has its drawbacks too. The worst of these
is probably its high price, which is mainly attributable
to a fairly complicated mode of manufacture. Another
drawback is its high reaction activity, which means that
the plants using ozone, in the same way as those using
chlorine, have to be kept absolutely gas-tight. For the
same reason, ozone may easily damage the cellulose itself
P1205/EM8
CA 02l320~6 l998-02-2
if the amount of dosed ozone is not exactly correct or
mixing is not efficient enough.
For the above reasons, bleaching sequences have been
5 lately supplemented with both peroxide stages and EOP
stages, especially pressurized stages (cf. article
"Medium consistency pulp washer generates superior
washing efficiency", TAPPI Journal, June 1990;
PCT/FI92/00198, published as W093/00470, January 7, 1993;
EU patent 557,112, published August 25, 1993;
PCT/FI93/00222, published as W094/20673, September 15,
1994). However, it becomes apparent from the above
publications that the role of ozone is still of great
significance in bleaching sequences.
15 The present invention strives for paying attention to
both environmental values, which are turning the trend
away from chlorine chemicals, and economic points of
view, which are to at least some extent opposed to use of
ozone. The method of the present invention enables
20 bleaching of pulp up to a brightness of 83 to 85 ISO or
even to a higher brightness, with no need to use either
chlorine or ozone. If, however, a still higher
brightness is desired, one peroxide stage of the sequence
according to the invention is replaceable by an ozone
25 stage. In this way, a brightness of ISO 90 is readily
achievable. Correspondingly, it is also possible to use
much less chlorine dioxide than before for the production
of fully bleached pulp.
The characteristic features of the method of the
invention are best seen from the accompanying claims.
The method of the invention is described in more detail
in the following, by way of example, with reference to
the enclosed drawings in which
CA 021320~6 1998-02-2
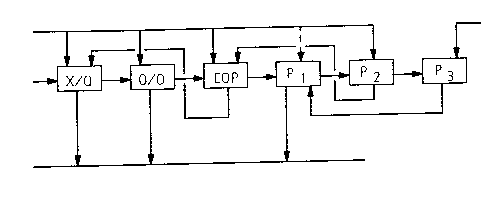
Fig. 1 shows a preferred embodiment of the bleaching
sequence according tc the invention;
Fig. 2 is a graphic illustration of a significant bene-
fit gained by the invention; and
Fig. 3 shows a preferred subprocess of the bleaching
sequence in accordance with the invention.
The bleaching sequence X/Q-O/O-EOP-P1-P2-P3 of the embodi-
ment shown in Fig. 1 is started with a previously known Q
stage, the purpose of which is to remove heavy metals
from the cellulose pulp by chelating (e.g., EDTA, DTPA or
equivalent compounds) or acidifying in order to prevent
them from impeding the peroxide stages (Pn) which follow.
In this stage, it is also possible to treat the pulp with
enzymes (denoted with X), which expose the lignin con-
sidering later delignification stages. The oxygen stage
O/O, which comes next, is actually formed of two or more
pressurized oxygen delignification stages or steps with
no wash therebetween, as described in greater detail in
connection with Fig. 2. The third stage, EOP ~alkalic
bleaching extraction), which is separated from the O/O
stage with wash, is disclosed, e.g., in article "Medium
consistency pulp washer generates superior washing effi-
ciency", TAPPI Journal, June 1990. The peroxide stages
(Pn) which come next in the sequence and which are prefer-
ably pressurized but may also be atmospheric, are corre-
spondingly disclosed, e.g., in PCT/FI92/00198 published
January 7, 1993. The use of a pressurized reactor is
advantageous especially when gaseous chemicals, such as
oxygen, are used or when gaseous chemical is generated in
the reactor, as happens when hydrogen peroxide splits
into water and active oxygen.
Fig. 1, on the other hand, illustrates an advantageous
arrangement for circulating wash liquid in the bleaching
sequence. In other words, washing after the last bleach-
P1205/EM8
CA 021320~6 1998-02-2~
~._
ing stage, P3 in this case, is effected with white water.
The filtrate thereby resulted is conveyed over the pre-
ceding bleaching stage P2 to serve as the wash liquid of
the washer in stage P1. The washer of stage P2 is supplied
with fresh water of about 75~C to serve as the wash
liquid therein. The filtrate from stage P2, or at least a
portion thereof, is conveyed to the washing stage after
EOP to serve as a wash liquid therein. The filtrate
received therefrom is taken to the washing stage follow-
ing the metal removal Q. The circulation of the filtratefrom the washing in stage P2 especially results in that
the peroxide which has come from the stage P2 and entered
the filtrate ends up in the oxygen stage and intensifies
its delignification reaction. If we go further in the
process, it is possible that the peroxide-containing
filtrate from a washer of some other bleaching stage,
e.g., Pl or P3, is taken to the washing stage of brown-
stock. Thus, peroxide is taken also via this route to the
delignification stages.
The reason for connecting two oxygen stages in sequence,
as in Fig. 1, is shown in Fig. 2. The vertical axis indi-
cates the kappa number and the horizontal axis the time
used for the process. Graph a) shows a conventional
advance of a bleaching process. In other words, as soon
as the chemicals have been mixed with the pulp, the kappa
number of the pulp declines sharply. Later it becomes
steady to such an extent that an extension of time does
not bring an essential change to the reaction result.
Naturally, there are various reasons for that. Firstly,
consumption of the chemical slows down the declining of
the kappa number. Secondly, more and more reaction prod-
ucts are formed in the suspension nearest to the fibers
all the time, whereby the unreacted chemical still pres-
ent in the suspension does not easily access the fibers.Thirdly, the amount of lignin present in the fibers
P1205/EM8
. CA 021320S6 1998-02-2~
',",,~..
decreases, whereby its detachment from the fibers in
proportion takes place more slowly. And fourthly, because
pulp always contains some amount of gas, the gas which is
contained by the pulp after mixer and being in the form
of micro bubbles tends to accumulate by nature and pro-
duce bigger bubbles, which impede mass transport in the
pulp suspension. Bubbles are also generated as product of
bleaching reactions. Such reactions may be, for example,
the splitting of hydrogen peroxide into water and active
oxygen. A new mixing stage breaks big bubbles into micro
bubbles. Thus, it may be estimated that mixing alone
somewhat improves the treatment result. Graph b) indi-
cates what happens if the first reaction is in a way
interrupted at point tl, a new portion of chemical is
dosed to the suspension, and is mixed therewith evenly
and efficiently. It can be seen that the shape of graph
b) is equal to the shape of graph a) after the chemical
addition. A comparison between the kappa numbers indi-
cated by graphs a) and b) when time t2 has passed reveals
that the procedure in accordance with graph b) brings a
considerably lower kappa number than the procedure illu-
strated by graph a). It is naturally conceivable that
chemical could be added once more to the suspension, and
obtain a still lower kappa number in the same time and
practically with the same amount of chemicals.
Fig. 3 illustrates slightly more in detail an implementa-
tion of the O/O stage in a sequence of a preferred embo-
diment of the invention. Outermost in the left is shown a
washer, denoted with reference numeral lo, which effects
removal of heavy metals from the pulp. The washer may be,
e.g., A. AHLSTROM CORPORATION's DD-washer or Kamyr Inc.'s
pressure diffuser. After washer 10, the pulp is conveyed
to a steam mixer 12 for raising its temperature, and
further via an intermediate tank 14 by pump 16 into a
mixer 18, where pulp is mixed with at least oxygen as a
P1205/EM8
CA 021320~6 1998-02-2
bleaching chemical and sodium hydroxide for pH regula-
tion. If it is desirable to add a little peroxide to this
oxygen stage, it can be mixed with the same mixer 18.
Steam and/or magnesium, preferably magnesium sulphate,
may also be mixed by mixer 18. Mixer 18 is replaceable
with an MC pump provided with at least one chemical feed
conduit, in which case the pump 16 posterior to the steam
mixer 12 could substitute for mixer 18. A precept for
mixing chemicals, especially oxygen, is a division ratio
60/40. In other words, the first stage is supplied with
60~ of the total amount of oxygen and the second with
40%. In general, the chemical dosage for the first oxygen
step is 5 to 20 kgO2/admt and, if peroxide is fed to the
first stage, the dosage is relatively small, only about 1
to 10 kg/admt. From the mixer 18, pulp is discharged and
fed to a reaction tower 20, where the pH value is kept
within the range of 9 to 13, preferably lO to 11.5, the
temperature within the range of 75 to 110~C, preferably
85 to 95~C, and the pressure within 2 to 15 bar, prefer-
ably 3 to 10 bar. The retention time in the first reac-
tion tower 20 is about 10 to 60 minutes, preferably 25 to
50 minutes.
From the tower 20, pulp is discharged with a discharge
means 22 via a second mixer 24 to a second reaction tower
28. The discharge means may be means which is capable of
gas separation, thereby eliminating the above described
gas problem (e.g., means disclosed in WO patent
applications PCT/FI90/00085 published as WO93/01875
February 4, 1993 and PCT/FI92/00216 published as
WO90/13344 November 15, 1990). Preferably pulp is fed to
the reaction tower 28 with a distributing means 26, which
ensures that the pulp column grows evenly in the tower 28
and that no channelling takes place. Said distributing
means is described, e.g., in U.S. patent 4,964,950
published October 23, 1990, and its use for the above
described purpose is dealt with in FI patent application
P1205/EM8 924805 published April 24, 1994. The chemical dosages in
mixer 24 are as follows: 5 to 20 kgO2/admt and
i
CA 021320S6 1998-02-2~
about 5 to 40 kg/admt peroxide; the whole peroxide dosage
preferably added ~o t~is second step. The pH value is
kept, by feeding sodium hydroxide, in this second oxygen
step within the range of 9 to 13, preferably 10 to 11.5,
the temperature within 75 to 110~C, preferably 85 to
95~C, and the pressure within 2 to 10 bar, preferably 3
to 5 bar. The retention time in the second reaction tower
28 is about 30 to 100 minutes, preferably 45 to 90 min-
utes. From the tower 28, the pulp is discharged via dis-
charge means 30, which separates gas if necessary, to a
blow tank 32, wherefrom it is conveyed by a pump 34 to a
washer 36. If gas is separated by discharge means, it is
advantageous to lead the gas into the blow tank 32.
A second embodiment of the bleaching method according to
the invention is a bleaching sequence, by which a pulp
brightness of ISO 90 is obtained. In this sequence, the
first peroxide stage of the sequence shown in Fig. 1 is
replaced with an ozone stage. In other words, the
sequence will be
X/Q-O/O-EOP-Z-P-P.
An alternative sequence is naturally
Q-O/O-EOP-Z-P-P
from which the enzyme treatment has been left out.
To the above sequence may also be applied a combination
of medium consistency ozone bleaching and pressurized
peroxide stage, which is described, for example, in WO
patent application PCT/FI92/00198 published January 7,
1993. In that case, the sequence will be
X/Q-O/O-EOP-ZP-P-P.
When the enzyme treatment is omitted, the sequence will
be
Q-oto-Eop-zp-p-p
P1205/EM8
q7a
CA 021320S6 1998-02-2S
Other applicable sequences, characteristic of the method
of the invention. are, for example, the following:
~~Q~~/~~Pn /
which is started with oxygen delignification and con-
tinued with the sequence described above. For example, inthis sequence a great benefit is gained if one of the
filtrates of peroxide stages Pn is introduced into a
washing stage preceding the oxygen delignification O. It
is also possible to add some peroxide to said oxygen
delignification stage for making this more efficient.
With this sequence, a brightness of up to ISO 85 is
obtained. If the brightness of ISO 90 is desired, an
ozone stage may be added before the peroxide stage as
follows:
O~Q~O/O~Z~Pn or
O-Q-O/O-Z/Z-Pn,
where Z/Z means more than one ozone bleaching step as
described, e.g., in FI publication 89516. In practice,
this is effected so that ozone is mixed with pulp by and
from a plurality of mixers sequentially, without any pulp
washing therebetween. Gas may be discharged from these
ozone steps, as disclosed in WO patent application PCT/-
FI92/00276 published April 29, 1993, so that a new
addition of ozone would bring in proportion a bigger
dosage of ozone into the pulp. Naturally, also the
following sequences are applicable:
O~Q~~/~~ZP~Pnl
O-Q-O/O-ZP,
O-Q-O/O-Z/ZP, and
~~Q~~/~~Z/ZP-Pn
Example 1
A pulp mill had the sequence
Do-o-Eop-Dl-E-D2
in use, where D denotes a chlorine dioxide stage in gen-
eral. A normal dosage in this case is about 40 kg active
P1205/EM8
~'
CA 021320~6 1998-02-2~
chlorine/admt, i.e., about 15 kg ClO2/admt. When the
oxygen stage O was replaced with a two-step oxygen stage
O/O, in other words
Do-o/o-Eop-Dl-E-D2
the chlorine dosage to the stage Do could be reduced to a
value 15 kg active chlorine/admt, i.e., to 5.7 kg ClO2/-
admt, in other words over 60%. Regarding the whole
sequence, the change in the consumption of active chlor-
ine was about 70 -> 45 kg Cl/admt, i.e., of the order of
35%. The AOX reduction which is often used as a measure
of environmental loading, is over 50%, i.e., < 1.0 kg/-
admt. The O/O stage may also be safely supplied with
peroxide because the Do stage is as such a highly effi-
cient remover of heavy metals.
Tests performed have given the following kappa numbers
and brightness values in various stages.
Do O/Op EOP D2
Kappa 16 3 3 <0.1
Brightness 50 83 go
The above results indicate that the Do stage may be mod-
ified so that it only removes heavy metals and activates
lignin, whereafter the O/O stage in accordance with a
preferred embodiment of the invention, intensified with
peroxide, is very efficient.
Example 2
A pulp mill used the bleaching sequence
Dl/O-O/O-Eop~D2~E ~
by which a brightness of ISO 90 was achieved. However,
since the sequence further had two chlorine dioxide
P1205/EM8
CA 021320S6 1998-02-2S
",~,", . ~...
stages, the mill wanted to substitute some other chemical
for chlorine. They ended up to replace the second chlor-
ine-using stage, D2 with one or more peroxide stages and
the first chlorine-using stage Dl with a stage X/Q, which
activates lignin and removes heavy metals, and in which,
for example, EDTA, DTPA or equivalent and enzymes (X
stage) are used. Thus, the resulting bleaching sequence
was
X/Q-O/O-EOP-pl-p2-p3 -
The dosages (kg/admt) in the test performed were in ac-
cordance with Table 1 below. Table 2 shows the correspon-
ding process parameters. The production rate in the test
was 500 admt/d. NaOH is not always added to the P stage
(P2 in Table 1) preceding the last P stage. When the pH is
remains lower, pulp is activated and delignified prior to
the last P stage. An appropriate pH in such a stage may
be 3-7, most preferably approximately 5.
Table 1
X/Q O/O EOP Pl P2P~ ¦
NaOH 25 5 11 7
~2 20 6
H2~2 5 7 40 5 21
H2SO4 5
EDTA 3
DTPA 2 2
MgSO4 3 2
Enzyme 5 - 10
SO2 3
; P1205/EM8
--'
CA 021320~6 1998-02-2~i
11
Table 2
X/Q ~/~ EOP P1 P2 P3
Temperature 40 95 80 85 70 85
Retention 50 150 25+100 180 60 180
Kappa 15.2 <8 <~ <3 ~2
Brightness 34 45 65 75 77 >83
pH 5.5 11 11
In the best succeeded trial, even > 85 ISO brightness w s
achieved, which was much better than expected. The addi-
tion of peroxide to the oxygen stage 0/0 raised the
brightness values considerably, i.e., from 45 to 55 ISO.
The kappa number again declined from 15 to 5 without an
addition of peroxide, but when peroxide was added, from
15 to 4.
Generally speaking, a bleaching sequence Q-O/O-EOP-P1-P2-
P3 with dosages (kg/admt) shown in Table 3 below, results
in pulp the quality of which is good and the manufactur-
ing costs of which are not high. Below each range isshown a dosage which has proven especially advantageous
in our tests, but such values have to be considered
approximate.
Example 3
In tests with pressurized peroxide bleaching, it was
found, among other things, that the same bleaching
result, with regard to both brightness and kappa number,
as was earlier received unpressurized with three hours'
treatment was now received in one hour when the pressure
was about 5 bar. Further, the tests revealed that pres-
P1205/EM8
A
CA 021320~6 1998-02-2~i
12
surized treatment also considerably improved the effect
of chelating agents, such as EDTA and DTPA. The improve-
ment even seems to make it possible to totally give up a
separate discharge stage of heavy metals and to effect
chelating of heavy metals in connection with each perox-
ide-using delignification/bleaching stage. Thus, the
nowadays very popular unpressurized sequence
Q-P-Z-P
could be replaced with a sequence
P-Z-P
in which the P stages are pressurized and they, or at
least the first of them, could be supplied with chelating
agents for binding heavy metals.
Pressurized peroxide-stages also facilitate replacement
of the above described sequence Q~O/O~EOP-Pn by a sequence
Q~~/~~Pn /
by which a brightness as high as ISO 85 is achieved. When
a brightness of ISO 90 is desired, the peroxide stage may
be replaced with a combined ozone and peroxide stage
Q-O/O-ZP.
As can be seen from the above description, a novel envi-
ronmentally friendly bleaching method has been developed,
which facilitates removal of the earlier used bleaching
stages, which employ both elementary chlorine and com-
pounds of chlorine, and lessens the use of expensive
ozone. However, it is to be noted that the above descrip-
tion handles only a few exemplary embodiments of the
bleaching method of our invention, and it is by no means
intended to limit the invention from what is disclosed in
the accompanying claims. Thus, it is for example self-
evident that also in those sequences where the feed of
enzymes to the metal removal stage has not been separate-
ly disclosed, such feed is possible.
P1205/EM8
CA 02132056 1998-02-25
~.,~ ' ~ .
O O
I O I O
~4 o ~ In ~1 ~1
O O
,~
I O
U~ ~1 ~ U)
O
~ i ~
~ O O I o
,1
O o
O l l l
O ,~
~o ~
~ i I ~ I
O ~J ~ In ~ ~ ~r
O o ~
I CO
~ I O I o o
O ~ ~I r~ ~ ~1 ~ O ~~
o
O o
O! o ~~ o ~ o o t~ ~ IO ~1
~~ ~
i~ ~r ~r
O ~ ~ O
~I E~ ~ N r~ O O rr,
:r~ o 5: :~
~
~, --