Note: Descriptions are shown in the official language in which they were submitted.
. W O 93/22302 ~13 3 ~ ~ 4 P ~ /GB93/00801
N-ARYLOX~(THIO)ALKYL-AZACYCLOALKANES USEFUL AS CALCIUM CHANNEL ANTAGONISTS
~eFresentLnven~onrela~sto aryl~%yaLkylam ulo and aryl~oa~kyla~no de~va~vcs,
prccesses~r~he~ prepala~on,ph~L~eu~e~ cvmposi~onscon~n~ng ~h~m andthe~ use
S in ~ho~a~y, ~n par~cular in the b~a~nentof ischae~c s~k~
Stcoke is ~xt~dly the thixd rnos~ ~mmon callse of death h the developed
woQ~ld. ~r~nt ~erapies f~ ischae~c s~ke are lirDl~d ~nd hav~ a ~L~ber o~
disadvantages, such as tho sisl~ of exacerb~ hao~age. Th~e is th~fore a need fornew and ~proved trea~ellts ~ is~ha~c s~l~e.
~o EP-A-103252 disclo~s a broad class o~ aryloxy~ylam~no d~a~ves. Th~se compounds arc said t~ h~ve udiir5r as h~Dicides.
French Pa~nt Applica~on ~o. 1601$gl d~scnb~ a class of n~gen~oll~g
het~cyclic com~ounds denved ~m phsn~y~l~l al~hols, which arc gald t~ be
cholesterol~lower~ng agents.
We have now ~Imdl that ce~ arylo~ya~amino ~d arylthioalkyl~o
der~va~ves exhibit acdvi~ as calciu~ cha~el an~g~n~sts.
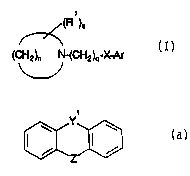
The present ~nvenGion ther~fare pro~r~des, Ln a ~rst aspect, u~e of a compound of
fo~mula (1): ;
"~ ). ;
~ '
(C~2)n N-(CH2k~X A
V
Forr~ula O
in which
nis3to 8;
qisSt~ 11;
Rlrepresen~ Cl~6aLkylor Cl.6alkoxy;
siszeno,1 or2;
X ~eprescn~ oxygen orsulphur,and
Arrepresents phenyl op~onally subs~tuted by 1-3 subs~nlents selccted ~om halo,
C~ ~aLkyl, Cl ~alk~xy,C1 2aLkylenedioxy e.g. me~hylenedioxy,~i~uoIome~hyl,
~iQuorom~thyloxy,oragToup Ph-(C~2)m-Y-(C~I2~p- whele Phisop~ona~ysubc~tuted
WO 93/22302 r~ 2 - P~'/GB~3/00801
phenyl, m and p are ~ndependen~y 0 to 4 and Y is a ~ondf O, S, or ~CH, provided that
m ~ p is not ~eater ~an 4, or Ar is all op~ona~ly subs~tu~ed
~icyclic hete~oaryl group:
~z~3
~n which yl is Y(CH2,)X whore ~c is 0 ~ 1 and ~ is C)~ S or ~ wher~ R is h~dro~en or
Cl q,~ll~l, Z is (~2~ or ~ , r ~ 0, 1 or ~ or Ar is ~he correspondin~ tricyclic
d~hydro n;la Sj'St~ r a ph~ceu~c~ly a~le s~ A~Of' in ths~ ac~ of
medicament fo~ th~ ~ent ~ eonditions rela~ t~ a~umula~ion of calcium ~n ~he
b~ain cells of m~ls.
Prçfe~ably n is 4f 5 or 6f most p~erably 5.
Proferably q is 6 to ~, most pr~erably 7.
Wnen s is o~ner ~an ze~ Rl preIe~ablg repre~nts C:1~6alk$ 1, such as m~nyl.
X pT~er~lv represe~s oxyge~
bs~e~ r~p Ph(-~2~ 2~P~ St ,~ p~ably ~x~
o or a ~on~ When ~ is oxyg~n p is pre~erably zero and m is prefe~ably z~ro or 1, When Y
is a bond the sum of m+~ is preferably 1 or 2. ~hen Y re~sents C~, m ~nd p are
prefcrably ~th ~ro.
Preferably Ar is phenyl mon~subsdtuted by phenoxy, ben~yl, benzyloxy or halo;
phenyl disubs~tuted by halo; or Ar is 2 dibenzofuranyl. M~st pxef~rably A~ is phcnyl
~s subs~tuted by ben~yl or benzyloxy.
~ .~ampies or ~sicyciic het~aryl ~çroups includ~ aibç~wIuraIlyl~ ai~nwti~icnyi,
carba~ole, N-n:lethylcarbazole, ~dine and dibcrl~oxep~ne. The tricyelic moic~ can be
linked to the remainder of formula (I) via any suitable r~ng atom.
Suitable subs~t~le~ for Ph, and triic~lic hoteroaryl groups ~ncludol for example,
30 1 to 3 subs~tuents selected f~m halogen, ~uorometbyl, ~fluo~ome~hoxy, C~ 4alkyl
and Cl Aalkoxy.
~ 1 ~oups present in the compounds of fo~mula (I), alone or as par~ of anothcr
group, can be straight or branched. Thus, a Cl 6aL~ oup may be for example methyl,
e~yl, ~-propyl, n-bu~yl, n-pcn~l, n-hexyl or any branched isome~ ~er~f sucb as
35 isop~opyl, t-butyl, ~ C-pCDyl.
Wo 93/22302 ~13 3 9 8 '~ PCr/GB93/00801
~ 3 -
It will be apprecia~ed that for use in medicine a salt of a compoulld (I) should be
pharmaceu~ically acceptable. ~amples of phalmaceuticall~ acceptable salts include
~ganic and o~ganic aad a dition salts such a~ h~hl~ide, hydrobromide, sulphate,
pllosphate, acetatc~ fuma~ate, maleate, citratc, l~ctate, ~ate, o~alate, methanosulphonate
S o~ similarpha~maceutically acceptable in~gan~c or otga~lic acid add~on ~alts. Other non~
pha~ac~utically aecepta~le salts m~g b~ used ~ exa~ple ~ the isolation of th~ final
produat and are included w~thin tho sc~po o~ inven~on~
It ~ be appr~cia~l ~1 the cox~po~ds o~ fc rmula ~I) maS~ contain one or more
asy~e~ric cen~res. Suah compoh~ds will ~ist as ~pdc~l isom~rs (enan~orr ers). Bo~b the
10 pure ~n~ome~ m~c mix~res ~$0% of ea~h ~n~tiame~ d lmeqllal rnixnL~s af the
two arc ~ncluded wi~n~n t~e seope of the in~o~on. F~ ;e~l all diaste~or~:~r.c ~ms
possible (pure enanliomels a~d mi~se~ ther~of) a~ within the ~copa ~ the ~nvention.
Ce~ compounds of ~or~ula (I) are ~lieYed to be n~vel. Thus, in a f~er
aspect the invention pro~ides a ~ompo~nd of ~ a (~
.
~ 1
(~Ha)n Nu~12)q~X Ar
Formllla (IA)
in which
n, q, Rl, s and X are as defincd for formula (I) and Arl is an op~onally substituted
t~icyclic heteroaryl group as d~fin~ for formula (I);
or a sa}t thereof.
^5 ~ ~ y~t ~es ~t t:he L~ve~on ~ provides a comDound of ~ormllla (l~
f~k )~
~N~ 2),X4
F~rmula (IB)
3~
wherein Rl, s, n Md X are as de~ed for farmula (I) and Ar2 repTesents phcnyl op~onally
subs~ted by a group Ph-(CH2)mY(CH2)p- o~ a aicyclic heteroaryl ~roup as defined for
fo~ula (I)? C~ a salt thereof.
wo 93/22302 ~13 3 9 g ~ pcr/GB93/oo8o~
-4-
In the compounds of fDImula (DB) n is preferably ~om 4 to 6~ most preferably 5.
X p~erably re~resents ~xygen. When s is other than z~r~, Rl prefe~ably rcpresents
Cl~l. Ar2 prefe~ably re~resents phenyl subs~tuted by a ~oup Ph(~I~)mY(CH2)p-.
Most prefesably Ar2 represents phenyl s~bs~cd by phenoxy, berlzyl or bcnzyloxy. In
s gen~al the phenyl subs~cn$ ~rill pre~cxlbly be at the 4-position of the phenyl ring
r~la~v~ e group ~C.
~ mpo~ds of f~rmulae ~) and (~B) represent n~vel ~nd advantageo
selee~ons on the basis o~ ~heir a~vi~ as calc~um ch~cl an~go~sts.
Pa~cul~r compouD~ ~f tbe inven~on, which are believed to be novel cotnpounds
O includc:
7-phenoxy-l~pip¢ridinoheptan~,
7-(~fluvrophenoxy)~l-piper~dinohep~ano,
7~(2,~diehloroph~noxy)~1-pipendinohep~net
7-t~phenox$fphenoxy)~1~pipexidinohep~e,
7-(3-phcnoxyphenvxy~ pipeddinohcp~anc,
7~(2~dibenzo~nyloxy)~1-pipendinohep ~ e,
7-(4~nzyloxyphenoxy)~l~piperidinoheptane,
N~ (~benzyloxyphenoxy)hepty~-hexamethylen~im~rle,
20 9-(3,4-dichlornphenoxy)-l-pipcridinononan~,
9-(4-benzyloxyphenoxy)-l-pipe~dinon~nane,
8-(~benzyloxyphenoxy)~l~piper~dinoootane,
8-(4-phenoxyphenoxy)-l-pipe~dinooctarle~
benzyloxyphenoxy)-l-piper~inohe%ane,
2s 7-~4-[2~ hlorophenyl)ethylJphenoxy)~l piperidinohepta~e,
srans-7-{4-(2-phenyledlenyl)phonoxy]~l-piper~dinoheptane,
7~(2-phenylethyl)phenoxy]-1-pip~ridinohcptane~
7-(4 benzylpn~noxy)-l~pipendinoheptanc,
7-(2-benzylphenoxy~-l-piperidirlobeptane,
30 7-(~med~oxyphenoxy~-1-pipe~idinoheptane,
7-(~1ert-buylphenoxy)-l-pipendinoheptane,
WO 93/22302 ~13 3 ~ ~ ~ PCr/GB93/00801
7-(3,~-methylenedioxyphenoxy) l-pipe~idinohept~ne,
7-[~(3,~dic~ benzyl~xy)phenoxy~-1-pi~dilloheptane,
7-~(~methoxybenzyloxy)phenoxy~ pipcndiIloheptane,
7-~(~fluo~o~nzyloxy)phonox~]~l-pi~L;noh~ptan~,
5 N-p-(~benz~lo~ypheno~y~hep~ 2-meth~rlpi~fidine,
N-r7-(4-ben~loxyphenoxy)hep~ 3-methylpip~ridine,
N-~7-(4-benzyloxyphcnoxy3h~ 4~methylpip~idirle,
N~7-(~benzyloxyphenoxy)h~pt$ l~ thylpiper~dinc,
N-~7-(~benzylo~yphelloxy)h~lJ~methoxspip~r~dine, and
0 $-(4-benzyl~yph~noxy)~l~piper~din~pentane,
and phar~aceu~ca~y acceptable s~ hereof.
~ he compolmLt.~ of the prese~t lnverl~on aan be prepared by p~cess~s analogous
to those ~cnown ~ ~he a~ Th~ present ~nvention the~fsr~ prov~des in a fur~her aspcc~, a
process for dle preparadon of a novel ~ompound of fo~mula ~I) in par~culas a compo~md
15 of formula (~A) or (lB) which compnses:
(a) reac~aon of a compound of ~o~ula (II):
~,~(~ )O
N ~CH2~-L
Formula (11)
in which n, Rl, s and q a~e as dcfined in fonnula (LA) and L~ is a ~roup displaceable w~th
a nucleophile wi~ a compound of fo~nula (XlI):
2~
Ar3-a~H
Fo~mula (m)
30 in ~hich X is as de~incd in fonnula (IA) and Ar3 rcpresents Arl when q is as deined in
folmula (IA~ or Ar2 when q is 7;
Wo 93/22302 ~, 13 3 .~ ~ 4 pcr/GB~3/oo8o1
6-
(b) reac~ion of a co~pound of formula Cl~):
Ar3~ L~
Fo~mlla (XV)
~r~ which ~ and q are as defined ~r fo~lu1a (~A), Ar3 is as de~ined Por f~ula (m~ and
L~ nng ~oup, ~th ~ compowid of ~aul~ ~):
~1
NH
Formtlla ~V)
h which n, Rl and s are as defin~ in f~rmula CLA); or
(c) reduc~on of an am~de of f~mllla (~) or (~):
` 1
f~,(R )4
~C~H2),t.1 /N (C~)qXAr
\~0
: ~0 Formula ~1)
~ 2)n N~Cff2)q~1Xa~r
Formula (V~)
2~
wherGin Rl, s, D, q, X and Ar3 ue a~ defincd abo~re;
d) Reduc~vo a~n25On of an ~Idehyde of fo~ula (Vm):
wo 93/223n2 2 ~ 3 3 9 ~ ~ PCI/GB93/00801
-7 -
~C~(C~2,)q l~3
~nnula ~m)
S whe~ein q, X and ~3 ar~ as herv~nbcfore de~ed, ~n thc prcsen~ of a compouIId of
fo~ula (V) as defined above.
e) Ib prepa~e a compo~d where~ Ar3 r~prexents phonyl substitut~d by
Ph(~2)mO-~ alkyla~on of a cv~po~d o ~ul
(R )~
~N~(CH2)~,X~
~ormul~
where~ Rl, s, n, q and X arv as h~re~nboforo defined; w~th an alkyla~ng agent of formuln
(X):
Ph(C~2)~L
Iformul~
where~ Ph, r~ and Ll are as h~befor~ d~iinod.
f) To prepaTe a compound wh~xe n is 5, redu~on of ~ pyridine deri~a~dve of
f~lmula (a~
/
~,N~ 2)
f~rmul~
whe~ein Rl, s, q, X and ~Ar3 a~e as he~in~for~ defined and A- is a counter anion;
g) L~lterconversion of one compound of formula (I) to a di~ent compound of
fo~mula (I) o.g. ~educ~rl of a compound whe~e~ Y represents CH~ ~o a compound
3~ wherei~ Y ~es~ 2~H2-;
WO 93/22302 PCr/GB93/00801 . i~
~ ~ 3 ~ 8 -
followed if desired by salt f~a~on.
~ proeess (a) th¢ ~on betwce~l a compound of fom~ula ~Il) and a compound
o~ fa~ula (~I) can bo cam~d ou~ a~nder s~andar~ condi1dons. Por example when Ll is
S hydroxy, the ~on is carr~ed out o p~esellce of diethyl azodiearboxylatc and ~ .
~iphcnyl phosphine. Such a xcac~on is hlown as ~ ~itsunobu reaction (as descnbedSynthcsis 1981, 1). This r~a~don may op~onally be e~ected ~n the presencc of a solvont
such a5 tetrahydrofuran. Al~a~voly tho lea~ng ~up Ll may be f~r examplG a balogen
atom o~ a sulphorlyloxy group eg. methanc-su:lphvnyloxy or p-~luene s~lphonyloxy. In
lo ~is case the r~on may be e:~ctcd ~ ~he abs~nce ~ ~esence ~ solvcnt such as
dimclhyl~G~amide or methyle~hylkc~ne i~ thG presonee sr a base such as sodi~n hyd~ide
potassium c~oaate ~d at a temp~a~ ~ the range O to 200~C.
The ~eac~oa of a compound o~ f~ula (~) ~th ~ compound of ~ormula (V)
acco~ding to p~cess ~ e ef~ccted in comen~onal manner, for example using
excess am~ne as solvent or ~l~rna~voly using an o~an~c sol~en~ such ~s e~hanol or
dimethylfo~mam~de. The lea~ing ~Lp L~ rtsay be ~or examplc a halide such as ~romlde
or ~hlo~xde, an a~yloxy ~up such ~ acetoxy or chl~raacetaxs~ ~ ~ sulphonylox~ group
such as methanesulphonyloxy or p~luenesulphonyloxy. The reaction is prefe~ablSI
Carrled OUt h ~e preserlce of a base suGh as potasshlm carbaxlatc~ sodi~ hydzide or
~eaucnon o~ an amide according t~ p~CSS ~C) may oe errec~ea usmg a sul~able
redu~ng agent sueh as li~ium aluminium hydride.
I~ proc~ss ~d) redu~vo am~na~n of a~ aldehyd~ (Vm) may be effeet~d us~ng a
reducing agont such as sod~um cyanoba~hydride in the presence of a compound of
2s formula (V), according to pr~cedur~s well ~nown ~n the art.
In process (o) the rcac~don o~ compounds (IX) and (~) may be effected in ar
analogous manner tv process ~a) desc~ibed above.
Reductlon o~ a py~dinium dcnva~ve (XI) acc~rding to pr~cess (f) may be
ef~ected for example by hyclrogena~on, using a noble metal catalyst such as palladium on
charcoal, pla~num or plat~um o~de (Adam's catalyst)~ su~ably ~n a solvent such as an
alcohol e.g. ethanol. ~ :
Interconversion reac~ons according to pr~ess (g) may be cani~d aut using
standard methods. Thus ~or example conversion of a compound (I) wherein ~ represents
into a compound (r) wher~n Y rep~sents -CH2CH2- may be effected by
3s cataly~ hydrogena~on.
A c~mpound of formula (lI) can be prepa~ed und~ standard alkyla~on c~nditions
by rea~ting a compound of formula (X~
.
W093/22302 ~ pcr/GB93/oo8ol
L~(CH2)q~L
Fo~nula (~
5 in which Ll. L2 and q a~ as heroinbeor~ defined, ~i~ a c~mp~lmd of f~mula (V) as
he~einbef~rc def~ned. Th~ reac~on is su~tably ca~r~ed out ~nder analogolls condi~o~s to
those de~d a~ove fo~process ~b).
It l1vill ~ apprc~ia~od that ~n ~mpolmds of ~ a ~) thc l~a~g ~roups L~
and L~ arv prçfesably s~ ~ s~ that tho compo~d ~ formula ~) ~eac~s s~lec~vely ~vith
10 L~. For e~camplet in a c~d of ~r~ula ~) L 1 is su~tabl~ h~rdroxy alld L~ is
sui~ably halo.
(:~mpounds of ~o~mula ~I) ar~ cammerciall~f ~vailable ~ may b~ prepared u~g
stand~d proced~as welI ~no~rn ~n the a~
Compo~nds of f~rmula (rv~ c~ b~ ~pared by ~ea~g a compou~d of f~ul~
lS (IrI) as he~ fore d~f;ned w~th a compoulld c~ mula ~) as hereinbefc ro de~ined.
In tbis rea~hon bo~ Ll and L2 can b~ id~ntical, ~ example halo. Th~ reac~on is sul~ably
camcd out in the presence o~ a wca~ bas~ such as ~sium c~nate. Al~natively the
reac~on may be ca~Tiçd out unde~ phas~ erans~r condi~ons us~ng a s~ng bas~ such as
potassium hy~x~d~.
2~ Compo~ds of ~ula ~V) as~d ~) ase ~^~.. 1211y ~ai~ble c r ~ay be
~epared by stan~ara me~nods.
Compounds of ~ula (V~ y be ~pa~d ac~ding to ger~ral p~esses (a)
and (b~ descnbed her~in esnplo~g a~ appr~ de co~s~ g to f~llla ~) ar
(~r).
Compounds of fo~ula (Y~) n~y be prepa~ed by acyl~on of a corllpound of
fo~ula ('V) for example w~th an appropnate ac~d chl~r~dc or es~, whieh may itself be
prepa~cd ~m a compound of formula (m~ by r~ on w~ an appropriate, commere~nlly
available bromo~yl ~s~ c r acid, followed if neccssary or dosired by conversion to an
a~id chlo~ide. Alterna~ely a compou~d ~ may be pr~pared by a method ~nalogous toprocoss (a).
AI1 aldehy~e of fon~lula ~ may be prepa~d for ex~mple by reduc~on of the
coq~esponding ni~ile using a ~du~g agont such as diisobutyl alu~nium hydride, an the
prosence of an inert so~vcnt such as toluene. Conven~cntly reduc~ve amina~on o~ the
aldehydc is car~ied out ~r ~. i.e. the compound of fo~mula (I) is obtained from the nitsile
3s in a one-potreaction wi~out isola~on of the intesmediate aldehyde. The r~i~rile may itself
bc p~pasd by ~eac~ng a compound of fon~ula (IY) whesc~n L2 is halo with potassium
cyax~ide. Compounds (Vm) may also be prepared by other standard procedu~es such as
redu~on of an estcr or o~idation of an alcohol.
WO 93t22302 PCr/GB93/00801 ~ :
~ ~33~ o~ ` ;
Compounds of f~mula (I:;~) may be p~epared by me~hods analogous to any of
p~cesses (a) ~ (d) desc~ d he~ein. Altema~vely a compou~d (IX) may be obtained by
catal~c hydrogena~on of a corresponding compolmd of formula (I) where~n Ar represents
a benzyloxyphenyl group. This theref~re p~ov~des a furthor method of convs~ng a
s compound of formula a) t~ a different compo~d of ~rmula (I).
Compounds of fa~mula (~ nay also ~e prepared ~ a similar manner to
p~esses (a) to (d) described above.
When a compound o~ formula (~A) or (~) is ob~d as a m~xr~ of
en~n~om~s, these may be s~p~d by conv~n~onal meth~ds su~h as cr~stallis~on Ln the
0 pz~sence ~ a ~sol~Lng ag~ntt or chr~r~at~raphy, ~or cxample us~ a chiral ~PLC
colu~nn.
It ~All be apprecia~ ~hat w~t the u~ mcdiatos of ~ormulao (~) to (~ e
d~ined w~th rc~ésencç to formulae ~) and (~3), the abovo p~cesses can ~e used toprepare any othcr n~vel compoun e scope of ~mula ~ and the prescnt
15 inv0ndon extends to the p~eparati~n of such compo~ds.
Thc ~nventi~n also encompass~s any n~el lnt~cdiates des~bed he~ein, la
par~cular thosG of ~ormulae ~), (~), (VI), ~, (~X) and (~.
Com~ounds of ~e invcnti~n havc b~en found ~ cxhibi~ high cal~u~ influx
blocl~lg a~rity, f~ example in neu~ons. As such the comp~unds arc oxpectcd to be of
20 use ;~ thc~ ;~ ;r~r.g ~nd~ d dlsc~sGs r~at~d t~ a~ acc~ lætion ~f c~!cillm ~nthe b~ain cells of mamma~s. m p~cuiar n~an~. ~or exampio, ~ile c~mpolmds ~re
expected to be of use Ln the ~eatmeslt of anox~a, ischaem~a ~cluding for ex~mple s~ke,
mi~e, viseeral paLn, cpilcpsy, ~ c head or sp~:naI ~njury, AIl)S~sela~d demen~,
neurodegcnera~ve dis~os such as Alzheimer's discase and age rcla~cd memor~ disardels,
~S mood diso~dcrs and drug addiction w~thdrawa~ such as e :hanol addic~on w~thdrawal.
Par~eu1arly prefe~ed compounds ac~ording to the present invention a~e 7-t4-
benzylphenoxy)~l-pi~dinoheptane and 7-t~bcnzylox~phenoxy)-l^pi~dinQhcp~e
(which may also be named as l~p~ ben~rlphcnoxy)hoptyI~piperidine and l-p-(~
bcnzyloxyphenoxy)heptyl]pipcridine) and pharmaceutically acceptable salts thereo~.
30 These compounds poten~ly inhibit Ca2~ c~ent par~cularly ~n neur~nal cba~nels. The
comp~unds also demons~te neu~oprotectivc effects ~n ~arious animal models of
ischa~mia, when adminis~red post-ischacmi~
The in~fcn~on also pro~r~des a me~od of treatment of condi~ons or diseases
rela~d to (e.g. causcd or exac~bated by) the accumula~on of calcilL~ in ~c brain cells of
35 mammals which comprises administenng to a subjecs in need thercof a~l e~fec~ve amowlt
of a compound of formula (I) as her~inbefore defi~ed or a pha~maceu~cally acceptable salt
d~eof.
. .
Wo 93/22302 ~ l ~ 3 ~ PCr/GB93/00801
Thus for exan~le, the present invention pro vides a meth~ of ~eatment of
anoxia"scha~a includirlg for exaDIple stroke, mi~e, visceral pain, epilepsy,
~auma~c h~ ~ sp~nal injurSr, A~S-~elated demen~a, ncurodegcnera~e diseases such
as Alzheime~s diSeasG and age~related m&m~y disorde~s, mood disorders and drug ::
s addietion withdraw~l such as etha~ol addic~on w~thdrawal, which compr~ses admin~t~g
to a subject ~n need tbe~eoft an e~eeti~!e, amount ~ a compound of ~on~ula (I) or a
pharma~etl~cally ~ceep~ablc salt ~ereof.
Pot use in medi~ne, tho c~mpolmds af the presen~ L vention are usually
a~mirlistered in a stasldard ph~rmaceutical camposi~on. The p~s~nt ~llwn~on therefore
0 provides in a furth~ asp~bt pharmac6udcal eompos~tiatls c~mp~sing a novcl compaund of
fosmula (I) as he~inberore de~cd o~ a ph~ceudca~ly accep~blo s~lt th~ and a
phannaceu~cally acceptable ca~r~es or exclpient.
The compo~ads o the in~en~on may be ~inistered ~y any co~venient method :~
for example by oral, parenteral, buccal, reet~ or ~ansdermal administration and the
pharmaceu~cal composi~ons adapted accordingly. Parenteral admiDis~ation is generally
preferred.
The compounds of formula (I~ and their ph~rmaceu~cally accepta~le salts which
are acdve when g~en orally can be formlllat~ as liquids or solids9 ~r exa~ple syrups, -
suspensions or ~mulsions, table~, capsul~s a~d lozenges.
A li4uid f~a~on ~ ~ly ~ns~ss of s~s~on or salu~n of ~be
cornp~und ~r phan~e~cally acceptaole salt ~ a suita~ie ii~d cameT~s; ~ar ~la,
e~anol, glycenne, non-aque~us solven~, for cxample polycthylene glycol, oils, or water
with a suspending ag~t, proserva~e, ~ ~o~g or colou~g agen~ :
A composi~on ~n thc form of a tablet can b~ prepared using any slL~table
2s pharmaceu~cal ca~mes(s) rou~nely used for preparing solid formul~ons. Examples of
such ca~ie~s ~nclude magnesium stea~ato, starch, lac~se, sucroso and cellulosc.
A composition in the form of a capsule can be pr~pa~d us~ng rou~nc
encapsula~on p~ocedures. ~or cxample, ~llets containing the ac~ve in~ient can beprepared using stan~ ca~riers and thon filled into a hard gelatin capsule; alternatively, a
dispersion or suspensic~n can be prepared us~g any suitable phaTmaceutical ca~rier(s), ~or
example aqueous gums, celluloses, silicates or oils and the dispcrsion or suspcnsion then
f;lled into a soft gelatin capsule.
Compounds o the invention may also be ~istered parentesally, ~y bolus
injec~on or co~l~nuous in~us~. Typical pa~onte~al composi~ons consist of a solu~on o~
suspension of the com~und or ph~maceutically acceptabl~ salt in a stesile aqueous carrier
o~ p~enterally acceptablc oiL fo~ exasnple polyetbylene glycol, poly vinyl pyrrolidone,
lecithin, aIachis oil OI` sesame oil. Alterna~vely, the soludon ca~ be lyophilised and then
recons~nlt~d with a suitable solvent just prior to administration.
WO 93/22302 ~13 3 5 ~ ~ PCr/GR93/V0801
- 12-
Bo~ uid and solid composi~ons m~y contain other excipients known in thc
ph~eu~dc~l ~, such as cyclodex~ins~
Preferably the co~posi~on is ~rl un~t dose form such as a tablet, capsule or
ampoule.
s Bach dosage un~t f~ ~1 ad~nistra~on cantains preferably *om 1 to 250 mg
(and for par~ntczal adminis~a~;on cont~ins pre~bly ~m 0.1 to 60 mg) of a comp~ d~R ~he fo~u~ a~ or a ph~eu~cally acceptable ~t the~eof calculat~d as the frec base.
Ibe daily dosage neg~men for an ad~t p~ont may bo, f~ cxample, an oral ~se
of between 1 m~ and ~00 m~ ferably b~t~een 1 mg and ~50 rn~, e~. S ~o ~00 ~g or an
0 i~travenous, subcutaneous, or in~amuscular doss of ~tween 0.1 ~g and 100 mg,
~rably betwe~n 0.1 mg and 60 mgl e~. 1 to 40 mg Ot ~e compo~d of ~he form~a (X~
ar a pha~ceu~cally a~c~table salt th~f aa~culatad ~ thc ~ec base, thc compound
bc~ng adminis~d 1 to 4 ~mos pe~ day. Alt~ vely the co~npounds of thc in~ention
~y ~ adminis~ by corltinuous ~nt~ensus infusion, p~e~erably at a dose of up t~
~ mg per day. Thus d~o to~l daily dosage by ora~ administra~oll c~d be ~n ~he ~ge 1
to 2000 mg and the total daily dosage by pa~enteral ad~nis~ation cotlld be in the r~nge
0.1 to 400 mg. The compound~ may bo ~ru~t~red for a peAod of con~nuous therap~,
for example for a we~k or m~re.
desi~ed a compound of ~smula a) or a ph~rmacou~ally acceptable ~alt th~f
~y ~c a~rl~s~ sbi~on o~ c3n~ ntly w~th on~ ~ m~ ath~ ~e~c
agents, f~ example a tns~m~iyi~c ag~n~ sucn as anistrcplase, s~rGptoK~ase o~ a ~ssuo
~: plasm~nogen a~ator, an excitatory am~no acid ant3gon~t su~h as an ~A an~gon~sts;
a ~ree radlcal ~hibi~or, or a calpah~ ~hibitor.
WO 93/22302 ~ 1 3 3 ~ ~ ~1. PCI /GB93/008Q1
~ 13-
BIOLOG~CAL l)ATA
l~ Vitro
S Ca2~ Curr~nt M~rement
Cell pFepar~tia~s
SensoIy neurons f~m d~al root gall~lia we~ di~ ~ated f~m 1 day old ~at pups (Forda
et al, l:~cvelopme~ Bra~n E~sea~h, ~2 ~l985~, SS-65). (:~lls w~ plat~d out onto glass
c~v~rslips and used withi~ 3 days to ~t e~foe :iv~ voltag~ alamp o~ Ca~ currents,
Solu~ons
The pipettc (inte~nal s~lu~on) ~n~ined ~n n~[: CsCl, 130; E~ES, la; EGT~, la;
Mg~, 4; ATP, ~; bufercd t~pH 7.2 v~ CsOEI. ~ells wc~e bathed in a normal
Ty~des solu~an bef~e establishment of whola c~l reaording when the bathing s~ution
was changcd ~ onc allow~g isolation of ~a~ curren~s. The çxtemal s~lu~on for
r~cc1rding Ca~ channcl c~rents cantai~cd ~n mM: : 3aCL2, 10; TEA~C1, 130; glucose,
10; HEP13S, 10; MgCL2, 1; buifer~d to pH 7.3 w~th l~;A~OH. Ban~n was used as the ~;
chargo ca~r~er as this assists ~n current isola~n and calc~uxn depondant ~nac~va~ion of
Cl~eM iS aYoided. C~mpoU;ldS W^._ ~ssol~ d in D~ o m~ ~ ~ mM s~ck
ss: lu~on. At thc drug concenmltlon us~ ~no venicie ~0.i~/oj i~aa no ~.,~ ~ n
Ca2~ c~Tell~s. All e~ents wem pc;f~ed at ~ ~ ~o 24C. Whole eell currents were
reco~ded us~ng List ~ 7 ar~lifiers and stored, d~gi~sed f~ lat~r a~lys~s us3ng PC
based sof~wa~ similar to dlat descr~bed prg~iousl~ ~Benham & Tsien, Jo~nal of
2s Physiology (1~88j~ 404, 767~784).
~Ca~ currents
Peak voltage gated Ca2~ chaMel cu~ents of up to 10 nA ~m d~sal ~o~ ganglion
~eurons were recorded us~ng 10 ~ Ba2~ as charge c~er. ~rents we~ evoked from
30 a holding poten~al of -80 mV to a test poten~al of 0 or ~10 mV e~ery 15 seconds. This
test pot~dal was at the ~ of the current ~oltage relationship and assessing block at this
poht ~uced any e~rors due tO d~if'ting hol~g potential. Some cells showed 51OW
rundown of c~scnt as is commonly se~n when recording Ca2~ currents. The $undown
~ate was measured in control condi~ons and ext:rapola~d through the ~me of drug
3s applica~on to denve a con~ol value to :rclate the drug affected current to. Block by
20 ~1 drug was assessed 3 minutes a~es drug applica~on.
.
~,~33g~
WO 93/2~302 pcr/cB93/oo8
Compounds of the inYen~on gave perc~n~age ~hibition of plateau Ca2~ cur~ent Ln the
range 35~100%
~ o
s Test ~ompound A - 7 (4~B0n~yloxgpheno~y)~1~ pi,wridinoh~ptan~ hydrwbloride
Test c~mpound B _ 7~(4~Be~ylphealo~y)~l~p~p0ridilloh~pt~sle hydr~hloride
Gerbil B(:AO Model
M~le Mong~liaD g~rbils wG~gh~na betwecn ~80 g w~e anaesth~ised with h~lothane,
10 placed on a hea~l mat and the ~arodd ~cs ~cluded. Aft~ repe~fusion, the alli~al5
wcre su~red and pla~ed in an ~ncubator malllt~ed at ~dy ~empera~e until r~cry.
Thc animals we~ then caged sepa~tely and on the 4tb day a~t~r the day of surgery, thcy
wcr~ assesscd for loGomot~ a~ty using an autoxr ated locomotor acti~y mon~tonng
system. The dosLng p~otocol ~ th~se ex~ents was 3û :ninutes post~ischaemia then
lS b.i.d. f~r 3 days, Lnjections be~ng g~vcn ~ ho i.p. xoute. Two sets of experiraerlts we~
c~ied out. In the ffrst thG duration of occlusian was 8 minutcs and test compound A was
gi~en 10 mg.kg-~ or 3 mg.kg-l using the abovo dosing regime. Ln tho second exporimontl :
10 minutes of occlusion was used alld 30 mg.kg~l of tcst comp~unds A and B
admil~LiSte~ed 30 ~utes post-ischaemia ~l~wcd by 10 mg.K~l b.i.d. ror 3 days.
Ln the 8 minu~ iod of ischaerc~a expe~imont test compound A at 10 mg.kg-l pr~duced
~ Si~iflCa~lt mtersal of the histolog.ical impalrmcnt in the t: Al reg.ion of dle hippocamplls
seen ~n the ischae~c vehicle~ated animals. This dose also p~duccci a slight, though
s~a8stically non-significant, revcrsal of the ischa~m~a-induccd hypeslocomotion. Iho
25 highe~ dose of tcst compouulds A and B used in the second ox~ent pr~duced a
si~ihcant reversal of the lo~oD:~otvr dehcit hlduced by the 10 m~slute period of ischaem~a,
but did not pr~duce a stads~ically significant effect on his~ology.
Rat Rose Bengal Mbdel
~fale Listes Hooded rats (250-280 g) anaesthe~sed with halothane were posi~oned in a
st~reo~c fs~me. Rectal tempesature was ma~ntaLned at 39t~ skull was exposed ;~
and a bif~ated fibre op~c light g ~ide (3.0 mm diameter3 ~om a 300 w xenon arc lamp
was posi~on~ to the sl~ull at Bregma in the antcr~or-pos~es~or plane in a holder desi~ed
35 to cont:re the heads o~ the Ught guides 2.5 mm to the left and r~ght of the m~dline. Rose
.
Wo s3~22302 ~ PCr/GB93/00801
-15
bengal (20 mg.kg~l) was Lnje~ted into a latc~al tail ~ein~ The skull was iLl~ated for
S minutes, aftcr which the wound was sutured and anaes~hesia discon~nued.
Test eompounds were administer~ i.p. at 30 r~g.kg~l 10 ~x~nut0s po~t-opera~vely,S foll~wed by a ~urthes dose of 10 mg,~g~ l onç hour pc st surgery and then tw~ce d~ily f~r
three days. A 75% I~dUGtiC~n 1~ lesion volumo was ~bserved ~ mpound A.
At th~ aboYe ~oses of Comp~unds A ~d ~ ~s~ed, no ad~e~se ~o~colog~Gal effects were
obscr~
Cardiovassular S~r~ll
Male Lister ~I~dGd ~ats we~ znaosth~tised w~th sodium pentobaJrbitone (~ mg.kg-l i.p.).
The effec~s on diastolic blood p~ssur~ a~d hcart rat~ w~re rn~sured OVG~ the dsug
in~usîon. Test compound A ~10 mg.kg~l dissolvcd ~n 10% ~ l)), was i~used ove~ 3015 minutes. Minor press~r effe~tsf 8imilaI to thOBe æon in v~h~cle ~eated nnimals, wese
observed, tc)g~ther w~th min~ ~u~ons i~ h~art rate. These results indica~e ~hat the
compound was w~thout signi~cant cardiovascular ef~c~ at the dose tested.
C~mpounds of formula (I) have been fo~nd tv exhibig ~d brairl-ponetrant prope~os.
W~ 93t22302 .~ 1~ 3 9 ~ ~ pC~ 93/OO~Ol l--
- 16-
PHAIRMAC~E~JTIC~ FORMULATXC)N8
1. Fo~mllation Sor inh a~now in~u~ion
~mpound of ~oImula (I) 0.1 ~ 60 mg
~odium hydrox~de/hy~hlcn~a acid ~n pH ca 7
po1yethy1ene g1yco1 0 - 30 ml
~pylene dycol O ~ 30 ml
alcohol 0 ~ 10 m~
watcI to 10a ml
2., Formul~tion for b~lus in,~ection
Compound ~f f~ la ~I) 0.1 ~ 60 mg
sodium hydro%ide ~ hy~chlc~c ~ to p~ c~ 7
polye~yl~ne glycol 0 ~ 2.5 ml
alcohol O ~ 2.5 ml
waler tO 5 ml
~, ~
A tonici~ a~ent e~ di~ chlQ~dc, d~x~se ~ ~nas~1 may a~so bc
~ ,
3. Tablet t~r oral admi~istratio
.
m~ltablet
Compound of fo~mula (I) 25
lac~se 1~3
starch 3~
crospovidone 12
; ~ 30 mi~crystalline cellulose 30
magrlesium stearate
::
Wo 93/22302 PCr/GB~3/0080l
~133~ 17-
~xamples
Lntermediate prep~rs~on~
5 1) 7~PiperidiYIohep~nol
7-Bromohep~ol (S.ûg) was added ov~ 30 mirlu~es to pipe~ (20ml) s~rred at ro~m
temperature. The resul~ng mi~ w~ fc to s~nd ~or 60 hau~ and dl~ disso1ved Ln
chl~ofo~. This solu~on was washed ~nth dllu~ s~dium hyd~ox~de s~u~on, ~rino
0 c~ntaining a few dr~p~ of dilute sodium hy~d~ solu~n alld dried ov~r magnesiumsulphate. The solv~nt was ~emovcd and ~c r~sidue was ~ugeirohr dis~ll~ tO ~e ~e
~tle compollnd as a solid ~4.53g) m.p. 37~3gC
Bromo~7 (~ Dibenzofi~ lo~y)hep :ane
A m~xture of 1,7-dibromoheptane t8.5g), 2-hydroxydibenzofuran ~4.66g), potassiumcar~nate (6.91g) and butan-2 one (lOOml) was he~ted at reflux temperature for 18 hours.
The m~xture was filtered and tbe filtrate was evap~ted. ~e r~sidue was pax~nonedbe~vee~ ehlo~fo~m and dilute s~um h~rdroxide. The cb~a~m laycr was separated,
20 wasned wi~ D~ine, drie~ aver mag;nesi~ s~ph~ d ~ ~lve3~ vas ~m~ e
~sidue was suspos~d~ ~n boiling hexano ~l~)t and filterea, an~ ate was a~iow~a
tO cool. The precipi~ate was ~llcc~d by ~ on to g~ve tho title, compound ~1.89g) m.p.
65-70~C which was us d wnhout ~cr puri~ca~ion. The ~l~ate was e~apo~ated tO giVCa secon~ c~op of ~e ti~e compourld (5.82g).
2s
3) 7~(4 Benz~lo~ypheno~ bro~heptane
1,7-Dibr~moheptano (12.9g) was ~ed dropw~se tO a stirrcd soluion ~f ~
be~loxyphenol (lOg), sodiurn hydroxide (2.5g~, benzy}~ethylammonium chlo~de
30 (0.4g) and water (30ml)~ The mixture was s~ at SO~ for 18 hours, water (50ml)
added and the solu~on extrac~d w~th dichloromethane (2 x lOOml). The combined
dichloro~ethane ex~wts wcre dried over magnesium sulphate~ solvent was removed and
the residue was cbrom~tographed on silica gel eluted w~th hexane/~ichloromethane to giYe
the ~tle compouIId (4.25g) as a soli~ m.p. 56 - 59C.
: ~ 35
:
WO 93/22302 ;~L33 ~ ~ PCr/GB93/00801
- 18-
4) 6- Piper~dinoh0xanol
The ~tle com~ound wa~s pTepared ir. a similar manner to ~nte~ediate 1 star~ng ~m 6~
bromohGxanol tlO.Og). Kugd~hr dis~llation (ovpn temperature 150~ @ 0.1 mmHg) gave
S ~ tle compound as a clear oil, (~.a7g).
S) 8 Piper~di~ooct~nol
The ~tle comp~und was ~epared ~n a s~nilar ~er to ~te~mediato 1 st~ng fr~m 8
10 ~omo~tanol (lO.Og). Kug~ la~on (o~en ternpe~ture 1~ ~ 0.1 mmHg) gave
the ~tle cornpound as a whi~e solid, (9.~) m.p. ~5-r7~C.
6) 9~Piperidinonanol
5 The dtle compc)und wa~ prcpared ~n a similar rnanncr to Intermcdiate 1 stardng ~rom 9-
bromononanol (S.Og). Eugclrohr dis~lIad~n (oven temperature 180~ ~ O.OS mmHg) gave
thc titlc compound as a white solid, (4.632g) n:Lp. 53~55C.
7) 7~(4~Hydro~phen~xy)~l piper~dinoh~ptan~
~i ~ure OI 7-~Dcnzyiox~ cnoxy) i~pîp~ainoncp~ane (i3.Z~g)l ~o pa~aaium or
c~on (0.5g), astd ethanol (250ml) was shaken ~der an atr~osphe~e of hydrogen a~ 50
p.s.i. for 16 hours. The catalyst was remored by fll~a~on a~d the ~ was evapora~ed.
The residue was recrystallis~ om aceton~trile, to g~re thc titlc eompound as a white
2S ~stalline solid (8.-38g), m.p. 106-106.5~C
Found: C, 74.03; ~I, 9.81; N, 4.93%
(ClgH2~N02~ICl) ~qu~es: C, 74.18; F~, 10~03; N, 4.81%
30 8) 1~[7-(4~Benzyloxypbenoxy)heptyl]~4~methoxypyr~dinumbrvmide
.A solu~on of ~methoxypyndine (3.21g) and 7-~benzyloxyph~noxy)-1-b~moheptane
(1 l.O9g) ir ethanol (lOOml) was heated at reflux fol 55 hours. The so1vent was removed,
the residue was treated with ether and the ~esul~}g solid was collec~ed and ~ecrystallised
3s ~om acetonitrile to give the ~tle cou~ md (5.23g~ which was used WithOUI fu~
purifica~on.
WO 93/22302 ; - 19 - PCr/GB93/00801
9~ 5~(4-Beslzyloxyphenoxy) l bron~pentane
,
Substi~uting 1,5~dibromop~ ane (12.~g) ~r 1,7-dib~moheptane ~n lnte~mediate
pr~para~orl 3 and using c~csponding molar p~p~ions of the o~her Teag nts gave the
5 titl~ c~mpound~ (11.60g) m.p. 45~C, which wa~ use~ without furth~s pu~calion.
10) 6 (4~Benzylvxypheno~r) l~lbromohe~ e
.
A mixtD of 4-benzyl~x~honol (17.47~), l,~b~omohexa~e (26.9ml),
10 ~en~yltrimethylarrmonium chlos~de (1~03g~, s~iu~ h~drox~de, water (OOml) and
dichloromethane (~5ml~ was s~sed and heatcd to 60~C and then reiluxed ~r 6 hours. The
cooled m~xt~ was ex~act~d w~th dlchl~m~t~ane. The dlchl~mothano cxt~s were
wæhcd w~th watcr, dried over s~iu}xl sulphate and ~c ~lvent was rorr oved. ~e residue
was recrystallised ~vice ~rom hexane to g~ve the ~e eompound, (17.83g), IlLp. 74-76C.
S
Found: C~ 63.07; H, 6.25; Br, ~1.68%
(C15H23~ reql~res: (:, 6~.8~; .H, 6.38; B~, 22.00~o
11) 7~(4~Benzyloxyphelloxy)heptanellitrile
~o
A m~xtur~ of 6~ ben~yloxyphcnoxy)~l~bramohexane ~lO.O~g), potassium cyan~de
(1.79g) and dimethyl sulphox~d0 was ~ and heatcd at 6~C ~or 16 hours. Ih~ c~oledmixnlre wa~ poured ~nto water (lL). ThG ~pi~a~e was collected, washed with water and
recrystal~ised f~m tolueno/hexane ~o ~e c~d~ compound (7.82g) -~
~5
Example 1
'
7 Phenoxy~l~pipe~dinoheptane hydrocbloride
30 A s~ution OI 7-pipendinohept~nol (l.Og), phenol (0.48g), ~iphenylphos~hine (1.31g) in
tetrahydrofuran (SOml) was treated wi~h died~yl awdicarboxylate (0.87g). ~le resul~ng
solu~oll was stirred at r~om temperature f~ 18 hours, the solvent removed and the residue
chr~matographed on silica gel eluted ~nth mothanoVdichloromethane. The sesul~g oil
was dis ved i~ ethyl acetate (5~) and trcated ~ith ethereal hydrogen cblor~de. The
35 ~ecipi~ate was collected by filtra~on and r~c~yst;~llised (methanol/e~hyl acetate) to g~ve
the dde compound (0.645g) as white needles, m.p. 154 - 155C.
wo 93/22302 ~, 13 3 ~ ~ ~ PC~/GB93/00801 .
- 20 -
Found: C, 6g.37; H, 9.62; N, 4.28; Cl, 11.11%.
(C1gH~gNO.HC1) requires: C, 69.32; ~I, 9.70; N, 4.49; Cl, 11.37%
E~mple 2
7 ~4~1Fluoropheno%y)~1~pip#riditloheptane ~drochloride
The ~ compolmd was prep~ ~n a ~imilaI ~n~ to ~xample 1 ~ ng ~m 7
pipcr~diDohept~ol (l.Sg), ~lu~ph~nol (0.84g), ~phenylphosphin~ (1.97g) and diethyl
0 a~dicarboxylate (1.30g,); Cl~n~togr~hy on s~llca ~el 01uted ~rlth
me~oUdichlor~me~ane asld ~ea~ent ~ e~hereal hy~gen chl~de ~ltowe~ by
res~stallisi~n ~m e~hyl aceta~o~etharlol gav~ a whi~e solid,(l.~7g), m.p. 126~127~C.
Found: C, 6S.ll; ~I, 8.49; N, 4~19; Cl, 10!83~o
5 (C18~28F~C) HCl) ~equires: C, 65.54; ~, 8.86; N, 4.~!5; Cl, 10.75~o
Exa~nple 3
7~(2941~1~ichlorophenoxy)~piperidi~aoheptane tl~drochlor~de
lne nu~ compouna was propar~ in a sim~ar ~ncr tC) ~Xa~lpiC: i S~g Ir~m 7~
pipesidinohcpt~ol (l.Og), 2,~dichlorophenol (0.81g), tr;phenylphosphine (1.31g) and
diethyl a~odicarboxyla~ (O.B7g). Chr~mato~aphy on s~lica g51 eluted wlth
methanol/dichlorom~thane and ~eatment w~th ethe~eal hydkogen chlor~de gave a white
25 soli~ which was r~crystallised f~m acoton~ile, n:~.p. 17~178~C.
Found: C, 56.54; H, 7.03; N, 4.00; Cl, 28 3~qo
(C18~7~2NQ-~ es: C~ $6.78; H, 7.41; N, 3.6B; Cl, Z7.93%
30 Example 4
7-(3,4~Dichlorophelloxy) l~piperidinoheptane hydr~chloride
The ~le compound was prepa~ed in a simila~ manner to Examplc 1 star~ng f~om 7~
35 piperidinoheptanol (l.Og), 3,~dichlorophenol (0.81g), tnphellylphosphirle (1.31g) and
diethyl azodicar~xylate (0.87g). Ch~mato~aph~ on silica gel eluted with
me~hanolldichlorome~atle and trea~ment w~th eth~real hydrogen chloride gave a white
solid which was recrysta~lised frorn medlaDoVethyl acetate, (1.27g)9 m.p. 139~141C.
WO g3~22302 PCI /Gs93/00801
~1~393A 21
FoLuld: C, 56.G2; EI~ 7.06; N, 3.57; Cl, 27.64%
(ClgH27C12NC).~Cl)~uiKs: C, 56.78; H, 7.41; N, 3.68; Cl, 27.93%
S E~ample S
,.
7~(q Phen~yphello~ piperidinoh~p~e hydrochlorids
Tho ~:itle c~mpound w~s prcpar~ in a similar ~ne~ t~ ~mplc 1 ~ ng fi~m 7
0 pip~r~din~heptanol ~l.Og), 4~phe~ p&onol ~0.93~), ~phenylphosphino (1.31g) a~d
dicthyl azodicarboxylate (0.87g). C~graphy on siiica gel clut~d with
methan~l/dichlc~aeth~ne ~d ~a~nt wi~ ethereal hydrogen chloride gav~ a whi~e
sobd which wa~ r~rystallised ~ methan~V~ cctatet ~1.238), ~p. 176~178~C.
Found: C. 71.13; H, 8.20; N, 3.4~; Cl, 8~8()~o
~C~33N02~HCl) reql~ros: C, 71.35; ~, 8.48, N, 3.~; Cl, 8.77Yo
.
E~ample 6
~: ~Q 7~(3 Pheno~ypheno~)-l~piper~dinoheptane hydr~ch30ride
l~e ude compound was ~ared ~n a similar ma~cr tO Example 1 s~ar~ng ~om 7~ ;
pipendirloheptanol ~1.Og), 3~phc~o~cyphenol (0.93g), ~phenylphos~ e tl-31g) and
diedlyl a20dicarboxylate (0.87g). C~matography on s~lica gel 01utcd wlth
2s methanoUdichloromctb~ne and trea~ent w~th e~crcal hydrogen chlo~de gave a white
solid which was re~ystallised f rom metha~ol/ethy~ acetate9 (0.8g4g)l m.p. 100~101C.
~: :
~ound: C, 71.23; H, 8.31; ~, 3.~; (::1, 8.94%
(C2~H33NO~-HCl~ ~lu~es: C, 71.35; H, 8.48; N, 3.47; Cl, 8.77Yo
E~cample 7
7-(2~Dibenzofuranyl~y)~l pîpeFidirloheptane l~ydrochloride
C~rude 1-b~omo~7-(2~ zo~yloxy)heptane (5.8g) was added ove~ 20 minutes to
piperi~e (lSml) s~rred a~ ~m temp~eran~re. The resul~ng mixture was left to stand ~r
. 18 hours and ~n dissolved Ln ehlorofoqm. This soludon was washed with dilute sa~ium
hydroxide solu~on, ~ine contahling a fow drops of dilute sodium hydroxide soludon arld
W0 93/2Z302 2, ~ 3 3 9 ~ 4 Pcr/cB93/no80 1
d~ied over magnesium sulphate~ The solrent was rernoved and ~e residue was ueated
with ctherea1 bydrogen chloride and rec~ystallised f~m acetonitrile to g~ve the title
compound as white nccdles. (l.9~Sg), m.p. 1~7 ~ 129C.
s Found: C:, 70.70; H, 7.77; N, 3.37; ~l, 9.03%
tC24H31NC)~C1Ø25 ~()) reqlL~res: Cl 70.91; ~, 7.81; N, 3.~; Cl, 8.7~%
Ex~mple 8
7al4aBenzyloxypherloxy~ pip~ Inoh~ptasle hydr~hloride
A m~xture 7~ cnzy1oxypheno~y)~1~b~mohep~e (S.Og), pipçri~tne (~.18g~, potassitLmcarbonau: (2.73g) ~nd ~thanol (S~) was s~Ted a~ reflwc f~r 18 h~urs. The rr~x~c was
filtered and the residue was washed witb ~l:hanol. The ~ t~s were comb~ned, the sclvent
was rem~ved and the residue was par~tioned l~twe~n dichloromc~ane and dilute s~
hydroxide solu~on. The dichlorornGthane layer was s~para~, ~ied o~er m~esium
sulphate and the solvent was remo~ed ~ g~ve an oil wbich was ~eated wi~ etherealhydrogon chl~de. Recr,Ystallisa~on ~m othyl as:0tate gavo tbe titlo compound, (1.9;~)
m.p. 171- 173C.
Founa~ i.25; *~ 7; ~ i; C, r)~ J4/o
(C2sE~3sNo~cl.o~s H20) seqt~r~s: C, 71.07; H, 8.70; N, 3.30; Cl, 8.30~0
Ex~mple 9
2s :
7 (4-Benzylo%yphenoxy) l~pyrrolidinobeptane hydrochloride
The dtle comp~und waæ prepared in a simi}ar maM~r to E~ample 8 s~ng from 7-(~
benzyloxyphenoxy)-1-bromoheptane (1.Og), pyrroli~ine (1.13g), potassium c~bonate30 (1~62g) and ethanol (~Sm~). Chrom~tography on sîlica gel elu~ with
methanoVdichlarom~thane and treatme~lt w~th ethereal hydrogen chloride gave a white
solid which was rec~ystallised from acetone, (0.45g), m.p. 132 - 134C.
Fuund: C, 70.89; H, 8.17; N, 3.45; Cl, 8.49~
(C2~33~ Q25H20) requlles: C, 7056i H, 8.26; N, 3.42; Cl, 8.69%
213~3~
WO 93/22302 PCrtGB93/00801
- 23 - i
Example 10
N~p~(4~Benzylo%~pbenoxy)heptyl]~he~amethylenelmine hydrochloride
S A mixture 7-(4~benzylaxyphcnoxy)~ om~ (1.8~g~, and 80% sodium hydride
~0.17g) and dimcthylf~de was s~d under n~gen ~r hve minutes.
Hexam¢thyleneimine (O.S63~nl) was addcd by s~in~ and the snix~e was ~sred a~ 60C
for 4 hours. The soludon was ~ted w~ wate~ ~3~1) and ex~ted ~vith
tichl~romcthane. The dichl~om~thanc layor wa~ washed consccu~vely with water and0 dilute hydrachlos~c acid and ~en dA~d over ma~os~u~n sulphste7 tho solvent was removed
and the rcsiduo was chr~maw~phed on silica gcl olu~ w~th dichl~ome~ rnetha~ol
and r~stalliscd l~om a~oton~ilc to ~e tho ~tle compound, (0.738g) m.p. 162 - 163~C.
Fou~sd: C, 71.92; H, 8.66; ~, 3.25; Cl, 8.1S~a
lS ~C26H37NO~Cl) rcquiros: C, 72.2~; ~, 8.87; N, 3.~; Cl, 8.~1%
Example 11
6~(3,4~Dichlorophenox~ piperidirlob~an0 h~drochlor~de
The ~ c~mpound was psepar~d m a s~mi~ar manner ~o i~;c; ~
pipc~idinohcxanol ~ g), 3~chl~phenol (1.32g~ riphenylphosphislc ~".12g~ and
die~yl azodicar~oxy1ate ~1.40g). C~ ato~raph5r on s~lica gel olu~ed ~th
: methanoVdichlorome~ane followed by ~eatment with c~ hyt~ogen chl~ride and
2S rccrystallisation f rom DthanoVothyl acetate, gave the ~tle comp~und as white needles,
(1.17g), m.p. 138-139C.
:` ~ ` : ~ :
Found: C,~55.60; H, 6.83; N, 3.90; C1~, 9.68%
(C17H2sCl~NO.HCI) rcqu~res: C, 55.67; H, 7.14; N, 3.81; Cl~, 9.66%
Examplel2 ~ ~
9~(3,4.Dichlorophenoxy) l~pi~dinonane hydrochloride
3S The ~dc com~ound wa~ p~epared ~n a similar manner to ~xample 1 star~ng ~om 9-pipaidinonanol (1.2g), 3,~dichl~ophenol (0.84g), triphenylphosphine (1.38g) and diethyl
azodicarboxylate (0.92g). Chsomatography on silica gel eluted with
me~anoUdicbloromedlane follo~ved by trea~nent w~th ethereal hydsogen chl~de and
~J~33~
WO 93/22302 PCI/GB93/00801
-24-
recrys~allis~tion ~rom acetoni~ile, gav~ the ~tle compound a~ white nee~les, (1.25g), m.p.
127-1~8C. .
Found: C, 58.65; H, 7.73; ~, 3.47; Gl, 26.21%
~C~5~31C12NO.I~C1) requ~s: C7 58.76; H, 7.89; N, 3.43; C17 ~6.01~ )
E~ample 13
9~(4~Be~zylo~pbenoxy)~1~piperidino~ h~drac~larid~
~e 7~de compou~d was p~cp~ in a ~imil~ ~ner to :E:xample 1 s~g f~m ~-
piperidinonanol ~1.2g), 4~$enzylox~phenol (l.OSg), triphenylph~sp~e (1.38g) and
dieth~rl a~dicarboxylate (O.9~g). C~m~o~aphy orl silica gel eluted with
rne~anoUdlchloromethane followecl by ~eatIn~nt vvith ethereal h~drog~n ~hloride and
recrystallisa~on f~ra a~etoni~rile7 g~vc the ~tle c~mpound as whi~e needles, (0.834g~, ~
m.p. 144 -145C. ; ;
Found: C, 72.39; H, 8l86; N, 3.16; Cl, 7.88~
(C27H3gN02~ICl) re~s: C, 72.70; ~ .04; N, 3.14; Cl, 7.95~a
E~ample l~
~4~Benzylo~ypheno~)-l-pîperi~inooct~e h~drochloride
The title compound was prepared in a simil~r manner to Example 1 star8ng ~om 8- :
piperidinooc~ol (1 3g)? ~benzyloxyphenol (1.22g), ~iphenylphosphine (1.60g) and
diethyl azodicar~xyla~e (1.06g). Chrornatography on silica gel eluted with
methanoVdichlorome~ane followed by ~ ment with ethereal hydrogen chl~ide and
re xystalLisation from ethyl acetate, gave the ~tlc compound as whit~ needles, tO.793g),
m.p. 141 -143~.
Four~ C, 71.63; H, 8.50; N, 3.16; ~1, 8.47~o
(~2~H[37N2~ 2) re~ulres: C, 71.54; H9 8.65; Nt 3.20; Cl~ 8.12%
~133~
Wo g3/2~302 PCr/G~93/0080l
-25
E~ample lS
~(4~PhenoxypheD~y) l~piperidinoo~e hydrocbloride
5 l~e ~e comE~und was prepared ~n a similar manne~ to ~ixample 1 stardng ~m 8-
piperidinooctanol ~1.3g), ~phen~ phenol (1~12g)~ phenylphos~hine (1.60g) and
diethsll azodicarbox~ylate (1.06g). Chromat~aphy o~l silica gel ~lute~ with
methanol/diehloromethane followed ~ nt ~th ethereal hydT~g~ll chloridc and
re~st~lli~tion ~rn othyl acetate, ~a~ e ~tle eompound as d white solld, (0.98g), ~p.
10 75 ~ 76
Foulld: C, 71.57 H, 8.41, N, 3.46; Cl, 8.11~
~C~H3sNO2~Cl) r~u~res: C, 71.83; H, 8.68; ~, 3.35; Cl, 8.~8~o
5 Example 16
6^(4 ~enzylox~pheno~y) l-piperidinohe%ane hydr~hlorid~
The ~tle compound was p~epared ~n a s~ar mann~r to E~L~ple 1 star~g ~m 6
~o piporiainohe~anol (1~5g), ~bo~loxypheI~ol (1.6"g~ h~ylphcsph~e (1.1 g) a~d
diethyl azodicasboxyla~ (1.40g~. C~ornatograp~y on s~ica gei ~iuted w~
methanol/L~hloromc~ane followed by trea~eIlt wi~ ~thesG71 hydrogen cblc~de and
recrysta~lisa~n f:~m ethyl aceta~, ga~e the ~e compound as a whit~ (1.33g), m.p.174- 17~C.
2s
FouIId: C, 71.16; lI, 8.~4; N, 3.67; Cl, 9.2%
(C24~33NO2 H~) ~equ~s: C, 71.35; H, 8.43; N, 3.46; C1, 8.80~a
.:
E~ample 17
3~
7~{4-12 (4Chlorophenyl)egbyl]pheno~y~ piperidinoheptane hydr~h1Oride
.
l~e ~tle com~ound was prepared ~n a similar mann~r to ~xample 1 sta~ng from 7-
pipesidinoheptanQl (1.Og), 4-~2~(4-chlo~ophenyl)sthyl]phenol (1.19g), tripheIlylph~ ine
35 (1.31g) and dietlhyl a~odicarboxylate (0.87g). (X~omasography on silica gcl eluted with
mcthanoVdichlorome~ane ~ollowed by treatmc~t w~th ethercal hydrogen chloride andrecrystallisa~on ~m acetonitrile, gave the title compound as white needles, (0.853g),
m.p. 167- 169C
:
WO 93/22302 ;!, - 26 - PCI`/GB93/00801
Found: C, 69.87; H, &.09; N, 3.10; Cl~ 15.43~o
(C~6H3~GINO~ICl) re~s: C, 69.32; H, 8.28; N, 3.11; CI9 15.74%
s E~x~mple 18
t~7~ Z~Phenyle~hengl)phenoxy]~1dpiper~d;nohept~n~ hydr~hloride
The ~ compound was prc pared in a ~imilar manner t~ l~arnpl~ 1 sta~ f~am 7-
pi~ noheptanol (3.0g), ~ans-4 hy~xys~lbene (3.01g), ~iphonylphosphine t3.93~g)
and dîe~yl a~dicar~oxylat~ (2.61g). Chr~mato~aphy Oll silica ~ol loluted ~dl
me~hanoVdichl~romethane gavo a white solid (4.0$6g~. A sample of this ~torial (1.75g)
W s ~eated with ethereal hydr~gen chlo~do to g~v~ a white solid which wa~ r~ystall!ised
i~om ethanoll to g~ve the title compound, (1.145g), m.p. ~19~20C.
Found: C, 7S.0; H, 8.47; N, 3~37, Cl, ~.S6%
(C26H3sNO.HCl) requ~res: C, 75.43; ~, 8.76;.N, 3.38; Cl, 8.S7%
E~mple 19
7~ Pnenyie~yljpheno~y]~i~piperînînon~p~an~ nyd~cnloria~
Amix~reof ~ s7^[4 ('~-phenylethonyl)phencxy~ pipcr~ ohep~c (~ g), 10~
palladium on carbon (0.35g), me~anol (50ml) and ethan~l ~S0ml) was shaken ~mder an
2s atmosph~e of hydrogen at S0 p.s.i. for 2 hours. The catalyst w~s removed by fil~uon
and the i~lltrate was evaporated. The residue was dissoIved ~n e~hyl acetate and treated
w~th e~hereal hydro~en chlonde to g~ve a white solid wh~ch was re~ystalliscd from
acetonitrile~ to give the titlo compound, (1.253g), m.p. 165-166~C
30 Fousld: C, 74.77; H, 9.05; N, 3.32, Cl, 8.27%
(C26H37N(:).H(: 1) reqli~res: Cl 7~.06; H, 9.21; N, 3.37; (: l, 8.52%
E~ample 20
,
35 7~ Benzylo~yphellQ~y)~lupiperidinoheptalle hydroclbloride
The ti~le compound was prepared in a similar manner to Example 1 star8ng from 7-pip~idinoheptanol (l.Og), ~benzylo%yphenol (1.14~ iphenylpho~hine (1.31g) ~d
- WO 93/22302 ~ }~ 3 ~ ~ ~ PCT/GB~3/00801
- ~7 -
diethyl azodicar~oxylat~ (0.87g). Ch~ma~gr~phy on silica g~l cluted with
m9~hallOVC~ ~ and treatment w~th ethe~eal h~drogen chl~r~dc followed by
secrystallision from a~etonil:rilc gave ~e ~e compound as white n~edles,(1.94g), m.p.
173-174C.
S
~;ound: C, 71.70; H, 8.S0; N, 3.38; C: 1, 8.26~o
(C2~3sNC)z~Cl) requ~es: C, 71.83; ~, 8.68; N, 3.35; C1, 8.48%
10 Exanrlple 21
7~Benz~lpheno~ piperidinoh~pt~n~ hydrochlo~id~
The title compound wa~ prcp~ ~ a sirrlilar ~nanner to Exarnple I star~ng f~om 7~15 pipaidinoheptanol (l.Og), 4-benzylphenol (0.93~ iphenylphasphine (1.31g) and diethyl
azodicarboxylate (~.87g). Chromato~aphy an sili~a ~cl elute~ w~th
methanoVdichlor~methanc and ~e~lme~t with ethe~eal h$~dragen chlor~de followed by
recrystall~sion f~m ethyl ace~t~/methanol gaYe the ~tle compound a~ a whi~e
solid,(1.94g), m~p. 95-96C.
2~
Found: C, 74.31; H, 8.7~; N, 3.42; Cl, 8.79% ~:
(C2sH3sNO.~Cl) requ~s: C, 7J,.69; H, 9.03; ~i, 3.48; Cl, 8.82~a
Example 22
7-(2~Ben~lpheno%y)~lapiperid~noheptane hydrochlQride
The ~tle compolmd was prep~ in a similar ma~ner to ~ample 1 stamng frorn 7
pip~idinoheptanol (2.0g), 2-benzylphenol (1.84g), triphenylphosp~aine (2.62g) and
30 diethyl azodicarboxylate (1.74g). Chro~tography on silica gel eluted with
metha~loUdichloromcthane a~d ~reatment with ethereal hydrogen chloride followed by
recrystallision ~rom e~yl ac~te/methanol gave the ~le compound as a whi~
solid,(1.17g), m.p. 11$-120C
35 ~ound: C, 73.96; H" 8.80, N, 3.71 Cl, 8.74%
(C2s~3sN(3.HClØ~2O) reql~lres: C, 74.02; ~ 9.Q4; N, 3.45; Cl, 8.74%
W0 93/Z2302 ~3~9~, ~ 28 - pcr/GB93/oo8ol
E~mple 23
7-~4 ~1eth~xyphenoxy) 1~pipefidinolbepta~e hydrochloride
S Tho ~dde ~ompoulld was p~pared in a ~imilar ~annGr to ~ample 1 s~ng i rom 7-
pi~dinoheptanol (1.Og), ~methox~h~nol tO.~2g), ~iphenylpbosphi~e (1.31g) and
diethyl azodicarbo~ylate (0.87g). Cbr~mata~aph~r a~ ~ilica gel oluted with
me~ oVdichlo~r~e~an~ and ~atrnent ~h e~aeal hyd~gen chl~de followed b~
rstallision fr~m ethyl acetate/methanol gaYe th~ ~tle eo~auIld as white
lo needles,(1.143g~ Lp. 128~130C.
Pound: C, 66.71; H, 9.30; ~, 4.1S; ~ .37~b :
(C1~31N02~C~ rss: C, ~6.74; ~ 9.43; N, 4.10; ~, 10.37~o
5 Example ~4
7 (4~ Butylpheno~y).l~p;p~ridinoheptaneb~drochloride
The ~tlc compound was prvpared in a similar ma~er to :Example 1 st~r~ng fr~m 7-
inohep~ol (1 Og), 4 ~ers~ he;~cl ~0.7Sg), ~ he~y~phos~hine (1.31~ d
diethyl a~odicar~oxylate ~0.87g). ~ ma~aphy on silica gd eluted with
methanolldichlorome~ane arld treat:ment wi~ cth~eal hydrogc~ chlonde ~ollowed by~crystallision ~m ~c~anoVe~yl ace~te gave the ~o c~polm~ as a white sclid,
(0.779g~, m.p, 17~171C.
~s
Found: C, 71.69; H, 1O.OO; N, 3.88; Cl, 9.57%
(C22H37NO.HCI) reqlLires: C, 71.80; H, 10.41; N, 3.81; C1, 9.63%
E~ample 25
7~(3,4 Meth~lelledio~:yphen~cg) l~piperidinoheptane hydrochloride
The ~tle c~mpolmd was prepa~ed in a s~ manncr to Examplc 1 star~ng f rom 7-
pipcridinoheptanol (l.Og~, sesamol (0.69g), ~iphenylphos~hine (1.31g) and dic~hyl
3s a~odicarboxylate (0.87g). Chromat~graphy on s;lica gol eluted with methanoVchloroform
and ~ea~nent with ethcreal hydrogen chlondc ~o)lowcd by ~ecrystallision ~m
mo~hanoVethyl ace~ate gave the ~tle compouDd as a white solid, (1.16g), m.p. 141-142C.
wo 93~22302 ,~ ~ 3 3 ~ ~ 4 Pcr/GBg3/00801
~ 29 -
Fo~ndi: C, 63.9~; H, 8,18; N, 3.g9; Cl, 10.38%
(C1~I2gNO3.HCI) requ~es: C, 64.11; Hl 8.4g; N, 3.93; C1, 9.965'o
5 Exampie 26
7 t4~(3,4~1)ichloro~enz~1Oxy)phen~x~ pip~;dinoheptane hydr~hlaride
The ti~lG compound was prep~red ~ a similar manne~ t~ Example 1 s~ng frorn 7~
hYdk~XYPhCnXY)~1-PiPO~ ~ePtan~ ~1.45g), 3~ Oh1~b~nZY1 a1COhOI (1.885g),
l:nphCnyiphosphine ~1.31g) and di~yl a~dic~Xyla~e (0.87~). The SOI~rent W~5
I'emOYed and th~ residUe W~S diss~lYed ~ ~Ch1~eth~e~ ThiS SO1Ut;~n WaS WaSh~d
thOr~Ugh]Y With dilUte h~r~Ch1~iC acid, driCd OV~ SO~itlln SU1Phate and ~aP~ated.
Chromatography on silica gel eluted w~th me~ oVdichlorometh~n~ and r~ystallis~onlS from ethanol, gave the ~le c~mpo~d as a white solid, (0.827g~, m.p. 184-186C.
;: ~ Found: C, 61.62; H, 6.87; ~, 2.98: Cl, ~1,759~
~: (C25~33~2N~ ) rcquireg: C~ 61.67; H, 7.04; N7 ~.88; Cl, :~1.84qo
: ~xample 27
7-~4 (4~Metho~ben~loxy)pbenoxy~ l-piperldin~heptane hydl~chloride
2s The ~tle compound was prepared ~n a similar ma~ner to Example 26 star~ng i~ :n 7-(~
hydroxyphenoxy)-l-pi~l~di.loheptane (1.45g), 4-methoxybenzyl alcohol (0.69g),
tdphenylphosph~ne (1.31~) and diethyl azodicar~xylate ~0.87g). Recrystal~ on from
acet~nitrilQ gave the d~ compound as a white crystalline solid, (0.519g~, m.p. 17Z-
176C.
~ ' ! . , . .
Found: (:, 69.20; H, 8.21; N, 3.23; C 1, 7.73%
(C26H37NO2~c~ 2o) zcq~ es: C, 69.40; EI, 8.51; N, 3.11: Cl, 7.88qo
W093/22302 ~$ P~/GB93/01)8UI,--
Example 28
7 l4 (4 ~luorobenzyloxy~phenoxy~ piperid;noheptanehydrochloride
s The dtle compound was pr~pared in a s~ilar manner to ~xample ~6 sta~ng from 7-~hydroxyphenoxy)- 1-piperidirloh~pta~e (1.45g), ~flu~benzyl alcohol (0.63g),
~iphenylphosphin~ (1.31g) and di~thyl a~ca~x~lat~ (0.87g). ~ecrystallisa~on fromacetonitrilc gave the ~tic compound as a white c~s~e solid, (0.782g), m~p. 167-
168C.
Found: C, 68.37; ~I, 7.6~, N, 3.~, Cl, 8.09~
(C~sH34FNO2.~ClØ1H2C)) r~qu~es: Ct 6~.S8; H, 8.07; N, 3.~; Cl, 8.10%
Exalmple 2g
lS
N~ (4 Be~ylo%ypheno~)heptyl~ mekh~lpip~ridine hydr~chloride
A mixture 7-(4-~enzyloxyphonoxy) l~bromoheptane ~1.8&g), 80q~ sodium nydAde
(0.17g) and dimethyl~rmamde (lOml) was s~ed undes ni~gen. 2~m~thylpiper~dine
~0 (0.6ml) was added ~y syr~g~ a~ ~ ~ ~as s~d 3~ 60C ~or 16 houss. ~he
soiurion was ~rea~ea wiul wa~r asld ~c soiid wa~ co~l~ ~nd od ~ ~. ~e
eth~ was ev~porate~, the residuo dissolved in dichloromethane, washed cons4cu~vely
vnth wat4r and dilute hyd20chlor~c acid and then dried over sodiurn sulphate. The solvent
was removed and the ~esidue was chrDmatographed cn silica g~l eluted with
25 dichloromethane-methanol and r~stallised ~rom acetotutrile to g~e the ~tle compound,
(0.66g) m.p. 128-129C.
Found: C, 72.OB; H, 8.h3; N, 3.~5, ~, B.O~qo
(C26H37NO~.HC~l) requires: C~ 72.28; Ht, 8.87; N, 3.:24; (:1, 8.21~o
, 30
Example 30
N~7~(4-Benzylo~ypheno~y)heptyl]-3-methylpiperidine hydrochloride
35 Subs~ ng 3-methylpiperidine for 2-methylpipendine ~n Example 29 and recIys~allis~ng
the product ~m acetonitrile gave title compound (0.989g) as a whi~e crystalline solid.
m.p.161-163C.
WO 93/22302 ~ 3 3 ~ PCr/GB93/00801
Found: C, 72.20; H, 8.S8; N, 3.20; Cl, 8.30'ro
(C2~H37N02~Cl) rcqu1reis; C, 72.28; H, 8.87; N, 3.~; Cl, 8.21'ro
E~ample 31
S
N~7~(4~Benzglo~gpheno%~)heptyll~ methglp3pei~ e hydr~hlo~de
Substitu$ing ~methylpip~ridine f~ ~methylpi~dins In l~xamp~e ~ a~d ~y~tallis~ng
~e pr~duct ~r~m aceton~trilo gave ~ ~mpound (O.~g8g~ as a whit~i ~rystall1na solid.
m.p.1~8-170C. ~;
Found: C~ 7~.~0; H~ 8.6l; N, 3.32; Cl, 8.12~o
(~ 2~37~ es: C, 7~.~8; H, 8.87 N, 3.24; Clt 8.21~o
i
S E%~mple 32
N [7~4~BenzyIoxgphenoxg)beptyl]~2~6~dimethyIpiperidine hydrochIoride
Subsdt~ng 2,~dimethy1pip~ ~ 2~meshy1pipesidine ~n ~xa~ple 29 and
20 rc~ys~aIlismg ~ne ~c~ ~m o~hy1 acetate~othar~o1 g~o ~le c~mpou~d (0,1 6g~
white cr~stallil~e solid. sn.p.127~128C.
Folmd: C, 71.13; H, 8,63; N, '3.~qo
(c~7H3gNc)2~Hcl~o~5H2oj rcquirgs: C, 71.26; H, 9.08; N, 3.~7%
E~ample 33
N~17~(4~Belozyloxyphenoxy~heptyI]~4meth~ypiperidine hydrobrom~de
; "
30 A mix~e of 1-~7-(A-BenzyIoxyphenoxy)hepty1~4~methoxypyndinum bro~de t2.0g)
and Adams catalyst (O.lg) in etharlol (SOml~ wa~ shaken under an atmosphcr~ of hydrogen
at S0 p.s,i. for 24 hours. The ~xNro was fil~ered, the r~sidue suspended in
dichlo~omethane, filte;~ and the filtrate evap~ated. The residue was chxornatographed
on silica eluted w~th ethyl ac~tate/mcthanol ~d ~crystallised fr~m ethanol to gi~e the ~tle
35 comp~und as a white ~rstalline solid (0.242g), m~p. 14~14~C
:E;au~d: C, 63.21; H, 7.56; N, 3.18, Br, lS.95%
(C26H37NO3~Br) ~equires: C, 63.41; H, 7.78; N, 2.84; Br, 16.22%
WO 93/22302 PCr/GB93/00801
;~333~ - 32 - .
Example 34
5-(4~Benzyloxypheno~ pip~r~dinop~lltane h3~drochloride
.
A mixtu~ 5~ bo~zy1Ox$phenoxy)-1-brc~m~n~ (Z.Og), pi~r~din~ (0.43g), potassium
carbona~e (0.8g) and ~anol (SOn~ll) was sti~redl at r~ or 18 hours. I~e r:~ixt~ was
fil~e~ed and ~e residuo was wash~ with ~than~. Tho ~ s ~ve~ combi~dt the s~lven~was removed and the residue wa~ p~tiancd be~voen oth~ and dllute s~d~um hydraxlds
10 solu~on. Thc e~e~ layer was s~p~ated7 d~ied tSV~ ~agnÇsSillm sulphate and the solYent
was removed ~o give an oil wh~ch was ~ated wi~h edn eal hyd~gen chl~nde. The solid
was colleGted and re~s~sed fr~m ethyl acc~ate to g~vo a s~lid whlch wals fu~
~rstallised f~om watel to gi~rc tho titl~ c~m~d a~; a white solid, (0.265g) l:n.p. 202
2~4or~
Found: C, 68.23; H, 8.00; N, 3.72; Cl, 8.32%
(C23H31N02~I~1Ø75 H~,O) req~ res: C, 6~.46; ~, 8.36; N, 3.46; Cl, 8.78%
Example 35 ',
O
4-Benzy~oxyph~noxg)~l plpor~dirtonep~ e ~y~rocniori~e
solu~on of 7-~benzyloxyphenoxy)heptarlen~trile (O.lg) i~ toluene (5ml) under ~îtrogen
was treated Y~nth diisobu~l aluminium hyd~ide i~ toluene (0.7ml, l.5mola~ solutio~l). The
~: 25 m~xnlre was s~rred ~r 16 ~ours and then pi~di~e (lral) was added. The mixture was
s~d for 1 hour, when methanol was added ~nd tho m~xture was stirred for a further 1
hour. Water (lml) and sodium cyano~ohydride tl.Og) was added and the mixture wass~red for 3 hours and then pourcd into water and extracte~ w~th dichloromethane. The
dichl~romethane layer was washed with dilute hyd~echloric ac~d, dried over sodium
30 sulphatc and the s~lvent was removed to givç a white solid (0.109g) which wasrecrystallised to give the dtle compound as a white solid, (0.03g). Product idendcal to the
p~oduct ~om Ex~mple 8
(~IPLC, TLC~)
W093/22302 ~? ~ $~ Pcr/GBg3/00801
- 33 - I
,
Example 36
. . .
7 (4-~enzyl~xyphen~%y3~1~piperidinohept~ne me~ylate
S The produc~ of Example 8 ~1.Og) wa~ equilibrated between e~hyl acetatc and O.SN NaOH.
The aqueous ~ o~ was re-e3~ t~ with ethyl acota~e and ~ comblned orga~c
ex~ts washed (H20, b~ine), dried (anhy~us Na~S04) and waparated ~o dryness ~o
~î~e a colourlcss ~il. This ail was dissolved ~ mothanollc~hyl ace~ a~d methane
sutphot~ic acid (0.23g, 1 ~quivalent) in methan~ ~ ~as c~wen~ated ;0 and stood o~e~ight in the fridge ta produce the ~e compound ~0~86~) as white crystals,
m.p. 1~148'C.
Folmd: C, 6S.3j; H, 7.92; ~, 3.01%
tC23E131N02~CH3S03H) ~ es: C, 65.38; Hl 8.23; N~ 2.~3%
Example 37
7 ~4 Benzyloxyphenoxy)~l pipel~dinoheptane t~rtrat~
~ne pr~d~ of ~le 8 (1.0~) was e~ dichlorome~ane and N NaC~L
The aqueous ~c~on was ~o~x~c~ wi~n a~ciuaromem~c (~ d ~ 4V~
o~ga~ia cxtracts washed (H~,0, brlne),, d~ied (~IgSt:)4) a~d e~ra~ted to ness to ~iv¢ a
colouslcss oil. This oil was dissolved in boiling methanol a~d ~ t~c acid (0.36g? 1
equivalent) in rnoehanol added. Tho ~e was concent:rat~, hot ethyl acctate add~d and
urther concentrated to g~ve a white crys~e solid (l.lSg). This matcrial was
crystallised filrst from methanol/eth~l acetate and ~nally from methanoVwater to g~ve the
title compound (0.52g) as a white ~rystallline solid, m.p. 83-84C.
Example 3
7~(4-Benzyl~ypheno~y)~ l~pipe~dinoheptalle o~calate
A mixnlre 7~ benzylo~;yphenoxy)-1-~mohcptane (l.Og), piperidine (0.25g), potassium
carbonate (2.00g) and ethanol (25ml) was s~red at ~eflux for 48 hours. The m~x~ure was
35 filte~ d the rcsidue was wa~hed w~th etha~ol. The filtrates were comb~ned, thc solvent
was remo~ed and the ~sidue was par~doned between dichloromethanc and dilutc sodium
hyd~oxide solu~on. The dichloromethane laye~ was scparated, dried over magnesillm
sulphate and the solvent was rem~ved to give an: oil ~hich was t~atcd widl oxalic acid ~n
WO 93JZ2302 PCr/GB93/0080l
39Qv~ 34~ '~
mEthanol/ethyl acetate. The r~sul~dng .solid was re~rys~Jised frorn ethyl acetate/methanol
to give the title compoun~, (0.49g) m.p. 161~163C.
Fouuld: C, 67.67; H, 7.73; N, 3.~%
S (C~ 35NC)~ ~2H2C)4 0-5 ~2Q) ~qu~s: C, 67.47; ~, 7~97; M, 2.~1%
W093/22302 ~13 3 9 S 135 pc~r/GB93/oo8
Pharmaceut;cal Formula~oll~
The following rcprosent typical pharmaceu~cal f~mulations according to the present
inven~on, which ~ay bc p~par~ us~ng st~ rd methods.
S
IV Infusion
Compound ~ ~ula (1) 1~4~ mg
Buf~er tc) p~ ca 7
Sovent/c~mplexing t~ ~00 ml
Bolus Inje~tion
Compound of fo~ula (I) 1~0 mg
Buf~r t~ pH ca 7
Co-Solvent tO 5 ml
Bu~fer: Suitable bu:~fcrs include citIa~, phosphate, sodilim hydroxide/hyd~chlonc
acid.
.
Sol~rent: Typieally wat~r but may also inclllde aycl~dox~s (1~1~ mg) and c~solvcslts
such as ~ylos~e glyc~l, paly~hylene gly~l a!ld alcahal.
Tablet
Co~pound 1 - 40 mg
Dilu~nt/F;ller * 50 ~ 250 mg
Binder 5 ~25 mg
Disente~rant ~ S - 50 mg
l.ubricant 1 -Smg
Cyclodex~rin 1 ~ 100 mg
30 * may also Lnclude cyclodex~rins
.,
Diluent: e.g. Mi~stalline cellulose, lactose, starch
Binder: e.g Pol~ylp~solidone, hydroxypropymethylcellulose
Disinte~arlt: e.g. Sodium starch glycollate, crospovidone
: ~ ~ 3s Lubricant: e.g. Magnesium stearate9 sodium stearyl fu~rate.
wo 93/~2302 ~ 39~o 4 36 - PCr/GB93/Oo~o1
Oral suspension
Compound 1 - 40 mg
SuspendingAgen~ 0.1- 10mg
Diluellt ~0~60mg
s ~eserva~vc 0.01 ~ 1.0 ~g
Buf~ to p~ ca 5 ~ 8
C~solveslt O ~ 40 mg
Flavou;r 0.01 ~ 1.0 mg
C~lo~rant 0.0~1 ~ 0.1
Suspending ag~ent :e.g. ~anthan gum, m~yrst~e cellulose
l:~iluent: c.g. sor~itol solu~on, typicaLly water
Prese~va~ve: G.g. sodium benzoate
Buffer: e.g. ~ :rate
Co solvent: e.a. alcohol, pr~pylene glycol1 polyethylene glycol, cycl~ex :rin ;