Note: Descriptions are shown in the official language in which they were submitted.
~ 213~0~
BA~KGROUND OF T.~ INV~TION
1. F.;eld o~ the Inven~ion
The i~vention relate~ to the art o~ chlorine dioxide
produ~ion, ~nd in p~r~icular, to ~he con~inuous production of
çhlorine dioxide in a plu~ flow reactor by redu~tion o~ an alkal~
me~l chlora~e or chloric acid with a red~cin~ agent. Tn a
preferred e~od$ment, the procees of ~he invention uses hydrogen
peroxide a~ the redu~ing agent.
~0
2 ~escrip~o~ of the.Prior Ar~
~hlorine dioxide used in a~ueous solution is of considerable
commercial in~erest, mainly in pulp ~leachin~, but also ~n wate~
purificatio~, fat blea~hlng, remov~l of phenol~ from induætri~l
waste~, etc. It is t~erefore desirable to provide processes ~n
which chlorine dioxide can be effi~iently produced.
~n existing processe~ for ~he production of chlorine
dioxide, chlorine gas is o~ten form~d ~s a by-product, du~ to the
~æe of chloride i~ns a~ a re~ucing agent. T~e ba~io chemical
rea~ion involved in su~h pro~-esses is-
Cl03 + Cl + 2H' - ~ Cl02 ~ lt2Cl~ ~ H20 ~1
The ch~ora~e ions are provided by alkali metal chlorate~
preferably sodium chlo~te, the chlorid~ ions ~y alkali m~tal
chlori~e, pxefera~ly sodium chloride, or by hydrogen çhlori~e,
~S an~ the hy~rogen ion~ are provided by mine~al acids, gener~lly
sulfurir- acid and/or hydro~hl~ri~ ac~d.
In the produc~ion o~ c~lorine dioxide wi~h chloride ions as
the reducing agen~ accoxding ~o xeacti~n ~1~, h~lf a mole of
213~04~
chlorine i~ produced f~r each mole of chlorine dioxide. This
~h~orine gas by-pro~uct has previously been used as such in paper
mills as a bleaching agent in a~eous solution~ HoweVer,
lncreased environmental demands ha~e resulted in a ~hange-over
to pure chlorine dioxid~ bleaching. ~o achieve pure chlorine
dioxide bleac~ing the~e is an increasing demand for chlorine
dioxide manufac~uring pro~e~es which do n~t produce chlorine as
a by product.
one known way of redu~ing the chlorine by-produ~t is to use
redu~in~ agents which do no~ p~odu~e chlorine a~ a by-produc~.
one example i~ in the so-called "SolYay" px4cess, wherein alk~li
metal ~hlor~te is reduce~ in an acid medium with methanol as the
reducing age~t. Another example is in the "Ma~hie~on" p~ocess,
in which chlorate i~ reduced w~h sulfur dioxide in a ~ulfuric
1~ acid-containing medium~ These pro~esses use ~e~hanol and sulfur
dioxi~e, respecti~ely, as indirec~ reducing agen~s, ~nd h~nqe the
rate of r~a~tion is very slo~. In U.S. Pat~t No. 4,081,5~0, an
allegedly more e~fetiVe ~Solvay" process is de~cribed using a
reduced pre~ure and a hi~h acid normality in a single vessel
reac~or.
The direct reducing agen~ in the case o~ methanol and
~ulfuric acid reactions is chlo~ide ion reac~ acco~din~ to
~eac~ion ~1~. The chlorine produ~ed the~ react~ with meth~n~l
to regenerate chloride ions ac~ording to the reac~ion:
C~30H + 3~l2+H20~ 6~1+~02+~H~ ~2
213~04~
or with ~ulfur dioxide a~¢ording to the reaction:
~1~ + So2 + ~H2O ~ > 2~Cl + H2SO4
According to o~e prevale~t theory holding that ~hloride ion
~ust be present, i~ is o~ten nece~sary ~o continu~u~ly ~d~ a
small amoun~ of chloride ion in order to obtain a æteady
produ~tion. ~ue to the continu~d pxesence ~f ch~oride ion, even
with methanol or sulfur dioxide as th~ reducing ~gent, a ~ertain
amount of ~hlorine ~y-pro~uct is produced. Accordlng to U.S.
Patent No. 4,~81,520, operating ~ith methanol as reducing agent,
~he amount o~ chlorine ~y-product p~oduced is ~e~r~a~e~ wi~h
increa~ing acid normality in ~he reaction medium. The reac~ion
rate i~ also increased with in~reasing acid strength. At a low
a~id normality, ~he reactio~ i~ so slow that it i~ of ~o
commercial interest. However, the drawback ~ith a high acid
stren~th in the reaction medium is, in addi~ion to more corrosion
in the eq~ipment, ~he production 4~ an ~cid s~lt in the for~ Of
~esquisulfate (Na3H( S04) 2~ or bisulfate (~aHSO4~. This occurs a~
an acid no~ality of from above about 5N to ~bout 12N. An acid
sal~ results in loss of aq~d in prod~c~iOn and cost~ ~or
neutralization of ~he ~alt. From about ~N to ~out 5N acid
normality, a neutral al~ali metal ~alt ~alka~i metal sul~ate~ is
fo~med.
It is also known to speed up the reaction rate at ~ow
aciditieQ by us~ng cata~ys~s bo~ with chloride and m~hanol as
~S ~he re~ucing agent. U.S. Patent No. 3,5~3,702 di~clos~s
cataly~t~ for chloride reduction. However, cat~lyst~ are
expensi~e and thus incre~se the production costs.
21~50~
,
Anothe~ drawba~k with methanol as the reducin~ ~gent i~ the
possi~le formation ~f chlorinated organic compounds, from ~y-
products of methanol, in the downstream blea~hing proce~s. It
is well known that the effic~ency of th~ ad~ed methanol is
lowered ~ue to side reactions ~herein form~l~ehyde ~d form~c
ac~d are formed. ~lso, ~ome of ~he methanol le~ves the xeac~or
w~thout having p~rticipa~ed in the redu~tion re~tion. The
~rre&ponding ether ~nd ester are probably thexe as well. ~t
could ~e expected that reactions can occur in the bleachin~ t~ain
with aldehyde, a~id, ether and e~ter, re~ultin~ in chl~rinated
organic compound~.
In U.S. Patents No. 5,0~1,16~ and No. 5,0~ 7, the draw-
backs of using me~hanol as a reducing ~gent are addressed by
s~tltuting hydroqen peroxide for ~ethanol. These p~tents
dis~los~ production of ch~orine dioxide ~s$ng a single vessel
p~ocecs under ~u~atmospheric preæsure. Alkali metal chlorate i~
redu~ed with hydrogen peroxide as the reducing ~gen~ in an
~queous reac~ion me~ium contain~ng ~ulfuri~ aci~. The reaction
me~ium i~ maintained at it~ boiling point of between 50c and
~o 100~ ~u¢h that water is evapor~ted theref~om, fcrming s~eam.
A gaseous mix~u~e co~t~ining the steam, p~oduced ~hloride
dioxide, and by-produ~ oxy~en i8 withdrawn from the ~es~el~
In the rea~tion medium an alkali me~al s~lt cryst~ ze~ and
is removed. The type of ~alt crystallized is a function c)f the
~5 acid normality of the ~ea~tion me~ium. At an acid n~mality of
between 2 and ~, ~ neutral sodium ~ulfate s~lt, fo~ ex~mple,
213S04S
Na2SO4, formc. At higher acid normalities, a ~esqui~ulfa~e sAl~
or a bi~ulfate is formed.
While ~he prcce~se~ dis~losed in U.S. P~tents ~o. 5,091,1~
and No. 5,~ 7 are ~ great improve~ent over the prior art
~ethanol processes, they are performed in ~ ~ingle ve~el process
~SVP~ reac~or in whi~h the generation ~nd separ~tion of ~hlorine
dioxide are carried out in a single reac~i~n ves~el maintained
at the boiling poin~ of the reaction medium. Kinetic~lly, ~he
si~gle rea~ion ~essel functions a a con~tant fl~w ~tirred tank
rea~or ('ICFSTR" o~ ~'CST~"~. There a~e indeed numerous ~v~ntag-
es ~o this type of reac~ion ves~el. By m~intainin~ the rea~ion
medium at ~tc boiling po~nt, t-he evolved ~hlorine dioxide i~
~ilute~ wit~ ~team, thereby reduGin~ explosion risk. Alkali
metal ~alt concentrat~on in the reaction ~edium is maintained at
lS sa~uration, resulting in the a}k~li metal salt ~eing ~ontinuously deposited and easily removed.
~n the other hand, ~ingle ~e~sel processe~ require long
residence ~imes to obtain an a~ceptable r~e of conver~ion. Long
~esidence times require ~ither a low ~ow rate through the
rea~ion vessel (and ~ub~equent low pr~uction ~te~ or a l~rge
vessel size. Pro~uction re~uirefflents, at lea~t for lar~e
consumers of chlorine dioxide, di~ate that reside~e times be
maint~ined ~ia a l~rge reaction vessel. P~per pulp mill3, for
example~ re~uire between 15 and 60 tons per ~ay ~TPD) of ~hlorine
dioxide, and this pro~uction le~e~ ~equire~ large scale equip-
ment.
213~0~
A typiG~l SVP~ reaction vessel for 40 TPP 4f chlorine
dioxide i8 abou~ 10 feet in diameter ~nd has ~ volume of about
8800 gal~on~. Sin~e chlorine dioxide is produced on-site tfor
safety reasons), the SVP~ reaction vessel ~nd rel~ted proc~ss
equipment mus~ Pe shipped ~o and in~talled ~t the location of
use. Shipping costs are high ~ue to ~he wei~ht and Pulk
involved. Al~o, the size of the rea~tion vessel requires ~he
commitment of con~iderable plant spaae.
In addition to the costs and th~ ~pace re~uiremen~ of ~n
initial in~tallati~n, fur~he~ costs ~re in~ur~e~ if a~ upgrade
in chlorine dioxid~ pr~uction ~pacity using SVP~ ~echnology is
required. Su~h an upgrade would require removal of the existlng
generator ves~el and replaceme~ with an even l~r~er vessel,
essentially duplicating ~he initi~l ins~al~ation co~ts.
Alternatively, the upgrade would require the ad~ition of a
second~ry SVP~ genera~o~ havin~ additional inst~lla~ion ~oSt and
space requirements. In either ~a~e, the upgrade would be
expensive.
The çoncept of plu~ flow rea~to~s has heretofore ~een
applied to various che~i~al processes ~nd of~ers ~he advantage
of small ~ize with reasonable pro~uction ~ates. ~owever, plug
~low reac~ors were not believed feasible ~or prod~cing chlorine
dioxide r due to the rel~tively slow kineti~s of uncatalyzed
rea~tion s~heme~. Ca~lyzed systems were ~l~o deemed un~uitable
2S for plug flow processes due to a~cumul~ n ~nd clo~iny of the
e~uipment by the ~olid pha~e catalyst.
't ' 21~046
In very ~mall ~cale processes, non-~TR, continuo~s chlo~ine
dioxide reac~ions have been u~ed cu¢cecsfully. For example, in
U~S. Patent No. S,06~,471, ~here is disclo~ed a proce3s for
~ontinuou~ product~on of chlorine dioxide usin~ al~ali metal
chlorate, ~lfuric acid and sulfur di~xide as the red~ing a~ent.
Th~s proces~ is ~ulta~le for small s~ale chlorine dioxide
applica~ion~ ~uch a~ trea~ent of dr~n~ing water, etc. Thi~
p~tent does not teach plug flow, however, sin~ ~he concentration
profile in the rsactor is uniform, which approxlmate~ a CSTR.
lo There is accordin~ly a ne~d in the axt for a chlorine
dioxide proc~ss ~hich has the advantages of low ~hlorine by
product genera~ion and high produc~ion rate and which al~o has
reduced installation and upgrading costs ~o~pared t~ p~ocesses
u~ing single ve~sel proce~s generato~s,
SU~A~Y OF TH~ ENTION
I~ is acc~dingly an ob;ect of ~he invention to pxovide a
proces~ for producing chlorine dioxide havin~ reduced ins~alla-
t~on costs.
It i~ a~other object o~ the invention to p~ovid~ a process
for produ~i~g chlorine dioxide, as abo~e, whereby th~ sp~ce
reguirement~ of the chlorine dioxide plan~ are reduced compare~
~o a single vessel proces~ -
ï~ is s'cill another obj ect of ~he in~rention to p~oride a
pxo¢e~ for pxodu~lng chlorine dioxide, ~ above, which c~n ~e
used ~o upgrade ~he ~apacity of an existing c:hlorine dloxl~e
pl~nt with reduc:ed instal~ ation cost~ and ~pace requirem~ntC ~
213~046
It is yet another object of the invention
to provide a process for producing chlorine dioxide,
as above, having low chlorine by-product generation.
These objects are achieved by a process
for producing chlorine dioxide,~ wherein alkali metal
chlorate, sulfuric acid and a reducing agent are fed
to one or more reaction conduits which function as a
plug flow reactor. Chlorine dioxide is formed as
the process stream flows through the reactor and is
recovered from the stream after it exits the
reactors. Alkali metal salt is also recovered from
the exiting process stream, preferably while still
in solution in the reaction medium. Hydrogen
peroxide is the most preferred reducing agent.
The plug flow reactor preferably comprises
a conduit through which fluid flows in an orderly
manner with no element of fluid overtaking or mixing
with any other element ahead or behind.
The objects of the invention are also
Z0 achieved by a process for producing chlorine
dioxide, wherein chloric acid and a reducing agent,
preferably hydrogen peroxide are fed to the plug
flow reactor. One of the advantages of using
chloric acid compared to alkali metal chlorate is
that the formation of sulfate salt is eliminated.
This in turn eliminates the need for a downstream
salt cake filter. This process is particularly
adapted for use as a "stand alone" chlorine dioxide
génerating plant. Optionally the chloric acid feed
can contain alkali metal chlorate which is not
reacted and thus comprises a "dead load" in the
system. This reaction scheme allows the use of a
mixed chloric acid/chlorate product produced by the
partial electrolysis of alkali metal chlorate.
-- 8
213S046
The objects of the invention are also
achieved by a method for increasing the production
capacity of an existing single vessel chlorine
dioxide generator by installing a plug flow
- 8a -
213S04~
rea~tor upstream from, and in sPries with, a single ve~sel
gener~tor.
BRIE~ ~ESCRIPTION OF THE DRAWI~S
For a full understand~ng of the invention, ~he followin~
detailed de~ription sho~ld be read in conjunction w$~h the,
drawings, wherein:
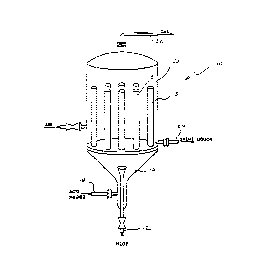
FIG. 1 illustrates one embodiment of a plug flow rea~tox for
use in the proce~ of the invention;
FIG. 2 iæ a ~chemati~ illustration of one embo~iment of ~he
process of the invention wherein a plug f~ow reactor is qonnected
in series to a single ve~el process gene~tor;
~ 3 i~ a graph of chlor~e conversion vs. reactant
concentration and temperature for operation of ~he plu~ flow
~e~ctor under adia~atic ~ondition~; ~nd
~IG. ~ is a ~aph o~ chlorate conver6ion v5. reactant
~oncentration for operation of the plug ~low reactor un~r
isothermal cond~tions.
O D~TAILEr~ 1:1ES~}~IPTION O~ THE pREF~ n P~l~ODIMENTS
The present invention provid~c for a plug flow process
havin~ high p~odu~ion rate ~hrough ~he use ~ ~elected reactant~
and hydrogen peroxide as a r~duoi~y agent. The plug flow process
of the in~enti~n can be uced as a -~tand alone chlorine dio~ide
generator, or in se~ie~ wi~h one or ~o~e additional pl~ flow
reac~ors, or wi~h one or more single vessel process ~SVP~)
gener~tor8. ~n one preferred em~odiment, the plug flow re~q~o~
_ g _
'21350~16
is u~ed to upgrade the produ~tion capacity of an existi~ SVP~
installation. In this em~odiment, a plug ~low reactor (pfr~ i~
installed upstream of and ~n series with an exi~ting S~P~. In
a highly preferred em~odiment, existin~ SVP~ installa~ion~ using
prior a~t technologies such a~ m~thanol, SO2 or ~1- ba~ed
rea~tionæ, can be upgraded by conversio~ to hydrogen peroxide
~echnology in conjunc~ion with use of the plu~ flow reactor.
In ano~her embo~iment, pure chl~ric acid i~ redu~ed with
hydrogen pe~oxide in a p~r to form ch~orine dioxide. This
process is p~rticularly adapted for 8 ~mall scale, s~nd alone
p~ug flow generator p~oducing chlorine dioxide for u~e in wate~
~re~tment and related applic~tion~ where~n stri~t environmental
regulations pre~-lude d~scharge of by-produc~s and unreac~ed
ætarting materials su~h ~ chlorate~, me~h~nol, meth~nol
~5 derivativ~s, ~ulfu~-~a~ed compounds, e~c.
A p~ug flow reac~or sui~le for use in the invention is
illustrated in FIG. 1 and s generally indicated by the number
10. ~ydro~en peroxide ente~s ~he reactor lQ ~hrough conduit 12,
~nd a mixtu~e of su~furic acid and either or ~oth chloric a~id
~o and alkali metal chlorate are fed thr~u~h conduit 14. Mixi~g of
the feed~ from conduit~ 12 and 14 occurs in ~he distribution
header 1~ of the reactor 10, forming a reaction medium. The
re~ion mediu~ enters a plurali~y of reac~ion ~ube~ 18 whexein
rea~tion tak~s place in a ~u~tantially plug f~ow manner.
Flow through ~he tub~ 18 i~ preferably in the turbulent
flow re~ion, bUt the level of tur~ulence is such that back mixing
or ~orward mixing of the flow ~ream is mini~i~ed ~n a~oo~dance
-- 10 --
213St) 16
wi~h plug flow req~irements. Each ~u~e ~unctions ~s an indi~idu-
al pfr. The ~iZe of each tube and the flow ~elocity through the
tubes is ~ function of the necessary ~esi~ence time to çonvert
a prede~ermined portion of the chlorate io~ to chlorine dioxide.
TypiGal tu~e velocities range ~rom abo~t 0. 0~ to about 1.0
ft/sec, desirably from about O.OZ to about 0.3 ft/~ec and are
preferably around 0.02 ft/se~. In general, the reaction tubes
have a length of ~om about O.S to about S ft, ~esirably f~om
~bout 1 ft to about 4 fk, and preferably from ab~ut l.S ft to
lo a~out 3.5 ft. Th~ inner diameter of the reacti~n tub~ 18 is
~etween about 0.5 in and ~bout ~ in, desirably between about 1
in and 3 in and preferably ~etween about 1.5 and about 2.0 in.
Chlorate ~onver~ion is typically in ~he range o~ 5 - 70~,
desirably between about 20% and 50% and preferably about 40~ of
the incoming chlorate feed.
After reaction, the reaction medium flows out of the
reaction tubes in~o ~he ~hell 20 of the pfr~ Gas - liquid
geparation occurs in the shell 20. Product chlorine dioxi~e gas
f~ows out of the reactor 10 via ~onduit ~2, and is recoversd by
~0 well-~nown means. Spen~ re~ctio~ liquor ~lows out of ~he tube~
18 and collects in the bottom of thP rea~or shell 20~ Thereaf-
ter, the liquor i~ pumped out o~ the chell ~ia ~ohdui~ ~4 and
into the SVP~ genexa~or tno~ shown).
The pfr can be op~rated isothermally ¢constant temperature),
2~ or adia~ati~ally (no energy input or lost)~ Because ~ulfuric
a~id is used in thiC embodiment, a heat of dilu~ion is generated
whi~h is ~uffi~ient to operate the proceæs adiabatic~lly.
11 --
213~046
Adi~atic operation has the adYantage of ~implifying the re~c~or
desi~n and conserving energy. Thu~, the energy needed ~o drive
the rea~tion in the pfr i~ provided exclusively ~y the he~t of
dilution ~o~ the a~id feed. The pfr i8 operated in a non-
crystalli~ation mo~e, whereby t~e ~ulfate sal~ is not cry~tal-
lized in the pfr.
The pfr c~n operate ov~r ~ range of pres~ures. Typically,
the total pres~ure in the reactor can be ~e~een about 20~ ~nd
~bout 760 mm Hg absolute. Desirably the total pre~ur~ is fr~m
abou~ Z~0 to about 310 ~m Hg absolute, with abo~t 300 ~m Hg
pre~erred. Air can Pe added to the pfr, if necessary, ~o dilute
~he chlorine dioxide produ~t ~as.
In a fir~t preferred embodiment, the pfr reactor is used in
series ~i~h an SVP~ generator, and emplo~ alkali metal chlo~ate.
The chemical feeds ~e split betw~n ~he pfr and the SYP~.
Generally from about 50% to about 80~ by weigh~ of the alkali
metal chlorate, from about ~0% up ~o 100% ~y ~eight of the
sulfuric acid and from about 30~ to about ~0% by weight of the
hyd~ogen peroxide is fed to the pfr, wi~h the ~emainder of the
~o chemicals fed directly to the S~P~ generator. Desirably, the
fraction of alkal~ me~al chlora~e fed to the pfr is ~etween a~out
~0~ and 70~ and preferably is about 65~ by weigh~. Des$ra~1y at
leac~ about 90% by weight of the total sulfuri~ d is fe~ to
the pfr. Hydrogen peroxide is fed ~o the pfr i~ an amount
~5 desirably be~ween about 40% and ~out 70% by weight wi~h abo~t
50~ ~y w~ight of the total peroxide ~eing pr~ferred. ~he purpose
,of using split feed is to maximize t~e acid concentration in the
- 12 -
. 21~04~
. pfr which in turn maximize~ chlora~e conversion ~o chlorine
dioxide .
In thi~ embodimen~, ~he spent liquor ex~ting the pfr and
pumped to the SVP~ ~enerator is still ~ich in reaction che~icals.
In the SVP~ genera~or ~he chlorate conv~rsio~ ~o chlorine dioxide
is at least about 25~ and can approach 100%.
In a ~econd preferred embodiment, a mixture of chloxi~ acid
~nd ~lkali metal chlorate is fed ~o the pfr. The rea~ion is
divided such ~hat chloric acid rea~ts in ~he pfr and the liquor
1~ exiting the p~r, containin~ the unreacted ~lkali ~etal chlo~a~e,
is fed to an SVP~ generator. ~n this embo~iment, no sulf~rio
acid i~ fed to th~ pfr, ~hus preventing rea~tion of k~e al~ali
metal chlorate in the pfr. The enti~e amount of ~ulfurio aci~
i~ instead fed to the S~P~ ~enerator far rea~tio~ of the alkali
metal chlorat~ in ~his later ~tage. Betwe~n about ~0% and 1~0~
by weight of the hydroge~ peroxide i~ fed ~o ~he pf~, and
desira~ly at lea~t 90% by weight.
This embodi~ent i~ especially advan~ageou~ for u~ing ~he
output from an electrolytic ~e~l for produ~in~ chloric acid,
whi~h i~ opera~ed ~or p~tial conver~ion of alk~ e~al ch~or~te
to chloric acid, leaving a considerable amoun~ C~f unreacted
alk~li metal chlorate in the ou~pu~ s~rea~n. Generally the feed
stream o~ chloric acid/alkali met~l ch~orate has a concentration
of chloric acid from abc)u~ 0. S ~o abou~ 6 M~ pre~e~ably from
~5 ~bout ~ . O to abou~ 3 . O M, while the amount of al~ali me~l
chlor~te ~n the feed is fro~ about 2.0 to abou~ 5~0 M, ~nd
preferably from about 3 . D to a~ou~ 4 . O M, It i$ ~dvantageou!c
-- 13 --
213504~
rom a process standpoint to maxi~ize the amoun~ of chloric a~i~
in the feed ~tream, but the upper limit of ~loric ~cid ~oncen-
tra~ion is de~ermined by the economi~ of the elec~rolytic cell
pxocess. Alterna~ively, in~tead of using ~n SVP~ genera~or to
react the alkali metal chlorate "~ead load" in the exi~ ~tream
from the pfr, the exlt ~tream ~an be recycled ~ the electrolytic
cell for fur~her conversion to chlaric acid.
FIG. 2 ~chemati~lly illustr~tes a pfr re~ctor co~nec~ed in
serie~ to an SVP0 generator. This arrangemen~ could ~e u~ed fo~
the ~ir~t and secon~ embod~nt~ descri~ed abo~t
As shown in FIG. Z, chemical feeds ~chlorate and/or chloric
a~id, reducing ~gent and ~ptionally ~ulfuri~ a~id~ are ~plit
between ~he pfr reactor and thq SVP0 ~o ~ptimize reaction
perform~nce, as described above. A plug flow re~ctor 100,
con~ain~ one or a plural~ty of reaction ~ubes ~not shown~. The
chemical feed en~er~ the pfr ~ia conduits 112 and 114. Unrea~ted
chemicals and chlorine dioxide exi~ via condu~t 11~ and ~re fed
to an SVP~ generator 118 where further reaction to approximately
100% ~onversion can occur. Sodium sulfate sal~ is cryst~llized
in the SVP~ generator 118. The solid sodium sulfate is ~hereaf-
ter removed via conduit 120.
In a ~hird preferred embodiment, the feed ~o ~he pfr is p~re
or 5ubs~ntially pure chloric acid~ Xn thi-~ embudim~nt, due ~
the high reac~ y of ~h~oric acid with ~ydrogen peroxide and
~5 the lack of a saltcake by-product, the pfr ~n function as ~
s~and alone chlorine dioxide ~nerator. Chloric a~id concentra-
tion is at least a~out 0.5 M and ~an ~e ~s hi~h as ~bout 6 M~
- 14 -
~13~046
Th~ limitin~ faqtor for chloric acid ~oncentration i- Q~ability.
Above about 6 M, the chloric acid solu~ion tends ~o decompose.
~e~irably the chloric acid cqncentration is at 7 e~st ~bout 2.0
M and preferably at ~eact abou~ 3.0 M.
In general, t~e plug flow reactor of the invent~on can be
either a ~ingle condui~ or a plurality o~ condu~s ~hro~gh whi~h
the process 6tre~m flow~ in par~llel.
While the invention is not to be limit~d ~hereto, kine~ic
studie~ have re~ealed tha~ the rate expression for a ~hlorine
dioxide pro~ess usin~ hydrogen peroxide, ~u~uric acid and alkali
me~l chlorate i~:
rclo2 k [N~S0"~ [H2~2l~ [N~103]
whe~e k=2.81 X 1~l2e~ 87 T)
The rate expression f ox processes usin~ either chlori~ a~i~
lS alone o~ a ~ixture of chloric acid and '~ead load" ~lkali metal
~hlora~ iS:
rc1O = ~3~2~o3~6~ H2o2~
w~re k = 1.93~x 10l~ 3~736)
Thu~ ~h~ reaction is essen~ially ~th order fox ~lka~i metal
2V chlora~e proce~se~ an~ 1~. 5 for chloric acid proce~se~.
- lS -
213~04S
The invention is illustrated by the following ex~mples:
~X~P~ ~ ~
A 40 ~PD SVP~ oper~ed with methanol, s~lfuri~ açid and
sodiu~ chlorate i~ converted to a 55 TP~ process by ~onnecting
a 15 TPD plug Ylow reactor in series to the SVP~ gener~r.
~ydrogen peroxide ic u~ed as the reducing a~ent for chlo~ate in
a ~ulfuric acid me~ium in both ~he pfr and the SVP~. To mini~ize
the pfr ~ize, the re~tant profiles in the rea~tor are optimized.
1~ Thi~ is achieved by splitting the total chemical feed~ to the
sys~em between the pfr and ~he SVP~ gener~tor. For this ~xample
70% by weight of the total chlorate feed and lO0~ by wei~ht of
the total acid feed ~re oombined and d~xected into the pfr.
Fifty percent of ~he tota~ peroxide feed is also dire~ted into
the pfr. ~he pfr xeact~nt feeds thoroughly mix in ~he ~hort
dis~rib~tion header of the reactor. The mixed re~tants then
flow thro~gh ~he individual reaction tube~ o~ the rea~or~ The
calculated production rate in the pfr is as follow~:
CtO2 T~ _(40% Convers on)(70% of Cl03 feed)(8~.9 tons Cl03~( 106 4~C10 )
l~Otons C~102
d~
To ac~ieve the~e con~ersions, the residen¢e time in e~ch tube i8
~pproxlmately 15 minutes. The expected rea~tant con~en~x~ions
a~d temper~ure profiles for this reactor are shown in FIG. 3.
~ 213~0~6
~X~ 2
A 40 TPD SVP~ uslng alkali ~eta~ chlo~ate, sul~urlc a~id and
meth~nol as the reducin~ agen~, operated in the su~t~ntial
absen~e of added chloxide ion, i~e., withou~ salt addition, iQ
converted ~o ~ ~0 TPD process by connec~ing a 20 TPI~ plu~ ~low
reac~or in ~eries to the ~VP~ gen~rator. ~he feed to the pfr is
a mixture of alkali me~l chlorate ~nd chloric a~id whiçh
aonstitutes ~he output from an electrolytic ~ell. Hyd~ogen
peroxide i~ used as the redu~ing agent both ~or chloric ~cid in
~he pfr, ~n~ for alkali me~al chlorate in the SVP0. Sulfuri~
acid is fed only to the SVP~ gene~tor. In this ex~mple, 100~
of an alkali me~al ~hl~r~te - chloric aci~ eed are co~ine~ and
directed into ~he pfr, The concentr~tion of these species in th~
feed is 3.5 M chlorate and ~.5 M chloric acid. One hundred
lS percent of the total peroxide feed is directed into the pfr
through the center distributor conduit. The p~r re~atant feeds
thoroughly mix in the short ~i~tribution he~er of the rea~tor.
The mixed reactants the~ flow throu~h ~he individual rea~tion
tubes of the ~e~tox. Each reac~ion ~ube in ~he p~ is 1.5
inches in internal diame~çr and l.S ft lon~. ~he total num~er
of tubes in the pfr is 8. ~hlorate conversion ~s 35% of the
incoming chlorate feed. The calc~la~d pxoduction rate in ~he
pfr reaction i~ shown below.
- 17 -
- 213SQ~6
C102 d,P~ =t3S% Conversion)(100% of C103 fec~ 4.~ ~ons Cl03)~ Ib I 2 )
20 0 tons Cl02
To a~hieve these conversions, the residence time in ea~h tube is
10 minutes. Each tube is open-ende~ at the top of the re~ctor
to allow for ~a~ uid separation and liquid overflow.
Product chlorine dioxide gas f 1QWS out of the ~op re~ctor hea~,
and spe~t re~ction liquor flow~ out of the tuPe6 and colle~t~ in
the bottom of the reactor shell. This liquor is rich in ~hlorat~
and peroxide and is pumpe~ out of thç ~he~l, and int~ the SVP~
genera~o~, In thi~ vegsel the alkali metal chlorate iæ con~erted
~o chlorine dioxide (approxim~te~y 100~ ~onverslon). The pfr is
ope~ated isothermally, with the energy needed to drive the
reaction in the pfr provided by steam. The pfr is operake~ in
a non-crystallizati~n ~ode at a pressure of 300 mm Hg. Air is
added to the pfr ~o dilu~e the chlorine dioxide product gas. The
expected reactant conçentra~ions and temperature p~ofiles for
this rea::~or ar~ ~hown in ~IG. 4.
EXA~PL~ 3
From an electrolytic cell, par~ially c~nverted ahlorate
solution containing chloric acid is fed to ~ pfr as described
above an~ reacted with hy~rogen peroxid~. since no o~her ~cids
or detrimental ~y-produ~s are present, only the chloric acid
~ 1~
2135Q~6
rea~s ~ form chlorine dioxide. Unrea~ted sodium chlora~e is
khen combined wi~h fresh alkali metal chlo~ate, reçy~le~ to the
e~ectrochemic~l cell, and further con~erted to chlori~ acid.
This proçe~ i8 optimized to give go~ curren~ efficiency ~nd
S chemi~al s~ability by keeping ~he per pass conversion o~ ~hlorate
low in the cell (i.e., 25~ conv~rsion of the to~al chl~rate feed
t~e cell) and the per pa~ conversion of t~e chloric a~i~ in
the pfr high (i.e., 50% conversio~ of the total chloric acid feed
~o ~he pfr).
,10
E~Al~spT~ ~
~ 40 TPD SVP~ uæing alk~li m~t~l chlorate, sul~uri~ ~cid and
methanol as the reduqing ~gent, opera~ed in the ~ubstan~ial
a~ence of added chloride ion, i.e., wit~out sal~ addition, is
~eplaced with a 40 TP~ pfr - ~hloric aci~ cell sys~em~ For this
ex~mple, 100~ of the total chlorate - chloric acid feed is
d$rected ~nto the pfr. One hundred percent of the ~ok~l peroxide
feed is al~o dire~ted $nto the pfr throu~h the center distri~u-
~ion ~onduit, The p~r xeactant feeds thoroughly mi~ ln the shor~
~o distribution header of the reactor. The mixe~ reactantQ then
flow ~hrough the individual reac~ion tu~e~ of the reactor. ~l~w
through the tubes i~ in the laminar f lo~ region . Each ~u~e
func~ions 8s an individual pfr, and has an internal diamet~r Of
2 inches and a leng~h of 2.5 f~. Chlorate conve~sion is 35~ of
~5 the in~oming chlorate feed. ~he calcu~ate~ production rate in
~h~ pfr is shown below.
21~504~
Cl02Td~ 35% C~onversion)(100~ of ClO3 ~ce~)(180tons C103)( 1064~ tO2 )
40 0 to7~s Cl02
d~
To achieve these conversions, residence times in each tube are
10 minutes. ~he chloric acid portion ~f the feed ic driven to
almost comple~e ~onver~io~. Product chlorine d~oxide gas flows
out o~ ~h~ top xeactor head, and is c~llected. Spen~ re~ction
liquo~ flows out of the tu~e~ and collects in ~he bo~om of the
reactor shell. ~hiS li~uor is still ri~h in çhlor~e ~ut low i~
acidity and peroxide. It i~ then pumped out of the ~h~ll, and
ba~ t~ an electxochemical cell. The pfr i~ operated i~her~
~o mally~ The e~er~y needed to drive the ~e~qtion in the pfr i5
provided ~y a sui~able heating medium ti.e., ste~m or hot water).
The pfr is operated at a pressure of about 300 ~m Hg, Air i-~
added to the re~ctor to dilute the chlorine ~ioxide product g~
Altho~h ~e pre~ent ~nvention ha~ been dec~ribed i~
connection with preferr~d embodiment6 of the inve~io~, it will
be appreciated by those skilled in the art that ad~i~ion~,
substitutions, modi~ications and d~letions no~ ~pecifically
~e~cribed, ~ay be m~de without depar~ing from the 6pirit and
scope of the i~vent~on as defined in the appendsd claim~.
- 20 -