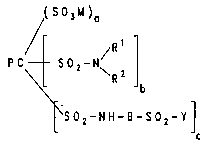Note: Descriptions are shown in the official language in which they were submitted.
213`~ ~ v ~
HOECHST AKTIENGESELLSCHAFT HOE 93/F 380 Dr.HU/do
Description
Water-soluble phthalocyanine dyes, preparation thereof
and use thereof
The invention relates to the field of fiber-reactive
dyes.
The users of fiber-reactive dyes have ever increasing
expectations of the quality of the dyeings and the
ecological compatib~lity of the dyes and of the dyeing
process. Consequently, there continues to be a demand,
especially with regard to the ecological safeness of the
use of the dyes, for novel, especially heavy metal-free,
fiber-reactive dyes which also have good dyeing qualities
and fastness properties. For example, U.S. Patents Nos.
4,237,050, 4,350,632 and 4,745,187 describe fiber-
reactive heavy metal complex phthalocyanine dyes, in
particular copper and nickel phthalocyanine dye~, which
have good dyeing properties and which pos~e~s, linked
through an N-arylsulfonamide or N-alkyl~ulfonamide
radical, a fiber-reactive group of the vinyl ~ulfone
series. As these heavy metal complex phthalocyanine dyes
decompo~e, for example in the course of the dispo~al of
dyehou~e waote water~ and in used textiles, their heavy
metals are liberated into the environment, ~o that the
heavy metals pas~ into the waste waters and sewage
sludges. Since there is already extensive legi~lation
limiting the levels of heavy metal ion~ in wa~te waters
and sewage sludges, a way has to be sought to replace the
prior art heavy metal phthalocyanine dye~ with other~
having no heavy metal ion content.
The pre~ent invention now provide~ novel, water-~oluble
phthalocyanine dyes of the below-indicated formula (1),
whose central metal atom is safe and which give high-
quality dyeings in brilliant green ~hades having good
. . . :: - , ,: , : : , . ~
2 1 3 ~
- 2 - :
fastnes~ properties.
( S 03M )a
~t \R2 ~b
~S02-NH-B-s02-y]
,:
In this formula:
PC is the radical of the phthalocyanine of the formula
(A) :
N ~N
N
~N M e N~[3 ( A ) ~
N ~ N ~:
where
Me is the bivalent metal radical ~`
- Z n ~ T i ( I V )
O ,.
2 13 ~
-V( I V)- or -S 1-
O R
where R is methyl, ethyl, hydroxyl or halogen,
preferably chlorine;
M i~ hydrogen or an alkali metal, such as ~odium,
potassium or lithium, or some other ~alt-forming
metal;
R1 is hydrogen, alkyl o$ 1 to 6 carbon atomo, prefer-
ably 1 to 4 carbon atoms, such as methyl and ethyl,
or is alkyl of 2 to 6 carbon atoms, preferably of 2
to 4 carbon atoms, such as ethyl and n-propyl, which
i~ substituted by alkoxy of 1 to 4 carbon atoms,
such as methoxy and ethoxy, alkanoylamino of 2 to 5
carbon atoms, such a~ acetylamino and propionyl-
amino, carboxyl, sulfo, phosphato, sulfato, hydroxyl
or dialkylamino having an alkyl each of 1 to 4
carbon atoms, ~uch as dimethylamino and diethyl-
amino,
R2 i~ hydrogen, alkyl of 1 to 6 carbon atoms,
pref~rably of 1 to 4 carbon atoms, such as methyl
and ethyl, or iB alkyl of 2 to 6 carbon atom~, pre-
ferably of 2 to 4 carbon atoms, such a~ ethyl and
n-propyl, which i~ su~stituted by alkoxy of 1 to 4
carbon atoms, euch as methoxy and ethoxy,
alkanoylamino of 2 to 5 carbon atoms, ~uch as
acetylamino and propionylamino, carboxyl, sulfo,
phosphato, sulfato, hydroxyl, phenyl, sulfophenyl,
carboxyphenyl or dialkylamino having an alkyl each
of 1 to 4 carbon atoms, suah as dimethylamino and
diethylamino, or is phenyl which can be sulfo-eub-
stituted, or0 R1 and R2, together with the nitrogen atom and an alkylene
of 3 to 8 carbon atoms, preferably 4 to 6 carbon
atoms, or with a further hetero group, such as a
- 2 1 3 ~
-- 4
nitrogen atom or oxygen atom or an -NH- group, and
two alkylenes of 1 to 5 carbon atoms, form the
radical of a 4- to 8-membered heterocyclic ring, ~or
example an N-piperidino, N-piperazino or
N-morpholino radical, or the radical -NRlR~ i~ cyano-
amino;
B is a radical of the formula (2a) or (2b)
R ~ :
R ( S 03M )m ( S 03M ) n
(2a) (2b)
where
R3 is hydrogen, alkyl of 1 to 4 carbon atoms, ~uch
as ethyl and in particular methyl, or alkoxy of
1 to 4 carbon atoms, Quch as ethoxy and in par-
ticular methoxy,
R4 i~ hydrogen, halogen, ~uch as bromine and :
chlorine, alkyl of 1 to 4 carbon atomo, ~uch as :.
ethyl and in particular methyl, or alkoxy of 1 to
4 carbon atoms, such a~ ethoxy and in particular
methoxy, -
M i~ a~ defined above, :~
m is zero or 1 (if zero, this group being
hydrogen), preferably zero,
n is zero, 1 or 2, preferably 1 or 2, and :: :
in the formula (2a) the -SO2-Y group i~ preferably
attached meta or para relative to the sulfonyl-
amino group and in the Xormula (2b) the bond
leading from the naphthalene radical to the
~ulfonylamino group i~ preferably di~po~ed in ~he
~-po~ition of the naphthalene radical;
Y is vinyl or i~ an ethyl group which, in the :~
~-po~ition, contains a substituent which is
eliminable by alkali to leave a vinyl group, or i~
2 1 3 ~ ~ ` 5
~ -sulfoethyl or ~-hydroxyethyl;
a i8 from zero to 3,
b is from zero to 2, and
C i8 from 1 to 4,
the sum of (a+b+c) being from 1 to 4.
The individual symbols in the formula can be identical to
or different from each other within the scope of their
definition.
The phthalocyanine dyes of the invention are generally
obtained in the form of mixtures of the individual
compounds of the formula (1), which individual compounds
differ from one another by the number of sulfo and
sulfonamide groups on the phthalocyanine radical. The
formulae of the phthalocyanine dye~ according to the
invention therefore have generally fractional indices.
Alkali-eliminable substltuent~ in the ~-position of the
ethyl group of Y include for example halogen atoms, such
as bromine and chlorine, ester groups of organic
carboxylic and sulfonic acid~, as of alkylcarboxylic
acids, ~ubstituted or unsubstituted benzenecarboxylic
acids and substituted or unsubstituted benzenesulfonic
acidR, such as alkanoyloxy of 2 to 5 carbon atom~
2specially acetyloxy, benzoyloxy, sulfobenzoyloxy,
phenylsulfonyloxy and toluylsul$onyloxy, also acid ester
groups of inorganic acids, a~ of phosphoric acid,
sulfuric acid and thio~ulfuric acid (phosphato, sulfato
and thiosulfato groups), eimilarly dialkylamino group~
having alkyl groups of in each ca~e 1 to 4 carbon atoms,
such as dimethylamino and diethylamino. Y is preferably
~-sulfatoethyl or vinyl, particularly preferably
~-8ul fatoethyl.
The groups "eulfo", "carboxyl", "thiosulfato",
"pho~phato", and "sulfato~ include not only their acid
form but also their salt form. Accordingly~ sulfo group~
are groups conforming to the formula -S03M, carboxyl
-- 6
groups are groups conforming to the formula -COOM, thio-
sulfato groups are group~ confor~ing to the formula
-S-SO3M, pho~phato groups are gxoups conforming to the
formula -OPO3M2, and ~ulfato groups are groups conforming
to the formula -OSO3M, in each of which M is as defined
above.
Radical~ of the formula -B-S02-Y include for example:
2-(~-sulfatoethyleulfonyl)phenyl, 3-(~-sulfatoethylsul-
fonyl)phenyl, 4-(~-sulfatoethylsulfonyl)phenyl,
2-carboxy-5-(~-~ulfatoethylsulfonyl)phenyl, 2-chloro-3-
(~-sulfatoethylsulfonyl)phenyl, 2-chloro-4-(~-aulfato-
ethylsulfonyl)phenyl, 2-bromo-4-(~-~ulfatoethylsulfonyl~-
phenyl, 4-methoxy-3-(~-sulfatoethyl~ulfonyl)phenyl,
4-chloro-3-(~-sulfatoethylsulfonyl)phenyl,2-ethoxy-4-or
lS -5-(~-sulfatoethylsulfonyl)phenyl, 2-methyl-4-(~-sulfato-
ethylsulfonyl)phenyl, 2-methoxy-5- or -4-(~-sulfatoethyl-
~ulfonyl)phenyl, 2,4-diethoxy-5-(~-sulfatoethyl~ulfonyl)-
phenyl, 2,4-dimethoxy-5-(~-sulfatoethyl~ulfonyl)phenyl,
2,5-dimethoxy-4-(~-sulfatoethylsulfonyl)phenyl,
2-methoxy-5-methyl-4-(~-~ulfatoethylsulfonyl)phenyl, 2-
or 3- or 4-(~-thiosulfatoethylsulfonyl)phenyl, 2-methoxy-
5 - (~- t h io ~u l fa to e t hy l ~ u 1 f o n y l) p he n y l,
2-sulfo-4-(~-pho~phatoethylsulfonyl)phenyl, 2-sulfo-4-
vinylsulfonylphenyl, 2-chloro-4- or -5-(~-chloro-
ethylsulfonyl)phenyl, 3- or 4-(~-acetoxyethyl-
sulfonyl)phenyl, 5-(~-sulfatoethylsulfonyl)naphth-2-yl,
6- or 7- or 8-(~-sulfatoethylsulfonyl)naphth-2-yl, 6-(~
sulfatoethylsulfonyl)-1-sulfonaphth-2-yl, 5-(~-sulfato-
ethylsulfonyl)-1-sulfonaphth-2-yl and 8-(~-~ulfatoethyl-
sulfonyl)-6-sulfonaphth-2-yl.
Preferably, in the phthalocyanine dyes of the formula
(1), R3 i~ hydrogen, methyl or methoxy, and R~ is prefer-
ably hydrogen or methoxy. Preference is further given to
compounds of the formula (1) in which Rl and R2 are each
independently of the other hydrogen, methyl or ethyl and
b is 1 to 2, and al~o to those in which b i~ zero.
Preference i~ further given to phthalocyanine dye~ of the
- ~ :-
' ' .
: -
2 ~
-- 7
formula (1) in which a i~ from zero to 3, preferably from
1 to 2.5, b i8 from zero to 2, preferably zero, and c is
from 1 to 4, preferably from 1 to 2.
Radicals of the formula -NRlR2 include for example amino,
methylamino, ethylamino, butylamino, benzylamino,
~-hydroxyethylamino, dimethylamino, diethylamino,
di-(isopropyl)amino, N-methylbenzylamino, N-piperidino,
N-morpholino, di-(~-hydroxyethyl)amino, ~-eulfoethyl-
amino, ~-carboxyethylamino, ~-(4-carboxyphenyl)ethyl-
amino, phenylamino, N-methylphenylamino, 3-aulfophenyl-
amino and 4-sulfophenylamino and cyanoamino.
The pre~ent invention further relates to a process for
preparing the phthalocyanine compounds of the formula
(1), which comprises reacting a phthalocyanine~ulfonyl
chloride of the formula (3)
( S 0 3 M ) p ( 3 ~
~( S O 2 - C I )q : .
,' :'.:
where PC and M are each as defined above, p is from zero
to 3, and q is from 1 to 4, the sum of (p+g) being from
1 to 4, or a mixture thereof, in an aqueous or non-
aqueous medium with an amine of the formula (4) ;~
H2N--B--S2--Y (4) ~ ~
where B and Y are each a~ defined above and Y mayadditionally have the meaning of ~-hydroxyethyl, and
optionally with a further amino-containing compound of
the formula H-NRlR2 where Rl and R2 are each ae defined
above, concurrently or in any de~ired order and with con-
current or successive partial hydrolysi~ of the ~ulfonylchloride group~ to eulfo groups. At the end of the
condensation reaction any sulfonyl chloride groups still
. .
.: - : ~ - :. :-
,, - . :
. .
.
- 213~5~5
- 8 -
pre~ent are hydrolyzed to ~ulfo groups. The reaction
according to the invention will generally inevitably
involve a partial hydroly~is of the sulfonyl chloride
groups to ~ulfo groupa. In the ca~e of the u~e of an
amine of the formula (4) where Y i~ ~-hydroxyethyl, the
~-hydroxyethyl6ulfonyl group $n the phthalocyanine
compound obtained can be converted by generally known
methodg into a group of the formula -S02-Y where Y i~ an
ethyl group Rub~tituted in the ~-position by an e~ter
group, for example into the ~-sulfatoethyl BUl fonyl group
by mean~ of ~ulfuric acid or sulfur trioxide-containing
~ulfuric acid or chloro~ulfonic acid.
The condensation reactions according to the invention
between the compound~ of the formula (3) and the amines
of the formulae (4) and H-NRlR2 are carried out at a pH
between 3.5 and 8.5, preferably between 4 and 8, in
particular between 5.5 and 6.5, and at a temperature
between 0C and 100C, preferably between 20 and 60C, in
particular between 45 and 55C. Generally they take place
in the pre~ence of a catalyat, auch as pyridine, a
pyridine~ulfonic acid, pyridinecarboxylic acid,
pyridinesulfonamide or pyridinecarboxamide compound,
preferably in the pre~ence of nicotinamide, analogously
to the procedure~ known from the U.S. Patents cited at
the beginning or from U.S. Patent 4,745,187.
The Rtarting phthalocyaninesulfonyl chloride co~pound~ of
the formula (3) are prepared analogously to known pro~
cedures, a~ discernible for example from statements in -
the above-cited U.S. Patenta and from German Patent No.
891,121. For example, a compound of the formula (3) i~
prepared by introducing phthalocyanine of the formula (A)
into chloro~ulfonic acid and ~tirring the batch at a
temperature of between 80 and 150C, preferably between
100 and 140C, for ~everal hour~, and then adding a
chlorinating agent, preferably thionyl chloride, and
further stirring the batch at 80 to 90C fox ~everal
hours. The batch i~ then ~tirred into cru~hed ice and the
~,
: .,
:
' ~ ~ . ' -
~ ~ 33~
precipitated phthalocyanineeulfonyl chloride i~ filtered
off with euction.
Another way of preparing a compound of the formula (3) i8
to react the phthalocyanine of the formula (A) with
~ulfuric acid/eul~ur trioxide in eulfur trioxide-contain-
ing ~ulfuric acid (oleum) having a eulfur trioxide
content rom about 50 to 65% by weight at about 80C.
After thie reaction, the batch is stirred into an ice-
cooled aqueoue solution of eodium chloride, and the
metal-containing phthalocyanineeulfonic acid obtained ie
converted in a conventional manner, or example by meane
of thionyl chloride in dimethylformamide, into the
phthalocyanine-eulfonyl chloride.
The metal phthalocyanine etarting compounde of the
formula (A) are known or are preparable by known methode.
Relevant literature includee US Patent 2,202,632,
British Patents 679,773 and 1,306,055, 3nd
US Patent 2,155,038.
The eeparation from the eyntheeie batches of the phthalo-
cyanine dyee of formula (1) prep~red according to the
invention, hereinafter referred to ae "dyee (1)", ie
effected by generally known methods either by precipitat-
ing from the reaction medium by means of electrolyte ,for example sodium chloride or pota~eium chloride, or by
evaporating the reaction ~olution, ~or example by epray
drying, in which case thi~ reaction solution may have
buffer eubstance added to it. The dyes (1) have fiber-
reactive properties and very good dye propertiee. Theycan therefore be u~ed for dyeing and printing hydroxyl-
and/or carboxa~lido-containing material, in particular
fiber material, and leather. 5imilarly, the eelutione
obtained in the eynthesie of the compounde according to
the invention can be u~ed directly in dyeing ae liquid
preparation with or without prior addition of buffer
eubstance and with or without prior concentrating.
: - .. - :
: . :- : :. . ...
. . . - -
. . . . .
" ' ~ ' ' . , ' '
213~v~
- 10 -
The present invention therefore also provides for the use
of the dyes (1) for dyeing (including printing) hydroxyl-
and carboxamido-containing material~, i.e. processee for
applying them to these sub~trates. The materials are
preferably employed in the form of fiber materials, in
particular in the form of textile fibers, such as yarns,
packages and fabrics.
Hydroxyl-containing materials are natural or ~ynthetic
hydroxyl-containing material~, for example cellulose
fiber materials, even in the form of paper, or their
regenerated products and polyvinyl alcohols. Cellulo~e
fiber materials are preferably cotton but al~o other
vegetable fibers, such ae linen, hemp, ~ute and ramie
fibere; regenerated cellulose fibers include for example
staple viscose and filament vi~cose.
, .
Carboxamido-containing materials lnclude for example
synthetic and natural polyamides and polyurethanes, in
particular in the form of fibers, for example wool and
other animal hairs, silk, leather, nylon-6,6, nylon-6,
nylon-ll and nylon-4.
The dyes (1) can be applied to and fixed on the sub-
strates mentioned, in particular on the fiber materiala
mentioned, by the techniques customary for water-soluble
dye~, in particular for fiber-reactive dyes. For
instance, on cellulose fibers they produce from a long
liquor by the exhaust method and by means of various
acid-binding agents with or without neutral salts, such
a~ sodium chloride or sodium sulfate, dyeings having very
good color yields and also excellent color build-up. They
are dyed at temperatures between 40 and 105C, if desired
at temperatures up to 130C under superatmospheric
pressure, and if desired in the presence in the agueou~
bath of customary dyeing as~istant~. One possible
procedure is to introduce the material into the warm bath
and to gradually heat the bath to the desired d~eing
temperature and to complete the dyeing proces~ at that
: .: .
' :. . : :: : . :
- :-: . : : . ,
~ ~ 3 ~
11 -
temperature. The neutral salt~ which ~peed up the
exhaustion of the dye can if desired not be added to the
bath until after the actual dyeing temperature has been
reached.
The padding processes likewi~e produce excellent color
yields and a very good color build-up on cellulose
fibers, on which fixing can be e$fected by batching at
room temperature or elevated temperature, for example at
up to about 60C, by steaming or with dry heat in a
conventional manner.
Similarly, the cu~tomary printing processes for cellulose
fiber~, which can be carried out either single-phase, for
example by printing with a print paste containing sodium
carbonate or some other acid-binding agent as well as the
colorant and by subsequent ~teaming at 100 to 103C, or
two-phase, for example by printing with a neutral or
weakly acid print paste containing the colorant and
subsequent fixation either by pas~ing the printed -~
material through a hot electrolyte-containing alkaline
bath or by overpadding with an alkaline electrolyte-
containing padding liquor with a ~ub~equent batching of -;~
this treated material or subsequent steaming or subse-
quent treatment with dry heat, produce ~trong prints with
well-defined contours and a bright white ground. The
appearance of the print~ is not greatly affeoted by
variations in the fixing conditions. Not only in dyeing
but also in printing, the degrees of fixation obtained
with the compounds of the invention are very high. ~;
When fixing by means of dry heat in accordance with the
customary thermofix processes, hot air from 120 to 200C
is used. In addition to the cu~tomary steam at 101 to
103C it is al~o pos~ible to u~e superheated steam a~d
high-pres~ure ~team at temperature~ of up to 160C.
The acid-binding agents which effect the fixation of the
dyes on the cellulo~e fibers include for example
,: ~ . . ~ ........................................................ .
.. . .
~ . ~: . ,
.. . . - ~ v"
~13S~ 5
- 12 -
water-~oluble basic salts of the alkali metals and the
alkaline earth metals of inorganic or organic acid~ a~
well as compounds which liberate alkali in the heat.
Especially ~uitable are the alkali metal hydroxide~ and
alkali metal salts of weak to medium inorganic or organic
acids, the preferred alkali metal compounds being the
~odium and potassium compounds. Such acid-binding agents
include for example sodium hydroxide, potassium
hydroxide, sodium carbonate, sodium bicarbonate,
potassium carbonate, sodium formate, sodium dihydrogen-
phosphate and aisodium hydrogenphosphate.
By treating the dyes (1) with the acid-binding agents
with or without heating, these compound~ are chemically
bonded to the cellulose fibers; especially the cellulose
dyeings have after the customary aftertreatment by
rinsing to remove unfixed portions of the dyes excellent
wetfastness propertie~, in particular since ~uch unfixed
portions are easily wa~hed off on account of their good
~olubility in cold water.
The dyeings on polyurethane and polyamide fiber~ are
customarily carried out from an acid medium. For
instance, the dyebath may have added to it acetic acid
and/or ammonium sulfate and/or acetic acid and ammonium
acetate or sodium acetate in order to bring it to the
desired pH. To achieve a usable levelneas of the dyeing,
it i~ advi~able to add customary leveling aid~, for
example ba~ed on a reaction product of cyanuric chlori~e
with three times the molar amount of an aminobenzene-
sulfonic acid or an aminonaphthalenesulfonic acid or
based on a reaction product of for example stearylamine
with ethylene oxide. Generally the material to be dyed is
introduced at a temperature of about 40C into the bath,
agitated therein for some time, the dyebath i8 then
adjusted to the deeired weakly acid, preferably weakly
acetic acid, pH, and the actual dyeing i8 carried out at
a temperature between 60 and 98C. However, the dyeings
can al~o be carried out at boiling point or at
:' ''' - , :
.
21~3~v5
- 13 -
temperatures up to 120C (under superatmospheric
pressure).
The dyeings and print~ prepared with the dyes (1) on
cellulose fiber materials have a high color strength, in
addition good lightfa~tne~ and good wetfastne~s proper-
ties, such as wa~h, milling, water, seawater, cross
dyeing and perspiration fa~tness properties, also good
fastness to pleating, hot pressing and rubbing.
The examples which follow illustrate the invention. The
compounds de~cribed in terms of a formula are indicated
in the form of the free acid; generally they are prepared
and isolated in the form of their alkali metal ~alt~ and
used for dyeing in the form of their salt~. Similarly,
the starting compounds mentioned in the form of the free
acid in the following examples, in particular table
examples, can be used in the synthesis as such or in the
form of their salts, preferably alkali metal ~alt~, ~uch ~
a~ lithium, ~odium or pota~sium salts. ~-
Part~ and percentages mentioned in the examples are by --~ -~
weight, unle~s otherwi~e ~tated. Part~ by weight relate
to parts by volume as the kilogram relates to the liter.
The absorption maxima (~) in the visible region
reported for the compounds of the invention were deter-
mined in a~ueous solution using their alkali metal salts.
In the table examples the ~ values are given in paren-
theses in the hue column; the wavelength un~t is nm.
Example A
25 parts of zinc(II) phthalocyanine are introduced into
120 parts of chlorosulfonic acid, and the temperature of
the batch rises to 50 to 60C. Thereafter the batch i~
stirred at 135C for a further 3 to 4 hours and then
cooled down to 80 to 90C, and 34 part~ by volume of
thionyl chloride are gradually added with thorough
stirring. After further stirring at 90C for about four
hours, the batch is then ~tirred out onto ic~, and the
: . , .'
~: , .: , - . , ~
.
.
2 ~
- 14 -
precipitated zinc(II) phthalocyaninesulfonyl chloride,
which has an average degree of eubstitution in respect of
the sulfonyl chloride groups of 2.5, is filtered off with
suction and waehed with ice-water.
Example B
To a mixture of 60 parts of 2-cyanobenzamide, 610 parts
of quinoline and 364 parts of 1,2-dichlGrobenzene are
gradually added, over about 5 minutes, and at about 20C,
68 parts of ~ilicon tetrachloride, the reaction mixture
i~ heated to 205C, and the reaction is continued for
10 minutee while stirring under reflux. Thereafter the
batch iB cooled down to 180C and the re~idue is filtered
off with ~uction at that temperature, suspended in 158
part~ of glacial acetic acid, filtered off again and
subsequently washed with 150 parts of aqueou~ 2N acetic
acid and 400 parts by volume of ethanol and dried.
:, '
30 parts of this dichloroeilicon phthalocyanine compound
are introduced into 440 parts of chlorosulfonic acid. The
reaction i8 carried out during about four hours at 130C
with thorough stirring, the batch is then cooled down to
85C, and 180 parts of thionyl chloride are gradually
added over about 30 minutes. The reaction is continued
under reflux at about 85C for a further ~our hour~, and
then stirred out onto 3000 parts of ice, and the result-
ing dichloroeilicon phthalocyaninetetrasulfonyl chlorideof the formula
- ~ 2~3~'3~
- 15 -
N =~3=N 1--( 5 2 - C I )
~N S\ C l 2 N~
L ~ _
,,''
is filtered off in the form of a moist filter cake.
Example C
To a mixture of 35 parts of dichlorosilicon phthalo-
cyanine and 220 parts of tetrahydrofuran are added at
20C 29 part~ of methylmagne~ium bromide and the reaction
is carried out under reflux (at about 67C) in the cour~e
of about 14 hours. Then in~olubles are filtered off the
cooled batch, and the filtrate i~ admixed with 100 part~
of aqueous 5% strength hydrochloric acid, the tetrahydro~
furan is distilled off, and the resulting dimethyl~ilicon
phthalocyanine is isolated by evaporating the aqueou~
pha~e to dryness.
18 parts of the dimethylsilicon phthalocyanine are
introduced into 520 parts of chloro~ulfonic actd. The
reaction is carried out during about three hours at 140C
with thorough stirring, the batch i~ cooled down to 85C,
and 100 parts of thionyl chloride are gradually added.
The reaction is completed under reflux at about 85C, the
batch is then poured with thorough stirring onto 2000
part~ of ice, and the precipitated dimethylsilicon
phthalocyaninetetrasulfonyl chloride of the formula
... .. .. . . .
213 ~3~
N=~ N ~-(52-C I )4
i8 filtered off in the form of a moi~t filtar cake.
Example 1
A pH 6.5 solution of 36 parta of 4-(~-sulfatoethyl-
~ulfonyl)aniline in 100 part~ of water i~ admixed with
the zinc(II) phthalocyanine~ulfonyl chloride moist filter
residue of Example A and 4 part~ of nicotinamide by
~tirring. The batch i8 sub~equently ~tirrsd for a further
two hours at 50C and thereafter at about 20C while the
pH of 6.5 i5 maintained. After the conden~ation reaction
ha~ ended, the batch i~ filtered and the phthalocyanine
dye of the invention i~ i~olated by ~alting out with
~odium chloride or by evaporating the reaction solution
to drynes~ under reduced pres~ure.
The phthalocyanine dye of the invention ha~, written in
the form of the free acid, the formula
N--~_ 11 , ~( S 0
~¢N Z n N~3
N~ _ 502-NH~502-CH~-CH2~050~N
~A,l~a" = 670 nm). ~:
.
~ ~ .
- 2:L35~
- 17 -
It has very good $iber-reactive dye properties, ia very
readily soluble in water and, applied by the application
and fixing techniques customary for fiber-reactive dyes
in industry, yields on the material~ mentioned in the
description, in particular on cellulose fiber materials,
~uch as cotton, brilliant green dyeings and prints having
good fastnees properties.
Example 2
Example 1 is repeated with the 4-(~-sulfatoethyl-
sulfonyl)aniline replaced by the same amount of 3-(~-~ul-
fatoethylsulfonyl)aniline. The resulting novel
phthalocyanine dye of the formula (written in the form of
the free acid)
11 ~ 11 SO~-~III ~ SO2-CH~-CH~-OSO~R ]
U~x = 670nm)
~ how0 good fiber-reactive application propertie~ and dyes
for example cellulose fiber materials, such as cotton, by
the dyeing and printing techniques cu~tomary in industry
for fiber-reactive dyeR in strong, brilliant, green
shades having good fastness properties.
Example 3
To a neutral solution of 30 part~ of 4-(~-sulfatoethyl-
sulfonyl)aniline in 300 parts of water are added with
thorough stirring at 35C and a pH between 5.8 and 6.2
the dichlorosilicon phthalocyaninetetrasulfonyl chloride
obtained in Example B in the form of a moi~t filter cake
and 3 parts of nicotinamide. The batch is stirred under
the stated reaction conditions for about a further three
hours and then the novel phthalocyanine dye of the
formula (written in the form of the $ree acid)
~ ~ -
: :
....
,,-.:
. . .
~ 2 1 ~ 5
- 18 -
--8=N , ~(SO~H)2.
~¢~1 SIIC12 1~3 ~
_ ~ _ --1SO2-UH ~ 3 SO~-CH2-CHI-OSOIII]
x = 677 nm)
iB isolated by evaporating the aqueous synthesis solution
to dryness under reduced pressure at about 60C.
The novel dye has very good $iber-reactive dye properties
and applied to the fiber materials mentioned in the des-
cription, for example cotton, by the printing and dyeingtechniques customary in industry for fiber-reactive dye~
produces ~trong, fast, green prints and dyeings.
Example 4
To a pH 6 solution of 16.3 part~ of 4-(~-~ulfatoethyl-
sulfonyl)aniline in 200 parts of water are added at atemperature of 35C and while maintaining a pH between
5.8 and 6.2, 1.66 parts of nicotinamide and the dimethyl-
silicon phthalocyaninetetrasulfonyl chloride obtained in
Example C as a water-moist filter cake. The batch i8
further stirred under these reaction conditions for some
time and then the resulting novel phthalocyanine dye,
which written in the form of the free acid ha~ the ~ -
formula ~
.:
~r ~ . . : : ,
,: ' ' :, .
' . ' " ' '
` ~' . ' ' ' ~' ~ : '
- 21~t~55
- 19 -
N~ /(SO~H),,, ~ .:
0~11 t~ CII~ N$~)
_ ~ _ --{SO2-~ ~so2-cH~-cH~-osolH ]
U~x = 670nm)
i~ isolated by evaporating the aqueou~ synthesi~ solution ~ :
to dryne~s under reduced pressure at about 60C. It has
very good dye propertie~ and applied by the usual tech-
niques customary in indu~try for fiber-reactive dye~ for
example to cellulose fiber materials produces strong,
fast dyeings and prints in dark green shades.
. ' :.
~,
- : ~, , , ,::
. ~ ' ' ~ :