Note: Descriptions are shown in the official language in which they were submitted.
vo 93/24494 2 l ~ ~ 8 l g PCT/US93/UJ4Kg
QUATERNARY NITROGEN-CONTAINING PHOSPHONATE COMPOUNDS, FOR TREATING
ABNORMAL CALCIUM AND PHOSPHATE METABOLISM.
BACKGROUN~ OF INVENTION
i This invention re~ates to novel quaternary, n~trogen-
containtng phosphonate compoun~s, including bisphosphonates,
- phosphonoalkylphosphinates, phosphonocarboxylates, and phosphono-
sulfonates. This inventton also relates to pharmaceuticalcompositions contatntng these novel compounds as well as to a
¦ method of treating or preventtng certatn metaboltc bone disorders
¦ charactertzed by abnormal calctum and phosphate metaboltsm by
uttl1ztng a compound or ph~rmaceut~eal compos~tton of the present
tnvention. Spectfically, this tnvent~on relates to a method of
treattng or preYenttng osteoporosis and arthritts, especially
rheumatotd arthr~tis and osteoarthrttts by utll1ztng a compound
or pharmaceutlcal composition of the present tnventton.
A number of pathological condtttons which can afflict warm-
blooded antmals ~nvolves abnormal calctu~ and phosphate metab-
olism. Such condittons may be dtvtded into two broad categortes.
l. Condtttons whtch are characterized by anomalous mobi-
ltzatton of calcium and phosphate leading to general or
I speciftc bone loss, such as osteoporos~s and Paget's
I ' 25 disease, or excesstvely htgh calctum and phosphate levels in
the flutds of the body, such as hypercalcemia of tumor
origtn. Such condtttons are sometimes referred to herein as
pathological hard tissue demineraltzattons.
WO 93/24494 ~ ~ PCI`~ ;93/0446
2l~6a~ -2- ,
2. Conditions which cause or result from deposit~on of
calcium and phosphate anomalously in the body, such as
arthritis, particularly rheumatoid arthritis and
osteoarthritis. These conditions are sometimes referred to
herein as pathological calcif~catlons.
The first category includes the most common metabolic bone
disorder, osteoporosis; osteoporos~s ~s a cond~tion in which bone
hard tissue is lost disproportionately to the development of new
hard tissue. Osteoporosis can be generally defined as the
reduction in the quantity of bone, or the atrophy of skeletal
- tissue. Marrow and bone spaces become larger, fibrous bind~ng
decreases, and compact bone becomes frag~le. Osteoporosis can be
subclassified as menopausal, sen~le, drug-1nduced (e.g.
adrenocort~cold, as c~n oceur ~n sterold therapy);
¦ 15 disease-~nduced (arthrit~e and tumor), etc.; however, the
manifestatlons are essentlally the same.
~ In general, there are two types of osteoporosis: primary
I and secondary. ~Secondary osteoporosls~ ~s the result of a
separate dlsease process or agent. However, approxl~ately 90X of
all ostsoporos~s cases are ~pr~ary osteoporosls~. Such primary
osteoporosls ~ncludes postmenopausal osteoporosls, age-associated
osteoporosls, dtsuse osteoporos~s (affectlng a ma~orlty of
¦ ind~v1duals over the ag~ of 70 to 80), and idlopath k
¦ osteoporosls ~ffectlng mlddle-aged and younger men and women.
For so - osteoporot~c ind~v~duals, the loss of bone tissue
ts su fflcl~ntly great so as to caus~ ~echanlcal f~llure of the
i bon~ structure- Bone fractures oft@n occur, for ex-~ple, in the
hlp nd splnQ of women suffertng froa post~enopausal
osteoporosls. Kyphos~s (abnormally lncre~s~d curvature of the
30 thoraclc splne) ~y also r~sult. r
The mechanis~ of bone loss 1n osteoporotlcs ls b~ Yed to
lnvolve an l~bal~nce ln tha process of ~bone re~odellng~. 80ne
remodellng occurs throughout l~fe, renewlng the skeleton and
matntain~ng th~ strength of bone. Th~s re~odeltng ~nvolYes the
35 eros~on and f~lltng of d~screte sites on the surface of bones, by
an organ~zed group of cells called ~basic multlcellular un~ts~ or
~ WO 93/24494 2 1 3 6 8 1 9 PCr/U~;93/04K9
~J~ .
-3- .,J,~
"BMUs". BMUs primarily consist of "osteoclasts", "osteoblasts",
and their cellular precursors. In the remodeling cycle, bone is
resorbed at the site of an ~activated" BMU by an osteoclast,
forming a resorption tav~ty. Th~s cavlty ~s then f~lled with
S bone by an osteoblast.
¦Nsrmalty, in adults, the remodeling cycle results in a small
¦deficit in bone, due to 1ncomplete fill~ng of the resorption
caYity. Thus, e~en in healthy adults, age-related bone loss
occurs. However, in osteoporot~cs, there may be an increase in
the number of BMUs that are act~vated. This increased act~vation
accelerates bone remodeling, result~ng in abnormally high bone
loss.
Although ~ts et~ology is not fully understoodl there are
many risk factors thought to be assoctated w~th osteoporosis.
These include low body we19ht, low calc~um tntake, phys~cal
inactiY~ty~ and estrogen def~c1ency.
Current osteoporos~s treatment conslsts prlmarily of catcium
and estrogen adm~n~strat10n.
The second category, ~nvolving cond~t~ons manifestet by
anomalous calcium and phosphate deposit~on, includes myos~t~s
ossif~cans progresstv~, calc~nos~s un~versalls, ant such
affl~ct~ons as arthr~tts (~nclud~ng, for example, rheumato~d
arthrltis and osteo-rthr~t~s), neurlt~s, burs~tis, tendon~t~s,
and condltlons ~hlch predtspose 1nvolved t~ssue to deposlt~on of
calc~u~.
In add~t~on to osteoporos~s, bone loss can result from
rh~u~ato~d ~rthr~t~s and osteoarthr~t~s~ Rheu~ato~d arthr~t~s is
a chron~c, systemic and art~cular ~nflammatory d~sorder
character~zed b~ ~eakening of the ~olnt capsules and l~gaments,
followed by dastruct10n of cart~lage, l~ga~ents, tendon and bone,
.and a decrease tn vlscos~ty and other alt~rat~ons ~n the synov~al
, flu~d. Rh~u~ato1d arthrlt~s symptoms ~nc~ude system~c weakness,
fat~gue, local~zed pain, stiffness and weakness and swelllng and
deformation of the ~o~nts of the body. Rheu~atoid arthritls is
most common in women in the fourth to s~xth decade of life.
WO g3/24494 ~ PCI'/US93/04469
~368~9 -4-
The pathogenesis of rheumatoid arthritis, leading to the
destruction of the joints, is characterized by two phases: l) an
exudative phase involving the microciroulation and the synovial
cells that allow an influx of plasma proteins and cellular
elements into the joint and 2) a ohronic inflammatory phase
occurring in the sub-synovium and sub-chondral bone,
characterized by pannus (granulation tissue) fomation in the
joint space, bone erosion, and cartilage destruction. The pannus
may form adhesions and scar tissue which causes the joint
~eformities characteristic of rheumatoid arthritis.
The etlology of rheumatoid arthrltis remains obscure.
Infectious agents such as bacterla and viruses have been
implicated. A current hypothesis ls that the Epstein-Barr (EBV)
virus is a causative agent for rheumatoid arthrltis.
Current rheumatoid arthritls treatment consists
predominantly of symptomatic rellef by administration of
non-steroidal anti-inflammatory drugs. Non-steroidal
antl-inflammatory drug treatment ls malnly effectlve ~n the early
stages of rheumatold arthritis; lt is unllkely it will produce
suppresslon of ~olnt inflammation 1f the disease ls present for
! m~re than one year. Gold, methotrexate, immunosuppressants and
-` corttcostero~ds hav~ been trled wtth ll~ted success.
On the other hand, osteoarthr1t1s, is an lnherently
non-inflam~tory disorder of the movable ~olnts characterlzed by
deter~or~t10n and abrasion of artlcular cartl~age, as well as by
for~t~on of new bone at the ~oint surface. As osteoarthritis
progresses, the surface of the artlcutar cart~lage is dlsrupted
~nd ~ear pàrt~cles gain access to the synovtal fluid whlch in
turn stimulates phagocytosis by macrophage cells. Thus, an
lnflammatory response is eventually induced ln osteoarthrttts.
Common cl~n~cal symptoms of osteoarthrttis include cartilaginous
and bon~ enlargements o~ the finger ~o~nts and stlffness on
I awakening and pa~nful movement.
i Common symptQmatic treatments for osteoarthritis include
analges1cs, anti-ini'la-~atories, steroids, and physical therapy.
~ WO 93/24494 2 1 3 6 8 1 9 PcT/us93/o446s
'- t ' ~ ~ r
-5~ ~:
A variety of phosphonic acid derivatives have been proposed
for use in the treatment and prophylax~s of diseases involYing
abnormal caloium and phosphate metabollsm. For example, numerous
references, all incorporated by reference herein, d~solose
composltions containing polyphosphonates, in particular
diphosphonates such as ethane-l-hydroxy~ diphosphonic acid
(nEHDP"), and their use in inhibiting anomalous deposition and
mobilization of calcium and phosphate ~n an~mal tissue: U.S.
Patent 3,S83,0Q0, issued August 8, 1972 and U.S. Patent
4,230,700, issued October 28, 1980, both to Francis, and U.S.
Patent 4,868,164 to Ebetino, issued Septe~ber 19, 1989. Numerous
other references describe heterocyclic subst~tuted d~phosphonic
acids useful for the treatment of osteoporos~s and/or arthritis,
and are hereby incorporated by reference herein: U.S. Patent
5,071,840, to Ebetino, et al., issued December 10, 1991; U.S.
Patent ~,868,164, to Ebet~no, et al., ~ssued September 19, 1989;
U.S. Patent 5,104,863, to Bened~ct, et al., issued
April 14, 1992; U.S. Patent 4,267,108, to Blum et al., issued
May 12, 1981; U.S. Patent 4,746,654 to Brel~ere et al., issuad
May 24, 1988; U.S. Patent 4,876,247 to Barbler, et al., issued
October 24, 1989, and European Patent Appllcat~on Publ1cation No.
100,718, of Brellere, publ1shed Februa~y 15, 1984; European
Patent Application Publlcation No. 170,228, of Boehringer
Mannhein G~bH, published February 5, 1986; European Patent
Appl1c~t~on Publlcation No. 186,~05, of Bened k t and Perkins,
publ~shed July 2, 1986;- European Patent Appl~cat~on No. 298,553,
of Eb~t~no, published January 11, 1989; U.S. 4,754,993, to
Bos~es, et 21.~ 1ssued November 15, 1988; U.S. 4,939,130, to
Jaegg~, et al., tssued July 3, 1990; U.S. 4,971,958 to Bosies, et
al., issued November 20, 1990; ~0 90/12017, Dunn, et al.
published October 18, 1990; ~O 91/10646, Youssefyeh, R., et al.
pu~lished July 25, 1991; AU-A-26738/88, Jaeggi, publ~catlon date
Jun~ 15, 1989; AU-A-45~67/89 of C~ba-Geigy, publicat~on date May
31, 1990.
WO 93/24494 PCI`/US93/04469
2~,36~ 6-
Finally, U.S. 4,208,401 to Bauman, issued June 17, 1~80,
discloses non-heterocyclic ring substituted quaternary ammonium
bisphosphonates useful as anti-calculus agents.
DE 40 11 777 to Jaegg~, K., disclosed ~ctober 18, l990; (DE
'777) discloses a heterocycl~c ring sub~tituted diphosphonate
wherein said heterocyclic ring can be lower alkyl substituted.
Said heterocyclic ring is bridged to the phosphonic acid group
via a quaternary non-ring nitrogen atom. OE '777 also discloses
that the compounds produce pronounced inhibition of bone
resorption and thus are useful 1n treating osteoporosis,
inflammatory and degenerative ~oint d~seases, peridontitis, and
hyperparathyroidism. The disclosures of these references are
incorporated by reference herein.
fhe compounds of the present invention have osteoprotective
activity at the s~te of joint destruction in arthritis conditions
and have that activity as an addit~onal benefit in the treatment
of arthr~t~s over the above merely relieving the symptoms of
inflammat~on. The term ~osteoprotec1ve activlty~ as used herein
means disease-modifying actlvity on bone and surrounding soft
tissue at the slte of ~oint destruct~on.
It has been surprlslngly d~scovered that the compounds of
~I the present 1nvent10n, where~n the nttrogen ~s quatern~zed, have
I more potent bone antlresorpt~ve actlv1ty and therapeut~c ut~l~ty
in treat1ng osteoporos~s and rheumato~d arthrlt~s and
osteoarthr1t~s than nltrogen-conta~n~ng compounds where the
n1trogen atom ~s not quatern1zed. Moreover, the compounds of the
present tnventton exhlb~t unusual solub~ttty propert~es. Thus,
the compounds of the present ~nvent~on may be more read~ly orally
absorbed compounds. The more read~ly absorbed a compound, the
more effect1ve ~t may be at lower doses. Lower doses are
generally preferable because undes~rable s~de effects are
decreased.
It ~s therefore an ob~ect of the present inventlon to
provlde new, more potent compounds wh k h are useful in
osteoporos~s therapy and anti-arthrit k agents espec~ally useful
1n the treat-ent of osteoarthrit1s and rheu~atoid arthr1t1s.
. ... .........
WO 93~24494 2 1 3 6 8 1 9 PCI`/US93/04469
-7-
It is a further object of the present invention to provide
pharmaceutical compositions useful for the treatment and
prophylaxis of osteoporosis and arthritis, especially rheumatoid
arthritis and osteoarthritis. In add~tion, it is an object of
the present invention to prov~de methods for treating or
preventing osteoporosis and arthrit~s, especially rheumatoid
arthritis and osteoarthritis.
These and other ob~ects of the present invention will become
apparent from the detailed disclosure of the present invention
prov~ded hereinafter.
SUMMARY OF THE INVENTIQN
The present invention relates to quaternary nitrogen-
conta~ntng phosphonate compounds, and the
pharmaceutically-acceptable salts and esters thereof having the
following general formula:
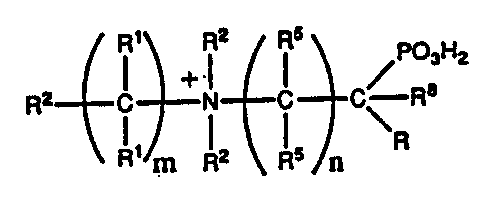
R2 ~ C ~ C \- R~
~hereln ~ ts an integer from 0-10; and n is an ~nteger from 1-10;
n ~s fro~ 1-10;
(a) Rl ls s~lected from the group cons~sting of nil; -SR6;
-R9SR6; hydrogen; substituted or unsubstltuted Cl-Cg alkyl;
-oR3; -Co2R3; -02CR3; -NR32; -N(R3)C(o)R3; -C(o)N(R3)2;
halogen; -C(o)R3; nitro; hydroxy; substituted or
, unsubstituted stturatet monocyclic or polycyclic
heterocyclic rings; substituted or unsubstituted saturated
monocyclic or polycyclic carbocyclic r~ngs;
(b) R5 ~s selected from the group eonsisting of -SR6;
-R9SR6; hydrogen; substituted or unsubst~tuted Cl-Cg alkyl;
.
WO93/~4494 PCI~/USg3/04469 -~
2~36~19 -8-
-oR3; -Co2R3; -02CR3; -NR32; -N(R3)C(o)R3; -C(o)N(P~3)2;
halogen; -C(o)R3; nitro; hydroxy; substituted or
unsubstituted saturated monocyclic or polycyclic
heterocyclic rings; substituted or unsubstituted saturated
monocyclic or polycycl~c earbocyclic rings; substituted or
unsubstituted unsaturated monocyclic or polycyclic
h terocyclic rings; subst1tuted or unsubstituted unsaturated
monocyclic or polycyclic carbocycl k rings and combinations
thereof;
I0 (c) each R2 is selected from the group consisting of
substituted or unsubst1tuted Cl-C3s alkyl, unsubst~tuted or
substituted phenyl; benzyl; or R9SR6;
(d) R3 is selected from the group consisting of H;
unsubstituted or subst~tuted Cl-Cg alkyl; R9SR6;
(e) R6 is selected from the group conststing of -H;
-C(o)R7; -C(S)R7; -C(o)N(R7)2; -C(o)oR7; -C(S)N(R7)2;
-C(S)oR7; where R7 is hydrogen or unsubst1tuted or
substituted Cl-Cg alkyl;
(f) R is selected from the group cons~st~ng of -COOH;
-SO3H; -PO3H~; and -P(o)(oH)R4, where R4 is an alkyl group
¦ having 1-3 carbons~
(g) R9 ~s substituted or unsubstttuted Cl-Cg alkyl;
(h) R8 is s~lected from th~ group consist~ng of hydrogen,
I halogen; SR6; R9SR6; amino; hydroxy; subst~tuted and
unsubst1tuted Cl-Cg alkyl;
In th~s general structure, the quaternary nitrogen atom must
b~ 11nked to the phosphonic acid ~ontalning carbon atom ~1a a
ltnk1ng chaln. It cannot be bonded directly to the phosphonlc
ac~d contain~ng carbon atom.
The present tnYention further relates to pharmaceutical
composit~ons cont~tning a safe and effective amount of a compound
of the present invention, ~nd pharmaceutically-acceptable
exciptents. F~nally, the present invent~on relates to methods
for treating or preYenting pathological conditions characterized
by abnormal calc~um and phosphate metabol~sm such as osteoporosis
and arthritis, espec~ally rheumatoid arthritis, and
WO 93/~2~494 `2 1 3 6 8 1 9 P~r/US93/04469
- . ~ n
_ 9
osteoarthritis, in humans or other mammals. This method
comprises adm;nistering to a human or other mammal in need of
such treatment a safe and effective amount of a compound or
composition of the present invention.
. 5
Definitions and Usaqe Qf Terms
The following is a list of definitions for terms used
herein.
"Heteroatom~ is a nitrogen, sulfur, or o%ygen atom. Groups
containing one or more heteroatoms may contain different
heteroatoms.
"Alkyl~ is an unsubstituted or substituted, straight-chain
or branched, saturated or unsaturated hydrocarbon chain, said
hydrocarbon chain may be saturated hav~ng 1 to 8 carbon atoms,
and preferably, unless otherwlse stated, from 1 to 4 carbon
atoms; said hydrocarbon chain may be unsaturated, having 2 to 8
carbon atoms, and preferably, unless otherwise stated, 2 to 4
carbon atoms. Accordingly, the term ~alkyl~, as used herein,
encompasses alkenyl hydrocarbon unsaturated chains having at
I 20 least one olef~n~c double bond and alkynyl hydrocarbon
unsaturated ch-ins having at least one triple bond. Preferred
alkyl groups 1nclude, but are not l~m~ted to, methyl, ethyl,
j
propyl, isopropyl, and butyl.
~Carbocycl~c ring~ or ~Carbocycle~ as used herein is an
I 25 unsubst~tuted or subst1tuted, saturated, unsaturated or aromatic,
hydrocarbon r~ng; Carbocycltc rings may be monocyclic or
l polycyclic: Monocyclic ring generally contain from 3 to 8 atoms,
I preferably 5 to 7 atoms. Polycyclic r1ngs conta~ning two rings
contain 6 to 16, preferably 10 to 12, atoms and those with three
rings generally contain 13 to 17~ preferably 14 to 15, atoms~
"Heteroalkyl~ is an unsubstituted or substituted, saturated
¦ chain having from 3 to 8-members and comprising carbon atoms and
one or two heteroatoms.
~ Heterocyclic ring~ or "Heterocycle~ as used herein is an
unsubstttuted or substituted, saturated, unsaturated or aromatic
WO g3/24494 PCI`/US93/04469
2~,368~9 -10-
ring comprised of carbon atoms and one or more heteroato~s in the
ring. Heterocyclic rings may be monocyclic or polycyclic rings.
Monocyclic rings generally contain from 3 to 8 atoms,
preferab1y 5 to 7 atoms. Polycyclic ring systems consisting of
two rings generally contain 6 to 16, preferably from 10 to 12
atoms. Polycyclic ring systems consisting of three rings
generally contain 13 to 17 atoms, preferably 14 to 15 atoms. A
heterocyclic ring moiety may consist of heterocycles or
heterocycles and carbocycles. Each heterocyclic ring moiety must
have at least one nitrogen atom. Unless otherwise stated any
additional heteroatoms may be independently chosen from nitrogen,
sulfur, and oxygen.
"Aryl~ is an aromatic carbocyclic ring. Preferred aryl
groups include, but are not limited to, phenyl, tolyl, xylyl,
cumenyl, and naphthyl.
~Heteroaryl~ is an aromatic heterocyclic ring. Preferred
heteroaryl groups include, but are not limited to, thienyl,
furyl, pyrrolyl, pyridinyl, pyrazinyl, oxazolyl, thiazolyl,
quinolinyl, pyr~midinyl, and tetrazolyl.
~Alkoxy~ is an oxygen atom ha~ng a hydrocarbon chain
substituent, where the hydrocarbon chain is an alkyl or alkenyl
(e.g., -O-alkyl or ^O-alkenyl). Preferred alkoxy groups include,
but are not 11~ted to, methoxy, ethoxy, propoxy, and alkyloxy.
~Hydroxyalkyl~ ~s a substituted hydrocarbon cha~n wh~ch has
2~ a hydroxy subst~tùent (e.g., -OH), and may ha~e other
subst~tuents. Preferred hydroxyalkyl groups include, but are not
11mtted to, hydroxyethylj hydroxypropyl.
~ Carboxyalkyl~ is a substituted hydrocarbon chain wh~ch has
a carboxy substltuent (e.g. -COO~) and may have other
substltuents. Preferred carboxyalkyl groups include
carboxymethyl, carboxyethyl, and the~r ac~ds and esters.
~ Am~noalkyl~ is a hydrocarbon cha~n (e.g. alkyl) substituted
with an amine motety (e.g., NH-alkyl-) such as aminomethyl.
~ Alkylam~no~ ~s an amino moiety ha~ing one or two alkyl
3~ substituents (e.g., -N-alkyl) such as d~methylamino.
WO 93/24494 2 1 3 6 8 1 9 Pcr/US9~o446~
"Alkenylamino" is an amino moiety haYing one or two alkenyl
substituents (e.g., -N-alkenyl).
; "AlkynalaminoH is an amino moiety having one or two alkynyl
substituents (e.g., -N-alkynyl).
', 5 "Alkylimino" is an imino moiety having one or two alkyl
substituents ~e.g., -N-alkyl-).
"Arylalkyl~ is an alkyl moiety substituted with an aryl
group. Preferred arylalkyl groups include benzyl and
phenylethyl.
"Arylamino~ is an amine moiety substituted with an aryl
~roup (e.g., -NH-aryl).
"AryloxyN is an oxygen atom having an aryl substituent
(e.g., -O-aryl).
"Acyl~ or "carbonyl" is a carbon to oxygen double bond, e.g.
R-C(~O). Preferred aeyl groups include, but are not limited to,
acetyl, propionyl, butanoyl, and benzoyl.
~ Acyloxy~ is an oxygen atom having an acyl subs~ituent
(e.g., -O-acyl); for example, -O-C(-O)-alkyl.
"Acylam~no~ 1s an amino moiety haYing an acyl substituent
(e.g., -N-acyl); for example, -NH-(C~O)-alkyl.
"Halo~, ~halogen~, or ~hal~de~ ~s a chloro, bromo, fluoro,
or iodo atom radical. Chloro, bromo, and fluoro are preferred
halides.
As referred to herein, a ~lower~ hydrocarbsn moiety (e.g.,
~lower~ alkyl) ts a hydrocarbon chain compr~sed of from, unless
otherwise stated, 1 to 6, preferably from 1 to 4, carbon atoms.
Also, as used herein, the term "thio-subst~tuent~ (SR6 or
¦ R9SR6) ~ncludes th~ols [-SHl where R~-H; thioesters t-SC(o)R7~
where R6-C(o)R7; d~thioesters ~-SC(S)R7] where R6~C(S)R7;
th~ocarbamates ~-SC(o)N(R7)2] where R6~c(o)N(R7)2;
dtth10carbamates [-SC(S)N(R7)21 where R6~C(S)N(R7)2;
thlocarbonates [~SC(o)oR7] where R6~C(o)oR7; and dithiocarbonates
[-SC(S)OR71 where R6~CtS)OR7. R7 ~s generally a hydrogen or
~ Cl-Cg alkyl. Any of the SR6 substituents may themselves be
1 35 substituted with an Rg moiety, i.e. R9SR6, where R9 is a
subst~tuted or unsubstituted Cl-Cg alkyl. Accordingly,
,
WO 93/~4494 PCr/US93/04469 `~.
2~,368~9 -12-
- additional thio-substituents denoted by R9SR6 are alkylthiols,
alkylthi~esters, alkyldithioesters, alkylthiocarbamates,
alkyldithiocarbmates, alkylth;ocarbonates, and alkyldithio-
carbonates.
The terms "bisphosphonate~ or "bisphosphonic acid" as used
herein relate to those phosphonate or phosphonic acid compounds
that have two phosphonate groups attached to the same carbon atom
and are u~ed interchangeably with the terms "diphosphonat2" and
"diphosphonic acids.~ Using the structures described herein, the
moietY R is P3H2-
As used herein, the term ~phosphonic acid carbon" refers to
the carbon atom to which a phosphonic acid group (P03H2) is
attached. When another phosphonic acid group is attached to said
carbon atom, the resulting compound is bisphosphonate. When a
sulfonate group is attached to said carbon atom, the resulting
compound is a phosphonosulfonate. When a carboxylate group is
-attached to said carbon atom, the resulting compound is a
phosphonocarboxylate. ~hen a phosphinic acid group is attached
to said carbon atom, the resulting compound is a
¦20 p~osphonoalkYlPhosPhinate.
iA ~pharmaceut~cally-acceptable~ salt is a catonic salt
-formed at any acid k (e.g., carboxyl) group, or an anionic salt
formet at any b~s~c (e.g., amino) group. Many such salts are
known in the art, as described in World Patent Publication
87/05297, Johnston et al., published September 11, 1987, hereby
incorporated by reference herein. Preferred catonic salts
~nclude the alkali-metal salts (such as sodium and potassium),
and alkaline earth metal salts (such as magnesium ant calcium).
Preferred anionic salts ~nclude the hallde (such as chloride),
acetate and phosphate salts.
!A ~biohydrolyzable ester~ is an ester of the quaternary
nttrogen-contatning heterocyclic phosphonate compounds that does
not interfere with the therapeutic act~vity of the compounds, or
that is readily metabolized by a human or other mammal. Many
3S such esters are known in the art, as described in World Patent
Publication 87/05297, Johnston et al., published
. - WO 93/244g4 rl 2 I 3 6 8 1 9 PCI`/US93/04469
September 11~ 1987, and hereby incorporated by reference herein.
Such esters include lower alkyl esters, lower acyloxyalkyl esters
(such as acetoxymethyl, acetoxyethyl, aminocarbonyloxymethyl,
pivaloyloxymethyl, and pivaloyloxyethyl esters), lactonyl esters
(such as phthalidyl and thiophthalidyl esters), lower
alkoxyacyloxyalkyl esters (such as methoxycarbonyloxymethyl,
ethoxycarbonyloxyethyl and isopropoxycarbonyloxyethyl esters),
alkoxyalkyl esters, choline esters, and acylamino alkyl esters
(such as acetamidomethyl esters).
I 10 As defined above and as used herein, substituent groups may
! themselves be substituted. Such substitution may be with one or
more substituents. Such substituents include, but are not
limited to, those listed in C. Hansch and A. Leo, Substituent
Constants for Sorrelation Analysis in ChemistrY and Bioloqv
(1979), hereby incorporated by re~erence herein. Preferred
substituents include, but are not limited to, alkyl, alkenyl,
alkoxy, hydroxy, oxo, amino, aminoalkyl (e.g. aminomethyl, etc.),
cyano, halo, carboxy, alkoxyacetyl (e.g. carboethoxy, etc.),
thio, thiol, aryl, cycloalkyl, heteroaryl, heterocycloalkyl
(e.g., piperidinyl, morpholinyl, pipera~inyl, pyrrol~dinyl,
etc.), imino, thioxo, hydroxyalkyl, aryloxy, arylalkyl, and
combinations th~reof.
¦ ~ETAILED DESCRIPTIQN OF THE INVENTION
Novel Ouaternarv N~troaen-Conta1nina
PhosDhonate ÇomDounds
The compounds of the present invention are quaternary
nitrogen-conta~ning phosphonate compounds, and ths pharmaceut~-
cally-acceptable salts and esters thereof, having a quaternary
nitrogen atom. The quaternary nitrogen atom is bonded to the
phosphonic acid containing carbon v~a a linking chain to the
phqsphonic acid containing carbon.
~ The carbon atom whlch has the phosphonic acid group attached
to it may be unsubstituted (i.e., a hydrogen atom) or sub-
stituted. The phosphonic acid carbon may contain two phosphonategroups, rendering a bisphosphonate compound; a phosphonate group
W o 93/24494 ; P ~ /US93/04469
2 ~ 3 6 8 ~ 9 -14-
and an carboxylate group, rendering a phosphonocarboxylate
compound; a phosphonate group and a sulfonate group, rendering a
phosphonosulfonate compound, a phosphinate group and a
phosphonate group, rendering a phosphonoalkylphosphinate
compound.
Thus, the quaternary nitrogen-containing phosphonate
compounds of the present invention, and the pharmaceuti-
cally-acceptable salts and esters thereof, have the general
structure:
In this general structure, Rl is selected from a variety
11 of non-r~ng moieties such as nil, -SR6, -R9SR6, hydrogen, alkyl
¦-¦ having 1-8 carbons, -oR3, -Co2R3, -02CR3, -NR32, -N(R3)C(o)R3,
-C(oJN(R3)2; halogen, -C(o)R3, nitro, hydro~y, substituted or
unsubstituted saturated monocycllc or polycyclic heterocyclic
25 rings, subst~tuted or unsubstituted saturated monocyclic or
polyc~clic carbocycllc rtngs.
Also, tn th~s general structure, the quaternary nitrogen
¦ ~to~ ts l~nked to the phosphonic acid carbon by a linking chain.
I Further, ~n th~s general structure, n, which is an inteqer from
1-10, represents sa~d linking chain.
Said l1nklng chain members are selected from a vartety o~ R5
moietles. R5 c-n be -sR6, -R9SR6, hydrogen, C1-Cg alkyl, -oR3,
-Co2R3; -02CR3; ~NR32; -N(R3)C(o)R3, -C(o)N(R32); halogen,
-C(o)R3; nitro; hydrogen; unsubst~tuted or substituted saturated
35 monocyclic or polycycllc heterocyclic ring, unsubstituted or
substitutet saturated ~onocyclic or polycyclic carbocyclic rings,
unsubstituted or subst~tuted unsaturated monocyclic or polycyclic
2136819
WO 93/24494 PCI'/US93/04K9
. ~
-15-
heterocyclic rings, unsubstituted or substituted unsaturated
monocyclic or. polyoyclic oanbocyclic rings and combinations
thereof.
Finally, in the quaternary nitrogen containing phosphonate
compounds of the present invention, R can be -COOH, -S03H~ -P03H2
and -P(o)(oH)R4 where R4 is Cl-Cg alkyl. Preferred R is P03H2
and P(o)(oH)R4. R8 is a substituent on the phosphonic acid
containing carbon selected from hydrogen, halogen, SR6, R9,SR6,
amino, hydroxy, substituted and unsubstituted Cl-Cg alkyl.
Preferred R8 is hydroxy, halogen and amino. R2 is substituted or
unsubstituted Cl-C3s alkyl7 substituted or unsubstituted phenyl,
benzyl; or R9SR6. Preferred R2 is subst~tuted or unsubst~tuted
Cl-Cg alkyl and R9SR6.
Preferred quaternary nitrogen-conta~ning phosphonates having
an Rl moiety selected from the Rl mo~eties described herein
before include,
CH3
20CH3(cH2)3cH2 Nl ~ O~H2
CH3 PO3H2
N-(3-hydroxy-3,3-diphosphonopropyl)-N,N-dimethyl-N-pentylammonium
chlorlde
CH
CH3 - N ~ CNH a
IH p~3Hk
30 v 3
N-(4-hydroxy-4,4-diphosphonobutyl)-N,N,N-trialkylammonium salt
WO 93/24494 P~/US93J0446
2~,36~9 - 16-
Ct13
CH3 1 f--OH Cl
CH3 P3H2
N-(3-hydroxy-3,3-diphosphonopropyl)-N,N,N-trialkylammonium salts
Also, in this general structure, the Rl moiety can also be
saturated monocyclic or polycycl~c heterocycle.
Preferred quaternary n1trogen-containing phosphonates having
a saturated monocyclic or polycyclic heterocycle as an Rl moiety
wherein the quaternary nitrogen atom is linked Yia a linking
chain to the phosphon~c acid carbon include:
¦ 20 N N ~ OH a
CH P(O)(OHl2
N-(3-hydroxy-3,3-diphosphonopropyl)-N,N-dtmethyl-N-(2-piperidine-
methyl) ammonium chloride
Add~t~on~lly, preferred compounds of the present inYention
include thsse co~pounds having ths following structures:
R2
R2- N+ ~f 03H2
R2 P03H2
--- ` WO 93/24494 2 I 3 6 81 g PCI`/US93/04469
-17-
R2 + I ~f 03H2
1 2 P3H2
Preferred compounds of the present inventisn also include ~he
thio-subst~tuted quaternary nitrogen eontaining phosphonates.
R2 R2
R3SR9 - N+ ~ O3H2 R5SR9 - N ~ po~3H2
~2 PO3H2 ~2 po3H2
.
._
R ~ 2 + ~ H2
Spec~f~c ~x2mples of compounds of the present inYention
include:
N^(4-hydroxy-4,4-diphosphonobutyl)-N,N~N-trimethyl ammonium
iod~de;
N-(3~hydro%y-3,3-diphosphonopropyl)-N,N-dimethyl-N-pentyl
ammon~um iodide;
1 35 N-(3-hydroxy-3,3-diphosphonopropyl)-N,N,N-trimethyl ammonium
i 10dide;
N-cycloheptyl-N,N-d~methyl-N-(diphosphonomethyl) ammonium iodide;
w o 93/24494 P ~ /v~s3/044Ks
3 6 ~ ~ g -l8-
N-(2-acetylthioethyl)-N-(4-hydroxy-4,4-diphosphonobutyl)-N,N-di-
methyl ammonium bromide;
N-(~-acetylthioethyl)-N-(3-hydroxy-3,3-diphosphonopropyl)-N-meth-
yl-N-pentyl ammonium bromide;
N-(4-hydroxy-4,4-diphosphonobutyl)-N-(3-mercaptopropyl)-N,N-di-
methyl ammonium chloride;
N-(4-hydroxy-4,4-diphosphonobutyl)-N-(mercaptomethyl)-N,N-di-
methyl ammonium chloride;
N-(4-hydroxy-4,4-diphosphonobutyl)-N-(4-methoxybutyl)-N,N-di-
methyl ammonium chloride;
N-(4-hydroxy-2-mercapto-4,4-diphosphonobutyl)-N,N,N-trimethyl
ammonium chloride;
N-(4-hydroxy-2-acetylthio-4,4-diphosphonobutyl)-N,N,N-trimethyl
ammonium chloride;
lS N-(3-hydroxy-2-mercapto-3,3-diphosphonopropyl)-N,N-dimethyl-N-
pentyl ammonium chloride;
N-(3-hydroxy-2-acetylthio-3,3-diphosphonopropyl)-N,N-dimethyl-N-
pentyl ammonium chloride;
N-(3-hydroxy-3,3-diphosphonopropyl)-N-methyl-N-pentyl-N-(2-(3-
pyridyl)ethyl) ammonium chlor~de;N-cycloheptyl-N-(2-mercaptoethyl) N-methyl-N-(diphosphonomethyl~
ammonium chlor~te;
N-cycloheptyl-N-(mercaptomethyl)-N-methyl-N-(d~phosphonomethyl)
ammonium chlorlde;
~5 N,N-d~methyl-N-(~,~-d~phosphonobutyl)-N-(2-(3-piperidinyl)ethylJ
am~on1u~ chlorlde;
In order to determine and assess pharmacological activity,
test1ng of th~ phosphonate compounds in animals ~s carr~ed out
using var~ous assays known to those skilled in the art. Thus,
the In vivo bone ant~resorptive actlvlty may be conveniently
demonstrated us~ng an assay designed to test the ability of these
compounds to inhibit the resorpt~on of bone, which bone re-
sorption is charactertstic of abnormal calc~um and phosphate
metabolism. One such test known to the art is the Schenk model.
35 Another useful art-known test is the adjuvant arthritis test.
Also useful is the in vitro hydroxyapatite crystal growth
WO 93~24494 ~13 6 819 PCI~/U~i93/04469
,,
..
-19-
inhibition test. Th~se and other appropriate tests for
pharmacological activity are disclosed and/or referred to in
Shinoda et al., Calcified Tissue International. 35, pp 87-99
(1983); Schenk et al. , Calcified Tissue Research. 11. pp 196-214
(1973); Russell et al., ~alcified Tiss~e R~earch. 6, pp 183-196
(1970); Muhlbauer and Fleisch, Mineral Electrolvte Metab. , ~ ,
pp 296-303 (1981); Nancollas et al., Oral ~iol., 1~. 731 (1970);
U.S. Patent 3,683,080, to Francis, issued August 8, 1972; U. S.
Patent 4,134,969, to Schmidt-Dunker, I~sued January 16, 1979; and
EPO Patent Application Publication No. 189,662, publlshed August
6, 1986; the disclosures of all these articles and patent
specifications being incorporated herein by reference in their
entirety. Certain of these tests for pharmacological activity
are also described in more deta~l in the Examples provided
15 hereinafter.
In addition to being useful for treating or preventing
pathological cond~tions characterized by abnormal calcium or
phosphate metabol~sm, the compounds of the present invention may
have other uses. For example, the compounds of the present
inventlon are bel~eved to be useful as bone scanning agents after
labeling with 99m-technetlum. In addltion, the compounds of the
-' pr~sent inventlon are useful as sequestering agents forpolyvalent metal ~ons, partlcularly dl-(e.g. calcium and
magnesium) and trlvalent (e.g. ind~um) ~etal ions. Thus, the
co~pounds of the present inventlon are useful as builders in
detergents and cleansers! or for treating water. They are also
useful as stablllzers for compounds. In addit~on, they may be
useful tn preventlng the formatlon of tartar (l.e., calculus)
and/or plaque on teeth~ Finally, the compounds of the present
inventlon may be useful as herbicldes which are non-toxlc to
animals.
- ~he quaternary nltrogen-contalning phosphonates to be
includad ln the pharmaceutlcal compositions of the present
i m ention can be made according to the following non-limiting
35 Examples 1 to S.
.
WO 93/244g4 PCI/U~i93/04K9
~ ~68fL9 -20-
Compositions Conta;ninq Novel_~uaternarv Nitroqen Containinq
Phos~onate Compounds
The novel quaternary nitrogen-containing phosphonate
compounds of the present invention may be administered to humans
or other mammals by a variety of routes, including, but not
limited to, oral dosage forms and injections (intravenous,
intramuscular, intraperitoneal and subcutaneous). Numerous other
dosage forms containing the novel quaternary nitrogen-containing
phosphonate compounds of the present invention can be readily
formùlated by one skilled in the art, utilizing the suitable
pharmaceutical excipients as defined below. For considerations
of patient compliance, oral dosage forms are generally most
preferred.
The term ~pharmaceutical compos~tionN as used herein means a
combination comprised of a safe and effective amount of the
quaternary nitrogen-containing phosphonate compound active
ingredlent, or mixtures thereof, and pharmaceut k ally-acceptable
excipients.
The phrase ~safe and effect~ve amountU~ as used herein,
means an amount of a compound or composition large enough to
signlficantly posittvely modtfy the symptoms and/or cond~t10n to
be treated, but small enough to avoid serious s~de effects (at a
reasonable benefit/risk ratio), with~n th~ scope of sound medical
judgment. The safe and effective amount of active lngredient for
use in the pharmaceutical composit~ons to be used in the method
of the lnvent10n here~n will vary with the particular condition
be~ng tre~ted, the age-and physical condit10n of the patient
be1ng tre~ted, the severity of the condttion, the duration of the
treatment~ the nature of concurrent therapy, the part~cular
act~ve ingredtent being employed, the particular
pharmaceutically-acceptable excipients utilized, and like factors
w1thin the knowledge and expertise of the attending physician.
The term ~pharmaceutically-acceptable excipients~ as used
herein includes any physiologically inert, pharmacologically
inactive material known to one skilled in the art, which is
compatible with the physical and chemical characteristics of the
WO 93/24494 2 1 3 6 8 1 9 PCI/U593/04469
-21- `
particular qua~ernary nitrogen-containing phosphonate compound
active ingredient selected for use. Pharmaceutically-acceptable
excipients include, but are not limited to, polymers, resins,
plasticizers, fillers; binders, lubricants, glîdants,
disintegrants, solvents, co-solvents, buffer systems,
surfactants, preservatives, sweetening agents, flaYoring agents,
pharmaceutical grade dyes or pigments, and viscosity agents.
The term ~oral dosage form" as used herein means any
pharmaceutical composit1On intended to be systemically
administered to an indiv~dual by delivering said composition to
the gastrointest1nal tract of an individual, via the mouth of
said individual. For purposes of the present invention, the
delivered form can be in the form of a tablet, coated or
non-coated; solution; suspension; or a capsule, coated or
non-coated.
The term ~injection~ as used herein means any pharmaceutical
composition intended to be systemically administered to a human
or other mammal, via delivery of a soluti.on or emulsion
containing the act~ve ingredient, by punctur~ng the skin of said
ind1vidual, in order to del~ver sa~d solution or emulsion to the
. circulatory system of the intivldual either by intravenous,
intramuscular, ~ntraperitoneal or subcutaneous in~ect~on.
j The rate of syste~c deltvery can be satlsfactorily
controlled by one skilled in the art, by manipulating any one or
mcre of the follow1ng:.
(a) the act1ve ~ngredlent proper;
(b) the ph~rmaceutically-acceptable exc~p~ents; so long as
the variants do not interfere in the activ~ty of the particular
act~ve ingredicnt selected;
(c) the type of the excipient, and the concomitant
desirable thickness and permeabil~ty (swelling properties) of
sa~d excipients;
(d) the t~me-dependent conditions of the excipient itself
and/or w1th~n the exc~pients;
(e~ the part~cle size of the granulated active ingredient;
and
WO 93~24494 PCI`/US93/04469 `-
2~36Qo~9 -22-
(f) the pH-dependent conditions of the excipients.
In particular, the solubility, acidity, and susceptibility
to hydrolysis of the different quaternary non-ring
nitrogen-containing phosphonate active ingredients, such as acid
addition salts, salts formed with the carboxylic group, e.g.,
alkali metal salts, alkaline earth metal salts, etc., and esters,
e.g., alkyl, aryl, aralkyl, may be used as guidelines for the
proper choice. In addition, suitable pH-conditions might be
established within the oral dosage forms by adding a suitable
buffer to the active ingredient in accordance with the desired
release pattern.
As stated hereinabove, pharmaceutically-acceptable
excipients include, but are not limited to, resins, fillers,
binders, lubr~cants, solvents, glidants, disintegrants
cosolvents, surfactants, preservatives, sweetener agents,
flavoring agents, buffer systems, pharmaceutical-grade dyes or
pigments, and vtscosity agents.
The preferred solvent ts water.
Flavoring agents among those useful herein include those
described tn Rem1naton's Pharmaceutical Sclences, 18th Edition~
Mack Publtshtng Cumpany, 1990, pp. 1288-1300, incorporated by
reference heretn. The pharmaceut k al compostttons suttable for
i use herein gener~lly contain fro~ 0-~% flavor1ng agents.
Dyes or ptgments among those useful heretn tnclude those
descr~bed tn ~n::~9~ L l lr aceutlcal Exci~içn~s, pp. 81-90,
1986 by the American Pharmaceuttcal Assoctatton ~ the
Ph~re~ceut1cal Soclety of Great Brtta~n, tncorporated by
reference heretn. The pharmaceuttcal compositions herein
generally contatn from 0-Z% dyes or ptgments.
Preferred co-solvents tnclude, but are not ltmtted to,
ethanol, glycer~n, propylene glycol, polyethylene glycols. The
¦ pharmaceuttcal composttlons of the present tnvent~on tnclude from
0-5~% co-solvents.
Preferret buffer systems include, but are not ltmited to,
acettc, bortc, carbontc, phosphor~c, succintc, malaic, tartaric,
citric, acet1c, benzoic, lactic, glyceric, gluconic, glutaric and
WO 93/24494 2 1 3 6 8 1 9 PCT/US93/04469
-23-
glutamic acids and their sodiu~, potassium and ammonium salts.
Particularly preferred are phosphoric, tartaric, citric, and
acetic acids and salts. The pharmaceutical composition of the
present invention generally contain from 0-5% buffer systems.
Preferred surfactants include, but are not limited to,
polyoxyethylene sorbitan fatty acid esters, polyoxyethylene
monoalkyl ethers, sucrose monoesters and lanolin esters and
ethers, alkyl sulfate salts, sodium, potassium, and ammonium
salts of fatty acids. The pharmaceutical compositions of the
present invention include 0-2% surfactants.
Preferred preservatives include, but are not limited to,
phenol, alkyl esters of parahydroxybenzoic acid? o-phenylphenol
benzoic acid and ~he salts thereof, boric acid and the salts
thereof, sorbic acid and the salts thereof, chlorobutanol, benzyl
alcohol, thimerosal, phenylmercuric acetate and nitrate,
nitromersol, benzalkonium chloride, cetylpyridinium chloride,
methyl paraben, and propyl paraben. Particularly preferred are
the salts of benzoic acid, cetylpyridinium chloride, methyl
paraben and propyl paraben. The compositions of the present
invention generally include from 0-2% preservat~ves.
Preferred sweeteners include, but are not limited to,
sucrose, glucose, saccharin, sorbitol, mannitol, and aspartame.
Particularly preferred are sucrose and saccharin. Pharmaceutical
compositions of the present invention include 0-5% sweeteners.
Preferred viscosity agents include, but are not limited to,
~ethylcellulose, sodium carboxymethylcellulose, hydroxypropyl-
~eth~lcellulose, hydroxypropylcellulose, sodium alginate,
carbo~er, povidone, acacia, guar gum, xanthan gum and tragacanth.
Particularly preferred are methylcellulose, carbomer, xanthan
gum, guar gum, povtdone, sodium carboxymethylcellulose, and
magnes~um aluminum silicate. Compos~tions of the present
invention include 0-SX viscosity agents.
Pr~ferred fillers include, but are not limited to, lactose,
mannitol, sorbitol, tribasic calcium phosphate, dibasic calcium
phosphate, compressible sugar, starch, calcium sulfate, dextro
WO 93/24494 PCI'/US93/04"69
2,13683~ -24-
and microcrystalline cellulose. The compositions of the present
invention contain from 0-75X fillers.
Preferred lubricants include, but are not limited to,
magnesium stearate, stearic acid, and talc. ~he pharmaceutical
compositions of the present invention include 0.5-27. lubricants.
Preferred glidants include, but are not limited to, talc and
colloidal silicon dioxide. The compositions of ~he present
invention include from 1-5% glidants.
Preferred disintegrants include, but are not limited to,
starch, sodium starch glycolate, crospovidona, croscarmelose
sodium, and microcrystalline cellulose. The pharmaceutical
compositions of the present invention include from 4-15%
disintegrants.
Preferred binders include, but are not limited to, acacia,
tragacanth, hydroxypropylcellulose, pregelatinized starch,
gelatln, povidone, hydroxypropylcellulose, hydroxypropyl-
methylcellulose, methylcellulose, sugar solutions, such as
sucrose and sorbitol, and ethylcellulose. The compositions of
the present inventlon include l-lOX binders.
Compounds of the present invention may comprise from about
0.1% to about 99.9X by weight of the pharmaceutical compositions
of the present invention.
Preferably the compounds of the present Invention comprise
from about 20X to about 80% by weight of the pharmaceutical
co~pos~t~ons o~ the present invention.
Accordlngly, the pharmaceutical compositions of the present
~nventlon lnclude from lS-95% of a quaternary n~tro~en-
conta~nlng phosphonate compound active ingredlent, or mixture,
thereof; 0-2% n avoring agents; 0-5~% co-solvents; 0-5% buffer
system; 0-2% surfactants; 0-2% preservatives; 0-5X sweeteners;
0-5% viscoslty agents; 0-75% fillers; 0.5-2% lubricants; 1-5X
glidants; ~-15X dlsintegrants; and 1-10% binders.
Su1table pharmaceutlcal compostt~ons are described herein in
Examples 9 to 11. It is well within the capabilities of one
skilled in the art to vary the non-limiting examples described
herein to ach~e~e a broad range of pharmaceutical compositions.
WO 93/24494 ~ 1 3 6 8 1 9 PCI/US93/04469
-25- `
The choice of a pharmaceutical excipient to be used in con-
junction with the quaternary nitrogen-containing phosphonate
compounds of the present compositions is basically determined by
the way the phosphonate is to be administered. If the compound
is to be injected, the preferred pharmaceut kal carrier is
sterile, physiological saline, the pH of which has been adjusted
to about 7.4. However, the preferred mode of administering the
phosphonates of the present invention is orally, and the
preferred unit dosage form is therefore tablets, capsules and the
like, comprising from about 0.1 mg P to about 600 mg P of the
phosphonic acid compounds described herein. Pharmaceutical
carriers suitable for the preparation of unit dosage forms for
oral administration are well known in the art. Their selection
will depend on secondary considerations like taste, cost, and
1~ shelf stability, which are not critical for the purposes of the
present Invention, and can be made without d~fficulty by a person
skilled in the art.
The term ~mg P~, as used herein, means the weight of the
phosphorus atoms present in an amount of a phosphonic acid
compound of the present invention. This unit is used to
standardlze the amount of the phosphon1c ac~d compounds of the
present invent~on to be used in the pharmaceutical compositions
and methods of the present inventions. For example,
N-(4-hydroxy-4,4-d~phosphonobutyl)-N,N-d~methyl-N-(2-mercaptoeth-
25 yl)-ammonium chlorlde has a molecular weight of 373.5 g/mole, of
whtch 17% ( 62 g/mole) is due to the two phosphorus atoms present
~n this molecule. One milligram of this sompound is therefore
c~lculated to have 0.17 mg P. Thus, to prepare a pharmaceutical
compos~tlon containing 0.17 mg P of th~s compound, the
3~ compos~t~on should contain l ~9 of the compound; and to dose 0.17
mg P/kg of th~s compound to a 50 kg pat1ent, the pat~ent would be
dosed w~th 50 mg of this compound.
The pharmaceutically-acceptable excipient employed in con-
junction w1th the diphosphonates of the present invention is used
35 at a concentrat~on suffic~ent to prov~de a practical size to
WO 93/24494 PCI'/US93/044~9
~36S~ dosage relationship. Preferably, the pharmaceutically-acceptable
carriers, in total, may co~prise from about 0.1% to about 99.97.
by weight of the total composition and ~ore preferably from about
~0% to about 80%.
'
Method for Treatinq or Preventinq Diseases Characteri~ed bY
Abnormal Calcium and Phos~ha~e Metabolism
Another aspect of the present invention is methods for
treating or preventing diseases characteri~ed by abnormal calcium
and phosphate metabolism. Such methods comprise administering to
a human or lower animal in need of such treatment a safe and
effective amount of phosphonate compound of the present
invention.
The preferred mode of administration is oral, but other
known methods of administration are contemplated as well 7 e.g.,
dermatomucosally (for example, dermally, rectally and the 1ike)
and parenterally (for example, by subcutaneous injection,
intramuscular injection, intra-articular injection, intravenous
injection and the like). Inhalation is also included. Thus,
specific modes of administratton tnclude, without limitation,
oral~ transdermal, mucosal, sublingual, intramuscular,
intravenous, intraperitoneal, and subcutaneous administration, as
well as topic~l application.
The ten~ ~abnormal calcium and phosphate metabolism", as
used herein, means (l) conditions which are characterized by
ano~lous mobili~ation of calclum and phosphate leading to
general or spec~fic bone loss, or excessively high calc1um and
phosphate levels in the fluids of the body; and (2) conditions
which cause or result from deposition of calciu~ and phosphate
anomalously in the body. The first category includes, but is not
limited to, osteoporosts, Paget's disease, hyperparathyroidism,
hypercalcemia of malignancy, heterotoptc ossification, and
osteolytic bone metastases. The second category includes, but is
not limited to, myos~tis oss~ficans progress~va, calcinosis
universalis, and such afflictions as arthritis, osteoarthritis,
WO g3/24494 ~ 6 819 PC~/IJS93J04469
-27-
neuritis, bursitis, tendonitis and other inflammatory conditions
which predispose involved tissue to deposition of calcium and
phosphate.
The term "rheumatoid arthritis" as used herein, means a
S chronic systemic and articular inflammatory disorder of unknown
etiology. It is characterized by destruction of articular
cartilage, ligaments, tendons, and bone.
The term ~osteoarthritis" as used herein, means a
non-inflammatory disorder of the movable joints. It is
character ked by deterioration and abrasion of the articular
cartilage; and new bone formation at the joint surface.
The terms "person at risk~ and "person in need of such
treatmentn, as used herein, mean any human or lower animal which
suffers a signiflcant risk of abnormal calcium and phosphate
metabolism if left untreated, and any human or lower animal
diagnosed as being afflicted with abnormal calcium and phosphate
metabolism. For axample, postmenopausal women; persons
undergoing certain steroid therapy; persons on certain
anti-convulsant drugs; persons diagnosed as having Pa~et's
disease, hyperparathyroidism, hypercalcemia of malignancy, or
osteolyt k bone metastases; persons diagnosed as suffering from
one or more of .the varlous fonms of osteoporosis; persons
belonging to a populatlon ~roup known to have a significantly
higher than average chance of dev@loping osteoporosis, e.g.,
postmenopausal. wo~en, men over age 65, and persons being treated
~1th drugs known to cause osteoporosis as a side effect;
persons dlagnosed as suffering from myositis ossificans
progressiva or calc1nosis universalis; and persons affltcted ~ith
arthrttls, osteoarthrltis, neurltis, bursitis, tendonitis and
other inflammatory condltlons whtch predtspose ~nvolved tissue to
depositlon of calclu~ and phosphate.
The phrase ~safe and effective amount~, as used herein,
means ~n amount of a compound or composltton of the present
inventton high enough to significantly positively modify the
35 cond~tton to b~ treated, but low enough to avo~d serious side
effects (at a reasonable benefit/risk ratio), within the scope of
y` ~ :
WO 93/24494 PCr/US93/04469
-28-
2 ~ 3 ~ a ~ sound medical judgment. The safe and effective amount of,
phosphonate compounds of the present inventian will vary with
the particular condition being treated, the age and physical
condition of the patient being treated, the severity of the
¦ 5 condition, the duration of ~ the treatment, the nature of
concurrent therapy, the s~ecific phosphonate employed, the
particular pharma~euticallytàcceptable carrier utilized, and like
factors within the knowledge and expertise of the attending
physician. However, single dosages can range from O.Ol mg P to
~500 mg P, or from 0.0002 to 70 mg P/kg of body weight (based on
a body weight of 50 kg). Preferred single dosages are from 1 mg
P to 600 mg P, or from 0.02 to 12 9 P/kg of body weight (based on
a body welght of 50 kg). Up to four slngle dosages per day may
be administered. Daily dosages greater than 500 mg P/kg are not
required to produce the desired effect and may produce
undesirable stde effects. The hlgher dosages wlthin this range
are, of course, required in the case of oral admlnistration
because of limlted absorption.
The followlng Examples further describe and demonstrate the
preferred embodiments wlth1n the scope of the present lnventton.
The Examples are gtven solely for the purpose of ~llustration,
and are not to be construed as limitations of the present
inventton since many varlatlons thereof are poss~ble without
departing from ~ts splrit and scope.
ExamDle 1
Svnthesis of N-(4-hvdroxY-4.4-d~DhosDhonobutYl)-
- N.N.N-tr~methvl ammon~um iod~de
CH,--~,OHH,H2 r
w o 93/244~4~ I 3 ~ 8 1 ~ P ~ /US93/04469
-29-
I. Svnthesis o~_r4-(N.N-dimethvlamino~ hvdroxY
butYlidenelbis~Phosphonic acidl
A solution containing 4-(N N-dimethylamino) butanoic acid
(2.9 mmol) phosphorus trichloride (2.0 mmol) and
diethylphosphite (12 mmol) is stirred 30 minutes at room
temperature and then heated at 60 C for 24 hours. The reaction
mixture is then cooled to room temperature and concentrated
hydrochloric actd (iO ml) is added. The reaction mixture is then
heated an add1tional 24 hours at reflux; then cooled to room
temperature and filtered through celite and the filtrate is
concentrated under vacuum. The crude product is trlturated in
ethanol collected by filtration and then dried under vacuum.
II. SYnthesls of N-(4-hvdroxy-4~4-diDhosDhQnobutyl)-N~N~N-t
methvl ammon~um ~odlde
The blsphosphonic acld (0.30 mmol) is dissolved in water (10
ml) and eth~no~ (15 ml) and the pH ~s ad~usted to 7.0 by the
add~tion of lN NaOH. To thls ~s added methyl iod~de (1.50 mmol)
and the reactlon ~s heated at reflux for 24 hours. The mixture
is then cooled and concentrated under reduced pressure. The
solid res1due ts dlssolved ln a minimu~ amount of water and the
quatern1zed product is prec~pltated by the add~tlon of
~soprop~nol. The product is collected by flltratlon rinsed with
~cetone ~nd then further drled under vacuum.
ExamDle 2
Svnthesls of N-(3-hYdroxY~-3.3-dlDhosDhonoDroDYl)-
30-N.N-dlmethvl-N-Dentvl a~monlum iodlde
ctla
CH3(C~3CH2~ + ~PHH2 r
WO 93/24494 PCI/US93/04469
2136~Ig ~o-
I. Svnthesis of ~3-(N,N-dimethvlamino!~roPYlidenelbis~Dhos-
ahonic acidl
Using essentially the same procedure as described ln
Example 1, part I, hereinbefore, 3-(N,N-dimethylamino) propanoic
acid is converted to [3,-(N,N-dimethylamino)propylidene]bls
[phosphonie ac~d].
II. S~nthes~s of N-(3-h~droxv-3~-d~Dhosphono~roDyl~-N N-di-
methvl-N-DentYl ammonium .iodide
The bisphosphonic ac~d (0.50 mmol) is dissolved in water (15
ml) and aceton~trile (20 ml) and the pH is ad~usted to 7.0 by the
add~tion of lN NaOH. To th~s is added pentyllodide (2.50 mmol)
and the react~on mixture is heated at reflux for 22 hours. The
mixture is then concentrated under reduced pressure and the solld
res~due ~s tr~turated in acetone. The product can then be
. recrystall~zed from water and ethanol.
ExamDle 3
Svnthe~ls of N-~3-hvdroxy ~3-dlDhosDhQDo~roDyl)-
` N.N~N-trtmethylammon1um ~od~d~
r~ 25 CW3 N~ POHH2 r
CH3 P3H2
~, ~
Uslng essentlally the same procedure as descr~bed ~n
~ Example 1, part II, here1nbefore, ~3-(N,N-d~meth~lam~no)pro-
!` pyl ~dene~bls~phosphon k acld], prepared as descr~bed in
Example 2, part I, here~nbefore, ls converted to
,~.
w o 93/24494 P ~ /US93~04469
~136819
N-(3-hydroxy-3,3-diphosphonopropyl~-N,N,N-trimethylammonium
iodide.
f~3m~
¦ Svnthesis of N-t2-ac~ylthioethYl~-N (4-hYdroxv-4~4-
dlDhosphonobutyl)-N~N-dimethYl ammonium bromide
.......
CH3C(O)SCH2cH2 ~ N ~ 2 B~-
, -
s 15 [4-(N,N-dlmethylamlno)~1-hydroxybutylldene]b~s~phosphon k
acid] (0.75 mmol), prepared as described ~n Example 1, part I,
hereinbefore, ls dissolved ~n water (50 ml) and acetonitrile (35
ml). To thls ~s added S-acetyl-2-bromoethanethiol ~3.75 mmol)
and the react~on m~xture ~s heated at reflux for 12 hours. The
m~xture is then concentrated under reduced pressure and the solid
res1due 1s trlturatet ln acetone. The quaternized product can be
:r,~ recrystalllzed fro~ water and ethanol.
ExamDle 5
SYnthesis of N-(2-acetvlthioetbY1~
(3-hvdr~xv-3.3-d1DhosDhonoDroDYl) N-methY1-N-
. DentYl ~TmQnlum brQm~de
C~-13
CJ~3(C~3CH2--N f PO3H2
CH3c(o)s J F~3H~
I. Svn.thesls of r3~(N-methvl-N-~entYlani~Q)oroDYlidenelbis-
osDhonic ac~dl
~,
WO 93/24494 Pcr/US93/04469
~,~36a~g -32-
Using essentially the same procedure as described in Example
1 part I hereinbefore 3-(N-methyl-N-pentylamino)propanoic acid
is converted to [3-(N-methyl-N-pentylamino)propylidene]bis[phos-
phonio acid].
s
II~ Svnthesis of N-(2-acetvlthioethvl)-N-(3-hvdroxY-3~3-diDhos-
pho~oproDvl!-N-methyl-~-Dent~l ammonium bromide
IUsing essentially the same procedure as described in
¦IO Example 4 hereinbefore ~3-(N-methyl-H-pentylamino)prcpylidene]-
Ibis~phosphonic acid] is converted to N-(2-acetylthioethyl)-N-(3-
hydroxy-3 3-dtphosphonapropyl)-N-methyl-N-pentyl ammonium
bromide.
ExamDle 6
Schenk Model
The compounds are evaluated for ~n ViVQ bone resorption
inhibition and mineralization tnhibttion in an animal model
system known in the f1eld of bone metabolism as the Schenk Model.
The general pr1nc1ples of this model system are d1sclosed in
Shinoda et al. Cale1~. Tissue lnt. ~ 87-99 (1983); and in
Schenk et al., Calcif. Tissue Res~ 11 , 196-21~ (1973), the
dtsclosures of wh1ch are incorporated here~n by reference.
Mater~als ~nd Methods:
~n~ls
Pre~ean~ng 17-day-old (30 gms) male Sprague Dawley rats
(Ch~rles R1ver Breeding Laborator1es) are sh1pped w~th thetr
~others and placed 1n plasttc cages w1th the~r mothers upon
arr~v~l. At 19 days of age pups rece1v1ng Rat Chow and water ~g
lit~um are randoml~ ~llocated ~nto treatment or control groups
compr~s~ng seven anl~als per group. On day 1 and agatn on day 7
all an1m~1s are 91ven an lntraperltoneal (~IP~) ln~ection of
Calcetn (lX solut10n 1n O.g% saltne solution; dosed at 0.2 ml~100
g body we19ht~. On day ~ all antmals are gtven an IP in~ectton
of tetracycl1ne hydrochloride (1% solution in O.S% saline
~.,.
,.~
,~
, .
, . .
WO 93/24494 ~ 1 3 6 8 1 9 PCI/US93/04469
-33- ,
solution; dosed at 0.2 ml/100 g body weight). These co~pounds
label actively mineralizing bone and cartilage.
Dose Solutions and Dosing Procedure
All solutions are prepared for subcutaneous injection in
5 0.~% normal saline and adjusted to pH 7.4 using NaOH and/or HCI.
Dose solution calculation is made by considering the mass of
powder (based on molecular weight, hydration~ of the active
material in mg/kg (body weight) that corresponds to mgp/kg.
Concentrations are bascd on dosing 0.2 ml/100 9 body weight.
Typically, all compounds are administered at 0.01, 0.1, 1.0 and
10.0 mg P/kg/day for 7 days. Compounds showing activity at 0.1
mg P/kg/day are then tested at logarithmic deorements down to
O . 001 mg P/kg/day . Adjustments in dosage based on changes in
body weight are made on a daily basis.
NecroDsY. Tissue Pro~essinq and Hi$tomQr~home~r~
On day 8 after the start of dosing, all animal s ar~
sacrificed by IP overdose of pentabarbitol. Tibias are dissected
free and placed in 70X ethyl alcohol. One tibia is dehydrated in
graded ethanol solutions and embedded in methyl methacrylate as
described in Schenk, Methods of Calcified Tissue PreDaration
(G.R. D k kson, Editor; Elsev~er Sctence Publ., The Netherlands;
1984), the dlsclosures of which are incorporated herein by
re~erence in the~r enttrety. The tibia is sectioned
long~tud~n~ through the metaphyseal area. Specimens are
sta1ned on one surface with silver nitrate and mounted on
~croscope sl~des for evaluation with a Quantimet I~ge Analy2er
(Ca~br1dge Instru~ents, Inc.) us~ng both incandescent and
ultraviolet 111umination. Metaphyseal trabecular bone content is
measured in the reqlon bet~een the fluorescent label and the
growth plate: expressed as percent of total area (bone + marrow).
Epiphyseal gro~th plate w~dth is obtained as the mean value of 10
equalli-spaced measurements across the section.
Stat1stical evaluat~on of data is made using parametric and
non-parametric analysis of variance and ~lcoxons rank sum test
to determine 2 stat~st~cally significant effect compared to
WO 93/24494 PCr/US93/04469
36a~9 -34-
control animals. ~he Schenk model provides data for ln vivo bone
resorption inhibition by the compounds.
~ample 7
Ad.iuvant Arthritis Model
There are numerous animal models of arthritis, among these
is adjuvant-induced arthritis using ~ycobact eri um butyr7 cum .
This model in a number of ways mimics rheumatoid arthritis in the
human (joint swelling associated with cellular and pannus
invasion af the joint space, bone resorption, and release of
chemotaxic factors and lysosomal constituents into the joint
space) (1,2). A number of prophylactic and ~herapeutic studies
have indicated the potential use of anti-infla~matory drugs (3,4)
and diphosphonates in arthritis (5,6).
REFERENCES
1. Pearson, C., ~ood F. (1959), Studies of Polyarthritis and
Other Lesions Induced by Injection of Mycobacterial
Adjuvant. 1. General Clinical and Pathologtcal
~ Characteristlcs and Some Modifying Factors, Arth. Rheum.,
2:440-~5g.
2. Blacb~an, A., Burns, J.~., Framer, J.B., Radziwonik, H.,
~estwlck, J. (1977), An X-ray Analysis of Adjuvant Arthritis
in the Rat. The Effect of Predn~solone and Indomethacin,
ent~ and Actlons, 7 145-151.
3. ~lnter, C.A., Nuss, G.~. (1966), Treatment of AdjuYant
Arthr1t~s in Rats wtth Ant1-lnflammatory Drugs, Arth.
Rheu~., 9:39~ ~0~.
¦ 4. ~lnder, C.Y., Lembke, L.A., Stephens, M.D. (1969),
Comparative Bloassay of Drugs in Ad~uvant-Induced Arthritis
in Rats: Flufenam1c Acld, Mefen~mic Acid, and
Phenylbutazone, Qrth. Rheum., 12:472-482.
5. Francis, M.D., flora, L. King, W.R. (1972), The Effects of
Disodlum Ethane-1-Hydroxy-1-Dlphosphonate on Adjuvant
Induced Arthrltis in Rats, Calcif. Tiss. Re~., 9:109-121.
,. .
W093/24494 ;~I3S819 PCItUS93/044~9
35-
6. Flora L. (1979) Comparative Antiinflammatory and Bone
Protective Effects of Two Diphosphonates in Adjuvant
Arthritis Arth. Rheum 22:340-346.
Adjuvant arthritis is a severe cellulitis and synovitis
induced in male rats (either Sprague Dawley or Lewis strain) by a
single subcutaneous (SC) ~njection of Mycobacterium butyricum
(8 mg/ml) in mineral oil on day 0. The compounds are dosed once
daily either orally (PO) or parenterally (SC) and can be tested
in either prophylactic (from day O) or therapeutic (from day 9 or
lO or 14) protocols. Antiarthritic efficacy can be measured as a
reduction in paw volume body weight loss bone loss or reactive
new bone formation compared to the saline~treated arthritic
controls. Treatment can be stopped and the "flare~ response
(rapid increase in inflammation) examined which indicates a
compound s ability to maintain efficacy.
Materials and Meth~d~
A. Animals
Animals used are male Lewis rats (LE~). On arrival the
rats are rando~zed by computer generated rando~ numbers and
placed in indlvidual wire suspended cages. Fsod and water are
administered ad 1ibitu~, throughout the entire study. Routine
care and ma1ntenance of the animals are perfsrmed according to
State and Federal regulat~ons. Each rat is identified with a
nu~ber placed 1n front of the cage and on the tail of the rat.
B. ExDer~mental Des1an
On day 1 body ~eights (B~) and hind paw volume ~(PV)
recorded by a mercury dtsplacement method using a pressure
transducer llnked into a co~puterl measurements are taken on all
rats. On day 0 the induction of arthr~tis using MFA
[~ycobacteri~ but~ricu~ (Mb) 4.4 mg/kg ln o~l] is as follows:
rats are anesthetlzed and receive a stngle SC injection of MFA at
the base of the ta~l under aseptic cond~tions.
Paw volumes and body weights are measured thereafter on
various days usually tw~ce a week. For the prophylact k
`
WO 93/24494 PCI`/US93/04469
36S~9 -36-
protocol, rats are randomly allocated into groups of 8-10 rats
and treatment begins on day O and continues daily until
termination. for the therapeutic protocol, the rats are
randomized into treatment groups of 8-10 rats according to their
PV on day 10. Dosing begins on day 10 and continues daily until
termination. For both protocols, animals are placed in shoe box
cages with deep bedding on or before day 10.
~osinq Solutions
For ComDounds Unl~kel~ to Oxidize
Drugs are weighed out on a calibrated balance and then mixed
with distilled water in a volumetric flask. The solution is
adjusted to pH ~.4 w~th O.lH NaOH. Then the solution is filtered
through a 0.45 ~m st-erile filter ~nto a ster~le storage
container. ~hen not in use, the solution is stored in the
refrtgerator.
For ComDounds Likelv to Ox~dke
Drugs are wetghed out on a calibrated balance and then m1xed
with deoxygenated water ~n a volumetric flask. The stock
solution 1s f11tered through a 0.45 ~m sterile f11ter into a
ster~le storage contatner. ~hen not 1n use, the stock solut~on
is kept refr19erated.
On a dally bas~s, a spec~flc amount of solutton ~s removed
from the stock solutlon, put tnto small dosing beaker and then
ad~usted to pH 7.~ accordtng to a predetermined calculat10n.
Further d11ut~ons of the ad~usted solut10n can be made if
necess~r~ (wlth deoxygenated water).
Drug calculat10ns are made based on the molecular we~ght,
the purlty of the compound, the amount based on mg/kg (body
we19ht) and the deslred flnal concentrat10n ln ~gP/kg. The
volume dosed per rat ~s 0.1 ml/100 gm of body we~ght sub-
cutane~usly, 91ven as an 1njection in the ingu~nal fold of the
an~mal, alternat1ng s~des each day or 1 ml/200 gm B~ g~ven orally
us1ng a curved sta~nless steel dos1ng tube. At~ustments based on
changes ~n body we19ht are made weekly.
w o 93/2449~ ~ I 3 6 8 I 9 PCT/US93/04469
Radioqraphs NecropsY and Tissue Collection
At termination, each rat i5 sacrificed with l ml Socomb~
intraperitoneally (lP). Immediately a whole body radiograph is
taken by a Torrox 120D x-ray unit at MA=5, ISUP~50 and time=60
second on Kodak non-screen medical film. Hind legs are removed
from each rat and fixed in lOX buffered formalin along with a
piece of liver, kidney, spleen, and thimus. The tibiotarsal
joints are decalcified in 4X EDTA, pH 7.4 and processed routinely
in paraffin blocks and H+E sta~n. The organ parts also processed
in paraffin and stained H+E.
The histology sections are evaluated qualitatively for bone
and soft tissue lesions using ltght microscopy. Radiographs are
graded for bone resorption (BR) in 6 anatomical trabecular bone
sites in each hind leg and 4 sites in each front leg on a scale
15 of 0-3 giving an arbitrary score of 0-60 for all 4 legs. For
reacttve new bone formatton (RNB), radiographs are graded on a
sever~ty scale of 0-3 for the lateral and med kal surfaces of the
tibia and then 0-2 for all other areas mentioned above, giving an
arbitrary score of 0-44.
D. Statisttcal Analvsts:
Data analysts on paw volume, bone resorpt10n and react1ve
new bone for~atton ts performed by student's t-test and one-way
analysis of vartance wtth Tukeys (SAS) (12). Dtfferences are
constdered stgntftcant at p~0.05 cr less.
Thts ~cdel provides in vivo data for the efficacy of
ant1arthrtt~c compounds in terms of reducing paw swelling bone
loss and reacttve new bone formatton compared to the saltne
treated arthrit1c antmals.
Capsules are prepared having the following composition:
Acttve Ingredtent Mq Per ~aesule
35 N-(3-hydroxy-3,3-diphosphonopropyl)-
N,N,N-trtmethyl ammonium chloride 350.0
Z WO 93/24494 PCI`/US93/04469
i,6~9 -38-
Exci~ients
Lactose 90 0
Microcrystalline Cellulose 60.0
Magnesium Stearate l.0
S
The capsules having the above composition are prepared using
conventional methods as described below
The active ingredient ts mixed with the microcrystalline
cellu~ose in a turn shell blender for approximately ten (lO)
minutes.
The resulting mixture is passed through a hammer mill with
an 80 mesh screen.
~ he mixture is put back into the twin shell blender along
with the lactose and is then mixed for approximately fifteen ~15)
lS minutes.
The magnesium stearate is next added and blended for an
additlonal f~ve (5) minutes. The resulting blend is then
compressed on a piston-activated capsule filler.
Any of the compounds prepared according to Examples l to 5
may be substituted for the active ingred~ent in the capsule
prepared h~re1nabove.
ExamDle 9
T~blets are prepared having the following composition:
25 Acttve Inared1ent ~ r_Tablet
N-(4-h~droxy-~ ~-diphosphonobutyl)- 700.00
h-dlmetbyl-N-(2-mercaptoethyl)
ammon1u~ chloride
30 ExctDients
Lactose (spray-dried) 200.0
Starch (lS00) lO0.0
Magnes1u~ Stearate 25.0
Tablets are prepared having ~he above eompositiQn using
Z conventional methods as described below:
WO 93/24494 2 1 3 ~ 8 1 9 PCr/US93/o~9
-39-
The active ingredient ts ground in a ball mill for
approximately thirty (30) minutes. The milled active ingredient
is then blended in a twinblade mixer with the spray-dried lactose
for approximately twenty (20) minutes.
The starch is added to the mixture and is then mixed for an
additional fifteen (l5) ~1nutes. The blend is rompressed into
tablets on a standard tablet press.
Any of the compounds prepared accordlng to E%amples l to 5
may be substttuted for the act~ve ingred~ent in the tablet
prepared hereinabove.
amDle 10
Injectable solutions are prepared by conventlonal methods
using lO.O ml of physiolo~lcal saline solut~on and N-(4-hydroxy- -
4 4-d~phosphonobutyl)-N N N-trimethyl ammoniu~ chlortde
ad~usted to pH 7.~.
One in~ectton one ttme dally for 4 days results in
appreclable alleviatton of rheu~atoid arthritls in pattents
wetghtng approxi~ately 70 kllogra~s.
Any of the co~pounds prepared accordlng to Exa~ples l to 5
may be substltuted for the actlve tngredlent in the lnjectable
solutlon prep~red herelnabove.
ExamDle 11
A C~ucas~an ~ale welghlng approx1mately 92 kllograms
seventy-t~o ye~rs of age suffertng from ~oderate to severe pain
~nd occaslon~l swelllng of the rlght knee. After ~pproxtmately
o~e ~e~r of steadlly increastn~ dtscomfort he vis1ts ~ phys ktan
who renders a cltnlc~l d1agnos~s of osteoarthr~tts of the rtght
knee ~hlch was subsequently verlfled by X-ray dlagnosls.
Aftor a perlod of unelloratl~e therapy of var10us NSAIDs
lncludlng asptrln naprosen and ketoprofen hls symptoms
contlnue to worsen and hls condltlon appears to ~egenerate. He
returns to hls physlctan who then prescrlbes the tablets prepared
as descrl~ed in Exurple 9 twtce dally two hours befor~ or after
~eals for a perlod of three months. Hls cllnlcat symptoms of
WO ~3/244g4 PCI`/~JS93tO4469
36~9 -40-
pain and swelling, particularly with extended walking, improved
significantly after his 3 months of therapy. At the conclusion
of three months at a dosage of 2 tablets per day, the therapy is
continued at one-half the dosage or;ginally prescrlbed (i.e.
5 capsule, prepared as described in Example 8, per day)
indefinitely.
ExamDle l2
A black female, ~e19hing approximately 6~ kilograms,
fifty-five years of age, presents w~th swelling and deformation
of the finger joints of both hands, with partial loss of strength
and/or dexterity of her fingers and hands. Upon v1sual and X-ray
examinat10n and vartous appropr1ate cl1n1cal tests approved by
the American Rheumatolog1cal Assoc1at10n (ARA) she 1s dtagnosed
with rheumato1d arthrtt1s.
After an unsuccessful analges1c and ant1-1nflammatory
therapy, her phys k1an preser1bes the tablets prepared as
descr1bed ~n Example 9~ two t1~es da~ly two hours before or after
meals for a per10d of faur ~onths. After a month of therapy, her
symptoms of knuckle s~elling not1ceably 1mproves and her range of
finger mot10n tncre~ses s1gn1f1cantly; she cont1nues therapy for
the re~1nder of the four ~on~hs, after wh1ch her phys1c~an
cont1nues the prescr1bed dos- for an add1t10nal two ~onths.
A f~nl~ of H1span1c or~gln, twelve years of agè, weigh~ng
W roxt~t~l~ 3~ k110grans, presents to the phys1clan w1th
1~opathlc ~v~n11~ rhe~to1d arthr1t1s. Her sy~pto~s 1nclude
~arked 1nflaaoat10n of mult1ple ~o1nts, co~pl1cated by heat 3nd
tenderness and 1ndicattng r~p~d and patholog1cal degenerat~on of
~o1nt funct10n.
Her phys1c1an refers her to a rheumatolog1st ~ho 1~med1at~1y
prescr~bes aggress1vc therapy b~ IV ad~1n1strat10n of the
solutton prep~red ~s descrtbect 1n Exa~ple lO over ~ per10d of
thre~ da~s, ~t the r~ta of 1 1n~ect10n per t~y, ~d~ntstered over
two hours. At the concluston of the IV reg1men, the physician
w o 93/24494 2 1 3 6 8 1 9 PCT/US93/0446g
-41-
prescribes the ~ablets prepared as described in Example 9 for a
period of two months during which she exhibits marked
improvement with increased mob~lity and decreased pain. For the
succeeding two months the phys kian reduces her dose to 3/4 of
the original oral dose by prescribing 3 tablets over a period of
two days ~.e. one 2-tablet day alternating with one l-tablet
day. At the conclusion of th1s regimen the dosage is again
reduced to l/4 of the or19inal dose by glving her the tablets
prepared as described ~n Example 9 1 tablet every day for an
add~tional four ~onths.
Exam~le 14
A 60-year-old C~ucas1an female we19h1ng 62 kg exper1ences
severe back pa1n. Her physic1an wtth the aid of a radiolog1stt
d~agnoses her as havtng a crush fracture of the Ll vertebrae
presumably due to osteoporot~c bone loss. The patient is
prescr~bed a three month once-da11y dosage regimen of a 700 mg
tablet prepared descr1bed in Example 9. The 700 mg tablet is
taken either two hours before or two hours after any given meal.
After three months the dos~ge ls reduced to a 3S0 mg capsule
prepared accord1ng to the procedure descr1bed ~n Exanple 8 taken
every other day for a per10d of three months. Her phystc1an then
puts her on a ~1ntenance dos1ng regi~en wherein she takes a lOO
mg capsule prcp~red ~ccord1ng to the procedures descr1bed in
Exa~ple 8 ver~ day for s1x months. After slx ~onths on the
~a~nt~nance dos1ng regt~en the pat1ent ~s not exper1enc~ng any
furth r bac~ pa1n. Follo~-up x-rays reveal no ~dd1t10nal
~r~ct~.
Example 15
A 75-year-old Orlental fe~le we1gh1ng 53 kg suffers a
fractured htp ~ftor a fall. She 1s hospltal1zed ~nd dlagnosed as
havtn~ ost~oporosls. A treat~ent regt~en of calclton~n
1n~ect10ns ts prescr1bed. The calc1ton1n ~n~ect10ns are p~nful
35 to the pat1ent ~nd she ~s unable to comply w1th s~td calc1tonin
treat~ent. Her phys k1an then sw~tches her therapy to an oral
WO 93/24494 PCI`tUS93/04469
~36~ phosphonate regimen. She is administered a 700 mg tablet
prepared according to the procedure described in Example 9, twice
daily for one month. At the end of this one month of therapy,
she is given a ~00 mg tablet once daily for two months. At the
S end of this two month perlod, she is given a lO0 mg capsule
daily, prepared according to the procedure described in Example
8, for three months. A follow-up visit to her physic1a~ reveals
no apparent decrease ~n m~neral density of the forearm as
determined by photonabsorpt~etry.
Example 16
A 85-year-old Hative Amer~can male weighing 65 kg presents
to his phys k1an w1th severe back pain. X-rays reveal multiple
m~nor vertebral body collapse result~ng fro~ signtf~cant bone
loss due to osteoporosts. The pat1ent 1s prescr~bed a two month
reg~men of a ~00 mg tablet and a 350 mg capsule to be taken on
the same day, eight hours apart, prepared accordlng to the
procedures descr1bed ~n Examples 9 and 8, respect1vely. After
two months on th~s regimen, his dosage is reduced to a 350 mg
capsule once a day for two months. X-rays are then taken and an
add1t1On~1 crush fracture ~s noted. He is then put on a
~intenance reglmen of a lO0 mg capsule, prepared accord~ng to
the procedure descr1bed ln Ex-mple 8, once a day for s1x months.
At the end of th1s s1x months, no s19n~fkant apparent decrease
1n bon~ dens1ty ~s observed.
:
. 30
,
,