Note: Descriptions are shown in the official language in which they were submitted.
W094/00535 PCI/EW3/01669 ~
21~7~.30
Additives and Fuel Compositions
This invention relates to the use of additives for improving the cold flow
properties of crude oil, lubricating oil or ~uel oil, for example distillate petroleum
fuel such as middle distillate fuel oil bolling within the range of 11 0C ~o 5û0C. ~:
. ~:
When oils and fuel oils are subjected to low ambient temper~ures, wax may
separate out from the fuel and impair the flow properties of the oil. For example,
middle distillate fuels contain wax which precipitates at low temperatures to form
larg~ waxy crystals which tend ~o plug the small pore openings of fuel filters.
This problem is particularly acute wh~n the fuel is a diesel fuel bscause tha
nominal apertures in the fuel filter of diesel engines are typically of diameter ~ -
batween about 5 and 50 microns. Additives are known in the art for overcoming
the above problem and are called Flow Improvers.
Such additives may act as wax crystal mcdifiers ~hen blended with waxy
mineral oil by modifying the shape and size of crystals of the wax therein and
r~duoing the adhesive forces between the crystals and between the wax and the
oil to permit the oil to remain fluid at a lower ternperature than in the absence of
the additive.
.
EP-A-0 261 9~7 describes the use of additives for improving the cold flow
pr~perties of distiltate fuels, and exemplifies an additive (designated Additive C
therein) comprising the reaction product of one mole of phthalic anhydride with
two moles of dihydrogenated tallow amine, to form a half amide/half amine salt,
in combination with an additive (designated Addltives A thersin) comprising an
ethylen~-vinyl acetate opolymer or a mixture of two ethylene-vinyl acetate :~
copolymers. Specific examples of Additives A used with Additive C in
EP-A-~ 261 957 are: ~:
3o
Additive A1, which is a 1:3 ~w/w) mixture of two copolymers, one being A2 as
defined b~low and the other being A3 as defined below. Thus, the average vinyl
acetate content of A1 is 32.75 wt%, i.e. 13.7 mo!e%;
:
Additive A2, which is a copolymer consisting of ethylene and about 17 wt% vi~yl
acetate and having an Mn of about 3000 as measured by Vapour Phase
Osmometry (VPO), i.e. 6.25 mole% of vinyl acetate;
WO 94/00535 PCI'/EP93/016S9 ,_
~,~3~,3a :
Additive A3, which is a copolyrner consisting o~ ethylene and about 38 wt% vinylacetate and having an Mn Of about 1800 as measured by VPO, i.e. 16.6 mole%
of vinyi acetate; and ;~
5 Additive A4~ which is a 50:50 mixture of Additives A2 and A3 as defined above, the av~rage vinyl acetate oontent of A4 there~ore being 27.5 wt%, i.e.
1 1 mole%.
l~tn in this specification is the number average molecular weight and is as
10 measured by Gel P0rmeation Chromatography (GPC) aaainst polystyrene
starldards urlless otherwise stated.
:
A prcblem in the use of the abov~-exemplified additives is that the Additives A, ~;
although having good performance themselves for improving the cold flow
prope~ies of middle distillate fuels, do not necessarily give good cold flow
properties when used in combinGtion with Additive C. The present inveniion
me0ts the problem by employing additives that have a specified mole per cent of
a comonomer such as vinyl acetate, the use of additives having such specified
mole% giving rise to unexpected and surprising advantages over use of
20 cornparison additives in the cold flow properties of rniddle distlll2te f!lel oils as
will be illustrated in the examples her~inafter.
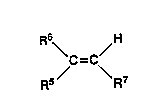
Thus, in one aspect, the invention provides an additive comprising one or more
oil-soluble copolymers of ethylene and an unsaturated monomer o~ the general
~5 formula
R6~ ~H
C=C
Rs / \ R7
wher~in 5~6 iS hydrogen or methyl,
30 R~ is a -OC)CR8 group wher in R8 is a hydrogen formate or a Cl to C2~, more
usu~lly C1 ~o C17, and preferably a Cl to Cg, straight or branched chain alkyl
group, or R5 is a -COOR8 group wherein R8 is as defined above but is not
hydrogen, and R7 is hydrogen or-COOR8 as defined above, and
wher~in
.
WO 94/00535 PCT/EP93/01669
2:1~7~3~
(i) where there is rr ore than one copolymer, the arithrnetic mean of the
content of said unsaturated msnQm~r in the copolymers is below
11 mole%, or
5 (ii) where there is ane copolymer, the content of said unsaturated monomer
in th~ copolymer is in the range of 6.5 to 11, preferably 10, mole/O.
In a s~cond aspect, the invention provides the use of an additiv~ of the first
aspect of the invention for improYing the cold flow properties of crude oil,
~o lubricating oil or a fuel oil such as a middle distillate ~uel oil. ; .;
, :
In a third aspect, the invention provi~es a cornposition oomprising an admixture : ;~
of a major proportion of a c~tde oil, lubricating oil or a fuel oil such as a middle
distillate fuel oil and a minor proportion of an additive of the first aspect of the ~;
5 invention.
In a fourth aspect, the invention provides a concentrate comprisin~ an admixture `:
of an additive of the first aspec~ of the invention dispersed in a liquid medium.ompatible with a crude oil, lubricating oil or a fuel oil such as a middle distillate
l Qil. :
The features of the invention will now be ~iscussed in furth~r detail. :~
~ ~ .
ADDITIVE
Said ~nsaturated monomer may include alcohol esters of C1 to Cs mono-
carboxylic acids, such as C2:to ~5 mono-carboxylio acids. Examples o~ the . `:
monomer include vinyl acetate, vinyl propionate, vinyl butyrate or isobutyrate,
vinyi hexanoate and vinyl octanoate, where vinyl ac~tate and vinyl propionate
3~ are preferred. It is preferred that the copolymers have a number avera~e
mo!ecular weight as measured by vapour phase osometry of 1,000 to 10,000,
preferably 1,000 to 5,000. If desired, the copolymer may be derived ~rom
additional comonomers, e.g it may be a terpolymer or tetrapolymer or higher, for ~:
example where the additional comonomer is an iso-olefin such as isobutylene or
; 35 ~i-isobutylene.
., ' , ' 'b ~ '
WO 94/00535 PCI/EP93/01~69 ~_ ~
~?~7P~, 4 ~
Wh~n there is more than one copolymer, the arithmetic mean of the ontent of
said unsaturated rnonom~r is preferably from 5 to 10 mole%, rnore preferably 6
to 10 mole%, such as 7 or 8 to 10 mole%.
When thare is one polymer, the content of said unsaturated monomer is
pr0ferably from 7 to 10 mole/O, more preferably 8 to 10 mole%, most preferably
8 to 9 mole%~
Such copolymers may be made by copolyrnerising ethylene and said
10 unsaturated monorner. They may also be made by transesterification, or by
hydrolysis and re-esterificationl of an ethylene unsaturated ester copolymer to
give a different ethylsne unsaturated ester copolymer. For example, ethylene
vinyl hexanoate and ethylene vinyl octanoate copolymers may be made in this
way, e.g. from an ethylene vinyl acetate copolymer.
t
OIL
:,
The oil rnay be a crude oil, i.e. oil obtained directly from drilling and beforerefining, the compounds of this invention being suitable for use as flow
20 improvers or dewaxing aids therein.
- The oil may be a lubricating oil which may be an animal. fruit, vegetable or
mineral oil, such as petroleum oil fractions ranging from naphthas or spindle oil
to SAE 30l 40 or 50 lubricating oil grades, castor oil, fish oils or oxidised mineral
25 oil. Such an oil may contain additives depending on its intended use; examples
are viscosity index improvers suc~ as ethylene-propylen~ copolymers, succinic
acid based dispersants, metal containing dispersant additives and zinc dialkyl-
dithiophosphate antiwear additives. The additives of this invention may be
suitable for use in lubricating oils as flow improvers~ pour point depressants or
30 dewaxing aids.
The oil may be fuel oil such as a petroleum-based ~uel oil, suitably a middle
distillat~ fuel oil. Such distillate fuel oils generally boil within the range of about
110C to about 500C, e.g. 150C to about 400C. The fuel oil can comprise
35 atmospheric distillate or vacuum distillate, or cracked gas oil or a blend in any
proportion of straight run and thermally andlor catalytically craoked distillates.
The most common petroleum distillate fuels are kerosene, jet fuels, diesel fuels,
WO 94/00535 2 1 3 7 ~ 3 0 PCI`/EP93/01669
heating oils and heavy fuel oils. The heating oil may be a straight atmospheric
distillate, or it may contain minor amounts, e.g. up to 35 wt%, of vacuum gas oil
or cracked gas oils or of both~ The above-mentioned low temperature flow
problem is most usually encountered with diesel fuels and with heating oils. The5 invention is also applicable to vegetable- or other plant-based fuel oils, for example rape seed oil (e.g. as its methyl ester).
The concentration of the additive in the oil may for example be 10 to 2,000 ppm
of additive (active ingreclient) by weight per weight of fuel, such as 50, preferably
10 ~ to 500 ppm, more preferably t00 tc 200 ppm. The ad~itive concentr2tion
may include co-additives such as described hereinafter.
."~
The additive should be soluble in the oil to the extent of at least 1000 ppm by
weight per weight of oil at ambient temperature. However, at least some of the
15 additive may precipitate from solution near the cloud point of the oil in order to
modify the wax crystals that form.
, "
The ratio of the additive to any co^additive such as described hereinafter may for
example be in the range of 20:1 to 1 :20, such as 10:1 to 1:10, preferably 5:1 to
20 ~ :5, a~ ti~s being weighi:wei~ht.
CO-ADDITIVES
The additives of the invention may, as indicated above, be used in combination
25 with onP cr more oil-soluble co-additives for improving the cold flow propeT~ks of
crude oil, lubricating oil or fuel oil. Examples of such co-additiv~s may be
s~lected from polar nitrogen compounds, comb polyrners, polyoxyalkylene
compounds, hydrocarbon polymers, and sulphur carboxy compounds. Such co-
additives will now be discussed in further detail.
(i) Polar Nitrogen Compounds
Oil-soluble polar nitrogen compounds may be ionic or dipolar and may comprise
for example one or more of the compounds (A) to (B) as follows:
WO 94~00535 c~ 3~ ?,3~ PCI'/I~P93/01669
(A) An amine salt and/or amide formed by reacting at leas~ one molar
proportion of a hydrocarbyl substituted amine with a molar proportion of a ~ ;
hydrocarbyl acid having 1 to 4 carboxylic acid groups or its anhydnide. q
Esterlamides may be used c~rltaining 30 to 300, preferably 50 to 150,
total carbon atoms. These nitrogen compounds are described in US
Patent 4 211 534. Suit~bl~ amines are usua!ly lon~ shain C~2-C,tû
primary, sècondary, tertiary or quaternary amines or mixtures thereof but
shorter chain amines may be used provided the resulting nitrogen
compound is oil soluble and there~ore normally c~r~tains about 30 to 300
total carbon atoms. The nitrogen compound preferably contains at least
~ne straight chain C8 to C40, preferably C~4 to C24 alkyl segment.
Suitable arnines include primary, secondary, tertiary or quaternary, but
pr~ferably are secondary. Tertiary and quaternary amines can only form
amine salts. ~xamples of amines include tetradecyl amine, cocoamine, `
and hydrogenated tallow amine. Examples of secondary arnines include
dioctacedyl amine and methyl-behenyl amine. Amine mixtures are also
suitable such as those derived from natural materials. A preferred amine
is ~ sQcor~ry hy~rogen~t~d tall~w camin~ f~ ~1-R2 W~ere
in R1 and R2 are alkyl groups derived from hydrogenated tallow fat
composed of approximately 4% G14, 31% Cl6, 59% C18.
Examples of suitable carboxylic acids and their anhydrides fer preparing
the nitrogen compounds inc!ude cyc,oh~xan~ t,2 dicarboxylic acid,
cyclohexene 1,2 dicarboxylic acid, cyclopentane 1,2 dicarboxyiic acid and
naphthalene dicarboxylic acid, and 1t4-dicarboxylic acids including
dialkyl spirobislactone. Generally, these acids have about ~-13 carbon
atoms in the cyclic moiety. Preferred acids useful in the present invention
are benzene dicarboxylic acids such as phthalic acid, isophthalic acid,
and terephthalic acid. Phthalic acid or its anhydride is par~icularly
preferred. The particularly preferred compound is the amide-amine salt
forrned by reacting 1 molar portion of phthalic anhydride with 2 molar
portions of dihydrogenated tallow amine. Another preferred compound is
the diamide formed by dehydrating this amide-amine salt.
WO 94/00535 PCl-/EP93tO1669
213723~
7 t
C)ther examples are long chain alkyl or alkylene substituted dicarboxylic !~
acid derivatives such as amine salts of monoamides of substituted f
succinic acids, examples of which are known in the art and described in
US-A-4 147 520, for example. Suitable amines may be those desGribed
above.
Other examples are condensates such as described in EP-A-327,423.
(B~ A chemical cornpound comprising or including a cyclic ring system, the0 compound carrying at least two substituents of the ~eneral formula (I) b~low on the ring system
-.A NRtR2 (1)
where A is an aliphatic hydrocarbyl group that is optionally in~errupted by
one or more hetero atoms and that is straight chain or brancned, and R~
and R2 are the same or d~fferent and each is independently a hydrocarbyl
group containing 9 ~o 40 carbon atoms optionally Interrupted by one or
more hetero atorns, the substituents being the same or different and the
2~ compound opt!onally being in the form of a sal~ ~hereof.
Preferably, A has from 1 to 20 carbon atoms and is preferably a
methylene or polymethylene group.
"Hydrocarbyl" in this specification means an organic moiety composed of
hydrogen and carbon wnich, unless tne context states otherwise, may be
aliphatic, including alicyclic; aromatic; or any combination thereof. It may
be substituted or unsubstituted alkyl, aryl or aralkyl and may optionally
contain unsaturation. Examples where it is substituted are oxy-,
.
halogeno- and hydroxy-hydrocarbyl. ~
,~.
The cyclic ring sys~em may include hornocyclic, heterocyclic, or ~used
polycyclic assemblies, or a system where two or more such cyclic
assemblies are joined to one another and in which the oyclic assemblies
may be the sarne or differen~. Wh~re there are two or more such cyclic
assemblies, the substituents of the general formula (I) may be on ~he
same or different assemblies, pr~ferably on the same assembiy.
WO 94/00535 PCT/EP93/01669 _,
2~3~ 8
Preferably, ~he or each cyclic assembly is aromatic, more preferably a
benzene ring. Most preferably, the cyclic ring system is a singl~ benzene
ring when it is preferred that the substituents are in the ortho or meta
positions, which benzene ring may be optionally further substituted.
The ring atoms in the cyclic assembly or assemblies are preferably
carbon atoms but may for example include one or more ring N, S or O
atom, in which case or cas~s the compound is a heterocyclic compound.
Examples of such polycyclic assemblies include :~
,: -
(a) condensed benzene structures such as naphthalene, anthrac,ene,phenanthrene, and pyrane;
.
(b~ condensed ring structures where none of or not all of the rings are
ben~ene such as azulene, indene, hydroindene, fluorene, and
diphenylene;
(c) rings joined "end-on" such as diphenyl;
. . :
(d) hetero~;yclic compounds such as quinoline, indole, Z:3
dihydroindole, benzofuran, coumarin, isocoumarin, benzothisphen,
c~rbazole and thiodlphenylamine;
(e) non-aromatic or partially saturated ring systems such as decalin
(i.e. decahydronaphthal~ne), a-pinene, cardinene, and bornylene;
ana
,' '.
(f) three-dimensional structures such as norborrlene, bicycloheptane
(i.e. norbornane), bicyclooctane, and bicyclooctene.
Each hydrocarbyl group constituting R1 and R2 (Formula 1) may for
example be an alkyl or alkylene group or a mono- or poly-alkoxyalkyl
group. Preferably, each hydrocarbyl group is a straight chain alkyl group.
The number o~ carbon atoms in each hydrocarbyl group is pr~ferably 16
to 40, more preferably 16 to 24.
W~94~Q535 2:~?7?..3a PCI/EP93/01669
.
9 ~, :
Also, it is preferred that the cyclic system is substituted with only two
substituents of the generat formula (I) and that A is a methylene group. '
Examples of salts of the chemical cornpounds are the ac~tate and the ;
hydrochloride.
The compounds may conveniently be made by reducing the
corresponding amide which may be made by reacting a secondary amine
with the appropriate acid chloride; and ~;
(C~ A condensate of long chain primary or secondary amine with a carboxylic
acid-containing polymer.
Specific examples include polymers such as;described in
GB-A-2,121,807, FR-A-2,592,387 and DE-A-3,941,561; and also esters of
telerner acid and alkanoloamines such as describ~d in US-A-4,639,256;
and the reaction product of an amine containing a branched carboxylic
acid ester, an ep~xide and a mono-carboxylic acid polyester such as i ;~
described in US-A-4,631,071.
(ii) Comb Polymers
Such polyrners are polymers iri which hydrocarbyl groups are pendant frcm a
polymer backbone and are discussed in "Comb-Like Polymers. Structure and
Properties", N A Ptaté and V P Shibaev, J. Poly. Sci. Macrornolecular Revs.,
8, p 1 t7 to 263 (1974~.
Advantageously, the comb polymer is a hornopolymer having, or a copolymer at
least 2~ and preferably at least 40, more preferably at least ~0, molar per cen~ of
the units of which have, si~e chains con~aining at least 6, and preferably at least
1 0, atoms.
As examples of preferred comb polymers there may be mentioned those of the
general formula:
WO 94/00535 PCr/EP93/01669 .~_ ~
'X,~;3~'1'3~ 1 o
tC--I H~ H~
E G m K L n
wher~i n D = R11, ~QoR11, OCOR1 1, R1 2COC)R1 1, or OR1 1,
E ~ H,CH3,D,orR12
G = HorD
J = H, Rl~, R12(: OOR11, or an aryl or heterocyclic group,
K = H, COOR12, OCOR121 OR1~, or COOH,
L = H, R12, t:~O :)R~, OCOR12, COOH, or aryl,
R11 2 ~1o hydrocarbyl,
R12 > ~1 hydrocarbyl,
and m and n represent mole ratios, m being within the range of from 1.0 t~ 0.4, n
being in the range of from 0 to 0.6. R11 advantageously represents a
hydrocarbyl group with frorn 10 to 30 carbon atoms, while 1~1~ advantageously
represents a hydrocarbyl group wi~h from 1 to 30 carbon atoms.
The comb poiymer rnay contain units derived from Qther m~nomers i~ desired or
required~ It is within the scop~ of the invention to include tv~o or more different
comb copolyrners.
n ~
These comb polymers may be copolymers of malelc anhydride or fumaric acid ~;
and another ethylenically unsaturated monomerr e.g. an oc-olefin or an
unsahJrated ester, for example, vinyl acet:ate. It is preferred but not essential that
equimolar amo~nts of the comonomers be used although molar proporti~ns in
25 the range of 2 to 1 and 1 to 2 are suitable. Examples of olefins that may be
copolymerized with e.g. maleic anhydride, includa 1-decene, 1-dodecene,
,
1-tetradecenP, 1-hexadecene, and 1-octadecene.
The copolymer may be esterified by any suitable technique and although
30 preferred it is not essential that the maleic anhydride or fumaric acid be a~ least
50% esterifisd. Examples of alcohois which may be used include
n-hexadecan-1-ol, n-dodecan-1-ol, n-tetradecan-1-ol, n-hexadecan-1-ol, and
n~octadecan-1-ol. The alcohols may also include up to one methyl branch per
chain, for~xample, 1-methylpentadecan-1-ol, 2-methyltridecan-1-ol. The
WO 94/00535 213 7 2 ~ ~ PCr/EP93/01669
"~
alcohol may be a mixture of normal and single methyl branched alcohols. It is
preferr~d to use pure alcohols rather than the commercially available alcohol ;;
mixtures but if mixtures are used the R12 refers to the average number of carbonat~ms in the alkyl group; if alcohols that contain a branch at the 1 or 2 positions
5 are used R1~ refers ~o the straight chain backbone segment of the alcohol.
These cornb polymers may especially be fumarate or itaconate polymers and
copolymers such for example as those described in European Patent
Applications 153176, 153177 and 225688, and WO 91t16407.
': '.
Par~icularly preferred fumarate comb polymers are copolymers of alkyl fumarates ;
and vinyl acetate, in which the alkyl ~roups have from 12 to 20 carbon atoms, -
more especialty polymers in which the alkyl groups have 14 carbon ~toms or in
which the alkyl groups are a mixturs of Ci4/C16 alkyl groups, made, for example,by solution copolymerizing an equimolar mixture of fumaric acid and vinyl
acetate and reacting the resulting copolymer with the alcohol or mixture of
alcohols, which are preferably straight chain alcohols. WhPn the mixture is usedit is advantageously a 1:1 by weight mixture of normal C14 and C16 alcohois.
Furtherrnore, mixtures of the C14 ester with the mixed C14/C16 ester m~y
20 advantageously be used. In such mixtures, the ratio of C14 to C141C1s is
advantageously in the range of from 1:1 to 4:1 j pref~rably 2:1 to 7:2, and mostprefarably about 3:1, by weight.
i. ,,
Othcr suitable comb polymers are the polymers and copoiymers of a-olefins and
25 esterified copolymers of sfyrene and maleic anhydride, and esterified
~opolymers of styrene and fumaric acid; mixtures of two or more comb polymers
may be used in accordanc~ with the invention and, as indicated above, such usa
may be advantageous.
.
30 (iii) Polyoxyalkylene Compounds
: .~ .
Examples are poiyoxyalkylene esters, ethers, esterlethers and mixtures thereof, ~-
par~icularly those containing ~t least one, preferably at least two C10 to C30
Iinear saturated alkyl groups and a polyoxyalkylene glycol group of molecular
35 weight up to 5,000 preferably 200 to 5,00û, the alkyl group in said
polyoxyalkyl~ne glycol containing from 1 to 4 carbon atoms. These materials
~,''~;
~'
WO 94/00535 P~/EP93/01669 ~ ¦
L3~3~
form the subject of European Patent Publication 0 061 895 A2. Other such ~;
additives are described in United States Patent 4 491 455.
The pre{erred esters, ethers or ester/ethers which may be used may be
5 structurally depicted by the formula
;
R-0(A)~0-R2 0
where R and R20 are the same or different and may be ~:
1 0
(a) n-alkyl
(b) n-alkyl- C- ;
o
(c) n-alkyl-O-C-(CH2)n
O O . .::~
(d) n-alhyl O-C-(CH2)n C-
n being, for example, 1 to 30, the aikyl group being iinear and saturated anb
containing 10 to 30 carbon atoms, and A representing the polyalkylene segment .
of the glycol in which the alkylene group has 1 ~o A carbon atoms, such as
polyoxymethylene, polyoxyethylQne or polyoxytrimethylene molety which is
substantially li~0ar; some dagree of branching with lower alkyl side chains (such
as in polyoxypropylene glycolj may be toler~ted bu~ it is preferred that the glycol ~:
shoLI!d be substantially linear. A may also contain nitrogen.
Suitable glycols generally are substantially linear polyethylene glycols ~PEG)
a~d polypropylene glycols (PPG) having a moleoular weight of about 100 to
5,000, preferably about 200 to 2,000. Esters are preferred and fatty acids
containing from 10-30 carbon atoms are useful for reaciing with the 9IYGOIS to
form the ester additives, it being preferred to use a C1g-C24 fatty acid, espeoially
behenic acid. The ester~ may âlsO be prepared by esterifying polyethoxylated
fatty acids or polyethoxylated alcohols.
WO 94/00535 PCI/EP93/01669
2137~30
1 3
Polyoxyalkylene dissters, diethers, ether/esters and mixtures thereof are suitable
as additives, diesters being preferred for use in narrow boiling distillates when
minor amounts of monoethers and monoesters (which are often formed in the
manufacturing pr~cess) may also be present. It is important for additive
5 performance that a major amount of the dialkyl compound is present. In
particular, stearic or behenic diesters of polyethylene glycolt polypropylene
glycol or polyethylene/polypropylene glycol mixtures are preferred.
Examples of other compounds in this general category are those described in
10 Japanese Patent Publication Nos 2-51477 and 3-34790, and the esterified
alkoxylated amines described in EP-A-117,108 and EP-A-326,356.
(iv) Hydrocarbon Polymers
Examples are ~hose represented by the following general formula
lI cL~c c
L ~ T I V L H U ~ W
where T = Horalkyl
U = H, T or Aryl
v = 1.0 to 0.0 (mole ratio)
w = 0 0 to 1.0 (mole ratio~ -
,
These polymers may be made dir~ctly from ethy!~nica!ly unsatur~ted monome!s
25 or indirectly by hydrogenating the potymer made from monomers such as
isoprene, butadiene etc
- .
Preferred hydrocarbon polymers are copolymers of ethylene and at least one a-
olefin, having a number average molecular weight of at least 30,000 Preferabiy
30 the a-olefin has at most 20 carbon ato m s Exa m ples of such olefins are
propylene, 1-butene, isobutene, n-octene-1, isooctene-1, n-decene-1, and
n-dodecene-1. The copolymer may also comprise small amounts, e.g. up to
~0% by weight of other copolymerizable monomers, for example olefins other
than a-olefins, and non-conjugated dienes The preferred copolymer is an
WO 94/00535 PC~IEP93/01669 __ i
2 ~ V 1 4
ethylene-propylene copolymer. It is within the scope of the invention to includetwo or more different ethylene-a-olefin copolymers of this type.
The number average molecular weight of the ethylene-a-olefin copolymer is, as
5 indicated above, at least 30,000, as measured by ~el permeation
chromato~raphy (GPC) relative to polystyrenë standards, advantageously at
least 6~,000 and preferably at least 80,000. Functionally no upper limit arises
but difficulties of mixing result from increased viscosity at molecular weights
above.about 150,000, and preferred molecular weight ranges are from 60,000
10 and 80,0û0 to 120,000.
. .
Advantageousiy, the copolymer has a molar ethylene content between 50 and
8~ per cent. More advantageously, the ethylene content is within the range of
from 57 ~o 80%, and preferably it is in the range frorn 58 to 73%; more preferably
15 from 62 to 71%, and most preferably 65 to 70%.
....
Preferred ethylene-a-olefin copolymers are ethylene-propylene copolymers with
a molar ethylene content of from 62 to 71% and ~ number average molecular
weight in the range 60,000 to 120,000, especially prefer~red copolymers are
20 ~thylene-propylen~ c4pQlyrn~rs with an ethyler.e c~nterlt of from 62 to 71% and
a molecular weight from 80,000 to 100,000.
The copolymers may be prepared by any of the me~hods known in the art, for
example using a Ziegler typ~ catalyst. The polymsrs should be substantially
25 amorphous, since highly crystalline polymers are relatively insoluble in fuel oil at
low temper~tures. ~ ` -
:;
The additive composition may also comprise a further ethylene-a-olefin
copolymer, advantageously with a number average rnolecular weight of at most
3Q 7500, advantageously from 1,000 t~ 6,000, and preferably from 2,000 to 5,000,as measured by vapour phase osmometry. Appropriate a-olefins are as giver
above, or styrene, with propylene ~gain being pref~rred. Advantageously the
ethylene content is ~rom 60 to 77 molar per cent although for ethylene-propyienecopolymers up to 86 molar per cent by weight ethylene may be employed with
35 advantage.
Examples of hydrocarbon polymers are described in WO-A-9 111 488.
WO ~4/0053~ 213 7 2 3 ~ PCI/EP93/01669
(v) Sulphur Carboxy Comp~unds , ~
Examples are those d0scri~ed in EP-A-0,261,957 which describ~s the use of ;;
compounds of the g~neral formula
;
A X--R13
C
B/ ~` Y--R21
;,
in which -Y~R21 is 503(-)(+)NR3R2,-So3()(~)HNR3R21,
-So3()(+)H2NR3R21~-so3(-)(+)H3NR21
-~;02NR3R~1 or-S03R21;
-X-R13is -Y-R21 or-CONR3R~3,
: 15 -CO2~-)(+)NR3R13,-CO2(-)(+)HNR2R13,
-R4-COOR13, -NR3COR13~
^R4OR13t -R4OCoR13, -R4~R13
~: ~ -N(COR3)R13 or Z(-)(+)NR3R13;
-Z(-) is S03( ) Or-Co2( );
R13 and R21 are alkyl, alkoxyalkyl or polyalkoxyalkyl containing at least 10 ::
carbon atoms in the main chain;
R3 is hydrocarbyl and each R3 may be the same or different and R4 is absent or
is C1 to C~ alkylene and in
A :
- /~
B
the carbon-carbon (C-C) bond is either a~ ethylenieally unsaturated when A and
35 B may be alkyl, alkenyl or substituted hydrocarbyl groups or b) part of a cyclic
WO 94/00535 PCrtEP93/al669 ~
~ ,3~ 16 ~ ~
structure which may be aromatic, polynuclear aromatic or cyclo-aliphatic, it is
preferred that X-R1 and y p~2 between them contain at least three alkyl,
alkoxyalkyl or polyalkoxyalkyl groups. r ...
.
M~lticomponent additive systems may be used and the ratios of additives to be ~:
used will depend on the fuel to be treated.
.
CONCENTRATE
.
The concentrates of th present invention are convenient as a means for .:
incorporating the addi~ive into bulk oil such as distillate fuel, which incorporation
may be done by rnethods known in the art. The concentr~es may also contain
o~her additives as required and preterably contain from 3 to 75 wt%, more
preferably 3 to 60 wt%, most preferably 10 to 50 wt% of the additives prcferablyin solution, in a carrier liquid as the liquid medium. Examples of carrier liquid
ars organic solvents including hydrocarbon solvents, for example petroleum
fractions such as naphtha, kerosene, diessl and heater oil; aromatic
hydrocarbons such as aromatic fractions, e.g. those sold under the 'SOLVESSO' . .
tradename; and paraffinic hydrocarbons such as hexane and pentane. The
zo carriar riquid must, of course, be selected having regard to its compatibllity with
the additive and with the oil.
The additives of the invention may be incorporated into bulk oil by o~her methods
such as those known in the art. If co-additives are required, they rnay be ;
incorporated into the bulk oil at the same tirne as the additives of the invention or
at a different time. ~
.:
The additives of this invention may be used in the presence of various other
additives that are commonly used in fuel compositions. Examples of such other
30 additives are rust inhibitors, dyes, detergents, demulsifiers, dispersants,
antioxidants, antis~atic agents, metal deactivators, cetane improvers and
antismoke ag~nts. Such other additives may be present in amounts of about
0.001 to 5wt%. ~ :~
WO 94/00535 213 7 2 3 0 PCT/EP93/01669
, ~:
17 :~
EXAMPLES
The invention will now be particularly described by way of example only, as
~ollows.
~
ADDITIVE5 :.
'.,:~'
The following additives were used. ~ `
: .
10 Ethylene-Vinyl Acetate Copolymers (Additives A~E and K S)
~ . ".,
Copolymer I Copolymer ll ;.
__ _
Additive Vlnyl % In Vlnyl % in Average Vinyl
Co d e Ac eta~e Add i tive Acetate Addlti ve Acetate Conten~
Content ~wt%) Content (wt%) ~mole%) ;
(mole~O) (mole%)
_ _ . _ .
A 15.5 100 ¦ 15.5 :~
_ _ . ~ I _ _ .~
B 4.6 100 4.6
_ _ _ .. ,
C - 15.~ ~ 7~ .6 L~ 25 ¦ 1~a
_ - ! -
D 15.5 50 4.6 1 50 9.7
__ _ _ _ j ,,.
E 15.5 2~ 4.6 75 7. 1
_ . _ _ _ - .
: .
Thus, Additives A and B comprise a single oopolymer whereas Additives C, D
and E comprise two copolymers.
Also, the following additives, each in the form of a single ethylene-vinyl aceta~e
copolymer, were used.
. - . ~
WO 94/0053~ PCr/EP93/01669
; 18
~..
Additive Code Average Viny~ Acetate
C:ontent (mole%)
, , .....
K : 16.6
M ~ . 11 1
N 8.5 .
o 7~7 :
P 6.5
Q 4.8 `
R 3.8
_ S _ 3.2
'.,".
The following were used as co-additives. -:
.
5 Polar Nitrogen Compound (Additive F)
An N,N-dialkylarnmonium salt of 2-N',N'-dialkylamido-benzoate, being the
reaction product of reacting one mole of phthalic anhydride with ~wo moles of
dihydrogenated tallow amine to form a half amide/half amine salt.
. . . . . ~ - ~ . .
' : :
C~rnb Polymer (Additive G)
~ : . .
An itaconate polymer of number average molecular weight about 400û as :
measured by Gel Permeation ~hromatography (GPC) prepared by polymerising :
a monomer in a cyclchexane:soivent using a fre~ radical catalyst, tne monomer
containing linear alkyl groups of 16 carbon atoms.
:
Com~ Polymer (Additive H)
As Additive G but wherein the monomer contains linear alkyl groups of 18 ~ -
carbon at~ms.
~ ' ,
Comb Polym~r (AdditiYe i) ..
:
2~ A copolymer of a dialkyl fumarate, having an Mn of about 9500, obtained by
reacting furnaric acid with a commercially available alcohol containing primary
~-.
WO 94/00535 ~ 1 ~ 7 2 3 0 PCI`/EP93/01669
1 9
n-C14 and n-C15 alcohols and a small quantity of ~he 2-methyl analogue of the .-~
aloohols, and then copolymerisin~ with vinyl acetate in a 1:1 mole ratio.
,:
Comb Polymer ~Additiv~ J) ;
i ;:
A copolymer of a dialkyl fumarate, having an Mn Of about 1 50ûO, obtained by
reacting fumaric acid with a n-C14 alcohol and then copolymerising with vinyl
aeetate in a 1:1 rnole ratio.
FUELS
The following fuels were used having the indicated characteristics:
~ _ _ '~`:
% Wax (wt/w~) CP (Cloud D-86 Distillation (C)
C:ode(at ~0C Point) (C) ,
below CP) IBP 20% 1 50% 9oo/o FBP
_ _ _ _ ,
1 2 4 -4 140 208 2~;0334 360 :
_ _ __ . _ _ ,_ _. -- __ , _ _ . ::
2 1 1 6 187 224 271370 392
_ _ _ _ , ~_ ,~
3 . _ 2.2 -8 138 202 238 327 366
15!~!: IBP is Initial Boiling Point -~
FBP is Final Boiling Point -
x% is the temperature at which x% of the fuel by volume had distilled.
T~STS
Additives were dissolved in ~he fuels and the following tests c~ d out on
untreated fuel and on fuel treated with additives, some of which were additives of
the invention and some of which were not, i.e. they were used for comparison.
.:
(a) Coid Filter Plugging Point (CFPP)
The test was carried out by the procedure described in detail in "Journal of
the Institute of Petroleum", Volume 52, Number 510, June 1966, pp. 173-
285. This test is designed to correlate with the cold flow of a middle
distillate fuel oil in automotive diesel engines. A lower CFPP value
WO 94/0~)535 c~ PCI-/EP93J01669
indicates a superior performance to a higher value in each table of test
r~sults below.
~b) Extended Prcgramme Cooling Test (XPCT)
The test is a slow cooling test designed to indicate whether the wax in the
fu~l will pass through filters such as those found in heating oil distribution
systems.
o In the test, the cold flow properties of the described fuels containing the
additives were determined as follows. 300 ml of fuel WQre cooled linearly
at 2C/hour to the test temperature and the temperature then heid constant.
Wax which had settled in th0 bottle was dispersed by aentle stirringl then a
Cold Filter Plugging Point (CFPP) filter asssmbly, which is described in
1 5 detail in "Journal of the Institute of Petroleurn", Volum~ 52, Number 510,
June 1966, pp. 173-2a5, inserted. The tap was opened to apply a vacuum
of 500 mm of mercury and closed when 200 ml of fuel had passed through
the filter into the graduated receiver. A PASS was recorded if the 200 ml
passed through a given mesh size or a FAIL if the filter became blocked.
- A series ~i ~F~P fitter assemrJ~ies w~h fitter screens uf different siz~s
including LTFT (AMS 100.65) and a Volkswagen Tank filter (part no
KA/4-270/5~.431-201-511) both intermediate between 30 ~nd 40 ~m were
used to determine the finest mesh the fuel will pass. The sizes of the filter
screens were as follows in order of increasing size, i.e. in prder of
decreasing severity as a test: 10 ~ 5 u, 20 Il, 25 ~, 500, LTFT, 3~0, VW,
250, 200, 150, 120, 100, 80, 60, 40 and 30. where figures alone indicate
mesh sizes. To assist comparison, results in each table are rated in
numerical order wh~re a lower number indicates a superior result to a
higher number.
RESULTS
The results are shown in the tables below, where additives are referred to by the
35 above-mentioned Additive Codes. EVA means an ethylene^vinyl acetate
copolymer in accordance with the Additive Code and VA means vinyl acetate.
Examples marked "oomp" are comparative examples.
~ ~ 3 7 2 :~ O PCr/EPg3/01669 ~
2 1
Table 1
Fuel 1; Treat Rates EVA:200 ppm; F:200 ppm; G:2Q0 ppm without H, y :.
100 ppm with H; H:100 ppm ~ .
.
_ _ T~sts
~ .,.
ExampleEVA; mQle% Co-additiYes CFPP ~C) XPCT
. VA ~ating No) ..
_ _ _ ~,
BASE -3.5 30#
1 (comp) A 15.5 F, G -7.5 500# (2) ~:
2 (comp) C 12.4 F, G g LTFr (3)
1 D 9.7 F, G -13.5 1 0~L (1 ) -
2 E 7.1 . F, G -10.5 VW (4) ;~ ~
l .
3 (comp) A 15.5 F, G, H 9 500# (4)
4 (comp) C 12.4 F, G, H -10 25~ (3) ;~
3 D 9.7 F, G, H -15.~ 1 5~,l (2)
4 E 7.1 F, G, H_ -18.0 t 0,U (1 )
: T~bie 2
Fuel 3; Treat Rates EVA:60 ppm; F:40 ,~pm; 1:100 ppm
___ , Tests ¦ ,,
Example EYA; mole% YA CFPP (C~ I XPCT
I (Rating No) :~
__ _ _ _ . _
~ASE : -10
7 (comp) K 1 6.6 -1 0.5 40# (7) ~:
8 (comp) L 11.1 -13 80# (1) :
1 0 N 8 ~ -15 80# (1 )
1 1 O 7.7 -1 4.5 80# (1 )
9 (comp) (; 4 ~ -12.5 80~ (1)
: I 10 (comp) R 3.8 -12.5 60# ~5~
11 (comp) S 3.2 _ -12.5 60#(~)
: ~ , ,;
. ~
WO 94/0053~ PCr/EP93/01669 ~ I
3 ~
2Z .;~
Table 3 ! '. ~' '
Fuel 2; Treat Rates EVA:6Q ppm; F:40 ppm
_ ~. i
Te ;ts
Exarnple EVA; mole% VACFPP (C) XPCT
(Ratiny No; I:
BASE -1 2û0#
12 (comp) K 16.6 ~4.5 200# (6)
13 (comp) L 1 1 .1 -3 200~ (6) . ~: :
12 M 10.2 -4 350~ (4)
13 N 8.5 -4.5 LTFl t3)
1 4 0 7.7 -5.;, 2~ (2~
1 ~ P 6.5 -4.5 350# (4)
14 (comp) R 3.8 -2.5 20~ ~1 )
.~ ,'
',~.
`:
..
"~
",',
WO 94/lD0535 21 3 7 2 3 3 PCI-/EP93/01669 ~1:
2 3 ' ::
~ ; ~
~able 4 5 :: IFuel ~; Tr~at Rates EV~:60 ppm; ~:40 ppm; J:100 ppm
_ _
Te sts
Example EVA; mole% V~CFPP (C) XPCT
(Rating No~
_ _ __ ~
BASE -1 200~
15 (comp) K 16.6 -4.5 200# (6)
16 (cornp) L 11.1 -15 200# ~6)
1 6 M 10.2 -17 ~oo~ (1)
17 N 8.5 -18 350# (3)
18 O 7.7 -18.5 500# (1)
19 P 6.5 -16 350# (3)
17 irom~_ Q 3.8 -17.5 350~ (3)
,:
5 The "Base" example is for the fuel as such ~i.e. containing no additives).
The examples marked 1 to 19 are examples of the invention and show an :-~
improvement in performanoe over the corresponding cornparative example.