Note: Descriptions are shown in the official language in which they were submitted.
213~'~62
Process for preparing metal hvdroxides
BACKGROUND OF THE INVENTION
The present invention relates to a process for preparing
low solubility metal hydroxides having the general
formula M~"~ (OX) X, where M - Co, Zn, Ni and/or Cu, and x
is the valence of the metal.
The metal hydroxides of cobalt, zinc, nickel and copper
are valuable intermediates for the preparation of
inorganic or organic salts of said metals and for the
preparation of the corresponding oxides or the pure
metals themselves. Starting from cobalt hydroxide, cobalt
oxide of defined composition can be prepared by
calcination, for example for application in electronics
for the production of varistors or in storage batteries.
Also a cobalt metal powder of defined particle-size
distribution can be prepared by reduction. Nickel
hydroxides serve as pigments or are used with various
dopings and particle structures for battery applications.
Zinc hydroxides serve as precursors for pigments and the
copper compounds can be converted into catalytically
active materials.
In the preparation of hydroxides for various appli-
cations, the objective of preparing as compact and flow-
able material as possible for further processing is
uppermost. As a result of its particle-size distribution
and particle structure, cobalt metal powder prepared from
cobalt hydroxide yields after being sintered together
with tungsten carbide, for example, Special hard-metal
tools.
For the newly developed foam anodes, which are used, in
particular, in nickel hydride storage cells, a nickel
hydroxide is needed whose physical properties are
optimized both in relation to the application purpose and
also to the processing procedure applied. While the
application in high-performance storage batteries re-
quires a high packing density of the active material, the
pasting process used for foam anodes requires a material
with high flowability, compact particle shape, narrow
particle-size distribution and constant quality.
STA 67-Foreign Countries - 1 -
CA 02137762 2004-06-14
23189-7724
Furthermore, the product should be capable of being mixed
well with the additives normally used, such as, for
example, cobalt metal powder and cobalt oxide.
A suitable material and basic features of the preparation
process are disclosed in the Japanese Patent JP Hei 4-
80513. In this process, nickel hydroxide particles having
a diameter of between 1 and 140 ~,m are crystallized by
continuously feeding a nickel salt solution and an
alkali-metal hydroxide in solid or liquid form into a
reaction vessel at a constant pH and at constant
temperature while stirring vigorously. A pH of 11 and a
temperature of 48°C are specified as favorable
experimental conditions. w
It is furthermore known that a sufficiently compact
nickel hydroxide can be prepared by precipitation in the
presence of ammonia or an ammonium salt. 3'hus, according
to Trans. Faraday Soc. 51 (1955), 961, a nickelamine
complex solution is prepared from nickel nitrate and
aqueous ammonia solution. A nickel hydroxide is obtained
from said complex solution by boiling at normal or
reduced pressure or by treatment with steam, which nickel
hydroxide, compared with those nickel hydroxides which
are precipitated in the absence of ammonia, has a
substantially lower specific surface (13 to 20 m2Jg).
The preparation of compact nickel hydroxiøe~ in the
presence of ammonia or an ammonium salt is known, ~.g., by
the precipitation of nickel hydroxide by adding an
alkali-metal hydroxide solution to a suitable solution
having a pH of at least 3Ø Electrochemical investigations
on material prepared in this way yielded particularly high
specific charging capacities compared with commercial nickel
hydroxides.
However, products of this type still do not fulfil the
above-mentioned requirements in relation to .particle
shape, particle-size distribution and flowability.
Essential features of the process for preparing a compact
nickel hydroxide and~its use in alkaline batteries are
disclosed in European patent document (EP-A) 353 837. A
nickel(II)tetramine salt solution is prepared by dissolv-
- 2 -
213762
ing nickel nitrate or nickel sulphate in dilute ammonia
solution and a decomposition is carried out by controlled
addition of sodium hydroxide solution in accordance with
the following reaction:
( I ) NI (NH3 ) 4S04 + 2 NaOH => Ni (OH) 2 + Na2S04 + 4 NH3
The reaction proceeds at temperatures between 40 and 50°C
in the pH range between 11 and 13. In this process, the
pore volume decreases with decreasing pH. It is
expressly found that a pore-free product can be
crystallized only at sufficiently low reaction rates.
Furthermore, the nickel hydroxide prepared by this
process has a high crystallinity, a low specific surface,
a low pore volume and, therefore, a high physical
density. The disadvantages of this product, which are
attributable to the high density, are also described.
The low specific surface results in a relatively low
proton conductivity and in a relatively high current
density which promotes the formation of the undesirable
Y-Ni00H, which leads to swelling of the electrode.
Although the nickel hydroxide crystallized at low pHs has
a high density, it has a greater tendency to form y-
Ni00H. A compromise between the required high density
and the porosity necessary to a certain degree can be
found by the choice of a medium pH. A nickel hydroxide
containing 3 to loo zinc or 1 to 3o magnesium in solid
solution is prepared by this process. These dopings
counteract the formation of 'y-Ni00H.
Japanese Patent JP Hei 4-68249 reveals a continuous
process for crystallizing a nickel hydroxide having
spherical particle shape. In this process, a nickel salt
solution (0.5 to 3.5 mol/1), dilute alkali-metal
hydroxide solution (1.25 to 10 mol/1) and an ammonia
and/or ammonium salt solution are continuously pumped by
means of a metering pump into a heated cylindrical
container provided with an overflow pipe while stirring
vigorously, in which process the ammonia can also be
supplied in gaseous form. The ammonia concentration is
specified as 10 to 28o by weight and the ammonium salt
concentration as 3 to 7.5 mol/1. In order to complex the
nickel, between 0.1 and 1.5 mol of ammonia per mol of
nickel salt solution are added. After about 10 to 30
hours, the system reaches a steady state, and then a
STA 67-Foreign Countries - 3 -
2137762
product having a constant quality can be continuously
drained off. The dwell time in the container is between
0.5 and 5 hours.
An essential feature of this process is that the reaction
is carried out at a defined pH which is kept constant to
within ~ 0.1 pH steps in the range between 9 and 12 by
pH-controlled addition of alkali-metal hydroxide
solution, and at a constant temperature in the range
between 20 and 80°C, in which connection the temperature
deviations should not be more than ~ 2 K. Under these
conditions, the compact spherical particles having a
particle size of between 2 and 50 ~,m are obtained. The
particle size can be adjusted, in particular, by varying
the supply of NH3, the dwell time and the stirring speed.
As the stirring speed decreases or the supply of NH3
increases, the particle size increases. As the dwell
time in the container increases, the product becomes
coarser and the particle-size distribution narrower. The
crystalline product is then filtered, washed with water
and dried. The product prepared by this process has the
properties mentioned at the outset, and it does not need
to be ground.
EP A 462 889 discloses a process for preparing nickel
hydroxide. In this case, the temperature range of the
crystallization is above 80°C. Nitrate or sulphate
solutions doped with cobalt, cadmium and/or zinc are
used. The cobalt content is between 1 and 8 o by weight
and the contents of cadmium and/or zinc are between 3 and
10°s by weight. Complexing is carried out with the aid of
an ammonium salt, the NH3/Ni molar ratio being between
0.3 and 0.6. In this process, a pH of 9.2 ~ 0.1 is
maintained. Furthermore, a three-paddle stirrer whose
diameter is half the container diameter and whose speed
is between 300 and 1000 min-1 is used.
As in the processes already described, the product is
filtered, washed and dried.
The disadvantages of these processes are, on the one
hand, the large amounts of neutral salts which are
inevitably produced and which are present in at least
twice the stoichiometric amount of the nickel hydroxide
and take the form of waste water. On the other hand,
STA 67-Foreign Countries - 4 --
2137762
231$9-7724
said waste water contains, in addition to small amounts of
complexly dissolved nickel, also large amounts of ammonia which
have to be disposed of.
SUMMARY OF THE INDENTION
The invention provides a process for preparing low
solubility metal hydroxides of the general formula M{x) fOH)x,
where M = Co, Zn, Ni and/or Cu, and x is the valence of the
metal, in which process reactive metal hydroxide is reacted, in
a first step, with a complexing agent L in the presence of
alkali-metal salts AY (e.g. NaCl., KC1, NaBr, KBr) to form the
metal complex salt of the general formula MLnYm and alkali-
metal hydroxide solution, and the metal complex salt is
decomposed, in a second step, by reaction with alkali-metal
hydroxide solution at pHs of > 7 to form sparingly soluble metal
hydroxides and complexing agent and alkali-metal salt.
In the process according to the invention, ammonia
and/or organic mono- and/or diamines having a chain length of 1
to 6 are preferably used as complexing agents (L).
The process according to the invention makes it
possible to avoid the waste-water problem in that reactive
hydroxides of the said metals are used as starting materials and
these are in turn converted into a solubi~ form. This is done
by complexing with a suitable complexing agent such as ammonia
or amines. Thug, for example, nickel hydroxide can be reacted
almost completely with the aid of ammonia to form the hexamine
complex in the presence of neutral salts in accordance with the
following equation.
5
2137762
23189-7724
(II) Ni(OH)2 + 6 NH3 + 2 NaCl => [Ni(NH3)61C12 + 2 NaOH.
The solid nickelhexamine chloride oan readily be
separated by filtering or decanting.
5a
23189-7724
CA 02137762 2004-06-14
Particularly good results are achieved if the process
according to the invention is carried out at temperatures
in the range from 30 to 85°C, preferably 45 to 80°C.
An essential feature of the process according to the
invention is that the metal hydroxide,.used.is in reactive
form since it is only in that case that it is dissolved
completely during the complexing. A particularly suitable
reactive metal hydroxide is a freshly precipitated metal
hydroxide. Also advantageous is the use of a reactive
metal hydroxide which has been obtained by anodic
_ oxidation of metal.
A process for preparing a suitable reactive nickel hydroxide
is disclosed in German Patent DE 4 239 295 C2. This is a
process for preparing pure nickel hydroxide by anodic
oxidation of metallic nickel in aqueous electrolyte solution
in the presence of sulfate ions and separation of the nickel
hydroxide formed, in which process a chloride- and
sulfate-containing nickel hydroxide is prepared which is
then converted into pure nickel hydroxide as a result of the
subsequent treatment with alkali-metal hydroxide solutions.
If, in the process according to the present invention,
the reactive metal hydroxide is to contain doping
elements such as, for example, is sometimes required in
relation to nickel hydroxide for the production of
batteries, the process according to the invention can be
carried out in the presence of cadmium, cobalt,
magnesium, calcium and/or zinc salts, these preferably
being used as sulfate and/or chloride salts.
It is also advantageously possible to carry out the
preparation of the reactive metal hydroxide in the case
of anodic oxidation in the presence of cadmium, cobalt,
magnesium and/or zinc, said elements being connected as
metal anodes.
BRIEF DESCRIPTION OF THE DRAWING
The invention is further illustrated by a single figure
of drawing.
- 6 -
23189-7724
CA 02137762 2004-06-14
~7ETAILED DES~tIPTI~N OF PREFERRED EMBODIMENTS
In a preferred embodiment of the process according to the
invention, the metal hydroxides are doped with one or
more of the elements Co, Zn, Mg, Ca and Cd in a total
amount of up to 10~ by weight.
The mother liquor produced in the process according to
the invention contains exactly the stoichiometric amount
of sodium hydroxide solution which is necessary for the
reaction to form compact, spherical metal hydroxide. It
is therefore preferred to carry out the process
continuously. In this case, the complexing agent and the
alkali-metal salt can advantageously be fed back to the
first step. The decomposition of the metal complex salt
can, for its part, be carried out with the alkali-metal
hydroxide solution formed in the first step.
The reaction of the initial stage proceeds with high
yield and rate if the components are vigorously mixed.
Particularly advantageously, the metal complex salts are
decomposed in a strongly turbulent flow which is
generated by vigorous stirring, by passive or active
mixing components or by flow nozzles. The decomposition
in a reactor is carried out with a defined dwell time of
0.5 to 10 hours, preferably of 1 to 4 hours.
A loop reactor has proved particularly advantageous as
reactor. The products ammonia and common salt produced
in the reaction to form metal hydroxide are fed back to
the first step and are again used therein to prepare the
metalamine complex, with the result that the entire
process can be run without waste water.
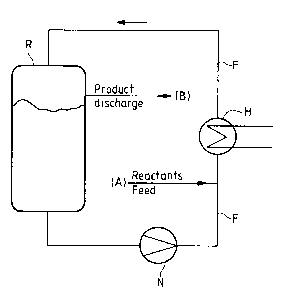
A loop reactor is described in its general embodiment in
Ullmann, vol. B4, pages 172-179. It is shown
diagrammatically in the drawing and comprises a reactor
vessel R, a heat exchanger H, a nozzle N and flow piping
F. Here, A denotes reactant feed and B denotes product
discharge. If the reactor is designed as a loop, a
continuous operation is possible and this allows, the
reaction of the solid amine complexes with the alkaline
mother liquor to be adjusted for the various metals as a
result of the choice of circulation speed so that the
2~~77s~
required physical properties of the products can be
adjusted by means of the dwell time, temperature and pH.
A stirred tank reactor of a given volume with a specified
stirring apparatus cannot, on the other hand, be adapted
flexibly enough for various metal hydroxides having
different morphology to be capable of being produced in a
reactor. The choice of circulation speed in the loop
reactor and the incorporation of various mixers such as
static mixers, active in-line mixers or nozzles (in
addition to, or in line of, item nozzle drawing) offer
the guarantee that the requisite mixing of the reactants
with the reaction medium takes place in a very short
time, which is not possible with the mixing intensity in
other embodiments of a reaction system having the desired
flexibility.
A nickel hydroxide obtainable by the process according to
the invention is suitable, in particular, for use in foam
anodes. Its typical characteristic data are:
- tap density (ASTM B 212): > 2.0 g/cm3
- BET specific surface (DIN 66232): 10 to 20 m2/g
- mean particle size D50: 10 to 20 um
- porosity: 47 to 53~
- swelling in 3-molar KOH solution: < 10~
- specific charging capacity in a foam anode (AA
cell): > 250 mAh/g
- water content: < 1~
The invention is described by way of example below
without any restriction having to be perceived therein.
STA 67-Foreign Countries - 8 -
2137762
Examt~les
1. Preparatiori of a reactive nickel hydroxide
a) Electrolysis
An electrolysis reactor comprising an
electrolysis cell (70 1) was filled with 200 1
of common salt solution (50 g NaCl/1) and the
electrolyte solution was circulated between two
containers by means of a centrifugal pump. Two
tantalum baskets whose side surfaces were
formed as a sieve and filled with Ni briquettes
were suspended in the electrolysis cell. The
tantalum baskets were connected as anode and a
pure-nickel sheet connected as cathode was
arranged opposite the side surfaces so that the
total electrode area was 0.5 m2. Electrolysis
was carried out at 4.2 V and 500 A, with a
current density of 1000 A/m2. During the
electrolysis, 200 ml of a solution of nickel
sulfate and cobalt sulfate (250 g NiS04
7H20/l; 250 g/1 CoS04 ~ 7H20) per hour were
continuously fed into the electrolysis cell.
After 5 h, 40 1/h of the suspension produced
were continuously discharged from the
circulation vessel and fresh common salt
solution was simultaneously pumped into the
electrolysis cell so that the liquid volume in
the electrolysis reactor remained constant.
The suspension was then filtered batchwise and
in the further course of the electrolysis, this
filtrate was fed back into the electrolysis
cell instead of the fresh common salt solution.
Consequently, the additional common salt
solution was used only in the initial phase of
the continuous operation, after which a closed
circuit was then established during the steady-
state operation of the reactor. The suspension
was capable of being filtered well and a gel-
like primary product was obtained which had a
water content of 90~ on average. The water
entrained therewith was fed back again into the
system as wash water for the gel-like primary
STA 67-Foreign Countries - 9 -
2137?6~
product. Chemical analysis yielded on average
a sulfate content of 1.8o and a chloride
content of 2o in the dried gel. The duration
of the experiment was 105 h in total. During
the continuous operation a total of 870 kg of
gel-like primary product was obtained during
this time.
b) Conditioning in NaOH
200 kg of the moist gel-like primary product
from the electrolysis reactor were finely
dispersed by vigorous stirring into a heated
double-jacket reactor containing 200 1 of
water. A pH of 13.7 was then established with
NaOH and the suspension was heated to 80°C
while stirring and kept at this temperature for
6 h.
Filtering was then carried out via the nutsch
filter and the product from the nutsch filter
was washed with water. After drying in a
drying oven, 19.7 kg of nickel hydroxide having
a Co content of to were obtained. The anionic
contaminants were less than 500 ppm. The bulk
density was 1.3 g/cm3 and the tap density was
1.8 g/cm3. The specific BET surface (measured
by the N2 one-point method) exhibited the very
high value of 88 m2/g. The half width of the
(101) reflection was 2Ø
2. Preparation of nickelhexamine chloride
a) Discontinuously using NHS (25%-strength)
1 kg of ammonia (25o-strength) was added to 1 1
of a suspension of 100 g/1 of highly active
nickel hydroxide gel prepared electrolytically
in accordance with Example 1 and 280 g/1 common
salt and stirred for 1 h while cooling. The
reaction mixture was filtered off by suction
via a laboratory nutsch and washed out
portionwise with a little concentrated NH3.
226 g of [Ni (NH3) ~] C12 were obtained with a
yield of 90.50.
STA 67-Foreign Countries - 10 -
z1377sz
The mother liquor contained 9.5 g of complexly
bound nickel hydroxide and 78 g~ of NaOH and
excess ammonia. The excess ammonia was
distilled off via a short packed column as 250-
strength aqueous solution.
In this process, 'the 9.5 g of complexly bound
nickel hydroxide was precipitated as a
filterable product which sedimented well.
After filtration and washing out with a little
water, 1.5 1 of filtrate containing 280 g of
NaCl (3.2 mol/1) and 78 g NaOH (1.3 mol/1) were
obtained.
b) Continuously with NH3 (25%-strength)
0.5 1/h of a suspension of 100 g/1 highly
active nickel hydroxide gel and 280 g/1 NaCl,
and also 0.5 1 of ammonia (25%-strength) was
pumped continuously while stirring and cooling
into a 21 beaker having an overflow. The
overflow was fed via a dip pipe into an
elutriator in which the nickelhexamine chloride
formed sedimented. The filtrate draining from
the elutriator contained 2 g/1 complexly bound
nickel hydroxide and 40 g/1 sodium hydroxide,
and also 140 g/1 NaCl and excess ammonia. For
a mean dwell time of 4 h, this corresponds to a
degree of conversion of the highly active
nickel hydroxide gel to nickelhexamine chloride
of 920. 1 1 of the filtrate containing 190 g/1
NaCl and 40 g/1 NH3 was worked up as in
paragraph al) using a short packed column.
25o-strength ammonia was obtained as condensate
and, after separating the precipitated nickel
hydroxide, 700 ml of process liquor containing
140 g NaCl (3.4 mol/1) and 40 g NaOH (1.4
mol/1).
c) Continuously with NH3 (50o-strength) as vapor
The reaction of highly active nickel hydroxide
gel and common salt with ammonia was carried
out continuously in a cooled 10 1 propulsive
jet reactor.
STA 67-Foreign Countries - 11 -
213??62
Ammonia was obtained from dilute process
solutions by evaporation via a packed column
and fed directly, without prior condensation,
as a 50%-strength NH3/H20 vapor mixture to the
propulsive jet reactor. 3 1/h of a suspension
of 100 g/1 highly active nickel hydroxide gel
were fed continuously into the propulsive jet
reactor along with 280 g/1 common salt and 600
g/h NH3 and 600 g/h H20 as a vapor mixture.
The overflow of the propulsive jet reactor was
connected via a dip pipe to an elutriator in
which the nickelhexammine chloride formed
sedimented. The clear filtrate draining off (4
1/h) contained 3 g/1 complexly bound nickel
hydroxide, 210 g of NaCl, 49 g of NaOH and 70
g/1 NH3. This corresponds to a degree of
conversion of 960.
3. Preparation of compact nickel hydroxide
a) Continuously in a 5 1 continuous-flow stirred
tank reactor.
A viscous suspension of nickelhexamine chloride
(400 g to 600 g of NH3, 7o-strength) was kept
pumpable into a feedstock container by
stirring. Of this suspension, 500 g/h was
continuously pumped into a 5 1 continuous-flow
stirred-tank reactor heated to 70°C. As a
second component, approximately 1.2 1/h pH-
controlled process liquor containing 60 g/1
NaOH and 140 g/1 NaCl were added.
The mean dwell time in the reactor was
approximately 3 h. After 21 h, a compact, very
readily filterable nickel hydroxide having a
packing density of 1.6 g/cm3 was obtained from
the suspension draining off after filtration
and washing with H20.
b) Continuously in a 100 1 propulsive jet reactor
A propulsive jet reactor in which optimum
mixing of the reactors and generation of high
shearing forces were ensured by means of mixing
nozzles and a high-capacity circulating pump
STA 67-Foreign Countries - 12 -
2137762
was used for the reaction of nickelhexamine
chloride and sodium hydroxide to form compact
nickel hydroxide. A suspension of 400 g of
nickelhexamine chloride in 600 g of NH3, 70-
strength, was kept pumpable in a feedstock
container by stirring. 20 kg/h of this
suspension were fed via a hose pump into the
propulsive jet reactor which was kept at a
temperature of 73°C. Approximately 43 1 hour
process liquor containing 65 g/1 NaOH and 200
g/1 NaCl were fed in continuously as reaction
components. The process liquor was added in a
pH-controlled manner, the pH being kept between
11.4 and 11.6. The mean dwell time was on
average approximately 1.6 hours.
After 16 h a very rapidly sedimenting, compact
nickel hydroxide which was extremely readily
filterable and was capable of being washed out
with approximately 7 1/kg of warm water to a
chloride content of < 500 ppm was obtained from
the overflow of the propulsive jet reactor.
The packing density of the nickel hydroxide was
on average 1.9 g/cm3 and the tap density 2.1
g/cm3. The mean particle size of the spherical
particles was on average 12 ~.m.
a
STA 67-Foreign Countries - 13 -