Note: Descriptions are shown in the official language in which they were submitted.
-2- 2 137983
BACKGROUND OF THE INVENTION
The instant invention relates to magnesite-carbon refractory
mixes for forming refractory shapes, particularly brick,
utilizing a metallic additive.
Carbon-containing refractory brick have long been in use in
metallurgical vessels where the lining is subject to slag attack
and where high hot strength and high slag resistance of the
refractories are desired and required. Improvements have been
made in such brick in which carbonaceous material such as pitch,
tar and the like which were used to form the bond of the
magnesite-carbon brick have been replaced with flake or vein
graphite.
While this has resulted in improvements, it was found that
loss of carbon in the brick was a factor which limited service
life. It was noted that the addition of metals such as aluminum,
silicon, magnesium, and alloys thereof increased hot strength and
oxidation resistance by forming carbides, lowering the
permeability of the brick by spinel formation, and by consuming
oxygen that would otherwise have oxidized carbon.
However, the addition of these metals has had in and of
itself a number of well known undesirable effects, one of them
being the troublesome problem of explosive dust cloud formation
which can occur with the use of the metallic powders. In some
instances, companies specify, for safety purposes that the metal
powders have a maximum of 5% of -100 mesh content. Thus, it has
been known to utilize coarse metal powders or metal alloys; that
~1~798~ ''J
-3-
is, those having a particle size larger than 100 mesh. While
eliminating the problem of explosion, this has resulted in
refractory shapes such as brick that contain magnesium having
undesirably high thermal expansion and static modulus of
elasticity. Use of such brick in metallurgical vessels such as a
Basic Oxygen Furnace (BOF) has resulted in excessive spalling and
cracking of the brick caused by thermal stresses which arise when
the constrained brick are heated to operating temperatures.
SUMMARY OF THE INVENTION
The instant invention overcomes the problems of prior art
magnesium-containing MgO-C brick and provides refractory mixes
which can be formed into refractory shapes without the risk of an
explosion during processing while also having low thermal
expansion, low static modulus of elasticity, and high 2000~F
modulus of rupture. The consequence of the positive improvement
in all three of the aforementioned properties is an improved
thermal stress tolerance for a magnesite carbon refractory.
Briefly stated, the present invention comprises a magnesite-
carbon refractory mix for forming refractory shapes comprising
magnesite, graphite and a fine particle size metallic additive
consisting essentially of a magnesium-aluminum alloy co-milled
with magnesite.
The invention also comprises the refractory shapes,
particularly brick, formed from such mixes which have lower
thermal expansion than conventional magnesium-containing MgO-C
brick and refractory linings formed using such shapes, which
213798~-'
--4--
during heat-up create less stress while also having the added
ability to tolerate higher stress levels than a conventional
magnesite-carbon lining.
BRIEF DESCRIPTION OF THE DRAWINGS
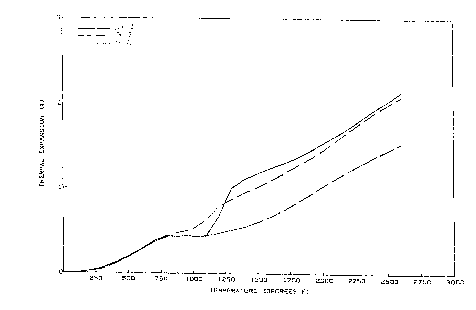
Figure 1 is a graph showing the thermal expansion of the
brick of Examples 1 to 3, and
Figure 2 is a graph showing the thermal expansion of the
brick of Examples 12 and 13.
DETAILED DESCRIPTION
The term "magnesite-carbon brick" is used herein as
generally defined in the metallurgical and refractory industries;
namely, brick that contain graphite in combination with a
magnesite such as deadburned magnesite and the like, with the
brick containing in excess of about 5 % by wt. carbon.
The magnesite used in the subject brick is most suitably
deadburned or fused magnesite, preferably one containing at least
97 wt. % MgO, most preferably at least 98 wt. % MgO. Although
lower levels of MgO content are still operative, the magnesite
should have a purity of at least 90% MgO.
As to the graphite, it is preferably flake graphite,
although vein graphite can also be utilized. It's sizing and
purity are not critical in this invention, but should be that
conventionally used in MgO-C brick making.
These two components are utilized in the usual proportions
forming mixes to be made into brick, ordinarily about 70 to 95
wt. % magnesite and, correspondingly, 5 to 30 wt. % graphite.
'~ 21'37g~
_ -5
Magnesite, as is noted, is the major component of the mix with
the only other essential component being the metallic additive of
the instant invention.
Such metallic additive is preferably a magnesium-aluminum
alloy co-milled with magnesite. It may be possible to use
discrete additions of magnesium and aluminum metal co-milled with
the magnesite to the fine size desired. For safety and reasons
of economy, it is preferred to use a magnesium-aluminum alloy and
preferably one that is a 50/50 admixture of the metals, although
various ratios of magnesium to aluminum may be employed. It is
envisioned that other metals or metal alloys, such as silicon and
boron, or mixtures thereof, may be co-milled with magnesite to
improve the thermal stress tolerance of the resultant refractory.
As to the magnesite co-milled therewith, it is preferred
that the total additive comprise at least 60 wt. % of the
magnesite with the balance being the alloy, although from 40 to
80 wt. % of magnesite can be utilized with the balance being the
alloy. The same magnesites as discussed above can be used
although it is preferred to use a magnesite containing at least
95 wt. % MgO. Of critical importance is the fact that the
milling must be such to give a resultant particle size which
imparts improved thermal stress tolerance to the refractory. The
particle size of the co-milled powder is 100% -28 mesh with there
being greater than 5% -100 mesh. Preferably, the co-milled
metallic additive has at least about 80% -200 mesh and most
preferably one in which the particle size of the metallic
7 9 ~3 -)
_ -6-
additive is at least about 50% -325 mesh. All mesh sizes
discussed herein are Tyler mesh sizes. It has been found that the
utilization thereof results in mixes which can be formed into
brick having unusually low thermal expansion for magnesium-
containing MgO-C brick.
As is conventional in making magnesite-carbon brick, it is
necessary also to include in the mix a carbonaceous bonding
agent, examples of which are novolak or resol resins. The amount
added is ordinarily about 1.5 to 6% by wt. based on 100 wt. % of
the mix.
Other than the fact that it is essential to co-mill the
metals and the magnesite in forming the metallic additive, the
method of forming the brick is not critical in that the
components noted are simply thoroughly admixed, pressed into
shape in the usual brick-making presses and then cured at the
usual temperatures; namely, about 250 to 500~F to form the cured
but unburned brick which can then be used as linings in
metallurgical vessels. When placed into metallurgical vessels as
a lining, the bricks become exposed to the high temperatures in
such furnaces and form carbon-bonded brick of high hot strength,
improved slag resistance, and most importantly of lower thermal
expanslon.
The invention will be further described in connection with
the following examples which are set forth for purposes of
illustration only.
i'- 213~983 '.-i
_ 7
EXAMPLES 1 TO 3
Three different mixes were made whose formulations, in wt.%,
are set forth in Table I below. Only the mix of Example 2 is in
accord with the present invention. The co-milled metallic
additive contains 3 parts by weight magnesite (- 97 wt. % MgO)
and 1 part by weight 50-50 Mg-Al alloy. Use of 12 wt. % of the
~' -8-2~'3~9~3 J
. .
o~o o~P
I I ~ A O ~ C~
r~ ~
o o~ O
u~ u
~P . . ~
a~ ,1 ~ r~ ~ ,~ ,~ I I o a~ ~ ~ o
L
Vl
O
~' ~ -rl ,.. .
a~ ~ ~1
o\O ~
~¢ V
~ ~ V ~ ~ V.., ~ 0 V
a ) ln a) Q)
a~ ~3 0 a~ r 1~ V ~ 13 .C .t
00 .C O ~ ~ V~
o ~ ~ a c -, u~ o ~ ~o VJ a)
~ I o ~ ~ ~ ~~
o + ~ v~ P~ o ~ c~ ~ + +
+ ~ + O U~
o oo ~ a,,~ ~ a ~ o~ Oo u~
- ~ ~ V~
X 'I ~1 0 ~ ~1
~ ¢ . U X
9 8 ~
h
o
a
o
., , "~
,
.. o
a ~,
al 5
--
~1
a al ~ O
a ~ co
,
, ~ ~ o o ~ ~o
o~
~U
~r ~
a,1 ~ ~ c~ E~
X ~:
H,~
O
E~ o
0 ~ O
u~ ~1 0
~l ~ 0
~ ......... m a~
v
V, ul
o :~ 0 u~ 0 u) o o o o u~ In
r~ O O Ul O 1
O ~- ~ ~ r~
~v ~ o ~
_V ~I ~
V ~ 11
~V J
~1~1gS3 --
The mixes were formed into brick by conventional means. The
brick were then tested to measure their physical properties. The
processing conditions and physical properties are set forth in
Table II below.
~. -11- 'J
-- 213'198~
a~
o o ~n
~ . . o o o ,~
o . CO ~ o ~. C~ o
CO CO o ~ ,' ,, o o ~ o
a~ ~ + ,~ ,1 ~ ~ + +
., ,,
~D ~ ~ O O
,~ ,~ o t~ I~ ., ~ ,~ , o
a~ ,~ + ,~ ,~ ~ ~ . + +
o
,, Ul
~o . . o o .,
O . 0~ m a~ ~ ~ o~ ~ -
O~ CO O ~ ~ I~ ~ ~ O
, o~ ~ + ~ ~ ~ ~ + +
U~
o ~
,, ..
q~O ~- ~
UX o\~ ~ X ~ ~-
~, ~, P, o
o o
~, ~o ~ ~ o\~ o
-ns~ o t~ o .~ ~ o
u n~o ~ v
r ~ ,4 ~q-~ n ~,
4 C 1~ V O
~) VJ
~ ~ v ~~ ~ a
s ~,~ n ~ 4 U~ -- ~- t~
U ~, ~0~ ) 0~ ~ O ~'
t ~ ~ ~L C.) ~1) a ~ ~I o ~ o ~ o ~, P:
C D 11 o ~ O s~ o ~
o ~ a ~ ~, ~ q~ o J~ oo al
ZSl-r~ ~3 r.~ (~J U . ~ O ~ ~ S' t,~
E~ al ~ 0-~
a ~ n
c~ P
X ~ X ~ a~
t~3 ~
Xtd ~ ~ :5 0 ~ 1 0 ~ ~ al
r~ ~ 5 ~ a X ~ ~ P4
J
- 2137983
The brick of Example 2 had a significantly lower linear
thermal expansion and permanent linear change as compared to the
brick of Examples 1 and 3 (see Fig. 1).
Figure 1 shows that brick of Example 2 did not show a rapid
increase in expansion between 1000 to 1250~F as did brick
containing discrete or alloyed metallics without being co-milled
with magnesite. The sharp rise in thermal expansion between
lOoO - 1250~F is believed to be very detrimental to brick
constrained in a furnace lining.
EXAMPLES 4 T0 11
A series of eight mixes having the formulations shown in
Table III below were prepared and formed into brick as set forth
13 J
2l~3~g83
o~O
,1 ~ ~ 1' 1 ,1 1 1 .
o\O
o\~ u~ m
O~o ~ a
a~ . 1
0~0 a~ r _
H + ~
~~,,
r ~ u~
o\~
4 ~ ~
~\~ ~1 ~J~ oD 1' ~ ~ I I I o\~ o~~ o\~
I I I ~ VU~ ~ o
O O O O
x - ~ - ~ u~ ~ ~
~ ~ O O O
a r
0 ~ -,¢ ,¢ ,¢ ~ X
a~ ~ 0 ~ ~X aiX X X
co ~ ,~: ~ 3 ~ 0 u~ Itl O O ~ ~:
~ O ~ Ul al ~ o a ~ o - o o o
--,1 0 0 0 P ~
o + ~ a~ o ~ ~ - I I I
Z ~ + ~ rlO O o
~ o oo ~ 0 0 ~ ~~ ~------o ~ I u~
,~ u I I I ~ ~ I ~ o 0\o 0~O
X ' 'I ~ ~ U
~ v
21~7983 'J
_ 14
The brick processing conditions and physical properties of
the brick are set forth in Table IV below.
1q~3
o o
o ~
o o~ ~ o ~
,~ o r~ o r1 ~1
+
~1 ~1 0 0
~ ~I N a~
O O ~ Ot~t' O ~ O~ CO
~ ~ ~~ ~ ~~ --~ ~ N r~
+ ~~
O O O
O ~1 0 r'rl a~ ~ N
O CO + ~1
O~ ~1 rl
O O O
~1 ~ 1'~1 a~ ~1 N
1' 00 0
oo al ,~ +
a~ o o
~In ~o o oo
O l~r' ~ ~ O N
H ~ O + ~1~1 a~ ~~7 N ~~
r~ ~ ~ oo
Ir L ~~ t~ ~ ~
~O ~ O t'
a~ o o
o ~ o
a~ I' o 1' l~ ~ ~ ~ ~1
o o
.1 ~ O111 N O ~D t'
0~ ~1 + ~1 ~10~ ~ N ~)
rl
~:~ vn
~~ ~ o
~n ~ ~,10
~ ~V~ ~ O ~
J ~!~ N In ~ F' r
11 :30 ~rl ~ aJ ~-~ ~ ~r,_ ~r
.~ , ...... n,u~ J~ ~ .~.rl U ~-- I J ~
~ ~ X ~ ~ u n x ~ ~
o ~ , una ~~ vu~ ~ u.
z ~~ u InL E3 ~ U ~~ V ~ O ~
o ~ ~ 4 C~ o C t~o C
a ~ o a ~ a . ~ ~un o-, ~ o-_
c a ~--~ c ~ o l ~, o ~
r~ r~ 0-~ ; CO-~
X ~ ~ ~ ~ Ei X ~ ~ ~ N ~: r t~l "C
x ~, ~ o ~ 10 ~J C ~l ~ C
m m E~ ~ m cl Pm~ X~CJ va~cJ
3 7 9 8
o
~ . ~ ~ .
,, oo o ,, ~ o
, . +
+
o
r1 1 1 1 1 1
OD ~ O~ O
~ o . ~ .
,, + o ~ o
a~ +
r- l l I
., I I I I
~ l I
r~
_, ~, ~I
r~ O
H ~ O ~ ~ ~
I L ~ + + ~I ~ O
~r
U) I I I I I
!
~ In
o In ~ o
~ O
O ~ ~
+ + r~l ~ O
r ~,1
c, , ~n
: o o~
o ~ o
~n rn,~
_ ~ J ~ X ) 4
r n~ o r
~- ~ o p
o
~I Q ~ C ~ O n
5 ~ o ~ n
Q~ ~ o4 ~.U Or a1
0 o
E~ o : ~ O r
Ql O 1 q~ n
~ ~ o ~ ~ q U~
C
~C r
J
721~7983
Comparing the brick of Examples 4, 5 and 6 to those of
Examples 7 to 11 again, the brick made utilizing the co-milled
metallic additive had lower linear thermal expansion, lower
permanent linear change, lower static modulus of elasticity
(MOE)at 2000~F, higher crushing strength at 2000~F, and a higher
thermal stress ratio* which i8 desirable. The thermal stress
ratio is determined by the following formula:
*Thermal Stress Ratio = Crushinq Strenqth
MOE x Thermal Expansion
Examples 12 and 13
Two mixes based upon use of higher purity raw materials were
made and their formulations in wt. %, are set forth in Table V
below. Example 12 contains aluminum metal plus a coarse metal
alloy. Example 13 also contains aluminum powder and co-milled
powder used in the prior examples. This imparts into the mix of
Example 13 2% Mg/Al metal, so it is a direct comparison to the
mix shown in Example 12. The mixes were formed into brick as
previously described and the brick were tested as set forth in
Table VI below.
A review of this data indicates the same trend as seen in
the prior examples. The mix with the co-milled alloy and
magnesite had lower apparent porosity, higher crushing strength
at 2000~F and lower thermal expansion (see Figure 2), than a
similar mix with the ~ame metal content, but without the co-
milled magnesite. The thermal stress ratio of Example 13 showed
a 13% improvement over the thermal stress ratio of Example 12.
~' ~J
2137983
-18-
TABLE V
Example No. 12 13
Deadburned Magnesite
(99% Purity)
-4+10 mesh 34.0% 34-0%
-10+28 mesh 33.0 33.0
Fines 16.5 10.5
Flake Graphite
-100 mesh, 99% Purity 12.5 12.5
Co-milled 50 Mg-50 Al
Alloy + MgO,
-28 mesh -- 8.0
Aluminum Powder,
-65 mesh 2.0 2.0
50 Mg-50 Al Alloy,
-10+65 mesh 2.0 --
Plus Additions:
Phenolic Resins 3.7 3.7
Calculated Screen Analysis
% Held on 10 mesh 30% 30%
-10+28 mesh 31 30
-28+65 mesh 11 13
-65 mesh 28 27
-150 mesh 23 22
-325 mesh 15 14
~1'37983 '
-- --19--
TABLE VI
Example No. 12 13
Mix Details:
Alloy Coarse 5OMg-5OAl Co-milled
50Mg-50Al
and MgO
Mixing Time, minutes: 4 4
Batch Temperature after Mixing, ~F: 92 94
Bulk Density at the Press, pcf: 184 184
Total Linear Expansion from Mold Size
After Curing at 350~F, %: +0.4 +0.4
Bulk Density after Baking, pcf 181 180
Data from Porosity (After Coking)
Unimpregnated
Bulk Density, pcf: 175 176
Apparent Porosity, %: 12.1 11.1
Apparent Specific Gravity . 3.18 3.16
Stress-Strain Tests
Static Modulus of Elasticity
(xlO6 pSi):
At 2000~F: 1.09 1.22
Crushing Strength, psi
At 2000~F: 4850 5160
Thermal Stress Ratio 0.32 0.36
Modulus of Rupture
At 2000~F: 1880 1900
Crushing Strength, psi
At 2800~F: 4360 4090
Thermal Expansion
% Linear Change at 2600~F: 2.00 1.71
~ ~ 3 ~ 9 8 3
-20-
While the invention has been described in connection with a
preferred embodiment, it is not intended to limit the scope of
the invention to the particular form set forth, but on the
contrary, it is intended to cover such alternatives,
modifications and equivalents as may be included within the
spirit and scope of the invention as defined by the appended
claims.