Note: Descriptions are shown in the official language in which they were submitted.
2138024
O.Z. 0050/43333
Simultaneous vroductioa of dicarboxylic acids and
diamines by s~littina polvamides into their moaomeric
constituents
The present invention relates to a process for
the simultaneous production of dicarboxylic acids and
diamines from
a) polymers based on polyamides of dicarboxylic acids
or their derivatives with diamines, or
b) compositions containing essentially such polymers,
by splitting these polymers into their moaomeric con-
stituents.
The splitting of polyamides such as nylon 66
(polyhexamethyleneadipamide, PA 66) into their monomeric
conatituents can be carried out in a neutral or acid
medium but in general it is preferably carried out in a
basic medium, inter alia because of the shorter reaction
time.
FR-A-926 873 describes the splitting of poly-
amides such as PA 66 and PA 610 with inorganic bases, for
example with a from 10 to 15% strength by weight alkali
metal hydroxide solution such as sodium hydroxide solu-
tion, at 200°C and about 15 bar. The resulting diamine is
then extracted or distilled out of the reaction mixture
and further purified by vacuum distillation. According to
this reference, the free dicarboxylic acid is obtained by
addition of a strong acid such as hydrochloric acid to
the diamine-free reaction mixture and subsequent precipi-
tation.
In IT-A-553 182 an excess of 20% strength by
weight of sodium hydroxide solution at 220°C and 25 bar
reduces the reaction time compared with the process of
FR-A-926 873. The diamine is extracted from the aqueous
solution with a-butaaol. One example concerns the removal
of insoluble titanium dioxide, previously present in the
polymer in the form of fibers, by filtration after
breaking dower. The dicarboxylic acid is likewise freed by
2138024
- 2 - O.Z. 0050/43333
addition of a strong mineral acid.
FR-A-1 070 841 describes the splitting of PA 66
with alkali metal or alkaline earth metal hydroxide
solutions. According to this reference, the reaction
mixture is initially worked up by acidifying with sul-
furic acid and than the precipitated adipic acid is
separated off. Thereafter the filtrate is admixed with
potassium hydroxide solution, and hexamethylenediamiae
separates as an oily layer which can be separated off and
purified. This reference also describes the splitting and
workup of polymers and copolymers that contain polycapro-
lactam (PA 6) .
DE-A-1 088 063 describes the splitting of PA 66
in a 10% strength by weight methanol Na08 solution. The
disodium adipate obtained is converted into the free acid
by acidification, while hexamethyleaediamine (~) can be
obtained in pure form by distillation.
US-A-2 840 606 describes the splitting of PA 66
into disodium adipate and HMD in a two-phase
C3-C,-alkanol/water mixture. According to this teaching,
the HMD is isolated from the alcohol phase by distilla-
tion. The adipic acid is obtained by acidifying the
aqueous phase with sulfuric acid and may be purified by
crystallization.
DE-A 39 26 642 describes a process and an
apparatus based on a four-compartment electrolysis cell
for obtaining an acid from its salt. However, no mention
is made of reaction parameters and examples in
DE-A 39 26 642.
A feature common to all these processes is the
isolation of adipic acid through acidification of the
respective alkali metal or alkaline earth metal salt
solutions. The inevitable inorganic salt by-product,
usually sodium chloride or sodium sulfate. not only
interferes with the attempt to purify the dicarboxylic
acid by crystallization, since it inhibits the latter,
AMENDED SBEET
2138024'
but also constitutes a considerable disposal problem.
A further disadvantage is that the processes
described cannot be suitably employed for working up
technical, for example fiber-reinforced, mineral-filled
and/or impact-modified, molding compositions that contain
PA 66, since the various additives would disrupt the smooth
running of the processes in question.
It is an object of the present invention to
provide a process for the simultaneous production of
dicarboxylic acids and diamines that shall be free of the
abovementioned disadvantages.
We have found that this object is achieved by a
process for the simultaneous production of dicarboxylic
acids and diamines from
(a) polymers based on polyamides of dicarboxylic
acids or their derivatives with diamines, or
(b) materials comprising essentially such polymers,
which comprises
(1) cracking these polymers into their monomeric
constituents using a base in an alcohol-water
mixture comprising from 5 to 40% by weight of
water to obtain a liquid phase and a solid
phase,
( 2 ) separating the solid phase from the liquid phase
to obtain a liquid phase comprising the diamine,
and
(3) electrochemically converting the resulting
dicarboxylic acid salts of adipic acid or
sebacic acid into the corresponding dicarboxylic
acids and bases.
Suitable polymers based on polyamides of
dicarboxylic acids or their derivatives, for example the
corresponding acid halides, preferably the acid chlorides,
with diamines are from observations to date poly-
hexamethyleneadipamide, polyhexamethylenesebacamide and
polytetramethyleneadipamide, preferably polyhexamethylene-
adipamide.
i
213824
3a
Suitable compositions containing essentially such
polymers, ie. at least 50% by weight of such polymers, also
include for example copolyamides with PA 66 and also PA 66
or copolyamides with PA 66 containing fibers and/or
additives.
The bases used for splitting the polymers are in
2138024
- 4 - O.Z. 0050/43333
general alkali metal hydroxides such as lithium
hydroxide, sodium hydroxide and potassium hydroxide,
preferably sodium hydroxide, or mixtures thereof,
preferably a mixture of sodium hydroxide and potassium
hydroxide.
It is preferable to use from 1.8 to 4.0, prefer-
ably from 2.0 to 3.0, equivalents of alkali metal
hydroxide per repeat unit of polymer, for example
- [- (CHs) s-CO-NH- (C8=) 6-NH-CO-] - in the case of PA 66 . If
less than 1.8 equivalents of base are used, the result is
in general as undesirably high proportion of oligomer. If
more than 4.0 equivalents of base are used per repeat
unit, this leads in general, in particular in the case of
glass fiber-reinforced and/or mineral-filled polyamide
molding compositions, to a high degree of degradation of
the glass fibers or of the mineral fillers.
In general. the alkali metal hydroxide is used in
the form of a from 5 to 25, preferably from 10 to 15, %
strength by weight solution is a C1-C,-alkanol. If
desired, instead of one alkaaol it is possible to use a
mixture of different alkaaols or an alkanol-water mixture
which contains from 0 to 50, preferably from 5 to 40,
particularly preferably from 10 to 30, % by weight of
water.
The Cl-C~-alkaaols used can be in general
methanol, ethanol, n-propanol, isopropanol, n-butanol,
preferably methanol, ethanol and isopropanol.
The reaction is is general carried out at a
temperature within the range from 100 to 300°C, prefer
ably from 140 to 220°C. The pressure for the reaction is
in general within the range from 0.08 to 15 MPa, although
it is also pos8ible to employ a pressure outside this
range. Preference is given to working under the autoge-
nous pressure.
Owing to the alkali metal hydroxide, the reaction
mixture pH is in general greater thaw 7.
A1~NDED SHEET
~13802d~
The duration of the reaction depends essentially
on the concentrations of the starting material, on the
temperature and on the pressure and will in general be
within the range from 0.5 to 15, preferably from 1 to 10,
h.
The splitting with a base can be carried out
continuously or batchwise.
It can be carried out in customary apparatus with
or without stirrer, preference being given to using a
pressure vessel equipped with a stirrer system that is
particularly suitable for solids dispersion, for example a
propeller stirrer or a cross-bar stirrer.
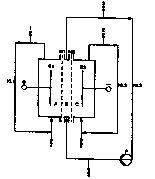
Brief description of the drawings
Fig. 1 is a diagram of a three compartment electrolysis
cell with three liquid cycles (KL 1 to KL 3).
Fig. 2 is a diagram of a four compartment electrolysis cell
with four liquid cycles (KL 1 to KL 4).
Fig. 3 is a diagram at a membrane stack cell with three
liquid cycles (KL 1 to KL3).
The present invention will be better understood
upon reading the non-restrictive description and examples.
Detailed description of the invention
In a preferred embodiment, the starting polymer
or polyamide-containing compositions are mechanically
comminuted to an average particle size from 0.1 to 50,
preferably from 1 to 10, mm before splitting. The
comminution can be carried out in a commercial mill, for
example in a cutting mill, or, preferably, in particular
when the compositions used contain hard materials such as
metal inserts, for example bolts, in a hammer mill.
j.
" i
2138024
5a
Metal parts present in the material thus
comminuted can be removed in a drying separation process
using an air table, preferably with subsequent induction
separation, using for example a free-fall tube separator,
for complete removal of the metal parts, or in a wet
separation process, for example by means of a hydro-
cyclone.
In a particularly preferred embodiment, the
polymer or composition feedstock is comminuted in a hammer
mill to a size of not more than 50 mm in length, any metal
parts present are separated off, and the millbase freed of
metal parts is then comminuted to a size within the range
from 5 to 12 mm in a cutting mill. If desired, the polymer
or polymer-containing composition thus pretreated can then
be additionally washed and dried before it is subjected to
splitting with a base.
The reaction mixture obtained on splitting the
'A
213802.
- 6 - O.Z. 0050/43333
polyamides consists in general of a liquid phase, which
contains the diamine, and a solid phase, which contains
the precipitated dicarboxylate .salt and insoluble con-
stitueats.
According to the invention, the solid phase is
then separated from the liquid phase.
Suitable processes for separating off the solid
phase are known processes such as filtration,
sedimentation or centrifuging.
Examples of insoluble constituents are glass
fibers, carbon fibers, carbon black, minerals and rubber
and any metals not removed or not completely removed
beforehand, unless dissolved by the base.
The removed solid phase may if desired be washed
in a further operation preferably with an organic sol
vent, particularly preferably with a Cl-C,-alcohol such as
methanol. ethanol, n-propanol, isopropaaol or mixtures
thereof, or with a mixture of a Cl-C,~-alcohol containing
from 0 to 30% by weight of water, very particularly
preferably with the solvent component used is the split-
ting, particularly preferably in a pure C1-C~-alcohol.
The washing can be carried out for example with
the apparatus used for the separation such as a belt
filter, a centrifuge, a filter press or a centrifugal
disk pressure filter, preferably a centrifuge or a filter
press. and/or other apparatus suitable for this purpose
such as a decanter. The washing operation is preferably
carried out in multiple stages and after each stage the
insolubles removed in the wash are preferably intimately
mixed with the particular washing medium used in order to
minimize the loss of solubles. Particular preference is
gives to using an apparatus in which insoluble matter can
be washed in countercurrent.
Ia a preferred embodiment, the liquid phase of
the as-split reaction mixture can be combined with the
liquid phases from the wash operations oa the solid phase
and used as solvent in the hydrolysis, in which case all
2138024
- 7 - O.Z. 0050/43333
or some of the diamine can if desired be removed before-
hand. Particular preference is given to recirculating the
liquid phases from the wash operations on the solid phase
directly into the hydrolysis stage.
Ia a further preferred embodiment. the diamines
obtained after the splitting are preferably separated off
after the solid phase has been separated off.
The separation of the diamiaes from the liquid
phase of the reaction mixture obtained is the splitting
can be carried out by known processes such as
distillation, preferably rectification, or extraction.
In general, the removal of the diamines is
preceded by the removal of low boilers, primarily the
alcohols or alcohol-water mixtures used as solvents,
preferably by distillation. This distillation can be
carried out in conventional evaporators. such as thin-
film or falling-film evaporators, single- or multi-
stagedly, preferably mufti-stagedly, or is a rectifica-
tion column.
The solvent obtained in this removal can be
directly used in the splitting of the polymers. However,
it can also be freed beforehand, advantageously by means
of a rectification, from impurities that interfere with
the splitting. It can also be desirable to separate off
water in this way in order to obtain in this way a
suitable composition for the alcohol-water mixture. The
rectification can is general be carried out in conven-
tional apparatus such as tray columns or packed columns
with arranged or dumped packing.
The diamines are is general isolated in conven-
tional apparatus, preferably tray columns or packed
columns if rectification is employed.
In a preferred embodiment, the diamines are
obtained in vapor form as a side takeoff from the strip
ping portion of the rectification column or, likewise
preferably, is liquid form as a side takeoff from the
enriching portion of the rectification column. The
. 2138024
- 8 - O.Z. 0050/43333
rectification is in general carried out at from 10 to 100
kPa, preferably from 50 to 80 kPa.
Any diamine still present in the rectification
residue can if desired be separated therefrom in a
further distillation step, for example using a thin-film
evaporator, at from 0.5 to 50, preferably from 2 to 30,
kPa, preferably in a force-cleaned paddle-type evapo-
rator, since it makes it possible to remove the
noavaporizable residues as solids.
The dicarboxylate salt present is the washed or
unwashed solid phase is in general dissolved therefrom,
for example from the filter cake, by admixing with water.
For this it can be necessary to use water at a
temperature that is higher than room temperature, for
example eves steam.
This operation can be carried out depending on
the choice of process for removing insoluble constituents
in the apparatus used for filtering off or washing the
insoluble constituents or using further apparatus suit-
able for this purpose such as belt filters, centrifuges,
filter presses and centrifugal disk pressure filters. If
desired, the aqueous phase is intimately mixed with the
insoluble constituents, for example by means of an
intensive mixer, before they are separated from one
another. If desired, this operation is repeated two or
three times in order to maximize the yield of dicarboxy-
late salt obtained.
Ia a particularly preferred embodiment, a very
concentrated aqueous solution of the dicarboxylate salt
can be obtained by dissolving the dicarboxylate salt out
of the solid phase in from 2 to 8. preferably 3 to 4,
stages, using a dicarboxylate salt solution. This will in
general concentrate the dicarboxylate salt solution used
and will save energy- and cost-intensive concentrating
processes such as evaporation. Preferably, the first
stage is carried out with a more concentrated dicarboxy-
late salt solution than the second stage, the second
2138024
- O.z. 0050/43333
stage with a more concentrated dicarboxylate salt solu-
tion than the third stage, etc. The last stage is is
general carried out with fully demineralized water.
Preference is given to using the most concentrated
solution for further processing (electrochemical treat-
ment), while the other, less concentrated solutions are
stored for a subsequent wash or, if the wash is carried
out as a continuous process, used at once. In general,
the wash solution of the first stage contains the
dicarboxylate salt is a concentration within the range
from 5 to 40, preferably from 15 to 30, % by weight.
The water-soluble extract, or the combined water-
soluble extracts, can then be subjected to a distillation
with or without reduced pressure in order that nay
residual alcohols and/or other volatile organic sub-
stances present therein may ideally be removed. Further-
more, it can be advantageous to concentrate the aqueous
solution prior to the electrochemical treatment by
removing the water by distillation.
The insoluble constituents optionally obtained
after the dicarboxylate salt has been dissolved can if
desired be further used as fillers when dry.
Impurities that interfere with the electrochemi
cal treatment such as alkaline earth metal cations,
silicate and polyphosphate anions or high molecular
weight organic amine compounds can advantageously be
removed from the aqueous solutions freed of iasolubles
and diamiaes and comprising essentially the dicarboxylate
salts, by treating these solutions with adsorbents and/or
suitable precipitants.
The adsorbents used are preferably activated
carbon, anthracite, calcined coke and macroporous organic
ion exchangers and also further inorganic adsorbents.
Suitable precipitants include carbonates of alkali metals
and/or ammonium carbonates.
From observations to date the manner of the
electrochemical treatment has in principle no bearing oa
2138024-
- 10 - O.Z. 0050/43333
the success of the process of the invention.
The electrochemical treatment may for example
take one of the following forms (a) to (f)
(a) In this version the splitting of the dicarboxy
late salt into the corresponding dicarboxylic acid
and the corresponding base can be carried out in a
two-part electrodialysis call using bipolar
membranes. Ia general, the electrodialysis cell has
between the anode and the cathode from 1 to 200,
preferably from 20 to 70, electrodialysis units
separated from one another by bipolar membranes. The
bipolar membranes are separated from one another by
cation exchange membranes. so that an electro-
dialysis unit has the following structure: bipolar
membrane (anode side) - acolyte compartment - cation
exchange membrane - catolyte compartment - bipolar
me~mbrsae (cathode side). The individual electro-
dialysis units are preferably electrically connected
in series.
In this version it is advantageous to feed the
aqueous dicarboxylate salt solution into the anolyte
compartment. In the electric field of as applied
direct voltage the alkali metal catioas generally
migrate through the cation exchange membrane into
the catolyte compartment. The hydroxyl anions re-
quired for compensating the separated charges are
formed by the dissociation of the water in the
bipolar membranes on the cathode side. Ia this way
the corresponding alkali metal hydroxide solution
collects is the catolyte compartment. In the anolyte
compartment the dicarboxylate anion can combine with
the hydrogen ions from the bipolar membrane on the
anode side to form the free dicarboxylic acid.
It is advantageous to feed the dicarboxylate salt
solution into the acolyte compartments in parallel.
The product streams from the anolyte compartments,
containing the free acid and unconverted
138024
- 11 - O.Z. 0050/43333
dicarboxylate salt, and the product streams from the
catolyte compartments are advantageously combined
with one another. The free dicarboxylic acid is in
general obtained by crystallization from the
combined product streams from the anolyte compart-
ment without coprecipitation of the corresponding
dicarboxylate salt, which is preferably subjected
again to the electrodialysis process.
The electrodialysis process can be carried out
not only continuously but also batchwise. A prefer-
red form of the continuous process involving a
plurality of electrodialysis cells comprises
dividing the total conversion between from 2 to 20,
preferably from 4 to 6, electrodialysie cells and
achieving only partial conversion is each electro-
dialysis cell.
It is particularly advantageous here to guide the
flows in countercurrent. The outflow from an acolyte
compartment forms the inflow into the next acolyte
compartment, etc., so that the outflow from the last
anolyte compartment is rich in dicarboxylic acid cad
lean in dicarboxylate salt. The outflow from the
last catolyte compartment, containing a low con-
centration of alkali metal hydroxide, forms the
inflow into the last but one catolyte compartment,
etc., so that the first unit has a high
concentration of dicarboxylate salt is the anolyte
compartment and a high concentration of alkali metal
hydroxide in the catolyte compartment. The result is
that the alkali metal hydroxide concentration dif-
ferences is anolyte and catolyte compartments are
small within a unit. This ultimately leads is gene-
ral to an energy saving due to a higher current
yield and on average to lower cell voltages.
The current densities are is general within the
range from 0.1 to 2, preferably from 0.5 to 1.0,
kA/m'. The cell voltage is is general from 3 to 8 V
2138024
- 12 - O.Z. 0050/43333
per electrodialysis unit.
The p8 is in general within the range from 2 to
is the anolyte compartments and within the range
greater than 13 in the catolyte compartments.
5 The compartment width is in general from 0.2 to
5, preferably from 0.5 to l, mm.
The electrodialysis temperature is is general
within the range from 40 to 110°C, preferably from
65 to 90°C.
10 The inflow and outflow velocities are in general
within the range from 0.05 to 0.2 m/sec.
The concentration of dicarboxylate salt used is
in general from 5 to 40% by weight, preferably from
10 to 20% by weight.
If desired, the conductivity in the anolyte
system can be increased by adding salts or acids
such as sodium sulfate or sulfuric acid. Substances
of this type are in general added within the range
from 0.1 to 10% by weight. preferably from 1 to 6%
by weight, based on the total weight of the solution
present in the anolyte compartment.
To the catolyte compartment it is advantageous to
add the substances which are obtained is the course
of the operation, preferably the corresponding
alkali metal hydroxide such as sodium hydroxide or
potassium hydroxide, preferably sodium hydroxide.
The inflow into the catolyte compartment general-
ly comprises fully demiaeralized water, but at the
beginning it is preferable to employ the from 1 to
25, preferably from 5 to 10, % strength by weight
alkali metal hydroxide solution formed in the course
of the electrodialysis.
(b) A three-part electrodialysis cell with bipolar
membranes has the advantage over the procedure
described under (a) that the feed materials need not
be very pure. Furthermore, in general, significantly
lower salt contents are obtained not only is the
2138024
- 13 - O.Z. 0050/43333
dicarboxylic acid solution obtained but also is the
corresponding alkali metal hydroxide solution.
The three-compartment system contains not only a
catioa exchange membrane but also an anion exchange
membrane, so that the structure of an electrodialy-
sis unit is as follows: bipolar membrane (anode
side) - anolyte compartment - anion exchange
membrane - center compartment - cation exchange
membrane - catolyte compartment - bipolar membrane
(cathode aide) .
The dicarboxylate salt solution is advantageously
introduced into the center compartment. Under the
influence of a direct current electric field the
dicarboxylate anions generally migrate through the
anion exchange membrane into the acolyte compart-
ment, where they can combine with the hydrogen ions
present there to form the free acid. Apart from
selectivity losses at the anion exchange membrane
the free acid can be withdrawn from the anolyte
compartment devoid of salt. As is (a) the catolyte
compartment yields the alkali metal hydroxide solu-
tion. The outflow from the center compartment, still
containing residual quantities of dicarboxylate
salt. can be disposed of or advantageously added to
the feed of the dicarboxylate salt dissolution stage
(where the dicarboxylate salt obtained in the crack-
ing is dissolved). Again as in (a) the flows can be
guided couatercurreatly in order to increase the
current yield.
To increase the conductivity the anolyte compart-
ment can have added to it for example an oxoacid
such as sulfuric acid, phosphoric acid or nitric
acid.
The catolyte compartment can advantageously have
added to it the substances which are obtained in the
course of the operation, preferably the correspond-
ing alkali metal hydroxide such as sodium hydroxide
_2138024
- 14 - O.Z. 0050/43333
or potassium hydroxide, preferably sodium hydroxide.
As for the rest, the process of (b) can be carried
out under the same conditions as described under
(a) .
(c) In principle it is also possible to use electro-
dialysis cells having four compartments. The layout
generally resembles that of an electrodialysis cell
with three compartments except that, to protect the
bipolar membranes from fouling, a further ion ex-
change membrane, preferably a cation exchange mem-
brane, is included. In general, an electrodialysis
unit will have the following structure: bipolar
membrane (anode side) - anolyte compartment - catioa
exchange membrane - anode-near center compartment -
anion exchange membrane - cathode-near center com-
partment - cation exchange membrane - catolyte
compartment - bipolar membrane (cathode side).
The dicarboxylate salt solution is advantageously
introduced into the cathode-near center compartment
with the dicarboxylic acid solution being withdrawn
from the anode-near center compartment and the
alkali metal hydroxide solution from the cathode
compartment.
Ia other respects, the process of (c) can be
carried out under the same conditions as described
under (b).
(d) The electrochemical splitting of the dicarb-
oxylate salt into the dicarboxylic acid and the
corresponding base can be carried out under a fur-
they embodiment in a two-part membrane electrolysis
cell known per se from chlor-alkali electrolysis.
The membrane electrolysis cell comprises is general
from 1 to 100, preferably from 20 to 70, elec-
trolysis units grouped together in a block. In this
block, the individual electrolysis units can be
electrically connected is series by electrically
connecting the cathode of one unit to the anode of
213804
- 15 - O.Z. 0050/43333
the next unit or by using internally connected
bipolar electrodes. The products generally flow in
and out via separate collector lines for each com-
partment type. The two-part membrane electrolysis
unit generally has the following structure going
from the anode to the cathode:
anode - anolyte compartment - catioa exchange mem-
brane - catolyte compartment - cathode.
The aqueous dicarboxylate salt solution is advan-
tageously introduced into the anolyte compartment.
Under the electric field of the applied direct
voltage the alkali metal cations generally migrate
through the cation exchange membrane into the cato-
lyte compartment, where they are converted into
alkali. The hydroxyl anions required for compensat-
ing the separated charges are released in the cath-
ode reaction. The cathode reaction can be for ex-
ample the cathodic evolution of hydrogen or a cath-
odic reduction of oxygen. The anolyte compartment
generally retains the organic acid radical which
combines with the hydrogen ions or their hydrated
forms released is the course of the anode reaction
to form the corresponding free acid. Aa example of
as anode reaction is the anodic evolution of oxygen
or the anodic oxidation of hydrogen. The anode
compartment will thus have in general become leaner
in the salt and richer in the free dicarboxylic
acid.
The membrane electrolysis process can be carried
out not only batchwise but also continuously. If it
is carried out over the continuous process, one
option is to divide the conversion between from 2 to
20, preferably from 4 to 6, cells and to guide the
flows countercurreatly (see (a)).
The dicarboxylate salt solution used, which may
contain a plurality of such salts, has in general a
concentration of from 1% by weight up to the
2138024
- 16 - O.Z. 0050/43333
saturation limit of the salt(s), preferably from 5
to 35, particularly preferably from 15 to 30, % by
weight.
The current densities are in general within the
range f rom 0 . 5 to 10 , pre f erably from 1 to 4 , kA/m~ .
The cell voltage is in general from 2 to 10 V,
preferably from 3 to 5 V, per membrane electrolysis
unit.
The pH is in general within the range from 2 to
10, preferably from 3 to 5, in the acolyte compart
ment and within the range greater than 13 in the
catolyte compartment.
The compartment width is in general from 0.5 to
10, preferably from 1 to 5, mm.
The temperature selected for carrying out the
membrane electrolysis process is is general within
the range from 50 to 110°C, preferably from 65 to
90°C.
To ensure mass transport, the compartment coa-
tents are is general recirculated either by means of
pumps or through natural convection, ie. through the
maa~oth pump effect due to gas evolution at elec-
trodes. The flow velocities in the compartments are
in general within the range from 0.05 to 0.5, pref-
erably from 0.1 to 0.2, m/sec.
(e) A particularly preferred embodiment is the elec-
trochemical splitting of the dicarboxylate salts
into the corresponding dicarboxylic acids and bases
in a three-part membrane electrolysis cell.
The three-part membrane electrolysis wait has in
general the following structure:
anode - anolyte compartment - cation exchange mem-
brane - center compartment - cation exchange mem-
brane - catolyte compartment - cathode.
The aqueous dicarboxylate salt solution is in
general introduced into the center compartment. To
increase the electric conductivity in the center
zmsoz4
- 17 - O.Z. 0050/43333
compartment, a mineral acid or a salt can be added
to the center compartment electrolyte. Examples are
sulfuric acid, nitric acid, sodium sulfate and
sodium nitrate.
The center compartment generally retains the
organic acid radical, which can react with the
hydrogen ions liberated in the course of the anode
reaction and which have migrated into the center
compartment through the anode-side catioa exchange
membrane to form the free acid. The acid is in
general removed from the center compartment system
together with unconverted salt. The anolyte used can
be as aqueous mineral acid such as sulfuric acid,
citric acid or hydrochloric acid, preferably
sulfuric acid. The anolyte's essential function is,
together with the anode-aide cation exchange mem-
brane, to protect the organic dicarboxylic acid from
anodic oxidation.
As for the rest, the process of (e) can be car-
rigid out under the conditions described at (d).
(f) The electrochemical splitting of the dicarb-
oxylate salts into the corresponding dicarboxylic
acids and bases can also be carried out in a four-
part membrane electrolysis cell.
The four-part membrane electrolysis unit general-
ly has the following structure:
anode - acolyte compartment - catioa exchange mem-
brane - anode-near center compartment - anion ex-
change membrane - cathode-near center compartment -
cation exchange membrane - catolyte compartment -
cathode.
The aqueous dicarboxylate salt solution is advan-
tageously introduced into the cathode-near center
compartment.
To increase the electric conductivity in the
center compartment, a mineral acid or a salt such as
sulfuric acid, nitric acid. sodium sulfate or sodium
2138024
- 18 - O.Z. 0050/43333
nitrate can be added to the center compartment
electrolyte.
The acid anion generally. passes from the cathode
near center compartment into the anode-near center
compartment, where it reacts with hydrogen ions,
which are evolved in the course of the anode reac-
tion and pass into the anode-near center compartment
through the anode-side cation exchange membrane, to
form the free acid. The acid is in general withdrawn
from the center compartment system in high purity.
The remaining salt solution is in general withdrawn
from the cathode-near center compartment and recir-
culated into the adipate dissolution stage in a
partial stream or disposed of. The acolyte used is
in general an aqueous mineral acid, preferably
sulfuric acid. The anolyte~s essential function,
together with the anode-side cation exchange mem-
brane. is to protect the organic acid from anodic
oxidation.
As for the rest, the process of (f) can be car-
ried out under the conditions mentioned at (d).
In the above-described alternatives the cation
exchange membranes used are particularly preferably
polymers based on perfluorinated olefins or copolymers of
styrene and divinylbenzene containing sulfonic acid and
if desired carboxyl groups as charge carriers. Very
particular preference is given to using membranes that
contain sulfoaic acid groups only, since is general they
are more resistant to fouling by multivalent cations than
other membranes. Membranes of this type are knows (for
example Nafion~ membranes of type 324). They consist of
a copolymer of tetrafluoroethylene with a perfluorinated
monomer that contains sulfone groups. Ia general they
have a high chemical and thermal stability. The ion
exchange membrane can be reinforced with a Teflon support
fabric . It is also possible to use copolymers based on
styrene and diviaylbeazene.
2138024
- 19 - O.Z. 0050/43333
Suitable anion exchange membranes are for example
the membranes described in detail in EP-A-449,071 so no
details will be given here.
The electrode materials used can be in general
perforated materials, for example in the form of nets,
lamellae, oval profile webs or round profile webs.
The oxygen overvoltage at the anodes is in
general set at less thaw 400 mV within the current
density range according to the invention in order that
the formation of ozone or per-compounds may be prevented.
Suitable anode materials of low oxygen overvol
tage are for example titanium supports with electrically
conducting interlayers of borides and/or carbides and/or
silicides of subgroups IV to VI such has tantalum
borides. titanium borides or titanium suboxide, doped or
undoped tin oxides, or tantalum and/or niobium with or
without platinum metal doping, whose surface has in
general bees doped with electrically conducting, noa-
stoichiometric mixed oxides of subgroups IV to VI and
metals or metal oxides of the platinum group or platinum
metal compounds such as platinates. On top of these
interlayers is is general the active electrode material,
which preferably consists of mixed oxides of tantalum
with iridium, platinum or rhodium and platiaates of the
type Llo.jPt3O,. To enlarge the surface area it is
customary to use superficially roughened or macroporous
titanium supports.
The cathodes are in general made of electrode
materials having a low hydrogen overvoltage in order to
avoid additional voltage losses in the membrane
electrolysis or electrodialysis cell. Suitable cathodes
are for example iron or nickel supports which have been
surface coated with finely divided cobalt, nickel,
molybdenum, tungsten, manganese, Raney metal compounds of
nickel or of cobalt, nickel- or cobalt-aluminum alloys,
or nickel-iron alloys or cobalt-iron alloys containing
from 65 to 90% by weight of iron.
~138024~
- 20 - O.Z. 0050/43333
To improve selectivity and membrane life the
cathode side can be equipped with cation exchange mem-
branes containing hydroxyl ion blockers. The selectivity
can be further improved by keeping the level of calcium,
magnesium and aluminum ions and also the silica contest
in each case below 5 ppm.
The dicarboxylic acid obtained by the electro-
chemical treatment is in general present as as aqueous
solution having a concentration within the range from 1
to 30, preferably from 4 to 30, % by weight. This solu-
tion can contain the conductivity salt, if present, in a
concentration within the range from 0.05 to 15, prefer-
ably from 0.06 to 6, % by weight and the mineral acid, if
present, in a concentration within the range from 0.05 to
15, preferably from 0 to 6, % by weight.
The alkali obtained according to the invention
generally contains as alkali metal hydroxide in a con-
centration within the range from 5 to 35, preferably from
10 to 25, % by weight.
Particularly preferably, the alkali metal hydrox-
ide solution obtained according to the invention can be
recirculated or otherwise used, in which case if desired
it can be concentrated beforehand in a conventional
manner, for example by evaporation.
To obtain the dicarboxylic acid in pure form, it
is is general crystallized out of the solution obtained
according to the invention, then separated off, for
example by filtration, and dried.
The dicarboxylic acid is preferably obtained from
the electrodialysis or membrane electrolysis solutions by
cooling or evaporation crystallizatioa. Then the dicar
boxylic acids are in general separated from the resulting
suspensions, for example by filtration, decanting or
centrifuging.
The cooling crystallization is customarily
carried out at from 0 to 50°C, preferably at from 10 to
40°C, advantageously at pressures within the range from
2138024
- 21 - O.Z. 0050/43333
Y
1 to 100 kPa, preferably from 4 to 20 kPa.
The dicarboxylic acids obtained can be preferably
obtained in a pure form by washing, for example with
water or Cl-C,-alkanols. and if desired by recryetalliza-
tion. If a plurality of dicarboxylic acids are present at
the same time, the individual dicarboxylic acids can be
isolated in pure form by utilizing the solubility dif-
ferences in a conventional meaner such as fractional
crystallization.
The aqueous solutions obtained by crystallization
and washing can be concentrated in a conventional manner
and resubjected to a crystallization, for example by
adding them to as-electrodialyzed. or as-electrolyzed
solutions that have still to be crystallized. They can
also ba for example added to the solid phase obtained
from the base treatment of the polymers used, or mixtures
obtained therefrom.
One advantage of the process of the invention
over known processes is that it eliminates the formation
and disposal of salts which are customarily obtained when
the dicarboxylic acids are freed from their salts by
acidification. A further advantage is that even fiber-
reinforced, mineral-filled and/or impact-modified molding
compositions can be processed. Furthermore, the sub-
stances produced by the process of the invention, such as
dicarboxylic acids, diamines and bases and also, as the
case may be, glass fibers and mineral fillers, can be
used for making new products.
EXAMPLE 1
300 g of a nylon 66 having a viscosity number
(VN) = 149 (unit: 1 cm'/g) (measured on a 0.5% strength
by weight solution of the nylon in 96% strength by weight
sulfuric acid at 25°C in accordance with DIN 53 727) and
comminuted to about 8 mm (average particle diameter) were
heated together with 780 g of a 15% strength by weight
solution of sodium hydroxide in methanol at 180°C for
4 hours is a pressure vessel with stirring.
pf3gp2,~ .
- 22 -
After this reaction mixture had been cooled down,
the precipitated sodium adipate was filtered off, washed
repeatedly with methanol and dried.
The mother filtrate and the combined methanolic
wash filtrates were subjected to a fractional distilla-
tion. Initially the low boilers such as methanol were
separated off at atmospheric pressure. At 128-131°C/
100 mbar 142 g of hexamethylenediamine were then obtained
in the form of a colorless melt.
249 g of the dried sodium adipate were then
admixed with 673 g of water, so that a 27% strength by
weight aqueous sodium adipate solution was obtained.
This concentrated sodium adipate solution was
then admixed with 0.5 g of pulverized activated carbon
per 100 ml of solution and heated to 50°C. After 1 h the
activated carbon was filtered off and 80 mg of sodium
carbonate per 100 g of solution were added with stirring.
After 1 h the stirrer was switched off and after a
further 4 h the solution was filtered. This pre-purified
sodium adipate solution was then subjected to a treatment
with a selective ion exchange resin (Lewatit TP 208* (from
Bayer) ) .
EXAMPLE 2
In a pressure vessel 300 g of comminuted nylon 66
(as described in Example 1) were heated with stirring
with 970 g of a 12.2% strength by weight solution of
sodium hydroxide in a solvent mixture consisting of 85%
by volume of methanol and 15% by volume of water, at
180°C for 4 hours.
After the reaction mixture had been cooled down,
the precipitated sodium adipate was filtered off, washed
repeatedly with a total of 750 g of methanol and dried.
The combined methanolic wash filtrates were reused as
solvent for the hydrolysis stage.
The mother filtrate of the reaction mixture was
subjected to a fractional distillation. Initially low
boilers such as methanol and water were separated off
* trademark
2138024
- - 23 - O.Z. 0050/43333
under atmospheric pressure. At 128-131°C/100 mbar 138 g
of hexamethyleaediamine were obtained in the form of a
colorless melt.
The workup of the sodium adipate to adipic acid
and sodium hydroxide solution was carried out analogously
to Example 1, the sodium hydroxide solution obtained is
the electrolysis being concentrated to 50% by weight and
reused is the splitting reaction (hydrolysis stage). This
again involved heating 300 g of comminuted nylon 66 (as
described in Example 1) with 232 g of 50% strength by
weight sodium hydroxide solution and 730 g of the methan-
olic wash filtrate at 180°C for 4 hours with stirring.
After the reaction mixture had cooled down, the
precipitated sodium adipate was filtered off and repeat
edly washed with a total of 750 g of methanol. The
methanol used for this washing of the filter cake had
been recovered pure from the rectification of the mother
filtrate.
(The rest of the workup was carried out analogously to
Example 1).
EXAMPLE 3
This experiment was carried out using a pigmented
(with carbon black), (thermally stabilized) glass fiber-
reinforced nylon 66 having a viscosity number (VN) - 140
(measured is accordance with DIN 53 727, see Example 1)
and a glass fiber content of 36% by weight (determination
of the calciaatioa loss of glass fiber-reinforced plas-
tics is accordance with DIN 53 395) which had been
commiauted to about 8 mm (average particle diameter). In
a pressure vessel 490 g of this composite material were
heated with stirring with 1180 g of a 10% strength by
weight solution of sodium hydroxide is a solvent mixture
consisting of 75% by volume of methanol and 25% by volume
of water at 180°C for 4 hours.
After the reaction mixture had cooled down, the
precipitated sodium adipate was filtered off together
with the glass fibers (and other insoluble constituents
2138024
- 24 - O.Z. 0050/43333
such as carbon black pigments) and repeatedly washed with
methanol. The mother filtrate' and the combined wash
filtrates were subjected to a fractional distillation.
Initially low boilers such as methanol and water were
separated off at atmospheric pressure. At 128-131°C/
100 mbar 140 g of hexamethyleaediamine were obtained is
the form of a colorless malt.
To recover the sodium adipate, the filter residue
of the reaction mixture was repeatedly admixed with a
total of 1000 g of water, stirred up and filtered. The
combined filtrate gave a 20% strength by weight aqueous
sodium adipate solution which was evaporated under
atmospheric pressure to a concentration of 27% by weight
of sodium adipate, methanol residues being removed as
well.
The sodiuat adipate solution thus concentrated was
then admixed with 0.5 g of pulverized activated carbon
per 100 ml of solution and heated to 50°C. After 1 h the
activated carbon was filtered off.
(The rest of the workup was carried out analogously to
Example 1).
EXAMPLE 4
Batchwise electrolysis in a three-compartment
electrolysis cell as per variant e)
The three-compartment electrolysis cell used was
that diagrammatically depicted in Figure 1 with three
liquid cycles (ICL1 to RI~3). All product-contacting parts
with the exception of the electrodes consisted of poly-
propylene, glass or quartz. The anode (E1) (ia compart-
meat (A)) was a titanium expanded-mesh anode having as
area of 100 cm' and a coating suitable for oxygen evolu-
tion. The cathode (E2) (in compartment (C)) likewise had
an area of 100 cm=. It consisted of a chromium-nickel
stainless steel (1.4571) which had been coated with a
nickel network activated for hydrogen evolution. The two
membranes (Ml and M2) of the type Nafioa~ 324 were posi-
tioned directly on the electrodes (El and E2,
2138024
- 25 - O.Z. 0050/43333
respectively) and were separated from each other by a
1 mm wide canter compartment (H) with a polypropylene
spacer.
The anode (RL1) and cathode (RL2) cycles ware
kept in natural circulation owing to the gas evolutions
at the electrodes. The cycle of the center compartment
(B), (RL3), was recirculated using a cycle pump (P). The
flow velocity is the center compartment (B) was
0.1 m/sec.
The anolyte used comprised 1131 g of 5% strength
by weight sulfuric acid introduced at location (1), the
catolyte comprised 1161 g of 5% strength by weight sodium
hydroxide solution introduced at location (2), and the
center compartment electrolyte comprised 995 g of 27%
strength by weight sodium adipate solution obtained in
Example 1 to which 21 g of 96% strength by weight
sulfuric acid were added so that 1015 g of a solution
containing 22% by weight of sodium adipate, 2.9% by
weight of adipic acid and 2.8% by weight of sodium
sulfate were introduced at location (3).
A temperature of 80°C, atmospheric pressure, a
current density of 3.0 kA/m', a cell voltage of 4.0 V (at
the beginning) and 5.3 V (at the end of the run) produced
with a current yield of 83% and after a reaction time of
2 h 26 min the following electrolytes:
anolyte (removed at location (4) ) : 729 g of 6.9% strength
by weight sulfuric acid,
catolyte (removed at location (5)): 1294 g of 10.9%
strength by weight sodium hydroxide solution,
center compartment electrolyte (removed at location (6)):
904 g of a solution containing 20.4% by weight of adipic
acid, 1.2% by weight of sodium adipate and 3.2% by weight
of sodium sulfate.
Batchwise crystallization
900 g of the center compartment electrolyte
solution thus obtained were introduced at 80°C into a
vacuum vessel with reflex condenser and then cooled dower
2138024
- 26 - O.Z. 0050/43333
over 100 min to 10°C (by continuously reducing the inter-
nal pressure (absolute) from 1013 mbar to 12 mbar). The
resulting adipic acid crystals were then separated off by
means of a vacuum nutsche at a filtration pressure of
450 mbar and washed with 700 g of water which had a
temperature close to 0°C. The crystalline product thus
washed was then dissolved is 420 g of water, giving a 30%
strength by weight adipic acid solution. Then the
crystallization process was repeated. Drying the crystal-
line product obtained in the second crystallization
process at 80°C and 100 mbar (absolute) left 175 g of
adipic acid having a purity of 99.8% and an ash content
of lass than 8 ppm.
Continuous crystallization
Example 4 was repeated except that the adipic
acid was purified by continuous crystallization. For this
two vacuum vessels (0.75 1 nominal capacity, with
stirrer) were connected in series. The absolute pressure
of the first stage (vessel 1) was 95 mbar (corresponding
to a boiling temperature of the adipate solution used of
45°C), the absolute pressure of the second stage was
12 mbar (corresponding to a boiling temperature of the
adipate solution used of 10°C). The liquid level was kept
constant in the two vessels by using a membrane metering
pump to pump 0.75 kg/h of adipate solution continuously
into the first vacuum vessel and decompressing under a
blanket of liquid. A level control valve was used to
likewise introduce 0.75 kg/h of the solution contained is
the first vacuum vessel into the second vacuum vessel,
the solution transported from the first into the second
vacuum vessel likewise being decompressed "dipped". A
charge (900 g) of the adipic acid crystallized out of the
second vessel was separated off by means of a vacuum
nutsche at a filtration pressure of 450 mbar and washed
With 700 g of water which had a temperature close to 0°C.
The crystalline product thus washed was then dissolved in
420 g of water, giving a 30% strength by weight adipic
2138024
- 27 - O.Z. 0050/43333
acid solution. Then the crystallization process was
repeated. Drying the crystalline product obtained in the
second crystallization process at 80°C and 100 mbar
(absolute) gave 175 g of adipic acid having a purity of
99.8% and an ash content of less than 8 ppm.
EXAMPLE 5
Batchwise electrolysis in a four-compartment electrolysis
cell as per variant f)
The four-compartment electrolysis call used is
diagrammnatically depicted in Figure 2 with four liquid
cycles (RLl to RL4). All product-contacting parts with
the exception of the electrodes consisted of
polypropylene, glass or quartz. Anode (E1) (in compart
ment (A)) was a titanium expanded-mesh anode having an
area of 100 cm' and a coating suitable for oxygen
evolution. Cathode (E2) (in compartment (D)) likewise had
an area of 100 cm'. It consisted of chromium-nickel
stainless steel (1.4571) which had been coated with a
nickel network activated for hydrogen evolution. The two
electrode-near cation exchange membranes (Ml and M3) of
the type Nafion~ 324 were positioned directly on the
electrodes (E1 and E2 respectively) and were separated by
two center compartments, (H) and (C), each 1 mm in width,
with a centrally disposed anion exchange membrane (M2) of
the type Tokuyama Soda~ AMB. The center compartments, (B)
and (C), were provided with two polypropylene spacers
which served to keep the flow chancel free and to prevent
direct contact between the membranes.
The anode (RLl) and cathode (RL4) cycles were
kept in natural circulation owing to the gas evolutions
at the electrodes. The cycles of the center compartments
(B) and (C), (RL2) and (RL3), were recirculated using the
cycle pumps (P1) and (P2). The flow velocities in the
center compartments (8) and (C) were in each case
0.1 m/sec.
The anolyte used comprised 1108 g of 5.1%
AMENDED SHEBT
2138024-
- 28 - O.Z. 0050/43333
strength by weight sulfuric acid introduced at location
(1), the catolyte comprised 1101 g of 4% strength by
weight sodium hydroxide solution introduced at location
(2), the electrolyte of the anode-near center compartment
(H) comprised 1097 g of 2.1% strength by weight sulfuric
acid introduced at location (3), and the electrolyte of
the cathode-near center compartment (C) comprised 1505 g
of 27% strength by weight sodium adipate solution
obtained in Example 2 introduced at location (4).
During the reaction a total of 900 g of water was
additionally introduced into the cathode-sear center
compartment (C).
A temperature of 80°C, atmospheric pressure, a
current density of 3.0 kA/m', a cell voltage of 7.0 V (at
the beginning) and 8.7 V (at the end of the run) produced
with a current yield of 75% and after a reaction time of
5 h, during which the p8 in the cathode-near center
compartment (C) was within the range from 10 to 12, the
following electrolytes:
anolyte (removed at location (5)): 793 g of 7.1% strength
by weight sulfuric acid,
catolyte (removed at location (6)): 1584 g of 13.3% by
strength weight sodium hydroxide solution,
product of the anode-near center compartment (H) (removed
at location (7)): 2034 g of a solution containing 15.1%
by weight of adipic acid, 1.1% by weight of sulfuric
acid,
product of the cathode-near center compartment (C)
(removed at location (8)): lOfil g of a 1.4% strength by
weight sodium adipate solution.
EXAMPLE 6
Hatchwise electrolysis in a membrane stack cell as per
variant b)
The membrane stack call used is diagrammatically
depicted in Figure 3 with three liquid cycles (RL1 to
RL3). All product-contacting parts with the exception of
the electrodes consisted of polypropylene, glass or
2138024
- 29 - O.Z. 0050/43333
polytetrafluoroethylene. Anode (El) (in compartment (A))
was a titanium expanded-mesh anode having an area of
320 em' and a coating suitable for oxygen evolution.
Cathode (E2) (in compartment (D)) likewise had an area of
320 cm'. It consisted of a chromium-nickel stainless
steel (1.4571) which had been coated with a nickel
network activated for hydrogen evolution.
The compartments (A) and (Hl), (Dl) and (B2),
(D2) and (B3) and also (D3) and E were in each case
separated from each other by a bipolar membrane (from
DE-A 40 26 154). The compartments (Hl) and (C1), (82) and
(C2) and also (83) and (C3) were kept apart from each
other by anion exchange membranes (Tokuyama~ Soda A1~C) .
The compartments (C1) and (D1), (C2) and (D2) and also
(C3) and (D3) were kept apart from each other by catioa
exchange membranes (Tokuyamam Soda C1~). The membrane
spacings were in each case 0.5 mm.
All liquid cycles with the exception of those of
the anode (A) and cathode (E) compartments were recircu
lated by mesas of cycle pumps, (Pl) to (P3), the flow
velocity being in each case 0.1 m/sec.
The electrolyte used in the acid medium comprised
10000 g of 1.5% strength by weight sulfuric acid intro-
duced at location (1), the electrolyte is the basic
medium comprised 5000 g of 1% strength by weight sodium
hydroxide solution introduced at location (2) sad the
center compartment electrolyte comprised 5000 g of 20%
strength by weight sodium adipate solution (obtained by
diluting the 27% strength by weight solution obtained) is
Example 3 introduced at location (3).
A temperature of 55°C, atmospheric pressure, a
current density of 0.31 kA/m~, a cell voltage of 13 V (on
average) produced with a current yield of 70% and after
a reaction time of 13 h the following electrolytes:
dialysis product (removed at location (4)): 11555 g of
6.4% strength by weight adipic acid which additionally
contained 1.3% by weight of sulfuric acid,
zl~soz4
- 30 - O.Z. 0050/43333
"basic" electrolyte (removed at location (5)): 6597 g of
6.9% strength by weight sodium hydroxide solution,
"depleted" center compartment .electrolyte (removed at
location (6)): a 1.8% strength by weight solution of
sodium adipate.