Note: Descriptions are shown in the official language in which they were submitted.
-
2138S3~
- o.z. 0050/43341
The preparation of 3-hYdroxyphenylacetic acid
The present invention relates to a novel process
for preparing 3-hydroxyphenylacetic acid.
It is known that a number of microorganisms is
able to hydroxylate phenylacetic acid.
A result of this is, besides various other
hydroxylation products, 3-hydroxyphenylacetic acid, but
this undergoes further metabolism by the microorganisms
(J.J. Anderson et al., J. Bacteriol. 141 (1980) 534-543).
Keisuke Kohmoto et al. (Phytopathology, 60,
(1970) 1025-1026) reported that the fungus Rhizoctonia
solani is able to hydroxylate phenylacetic acid in the
meta position. To prepare 3-hydroxyphenylacetic acid,
first the fungus was cultured and then the biomass was
isolated and incubated with a solution of phenylacetic
acid (replacement culture). The maximum concentration of
3-hydroxyphenylacetic acid obtained was 2.5 g/l.
This preparation is unsuitable for an industrial
process because of the low concentration of product and
the elaborate replacement culture.
It is an object of the present invention to
provide a fermentation process for preparing 3-hydroxy-
phenylacetic acid from phenyl compounds which is
straightforward to carry out and gives good yields.
We have found that this object is achieved
particularly advantageously by a process in which a
fungus of the genus Rhizoctonia, Ceratobasidium or
Pellicularia is cultivated in the presence of a
conventional nutrient medium to which is added a phenyl
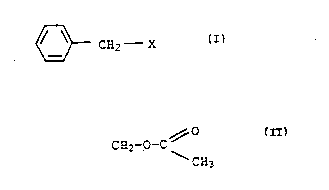
compound of the formula I
~ C32--X
where X is COOR, CH2OH, CH2NH2 or C~2-o-C ~
CN3
213~5~4
- 2 - O.Z. 0050/43341
R is methyl, ethyl, hydrogen, sodium, potassium or
ammonium.
Fungi of the genera Rhizoctonia, Ceratobasidium
and Pellicularia are known (D.L. Hawksworth, B.C. Sutton
and G.C. Ainsworth: Ainsworth & Bisby's Dictionary of the
Fungi, Seventh Edition, 1983 and issues cited therein).
The species which is particularly suitable for
the process according to the invention is Rhizoctonia
solani.
Nutrient media suitable for cultivating the fungi
are those which contain sources of carbon and of nitro-
gen, inorganic salts and, where appropriate, small
amounts of trace elements and vit~mins. Sources of
nitrogen which can be used are inorganic or organic
nitrogen compounds or materials which contain these
compounds. Examples are ammonium salts, nitrates, corn
steep liquor, yeast autolysate, soybean meal, wheat
gluten, yeast extract, yeast, urea and potato protein.
Examples of carbon sources which can be used are sugars
such as glucose, polyols such as glycerol, or fats such
as soybean oil.
Examples of inorganic salts are the salts of
calcium, magnesium, manganese, potassium, zinc, copper,
iron and other metals. The phosphate ion is a particu-
larly suitable anion in the salts. Growth factors are
added where appropriate to the nutrient medium, such as
biotin, riboflavin or other vitamins.
Success of the process does not apparently depend
on the nature of the nutrient medium.
A phenyl compound i8 added to the nutrient medium
for the process according to the invention. Phenyl
compounds which can be used in this process are phenyl-
acetic acid or its salts, especially its alkali metal
salts, with the sodium and potassium salts being particu-
larly preferred, or esters, preferably methyl or ethyl
phenylacetate. When esters are used as precursors in the
process according to the invention, there is not only
2138534
~ - 3 - o.z. 0050/43341
hydroxylation in the meta position but also cleavage of
the ester so that the product is 3-hydroxyphenylacetic
acid.
Other suitable phenyl compounds are 2-phenyl-
ethanol, 2-phenylethylamine and 2-phenylethyl acetate.
With these substrates in the process according to
the invention there is not only hydroxylation in the meta
position but also formation of a carboxyl group so that
the product is 3-hydroxyphenylacetic acid.
10The particularly preferred phenyl compounds are
sodium and ammonium phenylacetate~.
The phenyl compounds are usually added in amounts
such that the concentration is about 1-50 g, preferably
5-30 g, per liter of nutrient medium.
15The phenyl compounds can be added to the nutrient
medium at the start of cultivation of the fungus or dur-
ing the cultivation in several portions or continuously.
It is also possible to retain the cultivated
fungus after conversion is complete and to cultivate it
20further in fresh nutrient medium with the phenyl
compound.
This type of reuse of the biomass is a particu-
larly advantageous embodiment of the process according to
the invention because, for example, this saves the
biomass cultivation time.
Cultivation of the fungus does not require any
other special conditions. Thus, cultivation can take
place at from 20 to 40C, preferably from 25 to 35C.
The pH of the fermentation medium is maintained
30at from 3 to 9 and i9 advantageously from 4 to 7.
The fermentation times are normally from 1 to 10
days to achieve maximum accumulation of the product in
the fermentation medium.
The extent of reaction can easily be determined
35by taking a sample and analyzing by, for example, gas
chromatography.
The invention is illustrated by the following
213~53~
~ - 4 - O.Z. 0050t43341
examples.
EXAMPLE 1
Testing of various strains of fungi for conversion of
phenylacetic acid into 3-hydroxyphenylacetic acid
Medium 1
10 g/l glucose
40 g/l corn steep liquor
1.5 g/l KH2PO4
3.6 g/l K2HPO4
pH 6.8
The phenylacetic acid was dissolved in water with
the addition of little dilute NaOH and was sterilized by
filtration.
30 ml portions of medium 1 cont~ g l g/l
phenylacetic acid were introduced into sterile 250 ml
Erlenmeyer flasks with a baffle. The flasks were each
inoculated with a 0.5 x 0.5 cm piece of agar which had
been punched out of an agar plate on which a particular
strain had been grown. The flasks were shaken at 180 rpm
and 25C. After 3 and 7 days, 1 ml of the culture super-
natant was removed, mixed with 100 ~l of 5 N hydrochloric
acid and 800 ~l of ethyl acetate and agitated vigorously
for 15 s. 700 ~l of the ethyl acetate phase were removed
and evaporated at 50C under a gentle stream of nitrogen.
The residue was dissolved in 70 ~l of ethyl acetate, and
50 ~l of this was transferred into a gas chromatography
sample vessel. To this were added 50 ~l of N-methyl-
N-trimethylsilyltrifluoroacetamide (MSTFA) and mixed. The
samples were investigated by gas chromatography with an
authentic sample of 3-hydroxyphenylacetic acid for
comparison.
The results are compiled in the table.
-
21385:~4
~~ - 5 - o.Z. 0050t43341
TABLE
Conversion of phenylacetic acid to
3-hydroxyphenylacetic acid
Strain % conversion after
3 da~s 7 days
Rhizoctonia solani LU 6467 50.5 11.9
Rh. solani LU 6464 0.0 0.8
Rh. solani LU 6474 12.5 6.4-
Rh. solani LU 6473 22.3 55.7
Rh. solani LU 6475 14.7 80.2
Rh. solani LU 6476 48.1 28.0
Rh. solani DSM 852 (= ATCC 13250) 95.5 94.3
Rh. solani DSM 63010 (= IBM 11662) 1.5 4.7
Rh. solani DSM 843 (= ATCC 13249) 51.6 63.1
Rh. solani ATCC 10159 3.0 35.0
Rh. solani ATCC 16116 2.0 94.0
Rh. solani ATCC 48804 (= CMI 34886)57.0 79.0
Rh. solani ATCC 62153 32.0 86.0
Rh. solani ATCC 62154 0.0 25.0
Rh. solani ATCC 62156 80.0 97.0
Rh. tuliparum LU 6470 0.0 1.6
Rh. cerealis LU 6471 1.8 33.5
Rh. muneratii DSM 903 (= ATCC 13247)7.0 34.0
Pellicularia filamentosa ATCC 132892.0 61.0
Pellicularia filamentosa ATCC 132902.0 60.0
Ceratobasidium cornigerum 13.7 13.3
CBS 137.82 (= ATCC 13247)
Degradation of the product
The strains identified by LU originate from the
BASF collection of strains.
EXAMPLE 2
Preparation of 3-hydroxyphenylacetic acid in shaken
flasks
A small piece of Rhizoctonia ~olani (DSM 852)
mycelium was used to inoculate a 250 ml Erlenmeyer flask
2138534
- 6 - O.Z. 0050/43341
with baffle containing 30 ml of the following medium:
Medium A. 20 g/l glucose
5 g/l yeast extract (DIFCO)
5 g/l (NH4)2SO4
0.5 g/l MgSO4 x 7 H2O
0.05 g/l MnSO4 x 4 H2O
2 ml/l trace element solution
3 g/l Carbopol 946 (high molecular weight
carboxyvinyl polymer)
1.5 g/l KH2PO4
3.6 g/l K2HPO4
Trace element solution:
200 mg/l iron(II) sulfate 1-hydrate
10 mg/l zinc(II) sulfate 4-hydrate
3 mg/l manganese chloride 4-hydrate
30 mg/l boric acid
20 mg/l cobalt(II) chloride 6-hydrate
1 mg/l copper(II) chloride 2-hydrate
2 mg/l nickel(II) chloride 6-hydrate
3 mg/l sodium molybdate 2-hydrate
500 mg/l ethylenediaminetetraacetic acid
(EDTA)
5 ml of this preculture was u~ed to inoculate a
main culture. The main culture medium was 30 ml of medium
A without Carbopol but with the addition of 10 g/l
phenylacetic acid.
After shaking at 180 rpm and 25C for 4 days the
phenylacetic acid had been completely converted.
The bioma~s wa~ then harve~ted by centrifugation
at 4,000 rpm for 5 minutes and u~ed to inoculate fresh
medium A contA;n;ng 20 g/l phenylacetic acid. After con-
version of the phenylacetic acid was complete (7 days),
thi~ procedure wa~ repeated and the biomas~ was trans-
ferred into fresh medium A cont~;ning 30 g/l phenylacetic
213~524
- 7 - O.Z. 0050/43341
acid. The phenylacetic acid was completely converted
after 10 days. In this way the biomass was reused up to
4 times without detectible loss of activity.
Immediately after completion of the conversion,
the cultures contained about 0.5% 2-hydroxyphenylacetic
acid and up to 4% 4-hydroxyphenylacetic acid in addition
to 3-hydroxyphenylacetic acid. It was possible to reduce
the 4-hydroxyphenylacetic acid content to 0-1% by con-
tinuing cultivation for one day after the phenylacetic
acid had disappeared. This did not affect the
concentrations of 2- and 3-hydroxyphenylacetic acid.
EXAMPLE 3
Preparation of 3-hydroxyphenylacetic acid by repeated
10 l fermentations
Medium B: 20 g/l glucose
7.5 g/l yeast extract (Fould Springer, 65%)
5 g/l (NH4)2SO4
0.5 g/l MgSO4 x 7 H2O
0.05 g/l MnSO4 x H2O
2 ml/l trace element Rolution
1 g/l antifoam P 2000
1.5 g/l KH2PO4
3.6 g/l K2HPO4
Preculture:
3 x 330 ml of medium B in round-bottomed flasks
with baffle were each inoculated with 1/3 of an agar
plate on which DSM 852 had been grown and which had been
comminuted with an Ultraturrax, and were shaken at
180 rpm and 25C for 3 days.
10 l fermentation:
The preculture was used to inoculate a 10 l
fermenter contAining medium B plus lO g/l phenylacetic
acid. The fermenter wa~ stirred at 100 rpm and aerated
with 0.5 volume of air per reactor volume per minute
2138534
~ - 8 - O.Z. 0050/43341
(vvm). During the fermentation the stirring speed was
increased stepwise to 200 rpm and the aeration rate to
1 vvm.
After 88 hours, all the phenylacetic acid had
been converted. 9 l of the ferementer contents were
drained off and replaced by fresh medium B. The phenyl-
acetic acid concentration was adjusted to 10 g/l by
adding a 25% by weight phenylacetic acid solution (in
water, adjusted to pH 7 with NaOH).
During the fermentation, 50 g portions of phenyl-
acetic acid were added on 2 occasions. Glucose was
replenished if required. After 165 hours all the phenyl-
acetic acid had been converted. The final concentration
of 3-hydroxyphenylacetic acid was 26 g/l.
As described above, the fermenter was drained
apart from 1 l and replenished with fresh medium B.
During fermentation for 165 hour~, 4 x 50 g of phenyl-
acetic acid were added. The final concentration of 3-
hydroxyphenylacetic acid wa~ 30.6 g/l.
The fermenter was again pumped empty apart from
1 1.
Isolation of 3-hydroxyphenylacetic acid from the 3rd
fermentation broth:
9 liters of fermentation broth were centrifuged
(5,000 rpm, 20 min) and the precipitate wa~ washed with
1 l of water and again centrifuged. The supernatant from
both centrifugations (about 7 1) was adjusted to pH 2
with H2SO4 and extracted twice with the same volume of
tert-butyl methyl ether. The organic phase was evaporated
to dryness to yield 250 g of residue which wa~ 94% pure
3-hydroxyphenylacetic acid.