Note: Descriptions are shown in the official language in which they were submitted.
W094/~960 213 8 6 ~ ~ PCT~S94/0~06
--1--
SOLID WOVEN TUBUhAR PROSTHESIS
BAC~GROUND OF THE lN V~N-l'lCN
The present invention relates to tubular prostheses,
and, more particularly, to woven tubular prostheses having
increased wall stiffness.
In the past, tubular prostheses have commonly been
manufactured by, for example, weaving a plurality of warp
yarns and a plurality of fill yarns into a tubular fabric.
Such products, however, typically lack sufficient radial
stiffness to maintain an open lumen, i.e., if unsupported,
they will radially collapse. The tendency to radially
collapse or kink is particularly problematic to a surgeon
during implantation of the prosthesis.
Conventional weaves used for vascular grafts and
other medical devices are known as simple weaves, i.e.,
one-ply weaves. These types of constructions are
inherently strong with respect to burst pressure, but
suffer from the above-mentioned kinking and collapsing
tendencies, as well as the tendency to ravel at the ends,
making it difficult to properly hold sutures. Addition-
ally, the one-ply structure has inherent limitations as to
the properties which can be engineered into the final woven
product. For example, simple weaves do not stretch well
radially or longitudinally and, further, possess only a
simple pore structure due to the single layer.
To overcome these characteristics, prior art
prostheses are typically crimped at equidistant lengths
along their longitudinal axis. The crimping is believed to
provide the prosthesis with sufficient radial stiffness to
maintain an open lumen. The crimping additionally provides
a degree o~ longitudinal compliance to the prosthesis.
SlJBSTITUTE SHEET (RULE 26~
W094/~960 213 ~ ~ 4 0 -2- PCT~S94/0~0
Crimping, however, is not without its disadvantages.
For example, the crimps create a plurality of
irregularities along the inner wall of the prosthesis that
may create blood flow disturbances. These blood flow
disturbances become more pronounced and consequently less
acceptable as the diameter of the prosthesis is reduced.
In addition, thrombi can accumulate in the valleys of the
crimps, tending to form hyperplastic pockets.
An alternative prior art technique for providing
radial stiffness to a woven prosthesis involves the use
of a stiffening component. Specifically, the fabric is
woven around a suitable stiffener or, alternatively, such
a stiffener is secured to either the interior or exterior
of the prosthesis following weaving. The use of a
stiffener, however, creates its own drawbacks. ~or
example, tissue ingrowth may be hindered by the stiffener,
the porosity of the prosthesis may be affected by the
stiffener and, finally, the ability to suture the
prosthesis to the host vessel may be hindered by the
stiffener.
It would therefore be desirable to provide a woven
tubular prosthesis that contains sufficient inherent wall
stiffness so as to be radially self-supporting. Such a
graft would not require crimping or the use of stiffening
components, thereby providing a smooth, continuous inner
wall that better simulates the natural hemodynamics of the
connecting vessels, even with respect to those prostheses
having a relatively small diameter, e.g., down to about 4
mm. Additionally, the same prosthesis would be less prone
to pinching, kinking or other collapsing tendencies when
subjected to bending forces, as well as being resistant
to ravelling when cut to size during surgery. The
prosthesis would also have the ability to hold sutures
well. Finally, the pore structure would be more tortuous,
Sl BSTITUTE SHEET (R~LE 26)
W0941~960 213 8 6 4 0 ~; PCT~S9410~06
thereby providing better hemostasis at the time of
implantation and the ability to support long term healing
and tissue incorporation.
SUMMARY OF THE lN V~N'LlON
The present invention relates to implantable multi-ply
woven tubular prostheses, such as vascular grafts, intra-
luminal devices, such as endoprostheses, and the like. The
prosthetic devices of the present invention are fabricated
from a multi-ply solid weave construction which inherently
provides increased radial strength over traditional simple
one-ply weave patterns. The multi-ply solid weaves are
characterized in that the woven fabric has a plurality of
superposed plies including a plurality of circumferentially
extending fill yarns and a plurality of longitudinally
extending warp yarns. The warp or fill yarns must
continuously pass through at least two adjacent plies.
That is, at least two adjacent layers must have common
yarns which serve to interlock and integrate the plies
into a unitary structure.
Due to the potential for using materials having
different characteristics for each ply, a variety of
structural and property gradients can be achieved. For
example, a porosity gradient from the innermost to the
outermost ply can be incorporated into the prosthesis,
thereby reducing blood loss yet, at the same time,
encouraging tissue ingrowth and assimilation of the
prosthesis into the body. Similarly, different types
of yarns may be used in the interlocking plies to achieve
a gradient of properties such as stiffness, compliance,
texture, ravel resistance and fray resistance. This can
be achieved by using, for example, different yarns or by
subjecting the same or different yarns to different
treatments prior to incorporation into the fabric. In one
SUBSTITUTE SHEET (RULE 26)
W094/24960 ~ ' PCT,'US94/04606
embodiment, elastomeric yarns are incorporated, in an
elongated state, into the prosthesis in the warp direction
such that subsequent to the weaving process the fabric will
retract longitudinally, thereby providing longitudinal
compliance. Other means of providing longitudinal
compliance, such as heat-setting techniques, are also
contemplated.
The implantable prosthetic devices of the present
invention may be used in a variety of locations in the
body, such as intralllmi n~ 1 applications in the vascular
system, pulmonary system or gastrointestinal track. Of
particular usefulness, however, are vascular grafts that
are implanted surgically or by endoscopic means.
BRIEF DESCRIPTION OF THE DRAWINGS
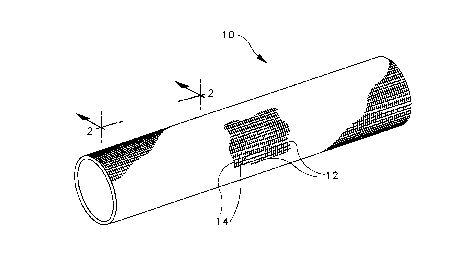
Fig. 1 is a perspective view of a solid woven tubular
prosthesis;
Fig. 2 is a cross-sectional view taken along lines
2-2 of Fig. 1;
Fig. 3 is a cross-sectional view similar to Fig. 2
wherein each of the outermost warp yarns float over three
fill yarns;
Fig. 3a depicts the prostheses of Fig. 3 after a
heat-setting process in which the outermost warp yarns
form a raised filamentous velour surface;
Fig. 4 is a cross-sectional view similar to Fig. 2
wherein a pair of elastomeric warp yarns have been
incorporated into the prostheses; and
SlJBST~TllTE SHEET (RUL~ 26)
WOg4/~960 ~138 6 4 0 PCT~S94/0~06
--5--
Fig. 4a depicts the prostheses of Fig. 4 after a
heat-setting process wherein the outermost warp yarns
form a raised filamentous velour surface.
DET~TT ~!n DESCRIPTION OF THE lNV~NllON
As mentioned above, weaving is commonly employed to
fabricate tubular prostheses. Woven prostheses are ideal
in that they provide strong, pressure-resistant vessels.
These same grafts, however, typically require radial
support through mechanical treatment, such as crimping,
or through the incorporation of radial stiffening yarns.
Referring to Fig. 1, a woven tubular prosthesis 10
fabricated in accordance with the present invention is
shown. As illustrated, the prosthesis may include
longitudinally-extending warp yarns 12 and circumfer-
entially-extending fill yarns 14. The prosthesis of the
present invention differs from prior art woven prostheses
(which typically were formed with simple, one ply weaves)
in that the present prosthesis includes a plurality of
solid woven plies (i.e., the plies are not separable into
discrete layers).
The use of several plies allows certain ideal
characteristics to be designed into the prosthesis.
Specifically, different yarns can be used in the different
plies. For example, depending on the chosen materials, a
porosity gradient can be created in the wall of the
prosthesis. Such a gradient resists leaking of fluid from
the inner wall, yet still allows ingrowth of natural tissue
into the outer wall. Moreover, because the prosthesis is a
solid woven unitary structure, the discretely-designed
plies are interconnected in such a fashion that the plies
become inseparable and also, at the same time, provide
sufficient inherent wall stiffness to the prostheses to
allow it to be radially self-supporting.
SUBSTITIJTE SHEET (RULE 26)
W094/24960 2 1 3 8 ~ ~0 -6- PCT~S94/0460
The solid woven prosthesis of the present invention
can best be understood by reference to Figs. 2-2a, which
depict a prosthesis having three plies. Referring to Fig.
2a, fill yarn 14a is located in the outermost ply, fill
yarn 14b is located in the intermediate ply and fill yarn
14c is located in the innermost ply. A plurality of warp
yarns 12 are solidly woven throughout these fill yarns.
Specifically, by definition each warp yarn (or fill
yarn) in a solid woven prosthesis must pass continuously
through, and therefore be part of, at least two adjacent
plies. Of course, the design of the prosthesis may require
that each warp yarn pass continuously through each and
every ply. In such a case, all of the plies will have warp
yarns in common to form a unitary solid structure.
In a preferred embodiment (as best shown in Figs.
2-2a), the prosthesis is fabricated with three plies.
The outermost ply preferably contains textured or
filamentous materials for enhanced tissue attachment.
The intermediate ply preferably contains a fusible
material to aid in ravel resistance. Finally, the
innermost ply preferably contains a bioresorbable
material to aid in healing, long term patency and zero
preclotting. Such designs, i.e., those having different
properties and property gradients, cannot be created with
simple, single-ply weaves as found in the prior art and
conventionally employed in medical devices of this sort.
The solid woven prosthesis of the present invention
has a wall thickness greater than that of the typical prior
art device, thereby providing a degree of radial support.
For example, wall thicknesses may range from 0.50 mm to
1.25 mm, whereas a conventional wall thickness ranges from
0.25 mm to 0.50 mm. Moreover, the solid woven design
provides greater wall stiffness and resistance to kinking
S~STITUTE SHEET (RlJL~ ~6)
W094/~960 21 3 8 6 4 0 PCT~S94/0~06
.
--7--
or pinching, as compared to single-ply grafts due to the
increased wall thickness. Notwithstanding the increased
wall thickness of the multi-ply unitary construction,
excellent longitudinal or axial flexibility for handling is
retained. Specifically, tubular products made from this
weave structure are sufficiently flexible and compliant
to meet the requirements of a prosthetic implant or graft.
Because the solid woven prosthesis is radially self-
supporting, crimping or the use of stiffeners is not
required. As a result, the inner wall of the prosthesis
can be fabricated as a smooth, continuous surface. In
contrast, the inner wall of a conventional crimped one-ply
prosthesis includes a plurality of irregularities or
corrugations that disturb the flow of fluid therethrough,
e.g., the flow of blood through an artery. This is
additionally problematic because debris may collect in
these irregularities causing further complications in
pa~ients with arterial disease. In larger-sized
prostheses, these disturbances have little effect on the
flow of fluid. However, as the diameter of the prostheses
decreases, the acceptability of the disturbances decreases.
The present invention, by providing a prostheses having a
smooth, continuous inner wall, overcomes this disadvantage
associated with the prior art and, as a result, is capable
of being employed to fabricate relatively small-sized
prostheses.
As described below in Example 3, a solid woven
texturized graft having raised velour loops can be
fabricated in accordance with the present invention.
Specifically, with reference to Fig. 3, prosthesis 10'
is woven such that the warp yarns located at the outer-
most surface pass externally over at least two fill yarns.
For example, warp yarn 12' passes over fill yarns 14a1,
14a2 and 14a3. The "loosel' surface weave allows these
SUBSTITUTE SHEET (~ULE 26)
W094/24960 ~ ;~ PCT~S94/04606
warp yarns to rise from the surface following heat-setting,
thereby forming a filamentous velour surface as shown in
Fig. 3a.
In an additional embodiment, as described below
in Example 4, at least one elastomeric warp yarn 16 is
incorporated into the fabric. Prosthesis 10" is woven
with the elastomeric warp yarns in a stretched state such
that subsequent to weaving the fabric will longitudinally
retract, thereby providing a degree of longitudinal
compliance. Longitudinal compliance assists the surgeon
in sizing the length of the prosthesis for implantation
and also provides a degree of flexibility to the prosthesis
following implantation.
Following manufacture and treatment, the prosthesis
is sealed in a package. The package, along with the
prosthesis contained therein, is then subjected to a
sterilization procedure, e.g., a radiation procedure, a
heat procedure, etc. Alternately, the prosthesis may be
sterilized prior to being sealed in its packaging or may be
sterilized by the physician performing the implant
operation at the time of such surgery.
EXAMPLES
Examples of polymeric materials useful in the present
invention include, without limitation, yarns made from
polyester, polypropylene, polytetrafluoroethylene,
polyethylene, polyurethane and resorbable polymers.
The following examples serve to provide further
appreciation of the invention but are not meant in any way
to restrict the effective scope of the invention.
SU~ST~TVTESH~T(R~iE261
W094/~960 ~13 g ~ 4 0 PCT~S94/04606
~ ~ ' .
_ g_
EXAMPLE 1 - 3 PLY SOLID WOVEN
The following specifications are used to fabricate a
solid woven prosthesis of the present invention.
Weave - 3 Ply Solid Woven (Triple Plain),
tubular
Warp Yarn - Textured 50 denier/48 filament
polyester
Fill Yarn - 2 ply/textured 50 denier/48 filament
polyester
Ends per inch - 160
Picks per inch - 200
Subsequent to weaving the prosthesis, the material
is scoured in a basic solution of warm water (e.g., 150~F)
and cleaning detergent. It is then rinsed to remove the
cleaning agents. Next, the prosthesis is heat-set on
mandrels of the final desired inside diameter. Typically,
the outside diameter of the mandrel is approximately equal
to the diameter of the final prosthesis. The woven tubing
is woven to be 5-15~ oversize so that it can be mounted
onto a mandrel and shrink fitted to an exact diameter.
Heat-setting can take place in a steam heated autoclave
at about 250~F for about 5-10 minutes or in a convection
oven at 250-400~F for about 10-30 minutes.
~T!rLJTE ~ Ul~ 26)
WO 94/24960 . , PCT/US94/04606
~g~ 10-
EXA~PLE 2 - 3 PI-Y SOLID WOVEN WITH LON~l-l UL~INAL COMPLIANCE
The following specifications are used to fabricate a
solid woven prosthesis of the present invention.
Weave - 3 Ply Solid Woven (Triple Plain),
tubular
Warp Yarn - Textured 50 denier/48 filament
polyester
Fill Yarn - 2 ply/textured 50 denier/48 filament
polyester
Ends per inch - 160
Picks per inch - 160
Subsequent to weaving the prosthesis, the material is
scoured in a basic solution of warm water (e.g., 150~F) and
cleaning detergent. It is then rinsed to remove the
cleaning agents. Next, the prosthesis is heat-set on
mandrels of the final desired inside diameter. Typically,
the outside diameter of the mandrel is equal to the
diameter of the final prosthesis. The woven tubing is
woven to be 5-15~ oversize so that it can be mounted onto
a mandrel and shrink fitted to an exact diameter.
Heat-setting can take place in a steam heated auto-
clave at about 250~F for about 5-10 minutes or in a
convection oven at 250-400~F for about 10-30 minutes. The
. A
SUBSTITUTE SHEET (RULE 26~
W094/24960 ~1~ 8 ~ ~ ~ PCT~S94/04606
heat-setting can be done in a two-step process. The first
step involves heat-setting the prosthesis in its fully
extended state to shrink fit the prosthesis snugly to the
mandrel. The second heat-setting step entails compressing
the prosthesis longitudinally. The compression is on the
order of 25-50~. The prosthesis is then heat-set a second
time using at least the same conditions as in the first
heat-setting cycle.
As a result of the heat-setting, the warp yarns buckle
and crimp. The heat locks the yarns in this geometry to
build in "spring like" or elastomeric properties.
EXAMPLE 3 - 3 PLY SOLID WOVEN WITH LON~llu~INAL
COMPLIANCE AND ~L~NAL VELOUR
The following specifications are used to fabricate a
solid woven prosthesis of the present invention.
Weave - 3 Ply Solid Woven, Tubular with 3
filling floats on outer ply
Warp Yarn - Textured 50 denier/48 filament
polyester
Fill Yarn - 2 ply/textured 50 denier/48 filament
polyester
Ends per inch - 160
Picks per inch - 160
SUBSTlTlJTE SH~ET (RULE 26)
W094/~960 2 1 ~ g 6 4 0 PCT~S94/0~0~
-12-
Subsequent to weaving the prosthesis, the material
is scoured in a basic solution of warm water (e.g., 150~F)
and cleaning detergent. It is then rinsed to remove the
cleaning agents. Next, the prosthesis is heat-set on
mandrels of the final desired inside diameter. Typically,
the outside diameter of the mandrel is equal to the
diameter of the final prosthesis. The woven tubing is
woven to be 5-15~ oversize so that it can be mounted onto
a mandrel and shrink fitted to an exact diameter.
Heat-setting can take place in a steam heated
autoclave at about 250~F for about 5-10 minutes or in a
convection oven at 250-400~ for about 10-30 minutes. The
heat-setting can be done in a two-step process. The first
step involves heat-setting the prosthesis in its fully
extended state to shrink fit the prosthesis snugly to the
mandrel. The second heat-setting step entails compressing
the prosthesis longitudinally. The compression is on the
order of 25-50~. The prosthesis is then heat-set a
second time using at least the same conditions as in the
first heat-setting cycle.
As a result of the heat-setting, the warp yarns
buckle and crimp. The heat locks the yarns in this
geometry to build in "spring like" properties. The warp
yarns in the outer ply which are floating over 3 picks,
would raise from the fabric, forming a filamentous velour
surface.
EXAMPLE 4 - 3 PLY SOLID WOVEN WITH ELASTOMERIC COMPONENTS
TO PROVIDE LONGITUDINAL COMPLIANCE
The following specifications are used to fabricate a
solid woven prosthesis of the present invention.
W094/~960 ~ 3 g ~ 4 ~ PCT~S94/0~06
-13-
Weave - 3 Ply Solid Woven, Tubular with 3
filling floats on outer ply
Warp Yarn - Textured 50 denier/48 filament
polyester & 140 denier Lycra Spandex
Fill Yarn - 2 ply/textured 50 denier/48 filament
polyester
Ends per inch - 160 of polyester, 20 of spandex
Picks per inch - ~60
The tubing is woven to include an elastomeric yarn
in the warp direction, such as Lycra Spandex from DuPont.
The elastomeric yarn is woven into the fabric by inserting
it between the first and second plies. The elastomeric
yarn is woven in a stretched state, so that after weaving
the fabric will retract longitudinally. The elastomeric
yarn provides the longitudinal compliance to the graft.
Subsequent to weaving the prosthesis, the material
is scoured in a basic solution of warm water (e.g., 150~F)
and cleaning detergent. It is then rinsed to remove the
cleaning agents. The scouring process allows the woven
tubing to fully retract by relieving the stress induced
by weaving the elastomeric warp yarns in a stressed state.
The prosthesis is heat-set on mandrels of the final
desired inside diameter. Typically, the outside diameter
of the mandrel is equal to the diameter of the final
prostheses. The tubing is woven to be 5-15~ over-size so
that it can be mounted onto a mandrel and shrink fitted to
an exact diameter. Heat-setting can take place in a steam
heated autoclave at about 250~F for about 5-10 minutes
or in a convection oven at 250-400~F for about 10-30
minutes.
SUBSTITUTE SHEET (RULE ~)
W094/24960 ~13 8 ~ 4 0 -14- PCT~S94/04606
The warp yarns in the outer ply which are floating
over 3 picks, are raised from the fabric, forming a
filamentous velour surface. Long-tudinal compliance on
the order of 25-50~ can be achieved by this method.
Thus, while there have been described what are
presently believed to be the preferred embodiments of
the invention, those skllled in the art will realize
that various changes and modifications may be made to
the invention without departing from the spirit of the
invention, and it is intended to claim all such changes
and modifications which fall within the scope of the
invention.
S~BSTITUTE SHEET (RULE 26)