Note: Descriptions are shown in the official language in which they were submitted.
' 'O 94/00492 2 1 3 9 1 0 S PCr/US93/06038
ANGIOTENSIN IV ~ )ES AND RECEPTOR
Field of the Invention
This invention relates to the polypeptide ligand VYIHPF (angiotensin IV or
AIV) and to related peptide ligands and poly~minoa~.id ligands that bind to, activate
S and/or antagonize a novel angiotensin AT4 receptor. The ligands comprise at least
three of the N-terminal arnino acids of AIV, or AT4 receptor binding equivalents or
analogs thereof. Engagement of the receptor by its ligand triggers acute physiological
effects (e.g., vasodilation) and long-term effects in cells (e.g., hypertrophic growth).
Background of the Invention
The renin-angiotensin system has wide-ranging actions on numerous tissues in
the body affecting blood pressure (pressor activity) and cardiovascular and electrolyte
homeostasis. It is ~;u~ ly believed that angiotensins AII and AIII are derived via
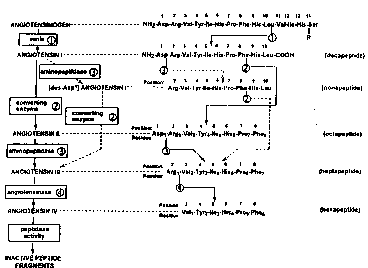
enzymatic cleavage in the cascade depicted in Figure 1, steps 1, 2, and 3 (l).
(Numbering herein of the amino acid residues in AI, AII, Am, and AIV is according
15 to that appearing in Figure 1.) The renin-angiotensin cascade is thought to begin with
the action of renin on angiotensinogen to release angiotensin I (AI), a biologically
inactive decapeptide. Angiotensin II (AII), the bioactive Oclai)eplide~ is thought to be
formed by the action of angiotensin converting enzyme (ACE) on circ ll~ting AI (2).
Des-AspAII (Angiotensin III; Am) is derived from AII, and certain reports have
20 suggested possible activities for Am in the adrenal gland (3) and brain (4). It has
been reported that AII and AIII are inactivated by el~ylllalic degradation through a
series of smaller inactive fr~gm~nts (5). F~ smaller than Am have been
thought, for the most part, to be biologically inactive and of little physiological
~ignifis~nce (6). This assumption has been based on the lack of pressor and certain
25 endocrine activities (i.e., aldosterone release) of small angiotensin fragrnlont~ (7) and
the finding that N-terminal deleted fraem~nt~ i.e., smaller than AIII, reportedly
WO94/00492 ~ OS -2- PCI/US93/060'~
exhibit low binding affinity for angiotensin AI or AII receptors (known as AT1
and AT2, respectively) as determined in radiolabeled ligand studies (8).
Certain studies have used AII(3 8) as one of several controls in structure-
activity studies of AT1 and AT2 receptors (9,10). An AII receptor having
components with molecular weights of 60-64kDa and 112-115kDa has reportedly
been cloned from adrenal cortical cells as well as rat smooth muscle (11).
In general, AII(3 8~ has been found to be much less active than AII or Am
with regard to typical angiotensin-dependent pressor activity or stim~ fing water
intake (9,10,12). However, certain reports have suggested that AII(3 8), while having
little pressor activity or ability to stim~ te aldosterone release, may under certain
circ~ ces inhibit renin release from kidney (12,13). Haberl et al. (14) reported a
possible effect of AII(3 8~ on endothelium-dep.onrlçnt dilation in rabbit brain.Braszko etal. (15,16) reported possible effects of AII(3 8~ or AII(3 7~ on motoractivity, memory, and learning when ~minict~red intracerebru~entricularly (icv) into
rat brain and s~lEEested that these effects should be considered "unspecific," i.e., not
metli~ted by receptors (Braszko et al. (17), p. 195).
The angiotensin field has often been fraught with complexity and conflicting
i,~"l,aLion, particularly with regard to the levels of di~l~"l AII and AIII peptides
required to elicit certain cellular responses, the concentrations predicted from receptor
binding studies to be biologically active, and the levels of angiotensin peptides that
may be measured in biological fluids. It has been reported that AII and AIII areremoved from, or destroyed in, circulation by enzymatic hydrolysis. Biological half-
lives of the di~ "l metabolic fr~Em~ntc are reportedly quite short. Semple and co-
workers (18) reportedly detected AIII, AII(3 8~, and AII(4 8~ in arterial and venous
blood in man with half-lives for AII, Am, AII(3 8~, and AII(4 8~ of 4.4, 2.0, 1.9, and
2.4 minlltes~ res~e~ ely. Blumberg et al. (19) reported that during transit through
the kidney 72-76% of AI and AII and 89% of Am was metabolized.
Confusion has existed in the art as to how metabolic products of AII and AIII
can exhibit certain biological activities (e.g., inhibition of renin release andenhAnce~ of cognitive function), while failing to bind to AI or AII recep~o~.
Fr~Em~.ntc of AII smaller than Am, e.g., AII(3-8) and other smaller fr~m~ntc have
not been reported to have specific saturable binding sites in tissues, and ,~ce~,lo,~ for
these fraEm~ntc have not been identified previously. The present invention provides
partial explanation for certain previous confusing and contradictory fintlingc, and
provides novel AIV receptors (AT4), AIV ligands, peptides, analogs, agonists andantagonists that bind specifically to the AT4 receptor and not to AI (AT1) or AII
~vo 94/00492 2 ~ ~ PCr/US93/06038
10 ~
(AT2) receptors. The AIV peptides and the AT4 receptor are labile and subject toproteolytic degradation. In other aspects, the invention provides a specific
angiotçnin~e enzyme that converts AII or AIII peptides to AIV peptides in a novel
pathway.
Summary of the Invention
The discovery, herein, of a unique and novel angiotensin AIV receptor (AT4)
and AIV ligand system for binding a small N-terminal h~;Aapeplide fragment of
Angiotensin II (referred to herein as AIV, with amino acid sequence
Vall-Tyr2-Ile3-His4-Pro5-Phe6) provides partial explanation for confusion in the prior
art. AIV binds saturably, reversibly, spe~ifis~lly, and with high affinity to me~ e
AT4 receptors in a variety of tissues and from many animal species. The AT4
receptor is pharmacologically distinct from classic angiotensin leceplo~ (AT1
or AT2) in that the AT4 leceptor displays no specificity for classic agonists (AII and
AIII) and antagonists (Sarl,Ile8-AlI). Thus, the disclosure details the
pharmacological and bioch~mic~l characterization of a newly discovered branch of the
renin-angiotensin system that employs an AIV ligand as the ~ ling agent, and theAT4 plasma membrane receptor as the detection mech~ni~m
Angiotensin AIV appears to specifically mobilize calcium in vascular
endothelial cells where AIV binding is evident. Binding to the endothelial AT4
receptor appears to trigger cellular proliferation. Binding of AIV to AT4 receptors in
kidney and brain increases blood flow. In addition, binding of AIV to AT4 receptors
in the brain f~.ilit~tes learning and memory retention. AIV has also been shown to
block the hypertrophic action of AII on cardiocytes despite its inability to bind AT2
receptors. Since cardiocytes possess large numbers of AT4 receptors this action of
AIV is most likely direct. Thus, in certain ~ pecl~ the action of AIV appears toneutralize, or act in apposition to the actions of AII and Am.
The invention provides an angiotensin AT4 receptor and receptor fragments
(inclllding the receptor binding site domain) that are capable of binding a VYIHPF
angiotensin AIV N-terminal peptide, and related AIV ligands, but do not bind an
angiotensin AII or AIII N-terminal peptide, i.e., DRVYIHPF or RVYIHPF,
respectively. The AT4 receptor from adrenal cortical cells has a molecular size of
about 140kD to about 150kD on SDS-PAGE following crosslinking a Kd Of about
0.5nM for AIV peptides, and is widely cAplessed on the surface of adrenal cortical
and me~ ry tissues in many ,.. ~.. ~li~n species. The receptor is t;A~Iessed in all
35 important organs and tissues in~ ling heart, lung, kidney, aorta, brain, liver, and
uterus.
W O 94/00492 PC~r/US93/0603~
213910S 4
The invention further provides processes for identifying angiotensin AIV
agonists and antagonists, and constructing diagnostic assays to specifically measure
AIV and AT4 receptors.
Brief Description of the Drawin~s
FIGURE 1 is a sçhPm~tic diagrarn depicting the amino acid sequence of
angiotensinogen and its conversion by renin to AI, by angiotensin converting enzyme
(ACE) to AII, by angiopeptidase to AIII, and by a novel AIV angiotçncin~ce, herein
disclosed, to angiotensin AIV (AIV).
FIG~RE 2A is a graphical leyresenlalion of the results of equilibrium binding
studies of l25I-radiolabeled AIV to AT4 lCCcylol~, isolated from bovine adrenal
cortical llltlll~l ~nes; as described in F.Y ~ ~ .ple 1.
FIGURE 2B depicts graphically the structural requilclllcllls and specificity forbinding of AIV ligand to the AT4 receptor from rabbit cardiac myocyte lllclll~l~nes;
as described in F.Y~mple 1.
FIGURE3 COllly~cS AT2 and AT4 receptor loc~li7~fion in the Habenula
region of the brain using receptor autoradiography with 125I-Sarl,Ile8-AII to localize
AT2 receptors, and l25I-AIV to localize AT4 leceplol~, as described in Example 2.
Panel A shows binding of l25I-AIV to cells in the habenula, th~l~mlls~ cerebral cortex
and hippocampus of guinea pig brain. Panel B shows that the binding of 125I-AIV is
spe~ ific~lly col.,yc~ ely inhibited by lOOnM non-labeled AIV co---yc~i~or. Panel C
shows that binding of 125I-AIV is not co...pc~ ely inhibited by l OOnM Sarl,Ile8-AII.
Panel D shows a pattern of binding of 125I-Sarl,Ile8-AlI to AT2 receptors that is
di~clcnl from the pattern observed with l25I-AIV in Panel A. Panel E shows that
binding of 125I-Sarl,Ile8-AII is specifically inhibited by lOOnM of non-labeled AII
25 coyc~ilor Panel F shows that binding of 125I-Sarl,Ile8-AII is not inhibited by
lOOnM non-labeled AIV colllyctiLor. Panel G shows a "pseudo-color" photograph of12~I-AIV binding. Panel H shows a "pseudo-color photograph of 125I-Sarl,Ile8-AIIbinding. Panel I shows a photomicrograph of a histology slide of a serial section of
the same tissue as in Panels A-I.
FIGURE 4 graphically depicts the pe.ccll~age change in renal blood flow after
infusion of lOOpmol of AIV (n=13 ~"~yclinlcllls); 0.15M saline (n=9); lOOpmol ofD-Vall-AIV (i.e., AIV with a D-valine residue in the 1 position); or lOOpmol of AII
(n=8) into the renal artery at a rate of 25ml/min, as described in Example 6.
FIGURES 5A and 5B are graphical epresclllalions of changes in blood flow
that result from binding of agonist, LyslAIV, to AT4 receptors in kidney, without
changes in systemic blood ples~-lre, as described in Example 4. Figure 5A shows
2139105
vo 94/00492 PCr/US93/06038
changes in arterial blood pressure following ~minictration of LyslAIV at
100pmole/25ml/min (open circles) or saline control (closed circles). Figure 5B shows
changes in renal blood flow following ~mini~tration of LyslAIV at 100pmole/25
I/min (open circles) or saline control (closed circles).
FIGURES 6A and 6B are graphical leplese,llaLions showing r.h~nges in blood
flow that result from ~tlmini~tering di~rellL doses of an agonist NorLeulAIV (i.e.,
NorLeuYIHPF) that binds to AT4 lecel,tol~ in kidney, without changes in systemicblood pressule~ as described in Example4. A Lllt;l~t;LItically effective dose for
increasing renal blood flow was achieved when doses greater than 50fmole/25~LI/min
were infilsed Figure 6A shows changes in arterial blood pressure following
~tlmini~tration of NorLeuYIHPF at 100pmole/25~1Vmin (open circles), 50fmole/25~1l/min (open squares) or saline control (closed squares). Figure 6B shows changes in
renal blood flow following a~ Lion of NorLeuYI~F at 100pmole/25~1/min
(open circles), 50fmole/25~1/min (open squares) or saline control (closed squares).
FIGURES 7A-7D, 8 and 9 are graphical leprese~ ;ons of AIV binding, as
described in Example 6. Figure 7A shows the results of kinetic analyses measuring
binding of AIV to coronaly venule endothelial cells (CVEC) showing Ill~illlal
equilibrium binding in about 60 mimltes with an appal~;llL Ka of about 9.3 x 107 M-l.
Figure 7B shows the results of kinetic studies measuring the dissociation of AIV from
CVEC endothelial cells with an appa,enL Kd Of about 0.3nM. Figure 7C shows the
results of equilibrium binding of AIV to 2 separable types of AT4 receptor sites in
corollaly venule endothelial cells (CVEC). One type of site with a Kd Of about
1.4+/-0.2nM and a second type of site with a Kd Of about 14.6+/-26.5pM.
Figure 7D shows the results of equilibrium binding of AIV to 2 separable types of
AT4 receptor sites in aortic endothelial cells: one type of site with a Kd Of about
4.4 +/- 0.8nM and a second type of site with a Kd Of about 26.9 +/- 9pM. Figure 8
shows c~ ,cLilion of 125I-AIV binding to colonaly venule endothelial cells (CVEC)
by non-radiolabeled AIV analogs. Figure 9 shows association of AT2 receptors with
G-protein in vascular smooth muscle cells (RVSMC), but non-association of AIV
with G-proteins in endothelial cells (BAEC), as evidenced by the inhibility of GTPyS
to inhibit AIV binding.
FIGURES 10A and 10B show e~-h~nce~ .l of cognitive function, i.e.,
learning, in AIV intraceleb.uventricularly (icv) injected animals but not in
AII-icv-injected animals. Testing of memory was con.i~lcted one day (Figure 10A), or
one, two and three days (Figure 10B), after the animals learned a passive avoidance
response; as described in Example 7.
WO 94/00492 2 1 3 g 1 0 5 -6- PCr/US93/0603P
FIGURE 11 is a graphical representation of the colllp~Li~/e stability of
l25I-AIV (closed dots) and l25I-divalinal (or l25I-VallVal3AIV, open squares)
following exposure to rat kidney, as described in Example 4.
FIGURE 12 is a graphical representation of the effects of divalinal AIV (open
squares), and divalinal AIV followed by LyslAIV (squares with dots), on blood
ples~ule (Figure 12A) and renal blood flow (Figure 12B), as colllpared to saline alone
(triangles), saline followed by AIV (closed circles) and saline followed by LyslAIV
(open circles), as described in Example 4.
Detailed Description ofthe Pler~lled Embodiment
As used herein the following terms are int~nded to mean the following,
namely:
"Angiotensinogen" is used herein to refer to a peptide having the sequence
AsplArg2Val3Tyr4Tl~sT-Ti~Gpro7phegHissLeulovalllIlel2Hisl3serl4
abbreviated DRV~ iLVIHS (SEQ. ID. NO. 1)
"AI" and "angiotensin I" are terms used to refer to the decapeptide fragment
of angiotensin having the N-terminal sequence
AsplArg2Val3Tyr4IlesHis6Pro7PhegT-TicgT eu1O,
abbreviated DRVYIHPFHL (SEQ. ID. NO. 2).
"des-Asp AI", "d-Asp AI" and "des-Asp angiotensin I" are terms used to
refer to an angiotensin polypeptide having the N-terminal sequence
ArglVal2Tyr3Ile4His5Pro6Phe7His8Leug~
abbreviated RV~ lL (SEQ. ID. NO. 3).
"AII" and "angiotensin II" are terms used to refer to an angiotensin, e.g., an
o~;~apep~ide, having the N-terminal sequence
AsplArg2val3Tyr4Ile5His6pro7phe
abbreviated DRVYIHPF (SEQ. ID. NO. 4).
"Am," "angiotensin m,~ "Des-Asp A~," and "AII(2 8~" are terrns used to
refer to the heplapeplide fragment of angiotensin having the N-terminal sequence ArglVal2Tyr3Ile4His5Pro6Phe7,
abbreviated RVYIHPF (SEQ. ID. NO. 5).
"AIV," "angiotensin IV," "A~1~3~)," "Am(2 7~," or "Des-Arg Am" are
terms used to refer to the heAapepLide Çl~l"en~ of angiotensin having the N-terminal
sequ~once VallTyr2Ile3His4Pro5Phe6, abbreviated VYIHPF (SEQ. ID. NO. 6). In the
context of usage herein "AIV" refers to physiological angiotensin II(3 8~ fr~gm~nt~
3 5 formed in a variety of animal species. An "AIV peptide ligand" is a ligand capable of
binding to an AT4 receptor. AIV is a ,~plese"La~ e example of an AIV peptide
ligand, as are AIV analogs.
2139105
vo 94/00492 PCr/US93/06038
--7--
"Des-x," also abbreviated "d-x," is used to refer to an amino acid sequence
that lacks the amino acid residue "x". Des-Asp AII is used to refer to an angiotensin
II lacking the N-terminal Asparagine residue; d-Val(l)AIV is used to refer to AIV
lacking the valine residue (position 1 ) at the N-terrninus of AIV.
S "N-terminal" and "N-terminus" are used interc.h~nge~bly to refer to theNH2-amino terminus of a peptide. The N-terminal amino acid is the amino acid
located at the NH2 terminus of the peptide.
"Peptide" and "~cl~,c~lide" are used hl~c~ e~bly to refer to a serial
array of amino acids peptide bonded one to another of at least three amino acids in
length to plcîclably six amino acids in length, but also up to many hundreds of amino
acids in length.
"AIV Ligand" as used herein refers to a compound that is capable of filling
the three-dimensional space in a Icceptor binding site so that electrostatic repulsive
forces are ...;.~;...;~e~, electrostatic attractive forces are l.,ax;...;7ed, and hydrophobic
15 and hydrogen bonding forces are ~,.;,;~ed Rep,esc,lL~lh~e ligands include "AIV
peptides" and "AIV analogs". T ig~n~ls bind to their specific receptor in a specific
saturable manner, e.g., specificity may dctclll hled by the ability of an AIV ligand to
bind to an AT4 receptor in a manner that is not co,lll)cLi~ ely inhibited in the presence
of an excess (e.g., 1000-fold molar excess) of a co",~ct-lor peptide (e.g., AI or AII).
"AIV peptide" is used interrh~nge~bly with "an~;iGt~-~ IV peptide" to
refer to an AIV ligand that is a peptide having, or co--c~l.onding to, at least three of
the N-terminal ten amino acid residues (preferably three of the N-terminal eight amino
acid residues, and most plcrel~bly three of the N-terminal six amino acid residues),
comprising three amino acids selected from among V, Y, I, H, P, F, L, K, A, H,
NVal, NLeu, or Orn; prcrcl~bly from among V, Y, I, P, K, NVal or NLeu; and most
plcrclably from among V, Y, K, NVal, or NLeu. Replcsenlali~e eAan",les of AIV
peptides have an amino acid seq~l~nce related to the AIV N-terminal sequence
VYIHPFX, i.e., by conservative and nonconservative sub~ ul;on~ of amino acids, or
by derivatization or covalent modification, (as desc,ibed below), and ~I,e[cin X is any
non-interfering amino acid. Re~,t;senl~ re AIV peptides are polypeptides from
3 amino acids in length to many tens of amino acids in length. Other rcplese~llali~/e
~Y~mrles of "AIV peptides" include peptides that are capable of antagoni,i"g binding
of "AIV" to its receptor, i.e., "antagonists" (as defined below), and other "AIVligands" are capable of binding to the AT4 receptor and exerting effects similar to
"AIV", i.e., "agonists" (as defined below).
WO 94/00492 PCI/US93/060~
- 213910~ -8- :~
As used herein the term "AIV analog" is inte.n~ed to mean a chemical
compound that mimics or improves on the electronic, steric, hydrophobic, and
3-dimensional space-filling requirements of the conctituent amino acid residues
involved in binding of the AIV peptide to the AT4 receptor (e.g., a mimetic chemical
5 AIV composition). AIV analogues may be polypeptides, i.e., having amino acids
bonded by peptidic linkages, or may be non-peptides, i.e., having amino acids not
bonded by peptidic linkages. Representative examples of AIV analogs include
chemical mimetic compounds that are capable of antagol i~ing binding of AIV to its
receptor, i.e., antagonists (as defined below), and other AIV ligands are capable of
10 binding to the AT4 receptor and exerting effects similar to AIV, i.e., agonists (as
defined below).
"Agonist" as used herein means an AIV peptide or AIV analog that is capable
of spacially collrul~ l-g to the molecular space filled by an AIV ligand and that is
further capable of colllbillillg with AT4 lecel)lol~ to initiate an action that is initi~ted
15 by a physiological AIV molecule when it binds to its specific AT4 receptors on cells
in vivo or in vi~ro. Represell~ e examples of actions initi~ted by AIV are illustrated
in the Examples. Agonists possess binding affinity for AT4 receptor(s) and intrinsic
activity for in~lçing the activities that are in~lced following the binding of AIV to
AT4 receptor. Represenlali~e examples of agonists include VYIHPFX, NvaYlHPFX,
20 and OrnYI~FX, wherein "X" is used to decign~te one or more non-hllelr~ling amino
acids. Representative examples of processes for recognizing agonists are described in
Example 4.
"Antagonist" as used herein means an agent that spacially conforms to the
molecular space filled by an AIV ligand and that is further capable of colllbinillg with
25 the subject AT4 rece~,lol(s) to inhibit, neutralize, impede or reverse, at least in part,
an action of physiological AIV when it binds to its specific AT4 receptors on cells.
Repleselllali~re examples of antagonists include KYI~FX, and NLeuYI~FX,
whelein "X" is used to deign~te one or more non-inle,Ç~,i"g arnino acids.
Representative examples of processes for recognizing antagonists are described in
30 Example 4.
"AII ligand" as used herein refers to a peptide having the N-terminal amino
acid sequence DRVYI~FX and capable of binding to an AT1 or AT2 AII receptor,
where X is any non-intelre,ing amino acid.
"Non-interfering amino acid" as used herein means any amino acid that
3 5 when introduced into the C-terminus of an AIV peptide ligand does not interfere with
binding of the AIV peptide ligand to its specific AT4 receptor.
~0 94/00492 21 39 1 05 PCI/US93/06038
._ ,
"ATl" and "ATl recc~Jlorll and are terms used interch~ngeA~kly to refer to a
receptor subtype capable of binding AII.
"AT2" and "AT2 rcc~,lor" are terms used interchangeably to refer to a
second receptor subtype capable of binding AII.
"AT4 f~c~;~.lor"is the term used to refer to a receptor capable of binding an
AIV ligand but not an AI, AII, or AIII ligand.
"AT4 rec~,lor fragments" is a term used herein to refer to portions of the
AT4 receptor that are smaller in size than an AT4 receplor isolated from a natural
source, e.g., tissues, biological fluids and the like, but remain capable of binding AIV.
FragmPnts may be pley~ed from an AT4 receptor isolated from a tissue and then
subjected to proteolytic degradation or trç~tmPnt with a ~.hPmic.~l such as cyanogen
bromide. In the latter case the rli~g.~ s of the receplor are conveniently purified
before use, e.g., by reverse-phase HPLC or immllne affinity chlol"alography.
Alternatively, fr~gmP.nts of the AT4 l~ceptor may be yley~ed by eAyiession of a
portion of a nucleotide sequence of a genomic or cDNA clone capable of eAylessing
the AT4 receptor, e.g., a portion of the AT4 nucleotide sequence in an c~ylt;ision
plasmid or vector introduced into a cell, wl,e~ei" the cell m~mlf~ctllres the AT4
receptor fragment and the fragment can be purified (as above). For example,
fr~gmPnt~ of the AT4 receptor that contain the AIV ligand binding domain of the
receptor may be soluble in biological fluids and aqueous solutions and may bind AIV
ligand with a greater or less Kd than AT4 receptor under these conditions. The
binding affinity",Ayl ~ssed as the Kd, of the AT4 receptor fragment for an AIV ligand
is about 30nM to about 0.003nM, plerel~bly about lnM to about 0.01nM, and most
preferably the binding affinity is about 0.5nM to about 0.01nM.
"Triggering the AT4 ~cce~,lor," "acti.ali,.~ the AT4 fece~tor," or
"activation of the AT4 r~C~;IJtOr" are used interch~ng~hly to refer to col~ll"aLional
and/or structural or activity ~h~l~ges resident in an AT4 receptor following binding of
an AIV ligand; e.g., col~""~Lional rhAr~es may be evident by cl,anges in the near
W spectra of the receplor or ch~nges in the circular dich,ois", (CD) spectra;
structural ~ h~nges may be evident as covalent modification of the receptor, e.g., by
phosphorylation; and, activity c.l-~np,es may be evident as an increase in enzyme
activity, e.g., an innate tyrosine kinase activity. A receptor that has interacted with an
AIV ligand and has undergone the process of "trigge~ing" is also referred to herein as
a "triggered AT4 receptor. "
"Substantially purified" as used herein refers to a prel)al~lion that colllaills a
peptide, ligand, or receptor that is enriched greater than about 10-fold from the
WO 94/00492 PCr/US93/0603Y
~ Z139i~5 -10-
natural source material, e.g., membrane plepal~ions of a tissue, and that also contains
less than 5% impurities detect~ble by one-dimensional SDS-PAGE. The subst~nti~lly
purified AT4 receptor approaches homogeneity at purification levels greater thanabout lOOOx.
The term "AIV angiot~ r~ e" as used herein refers to a dipeptidylpeptidase
capable of catalyzing hydrolysis of an arginine-valine peptide bond in an angiotensin,
e.g., AI, AII, or AIII, without catalyzing hydrolysis of any of the other peptide bonds
in the angiotensin.
"Pressor activity" is used to refer to blood pressure changes ind~lced by an
agent, e.g., AII.
As diccncsed above, the angiotensin field has often been fraught with
complexity and conflictinE h~rul.l.alion. During attempts to purify the angiotensin II
(AT2) receptor from bovine adrenal cortex, the curious observation was made that as
purification proceeded the appa~en~ spe~ifirity of the receptor ~.h~nged While
isolated ~--clll~ es bound stable AII analogs better than AIII, the solubilized
receptor c~ ed the opposite order of ligand specificity with AIII binding betterthan AII. Following purification it became app~rc..L that the receptor had all but lost
its ability to bind AII, and was slowly losing its ability to bind Am, despite taking
steps to inhibit proteases. Previously it had been reasoned that 10% hydrolysis of
20 ligand was h~conse4~1ential; however, considering the strange behavior of the receptor
during purification, the possibility was considered that a portion of the confounding
data could be explained by metabolic conversion of the active AII and AIII ligands to
previously l-nrliccP.rned metabolite(s) and interaction of the metabolite(s) with a novel
receptor(s). The N-terminus of both AII and AIII are reportedly labile to proteolytic
25 hydrolysis, but previous studies did not s~ticf~ctorily control for such hydrolysis.
Thus, the possibility was considered that previous studies may have been confounded
by the conversion of one ligand (i.e., AI or AII) into another (i.e., Am or AIV), e.g.,
medi~ted by renin, angiotensin co..~c l;ng enzyme (ACE), ~.i..opepLidases,
endopeptidases, and/or c~l,u~yl,c~,Lidases (e.g., through reactions such as those
30 depicted in Figure 1). Considering the previous studies in this light, physiological
activities reportedly triggered by the AII or AIII peptides, on rc-P-~,.,;n,.l;Qn, seemed
to require higher levels of peptides than would be predicted from the physical binding
propc.Lies of the rcspecLi~lre AT1 or AT2 receptors. However, if a previously
unrecognized receptor existed it might be reasoned that the relatively high levels of
35 AII or AIII are required to provide precursors for conversion to metabolites that
would bind to the novel receptor. To approach this problem an improved assay
' ~ 94/00492 ~ 2 1 3 9 1 0 S PCl/US93/06038
-1.1-
system was developed. After an exhaustive search, a rapid spin chromatographic
technique was developed to separate bound from free ligand, and an assay buffer was
discovered that ...;~ ed N-terminal degradation of angiotensins. In addition, a
stable N-substituted analog of AII was used as a control (i.e., Sarl,Ile8-AII), so that
S classic AII binding sites (20) could be identified. Under these conditions, the possible
conversion of AII to AIII or other metabolites is klimin~tefl, and AII binding sites are
accurately idçntified When assays were con-J~cted under these conditions to
determine what angiotensin fr~gmknts bound to bovine adrenal cells, it was
surprisingly observed that the 125I-AII or 125I-AII radiolabel "specifically bound" to
10 the cells was a hexapeptide fragment of AIII consisting of residues 3 through 8, i.e.,
AII(3 8~. Further, it was discovered that the amount of binding in a given AII (or
Am) prcep&l~lion was directly proportional to the amount of the hexapeptide in the
prel)~alion The use of 125I-radiolabeled h~,~?cl~Lide as the ligand in the receptor
assay dramatically increased binding. A reevaluation of binding in purified Illtlll~ e
15 plepal~lions demonstrated the presence of two di~erclll and distinct receptors, one
for AII and a second for AII(3 8~. Further, neither AII nor AII(3 8~ ligand effectively
displaced the other. The results, thus, SLl ol1gly suggested the presence of two distinct
receptors, one for AII and a second for AII(3 8~. HereinarLer AII(3 8~ is lerclled to as
AIV and the novel AII(3 8) cceptor is lercllcd to as the AT4 receptor. The notion of
20 two separate and distinct receptors was cor~,lled by solubilizing, isolating, and
subst~nti~lly purifying the AT4 receptor under conditions that did not solubilize the
AT2 receptor.
The experimental results described in the Examples, demonstrate for the first
time the ~xi~tknce of a distinct high-affinity cellular receptor that specifically binds the
25 h.,A~pcl~Lide fragment of angiotensin AII, i.e., AII(3 8~, termed herein angiotensin IV,
or simply AIV. The angiotensin AT4 receptor is characterized in the Ex~mples, with
respect to structural IC~IUilClllCllL~ for ligand binding, species and tissue distribution of
the receptor, physiological role of the AIV ligand-AT4 receptor system, intracell~ r
Illk~sçl~g~l ~ign~lin~ pathways activated by the receptor, conditions for isolation and
30 purification, and molecular size of the recel)tor.
In one embodiment of the invention, compositions are provided which
comprise subst~nti~lly purified angiotensin AT4 recep~or or rl;.~ thereof, that
are capable of binding an angiotensin AIV ligand but not an angiotensin AI or AII
ligand. The AT4 receptor binds AIV ligands, and does not bind to a peptide having
35 the AII N-terminal sequence, i.e., DRVYI~F. AT4 receptors of the invention are
specific for AIV and AIV ligands, and are more fully characterized by the following
WO 94/00492 ~ Q~ -12- PCr/US93/0603P
properties a) AT4 receptor has a Kd for AIV of about 30nM to about 0 003nM,
preferably about 3nM to about O OlnM, and most preferably about lnM to about
O lnM (representative examples of binding p-opellies of AT4 receptors are
summarized in Table 1); b) AT4 receptor binds to AIV ligands in a saturable and
5 reversible manner; c) the binding of an AIV ligand to the AT4 receptor is
compe~ ely inhibited less than about 1% to about 10% by an angiotensin AII
prep~lion (e g, Sarl,Ile8-AII) that contains less than 0 1% of an AIV ligand when
the competition of AIV binding is measured in the presence of about a 1000-fold
molar excess concentration of the co...pe~ing ligand using the assay conditions
10 described in Example 1
In a represe..L~Li~e embodiment, AT4 receptors having these plopellies may
be isolated from bovine adrenal cortical ...e...~ es (e g, described in Example 1)
Isolated AT4 receplo-~ from this source have the kinetic, equilibrium binding, and
physical propcl Lies set forth below in F~mple 1 The AT4 receptor of the invention
has a molecular size of about 120kD to about 200kD on SDS-PAGE, preferably
about 140kD to about 160kD, and most preferably about 140kD to about 150kD
For example, an AT4 receptor of the invention is present in ll~u~b-~ne p- ep~ alions of
adrenal glands of most ~ n species (e g, cow, pig, horse, dog, cat, rabbit, and
guinea pig) and, as purified from bovine adrenal ~c~nb~1es, the AT4 receptor has an
20 appale-.L molecular size of about 146kDa on SDS-PAGE AT4 receptors are also
cA~ressed in guinea pig aorta, heart, kidney, liver, lung, vascular smooth muscle,
pituitary, and uterus, as well as vascular endothelial cells and brain
''~94/00492 ~1i3~S PCI/US93/06038
-13-
TABLE 1
Bindin~s Plope~ lies of AIV Receptors
Animal T ssue Ple~ lion K~l nM) Bn~ ~xC Example #
Rabbit .-:eart ~ ~ f.C overal: 1.70 731 i~2
Guineapig .-.eart ~.. , .. i.i-~C site#: 1.33 144 i~l
Bovine A~renal ~ . .. ~ - c 0.54 1030 i~l
Cortex
Sol. Receptor~ 0.51 87.9 #1
Adrenal~ ' -- -- 397.3 #2
Medulla
Bovine HeanVasc. Endo.e overall: 0.7476 ~2
site#l: 26.5 6 i~7
site#2: 1.4 594 i~
Bovine HeartAorticEndo.~ site#l:26.910 ~~
site#2: 4.4 434 ;~
Guinea Pig Brain I-rr ~ ~ 0.1 306 #11
g
HSTAh 0.11 ~l~ j
Cerebellum 0.2''.3'' ~::
Brain Stem 0.9 :~
GuineaPig ~orta
.:.eart '~ y_
~dney " -- ''2.7 i~
_iver " -- "8.9 i~~
.,ung
~-.erus " -- 7 i~2
Pig ALrena ~.. ~.,.. ~c ~ 3~7.3 i~2
Horse ALrena " -- -0.8 ~2
Dog ALrena " -- -2.7 i~2
Cat ALrena " --- 199.6 ~2
Rabbh ALrena " -- 105.3 i~2
Guinea Pig ALrena " - 101.2 i~2
c.) BmaX= ~ 1 b nding under equilibrium b nding concitions, (fmol/mg
protein),
d.) sol. rec~lo,- solubilized receplo~,
e.) vasc. endo.= vascular endothelial cells CVEC;
) aortic endo.= aortic endothelial cells BAEC;
g.) hippocampus= hippocampal solubilized receptor; and,
h.) HSTA= hypoth~l~mllc~ th~l~ml~c, septum, a.,Leleo~entral third ventricular
areaofbrain.
- The invention further provides AT4 receptor ligands that specifically bind to,
activate and/or antagonize the AT4 receptor. The AIV ligands generally comprise at
- least 3 of the N-terminal amino acid residues of AIV, or analogues or AT4 receptor
binding equivalents thereof. The amino acid residues of the ligands may be bonded by
WO 94/00492 ` ~ ~j`~ PCT/US93/0603
-14-
peptidic linkages, or may be bonded by non-peptidic linkages. The ligands generally
have a Kd for the AT4 receptor below about 3 x 1 O~M.
Generally, the AIV ligands of the invention are based on the structure of AIV.
The AIV ligands may be obtained by constructing AIV analogs that have one amino
5 acid substituted for by another of like plop~lLies, i.e., a neutral polar amino acid for
another neutral polar (e.g., G, A, V,I,L,F,P, or M), a neutral nonpolar amino acid
for another neutral nonpolar (e.g., S, T, Y, W, N, Q, C), an acidic amino acid for
another acidic (e.g., D or E), or a basic for a another basic (e.g., K, R, or H). The
AIV ligands may alternatively be obtained by constructing an AIV analog that is
10 covalently modified, e.g., wherein an amino acid residue is substituted by amidation,
adenylation, methylation, acylation, phosphorylation, uridylation, fatty-acylation,
glycosylation, and the like to form a "s~sliluled amino acid residue". In addition, the
AIV ligands of the invention may contain one or more stereoisomers of the
cor..~ Pnt amino acids residues; i.e., may contain one or more substituted or
unsubstituted amino acid residues in the D-configuration.
In other embodimpntc~ the invention provides angiotensin AIV ligands and
ligand compositions that include AIV analogs, AIV peptide derivatives, and
covalently modified AIV peptides, all of which are capable of binding to an
angiotensin AT4 receptor. AIV ligands of the invention are generally defined by the
formula
Rl-R2-R3-X
wherein Rl is a substituted or ullsubs~ ted amino acid residue having a neutral or
positively ch~;ed ~liph~tic side chain Zl, said amino acid being selected from among
V,I,L,A, G, F,P, M, K, norvaline, norleucine, and ornithine;
R2 is a sub~liluled or unsubstituted neutral nonpolar amino acid selected from
the group concicting of Y, W, N, Q, F, or C;
R3 is a substituted or unsubstituted neutral polar amino acid sPlected from the
group co~ g of G, A,V,I,L,F,P, or M; and
X is nothing, R4, R4-R5, or R4-R5-R6, wherein R4 is a substituted or
unsubstituted basic amino acid residue sPIected from the group colls;sli,lg of K, R and
H, R5 is a substituted or unsubstituted neutral polar amino acid residue selected from
- the group concicting of G, A, V,I, L, F, P, and M, and R6 is a substituted or
unsubstituted neutral polar amino acid residue sPlected from the group Col,S.sli~lg of
G, A, V,I, L,F, P, M, and polyamino acid residues co..l~it~ g one or amino acid
3 5 residues which do not prevent binding of the AIV ligand with the AT4 receptor.
-'~94/00492 7~ 3910S PCI/US93/06038
-15-
Thus, the AIV ligands of the invention are generally amino acid chains that
contain 3, 4, 5, or 6 amino acid residues corresponding to the N-terminal 3, 4, 5 or 6
amino acid residues of AIV (the polypeptide, VYIHPF), or may optionally extendedat the C-terminal end with one or more amino acid residues that do not prevent
5 binding, due to spatial, co~ alional, electrostatic or other considerations, to the
AT4 receptor. The amino acid residues may be linked in the amino acid chain by
peptidic linkages to form peptides, or the AIV ligands of the invention may contain
one or more non-peptidic linlf~g~c, such as methylene or C-N linkages, to enhance
metabolic stability or other properties of the AIV ligands, as is hereinafter further
10 described. Rep.t;se~ /e AIV ligands of the invention include, but are not limited to
C-terminal trunc~ted forms of AIV, such as AIV(1 5), AIV(l 4), and AIV(1 3);
stereoisomerically modified forms of AIV, such as D-H4 AIV, D-P5 AIV, and D-F6
AIV; full or trlmc~ted forms of AIV with modified amino acid re~i~luee7 such as G4
AIV, G5 AIV, G6 AIV, Nlel AIV, Kl AIV, F AIV, Il AIV, Pl AIV, Nval AIV, Ornl
15 AIV, Y6 AIV, I6 AIV, NleYI, KYI, and MeYI, derivatives of AIV with one or more
non-peptide linkages belween amino acid rç~iduçc, such as Me all AIV (whereill the
dçeign~tion all refers to a methylene -CH2- linkage belween the amino acid residue in
position 1 (Nle) and the amino acid residue in position 2 (Y)), Me all Val3 AIV, Kall
Val3 AIV, Kall AIV, Vall AIV, Val3 AIV, and Vall Val3 AIV, and substitued AIV
20 ligands, such as propal1oyl-N oml AIV, O-me Y2 AIV, isobutyl-N oml AIV, N-me Il
AIV, NleYI amide, KYI amide, MeYMe amide, MeYNva amide, Me al N-me YI
amide, benzyl Cl AIV and the like.
The physical plope-lies of the AT4 receptol~ that dt;lelll~ e binding of the
AIV ligands were mapped using synthetic peptides and analogs, as described below in
25 detail in the e,.~nplEs. The structure of the N-temlinus of AIV is most important for
high affinity binding of an AIV peptide to an AT4 receptor. The AT4 receptor
binding site is a cool.li..aled multidomain binding site ~hereil1 binding in onesubdomain may be ~y~lllded by high affinity binding at a second subdomain through
an intluced co,~ulmalion change in the AT4 receptor binding site l-ydlophobic pocket
30 subdomain. At least three binding site subdom~in~ in the AT4 receptor were .napped
using syllllletic peptides and analogs. The binding site is stereospecific at a first
subdomain for L-Valine in N-temlinal amino acid position 1 (Vall) of AIV; at a
second subdomain for L-Tyrosine in position 2 (Tyr2) of AIV; and at a third site for
L-isoleucine (Ile3) in position 3 in AIV. The results suggest that Vall in AIV may
35 interact laterally with the walls of the groove of the receptor while Tyr2 in AIV may
interact with the receptor binding site through van der Waals forces and hydrogen
WO 94/00492 PCr/US93/0603
213gl0S -16-
bonding. AIV peptides having a weak hydrophobic amino acid at the N-terminus with
an aliphatic side chain (e.g., KYIHPF, NleYIHPF, OrnYIHPF) bind to the AT4
receptor with a higher binding affinity than AIV (binding of KYIHPF is 50-fold higher
than AIV, and NleYIHPF has a Ki Of about 1O-l2M). N-terminal extension of AIV is5 incompatible with binding, as is deletion of the N-terminal valine (Vall) residue.
Deletion of Vall reduced binding affinities 1000-fold; substitution of Vall with Sar
decreased binding affinity; addition of D-arginine to the N-terminal Vall reduced
affinity for the receptor by 100-fold. The receptor binding site domain of the AT4
receptor COllL~llS a hydrophobic pocket COl~llllillg closely to the space filled by
10 norleucine (i.e., çng~ging the Vall residue of AIV) and in close apposition with a
negatively chalged residue (i.e., çnp~ging the primary amine of the N-terminus of
Vall). Removal ofthe N-terminal amino group decreases by 1000-fold.
The C-terminus of the AIV peptide is relatively less important in the receptor
binding and C-terminal extension of AIV ligands of the invention with "X" is allowed.
15 However, removal of both the Pro5 and Phe6 residues from AIV reduced binding
affinity by about 21-fold to a Ki Of 500nM. The C-terminus of the AIV peptide may
determine receptor subtype specificity of binding.
In addition, it has been found that AT4 receptors isolated from bovine adrenal
cortical ",t".l"~nes do not effectively bind AIV peptides synth~ ed with an
20 N-terminal extension with Sar or GABA. Nor do the illustrative AT4 receptors
effectively bind peptides having the N-terminal L-Val replaced with D-Val or Sar.
Also, removal of the N-terminal L-Val from AIV all but elimin~tes binding to the AT4
receptor. AT4 receptors of the invention have a receptor binding site that is
stereospecific for L-Valine. In one illustrative example, D-VallYIHPF has 1000-fold
25 lower binding affinity for the AT4 rece~or than L-VallYI~F. The illustrative AT4
receptor icol~ted from bovine adrenal cortical ",e",~ es coll~ains a binding site that
prefers weak hydrophobic amino acids in the number 1 position (i.e., Rl) of the AIV
ligand, i.e., h.cleas,.,g hydlophobicity by repl~ing Vall with Phe (i.e., FlYIHPF)
decleases binding affinity 4-fold, but repl~cPmlont of Vall with another weak
30 hydrophobic amino acid (i.e., IlYIHPF) results in only a slight change (an increase) in
binding aff~nity. For high affinity binding of an AIV peptide to an AT4 receptor the
structure of the N-terminal neutral polar amino acid is most i...~,o"~. N-terminal
extension is incol..pa~ible with binding, deletion of the terminal valine residue
";~ es binding (Ki >10~), substitution with Sar decreased binding affinity,
35 substitution with Ile results in equivalent binding, substitution with Phe resulted in a
5-10-fold decrease in the affinity of binding, Pro-substituted AIV peptides bind with
21391o~
~~ ~ 94/00492 PCr/US93/06038
-17-
100-fold lower affinity, Lys-substituted AIV peptides bind with 10-fold higher
affinity, and AIV ligands having a norleucine in the number 1 position (also
abbreviated herein Me, NLe, NLeu, NLeul, or Mel) bound with 1000-fold higher
affinity.
The interaction belween the AT4 receptor binding site and AIV ligand may be
dictated by requilelllellls for an AIV ligand co"l~ ;"g a flexible aliphatic carbon side
chain, (i.e., as opposed to a relatively rigid aromatic ring), rather than by the degree of
hydrophobicity of the side chain. In a representative example, substitution of Vall
with Aspl (i.e., to form AlYIHPF) results in an analog with no binding affinity for the
AT4 receptor (i.e., has a Kd > lO~M). Further, the AT4 receptor binding sites of the
invention may prefer a flexible aliphatic carbon side chain having 4 carbon atoms that
lack a positively charged residue. Heptanoyll AIV with a 5 carbon side chain hasreduced affinity as colll?ared to Mel AIV. In a represellla~ e example, MelYIHPFhas higher binding affinity for an illustrative AT4 receptor than LyslYIHPF, which
was higher than NVallYIHPF, which is in turn higher than OrnlYIHPF. The AIV
peptide ligands of the invention having norleucine substituted for Vall (i.e.,
MelYIHPFX) are partial agonists of VYIHPFX binding to the subject AT4 receptor
and have an app~t;llL Ki Of about 1 x 10-12M.
The AT4 receptor binding site interacts specifically with the N-terminal amino
acid residue (i.e., Rl ), and the latter interaction is specific with respect to both
absolute space occ~p~ncy volume (i.e., of the receptor binding site) and charge (i.e.,
of the AIV ligand). In representative examples, methylation of isoleucine in Ilel of
IlYIHPF (i.e., to form CH3-IlYIHPF) reduces affinity of the illustrative receptor for
the peptide by 67-fold; substitution ofthe Vall primary amine (NH3) with a secondary
amine (-NH-; in this case by sllbstitllting Prol for Vall, to form PYIHPF) reduces the
affinity of binding to the illustrative receptor by 8-fold; substitution of Vall with
benzoic acid or 6-amino-hexanoic acid gives peptides with a Kj>lmM; and, replacing
Vall with GABA (gamma-amino butyric acid; to form GABA-YIHPF) decreases
binding affinity by 250-fold for the illustrative receptor.
The AT4 rec~lor binding sites of the invention also appear to be
stereospecific for Tyr2 (i.e., Y) in the R2 position of the subject AIV peptide ligands.
In lt;presell~ e examples, substitution of D-Tyr2 or Phe2 (with a benzyl ring) for
Tyr2 (with a phenolic ring) results in analogs (i.e., V[D-Y2]IHPF, or VF2IHPF,
respectively) with very low affinity for the illustrative adrenal cortical receptor.
Phenolic side chains in the Tyr2 residue may also interact with residues in the subject
AT4 receptors through hydrophobic and/or hydrogen-bonding.
WO 94/00492 ~39 -18- PCI/US93/060.~"
The AT4 receptor binding sites of the invention tolerate repl~rçm~nt of the
Vl-Y2 peptide bond with a non-carbonyl bond that has a similar bond length, but is
non-planar and has a non-rigid carbon-nitrogen bond. The latter repl~c~mP.nt bond
may preferably be lesis~ to proteolytic hydrolysis thereby cGl~lling additional
5 stability on the AIV ligand and enh~n~ing utility in therapeutic compositions for oral
delivery. In a represelllali~/e example, repl~c~m~nt of the Vl-Y2 peptide bond with a
methylene bond reduces receptor binding affinity by only 5-fold; and, repl~cem~nt of
both the Vl-Y2 and I3-H4 peptide bonds with methylene bonds results in
N-Vl-CH2-NH-Y2V3-CH2-NH-H4P5F6-C (also rerelled to herein as Vall Val3 AIV
10 or divalinal AIV) that has an affinity equal to or better than VYIHPF.
The binding site of the AT4 receptors of the invention is a coor(lin~te~7
mlllti-lom~in binding site wht;lt;ill binding in one subdomain ofthe binding site may be
enhanced or inhibited by binding at a distant second subdomain. In one representative
example, substitution of Ile for Phe at the R6 position of VYIHPF6 results in an15 analog (i.e., VYIHPI6) that binds to AT4 receptor (i.e., through the Vl subdomain
sites) with a higher affinity than the parent VYIHPF molecule. In a second
representative example, substitution of Ile6 for Phe6 in KYIHPF6 results in an analog
(i.e., KYIHPI6) that binds to the receptor (i.e., through the Vl subdomain site) with a
lower affinity than the parent KYIHPF6 molecule. The C-terminus of the subject AIV
20 peptide ligands appears to be relatively less important in receptor binding. In
replt;selllali~e examples disclosed below, deletion of the C-terminal Phe6 from
VYIHPF (i.e., to form VlY2I3H4P5) does not alter binding ~ignific~ntly; C-terminal
extension with hi~ti~ine does not alter binding (i.e., to form VlY2I3H4P5F6H7); and,
addition of both his and leu reduces affinity only 2-fold (i.e., VlY2I3H4P5F6H7L8).
25 Truncation of the C-terminus, i.e., at the R5 position decreases binding. In a
replesenlali~e example removal of Pro5 from VYIHP to give VYIH, decreases
binding 21-fold, and gives an analog with a Kj>500nM. The binding site domains of
the subject AT4 receptor of the invention recognize the N-terminus of the subject
AIV peptide ligands with a high degree of specificity and while the receptor interacts
30 less closely with the C-terminus this region of the subject AIV ligand may determine
recel,lor subtype specificity.
In another embodiment of the invention, antagol~sls of AIV are provided that
bind to the AT4 receptor. Plesenlly particularly plerelled antagonists of the invention
include the non-peptide divalinal AIV and the C-terminal substituted tripeptide MeYi
35 amide, as described in Example 4, although other antagonists will be readily appare
from the data and disclosure set forth herein.
. .
~ 94/00492 2 ~ 3 9 1 0~: PCr/US93/06038
Other aspects of the invention include processes for identifying AIV peptide
ligands, i.e., by structural ~ ;on of the receptor binding requirements of test
prepa,~Lions (e.g., with respect to both blocking and/or promoting binding of the
alternative peptide) to AT4 receptors such as those in heat-treated purified membrane
5 preparation that are free of peptidase activity and devoid of other angiotensin
receptors, i.e., AT1 or AT2 receptors. (Examples of such heat-treated membrane
plep~Lions and assay methods are provided in the examples, below.) Those skilledin the art will recognize that the binding activity of any AIV peptide can be tested,
e.g., using the receptor binding assays described herein, and that analogs, AIV peptide
derivatives, and covalently modified AIV peptide or non-peptide ligands may exhibit
activity as antagonists, agonists, promoters, or enhancers of AIV binding to its AT4
leceplor. C~n-lid~te AIV peptides may be prepared with substitution of other
L-amino acids having di~relll steric, electronic, and hydrophobic character for the
L-Val in the natural AIV ligand. Skilled artisans will also recognize that a similar
approach may be used to characterize further the role of C-terminal amino acid
residues in binding of a peptide to the AT4 receptor, (i.e., other than the C-terminal P
and F). Substitutions and modifications of internal amino acids (i.e., Y, I, or H) can
also be c~ ed by constructing the approp,iate series of D-substituted, covalently
modified, derivatized, or deleted peptides. The first or second meSse~er intracçlh.l~r
pa~h~lvays triggered in cells by interaction of an AIV ligand with an AT4 receptor may
be used to test a series of peptides, analogs, derivatives, or covalently modified AIV
peptides for their ability to bind to the AT4 receptor and trigger the intraCçl~ r
signal. For in.~t~nce7 activities such as tyrosine kinase, guanylate cyclase, Protein
kinase C, Ca~ flux Gh~ges7 phospholipase C (PLC) activity, or prost~gl~n~lin or
endocrine or exocrine hormone release from cells, may be monitored to determine
whether the peptide triggered the AT4 receptor, and the receptor then signaled an
increased or decreased activity in the cell.
In all cases, the AIV peptides, AIV analogs, agonists and antagonists, and
derivatives and covalently modified forms of the AIV peptides of the invention are
recognized by their ability to bind the AT4 receptor with an equilibrium dissociation
cons~ (Kd) below 3 x 10~M, more preÇt;,~bly below 3 x 10-8M and most
ple~lably below 3 x 10-9M, and to a low binding affinity for AT1 and AT2 recep~o,~
with a Kd greater than 1 x 10~M.
In still other embodiments of the invention, processes are provided for
identifying and characterizing a physiological effect of an angiotensin AIV peptide by
assaying the effect(s) of the peptide on a selected in vitro cellular process. For
2~ 39~0S -20- PCI/US93/0603~
imt~nce, to identify and characterize the physiological effects of an AIV peptide on
blood flow, it may be convenient to assay renal blood flow, or in vitro cellularprocesses of endothelial cells and/or vascular smooth muscle cells. To identify and
characterize a physiological effect of an AIV peptide on cardiac ventricular
hypertrophy, assays may eY~mine the effects of an AIV peptide on growth of a
cardiomyocytes in vitro. The processes disclosed herein are also useful in identifying
how the in vitro activities of physiological AIV may be blocked or promoted by AIV
peptides, AIV analogs, or derivatives or covalently modified forms of AIV peptides,
as well as AT4 receptor fr~gmPnts and the like. Representative examples of useful
assays for identifying the subject AIV peptide ligands and AIV ligands are provided in
the C;A~n~
As used herein the term "cellular processes" is inten~led to mean biological
activities that may be measured in vitro or in vivo by q~ e and/or q~ it~tive
assay. For example, cell growth or metabolism may be measured (e.g., radiolabeled
amino acid synthesis into protein, glycolytic activity, oncogene cAI,ression, and the
like); or, proliferation (e.g., 3H-thymidine synthesis into DNA); or, marker eAI.less;on
(e.g., }nRNA by Northern, protein by Western blot, antigen by immlmo~ss~y~ in vitro
selectable drug-re.cist~n~e marker by cell survival in toxic drug, and the like); or,
electrical activity (e.g., in neural cells).
Other aspects of the invention provide compositions and methods for
promoting or inhibiting cellular activity of neural cells, e.g., neural motor, cognitive or
analgesic activity of neural cells in the brain. The effect of the AIV compounds on
motor activity may be observed by e~ alterations in activity as measured with
open-field techniques. The cognitive activity may be observed by passive avoidance
testing, Morris :iWilllllling maze pe~ro~ ce, and various operant tasks. To assay the
effects of an AIV composition on a cellular process it may be useful, for example, to
measure cellular processes before and after addition of AIV peptides to make
colll,~ e observations in parallel cell cultures. In this manner antagonists,
agonists, inhibitors, promoters, enh~ncPrs~ and the like may be identified and
characterized with respect to their physiological effects in vi~ro and possible effects
in vivo.
When used for therapeutic purposes, the route of delivery of the AIV ligands,
AT4 receptor, AT4 receptor fr~gmrntc7 and AIV monoclonal antibodies of the
invention is determined by the disease and site where ~ is required. For
3 5 example, the compounds or compositions of the invention may be applied topically, or
by intravenous, intraperitoneal, intr~m~sc~ r, subcutaneous, intranasal and
) 94/00492 2 1 3 9 1 0 S PCI/US93/06038
intradermal injection, as well as by intrabronchial inctill~tion (e.g., with a nebulizer),
transdermal delivery (e.g., with a lipid-soluble carrier and skin patch), gastrointestin~l
delivery (e.g., with a capsule or tablet), intracelebroventricularly (icv) into brain, or
intraspinally into cerebrospinal fluid (CSF).
5 The prere"ed therapeutic compositions will vary with the clinical indication.
Some variation in dosage will neces~rily occur depending on the condition of thepatient being treated, and the physician will, in any event, determine the applopliate
dose for the individual patient. The effective amount of AIV ligand per unit dose
depends, arnong other things, on the particular ligand employed, on the body weight
and the chosen inoculation legh"e~l. A unit dose of ligand refers to the weight of
ligand without the weight of carrier, when a carrier is used. An effective tre~tm~nt
will be achieved in the microenvil~,lllllell~ of the cells at a tissue site as the
concentration of AIV ligand approaches a collcellllalion of 10-sM to 10-1lM. Since
the pharmacokinetics and pharmacodynamics of these agents will vary in di~lenl
species and di~lelll p~tiçnt~, the most prer~lled method to achieve the therapeutic
concentration is to gradually esc~l~te the dosage and monitor both the biological
effects and the concellL~lion in the biological fluids (e.g., through the use of a
diagnostic imm~-no~s~y, or radioisotopic or chemical label). The initial dose, for
such an esc~l~ting dosage regilllell of therapy, will depend upon the route of
a~mini~tration~ For intravenous a~lmini~tration, for an agent with an appro~illlale
molecular weight of 10,000 d~ltone, an initial dosage of appro~hllately 70mg/kg body
weight is ~tlmini~t~red and the dosage is esc~l~ted at 10-fold increases in
concentration for each interval ofthe esc~l~ting dosage l~hllell. Therapeutic efficacy
in this example is achieved at 0.7-70mg/kg body weight of the theoretical
10,000 dalton peptide.
The compounds may be ~d~ el t;d alone or in co,l-billa~ion with
ph~.,~celti.~.~lly acceptable carriers, in either single or multiple doses. Suitable
pharm~ce~ltical carriers include inert solid diluents or fillers, sterile aqueous solutions,
and various nontoxic organic solvents. The pharm~ce~ltic~l compositions formed by
colllbi~ g the AIV ligands or receptor f~gmf~nts ~ith the pharm~ce~ltic~lly
acceptable carrier are then readily a~mini~t~red in a variety of dosage forms such as
tablets, l07f'~eS7 syrups, injectable solutions, and the like. These pharm~ceutic~l
carriers can, if desired, contain additional ingredients such as flavorings, binders,
excipients, sweet~ning or flavoring agents, colored matter or dyes, emulsifying or
suspending agents, and/or. For palell~el~ on~ solutions ofthe AIV ligand
WO 94/00492 . ~, PCr/US93/060^
2~ 39~~ -22-
or receptor fragment in sesame or peanut oil or in aqueous propylene glycol may be
employed.
The present invention further provides processes for isolating inhibitors of an
AT4 receptor-AIV ligand interaction by: a) selecting a cell type that expresses the
5 AT4 receptor; b) adding an AIV ligand to a control culture of said cells; c) adding the
AIV ligand and a putative inhibitor to a second test culture of the cells; and
d) measuring the level of binding of the AIV peptide to the cells in said second test
and control cultures. In the case that an inhibitor is present in the plepal~lion, the
level of binding in the test culture is lower than that in the control culture. (An
10 example of such a process is provided in Example 1). Those skilled in the art will
recognize that this process may be used to identify an inhibitor of an AT4 receptor-
AIV ligand interaction in ch r olllaLographic fractions and the like during solubilization,
isolation, and purification of said inhibitors, and that the subject inhibitors may act as
agonists or antagonists of the action of AIV inril1ced when AIV binds to its specific
15 AT4 receptor.
In other embodiments the invention provides an AIV angiontçn.cin~ce enzyme
capable of hydrolyzing a peptide bond bt;lween an al~;lline and a valine residue in an
angiotensin polypeptide, e.g., a polypeptide with a DR\VYIHPF N-terminal sequence,
wherein "\" indicates the proteolytic cleavage site that gives rise to an AIV peptide,
20 i.e., with an N-terminal seql1çnce related (as described above) to the amino acid
sequence VYlHPF. Isolation and s~s~ ial purification of AIV angiotçncin~ce may
be conveniently accomplished, for example, by prepalillg an affinity resin having a
non-cleavable or slowly-cleavable AIV ligand covalently bound to the resin, e.g.,
chemically modified derivatives of a peptide in an amino acid sequence selected from
25 among DRVYIHPF, DRVYIHP, DRVYIH, DRVYI, DRVY, DRV, RVY, or
NRVYIHPF, NRVYI~, NRVYLH, NRVYI, NRVY, NRV. Operationally, the
peptide useful in this assay is selected based on its ability to bind the AIV
angiotç~ ce and to be l~;si;~L~III to cleavage by the enzyrne. A test prep~ ion of a
cellular or tissue extract (or a biological fluid sample) is next ch~ llalographed
30 through the affinity resin; the bound polypeptide(s) is eluted, e.g., at low pH and high
salt (e.g., pH2-3, and 2-3M NaCl, and the like), or the bound polypeptide is eluted by
adding an excess of AIV ligand. The presence of the AIV angiot~ .s;i~ce in the eluate
can be dt;lellll,led by assaying for the ability of the column eluate to catalyze
hydrolysis of an Arginine-Valine peptide bond in an angiotensin peptide (e.g., AIII),
35 and subsequently conrllllling that the seq~l~nce of the product of the reaction has a
Valine residue at N-tenninal amino acid and an AIV peptide sequence.
) 94/00492 2 1 3 9 1 0 5 Pcr/us93/o6o38
-23 -
The novel AIV ligand-AT4 receptor system of the invention is useful in a
comple,..~ ,y or antagonistic role to AII in metli~ting long-term effects of
- angiotensins, and in mod~ ting the effects of AI, AII, or AIII on cells. Although not
being limited by any particular theory of action, it is believed that: 1) AIV is derived
5 from AII (or AIII) directly (e.g., through the action of a specific AIV angiot~n~in~ e7
and other peptidases); 2) AIV is very labile and will accnmlll~te at physiologically
~ignific~nt concentrations only when high levels of AII are present at the target site;
3) the AT4 receptor is specific for AIV ligand (with accGll,,ually;ilg low affinity for the
parent peptide, AIII). Under certain conditions, AIV begins to acc lm--l~te at
10 angiotensin target tissues as the AII levels rise. When AIV concentrations rise to near
0.5nM (i.e., the Kd Of the receptor) auxiliary processes which modify the acute action
of AII will be ~ng~ge(l These actions will be mç~i~ted by an intracçll..l~r ~ign~ling
system(s) di~lelll from that employed by AII. The activation of such an intracelll-l~r
system may potentiate or antagonize the target cell's short-term response to AII. One
physiological function of the AT4 receptor-ligand system may be to impart a longer-
term response to high-level or chronic angiotensin stim--l~tion in a tissue. Studies
support the hypothesis that the AIV ligand-receptor system possesses the
characteristics set forth above and is, thel~fole, in a position to serve a short- or long-
term modulatory role on the activities of AII. Studies using bovine adrenal tissues
have shown that the AT4 receptor is specific, with almost no affinity for AII. In
addition, AIV is metabolized/hydrolyzed in bovine adrenal homogen~tes at 200 times
the rate of AII and 4 times the rate of AIII. Data suggest that AIV may be derived
directly from AII by action of a dipeptidylaminopeptidase, termed herein AIV
angioten~ln~ce.
The location of AT4 receptor sites in groups of cells in tissues allows a skilled
artesian to predict likely functions for the AT4 receptor in dirrelc;l" tissues. In
addition, it will be recognized that many activities previously attributed to the action
of AII (and/or AIII) may be triggered or re~ ted instead by the AT4 receptor-ligand
interaction. For example, it is likely that AIV ligand acts as a negative-feedb~c~ agent
thus enabling tighter control on the aldosterone release process. The AIV ligand-
receptor system may also be associated with a previously inexplicable up-regulation of
the angiotensin receptor seen following chronic AII exposure of cells in vi~ro. Still
other functions attributed to AII that may be medi~ted instead by the AIV ligand-
receptor system include altering the release of catecholamines from adrenal medllll~ry
cells or re~ ting adrenal blood flow. It is, the,erore, likely the AIV ligand-receptor
system modulates (i.e., increases or decreases) either the acute and/or the long-term
WO 94/00492 PCr/US93/0603
2~39~S -24-
synthesis and release of chromaffin catecholamines, e.g., by acting to stim..l~te
intracell~ r ~Apfes~ion of tyrosine hydroxylase (the rate limiting enzyme in thesynthetic pa~ ar)
E~e~ ,enls described below de",ons~ e that the AT4 receptor-ligand
5 system may have a role as a metli~tor of long-term angiotensin effects on endothelial
cells (e.g., cell growth; Example 5). AIV ligand-receptor interactions also appear to
activate processes in endothelial cells that are comple",e"l~y or antagonistic to those
activated by AII. For ;,.sl~llce, some of the AIV ligands that are embotlim~nts of the
invention are useful for increasing blood flow (e.g., renal blood flow as demonstrated
10 in the e~"~les).
Because of the widespread distribution of AT4 receptors in many tissues (see
examples, below) it is impossible within the scope of this specification to detail every
one of AIV's actions in angiotensin-sensitive tissues. However, l~presenla~ e data is
provided in the following examples (below), for the physical characteristics of AT4
receptors (see esp. Example 1), for AT4 receptor tissue distribution and speciesdistribution (see esp. Example 2), for physiological functions of AIV peptide ligands
and AT4 receptors in controlling renal blood flow, for the cellular biology of AIV
ligand-AT4 receptor interactions (e.g., second messPnger pathways, G-proteins,
phosphorylation, intrac~ll--l~r Ca++, phosphoinositide turnover, and guanylate cyclase
activity), for vascular effects on venular and aortic endothelial cells and vascular
smooth muscle cells and G-protein linkage of certain AIV-receptors, for endocrine
effects on adrenocortical cell catacholamine release for effects on cardiac myocytes
(i.e., cardiocytes), and for characterization of brain AT4 receptors (e.g., in
hippocampal cells and in cerebellum, hippocampus, pi,irO",. cortex, Par 1/2, Fr 1/2,
caudate put~m~n, HDB, th~l~mlls, and inferior cnll--c--l-ls), as well as, neurological
effects of intracelel~ rentricular injection of AIV (e.g., on le~l~ing and memory).
The disclosures made herein for assays and processes relating to endothelial cells,
adrenal cortical cells, cardiac myocytes (cardiocytes), and vascular smooth muscle
cells are ~i~cussed briefly below.
As sho~,vn in the examples AIV is active in endothelial cells in ~nh~n~in~
cellular proliferation (as evidçnced by thymidine incorporation) and stim--l~ting
production of endothelial cell relaxing factor (EDRF). These results also show the
non-interaction of G-proteins with vascular AT4 lece~,lo.~ in bovine aortic or
colon~y venous endothelial cells. The results set forth in the Examples further
identify a role for the AIV ligand-AT4 receptor interactions in triggering normal
and/or hyperplastic growth of endothelial cells in sites of tumors or traumatic or
) 94/00492 21 391 OS PCI/US93/06038
-25-
wound injury, and angiogenesis, and a therapeutic use for AIV analogs, agonists,antagonists, and derivatives and covalently modified AIV peptide ligands that are
capable of inhibiting vascular smooth muscle cell growth in such hyperplastic states
while at the same time promoting endothelial cell growth. The agonist compositions
5 are also useful for encouraging endothelial cell growth, e.g., in wound sites;~nt~goni~t~ for discouraging vascularization in tumor sites. In addition, the AT4
receptor-ligand system may play a role in triggering vasodilation through a selective
effect on subpopulations of endothelial cells that exist in particular vascular beds (e.g.,
in the heart, lung, liver, kidney, brain and the like). As shown in the examples,
10 increased renal blood flow occurs in rats following infusion of AIV ligands and taken
together with the demonstrated ability of AIV to stim~ te EDRF production in
vascular endothelial cells, the AIV ligand-receptor system metli~tes actions of
angiotensin that fall within the bounds of cardiovascular regulation and body water
homeostasis. Thus, Ill~;l~c;~ltic uses for AIV analogs, AIV agonists and antagonists,
15 and derivatives and covalently modified AIV peptide ligands include promoting renal
blood flow (e.g., in chronic kidney (liqe~ces) or, alternatively, inhibiting renal blood
flow (i.e., using inhibitors and ~nt~goni~ts of AIV), e.g., in conditions of hyperacute
renal dysfunction and water loss, or during renal surgical procedures.
In cardiac myocytes (also termed herein "cardiocytes") it has been specnl~ted
20 previously that angiotensin II may somehow be involved in the development of left
ventricular hy~elllophy since patients treated with angiotensin converting enzyme
(ACE) blockers to block blood pressure ~h~l~gec show less tendency to develop left
ventricular hy~elLlophy (2~,26). As shown herein, AIV antagonizes the hy~ ophic
action of AII. Accoldhlgly, the control of cardiocyte growth may be re~ ted
25 endogenously by a balance between the activating action of AII and the inhibiting
action of AIV. It is further believed that AIV and AIV agonists will be effective in
blocking the development of, and reversing the effects of, left ventricular hylJel Llophy
in p~ti~nt~ Additionally, it is believed that the action of ACE inhibitor is due not to
- their inhibition of AII synthesis but to their ability to enh~nce the synthesis of AIV
ligands such as results from the cl.. ~ of p.e~;ul ~u- ~ from the AII synthetic PalLW~r
into the AIV palllway. Contrary to current popular belief, the b~-nefi~i~l effect of
ACE inhibitors in lle;&~ g cardiac hypertrophy may be due to ACE inhibitor
~nh~nc~m~nt of the formation of AIV.
The data presented herein also indicates that AII and AIV operate by separate
35 receptors employing di~erellL intr~cçll-.l~r ~i n~ling systems. It has been reported that
ACE inhibitors may have a beneficial effect in red~ing cardiac hypertrophy through
WO 94/00492 2J~39~S PCI/US93/060
-26-
effects at the level of AII or AIII. Considering the results disclosed herein it is most
likely that the long-term effects previously attributed to decreased AII may in fact be
m~di~ted by the interaction of increased levels of endogenous AIV ligands with the
AT4 receptor. Further, it is most likely that the antagonists and agonists of AIV
5 ligands, disclosed herein, will provide improved pharm~ceutical compositions for
treating cardiac hypelLlophy attributable to the renin-angiotensin system, e.g.
ventricular hyl~clllophy. The inventors believe that the interaction between AIV and
the AT4 receptor may trigger the receptor and inhibit growth in cardiomyocytes.
In adrenal cells angiotensin AII's role in the regulation of aldosterone release10 from the adrenal cortex is reportedly well established (27). As shown herein, certain
activities (such as adrenocortical cell growth), previously attributed to AII or ATI, are
actually activated following AIV ligand binding to the AT4 receptor. AII (and AIII)
reportedly stim..l~tes aldosterone release from adrenal glomerulosa cells. The
disclosure, herein, of high levels of AT4 receptors in adrenal cortical cells
15 (E~ples 1-2) sllggests a possible role of AIV ligand (i.e., rather than AII or AIII) in
triggering AT4 receptors on adrenal cells to inhibit AII-m~di~ted aldosterone release.
Another role of AT4 receptors in adrenocortical cells may be to up-regulate the
threshold level of AII ligand required to trigger a cellular response by re~ ting the
levels of cellular AT1 and/or AT2 ccel~o~ and/or to regulate adrenal blood flow.
In addition to being found in high concentrations in the adrenal cortex, AT4
receptors are found at even higher levels in the adrenal n1e~Ullnry cells where AII has
previously been reported by others to potentiate catecholamine release. AIV ligand
may modulate release of catecholamines (i.e., increase or decrease the release) acutely
(or possibly even long-term, e.g., by triggering the AT4 receptor and thereby
stiml~l~ting increased or decreased t~-t;ssion of tyrosine hydlo~ylase, the rate-
limiting enzyme in catecholamine sylllhes;s.
In vascular smooth muscle cells the role of AIV and its specific receptor
appears to be similar to that articul~ted above for AIV in cardiocytes: AIV may act to
inhibit growth of the cells thus opposing the action of AII. Agonists of AIV binding
to the AT4 receptor will be effective inhibitors of vascular smooth muscle growth and
will be ~Lc~ ;c~lly useful in reducing neoillLilllal growth which often occurs
following angioplasty.
As disclosed herein, high levels of AT4 receplol~ are present in cardiac and
vascular tissue, inclurling cultured bovine endothelial cells. The disclosure, herein,
that AIV ligands and the AT4 receptors may function as growth factors of the
tyrosine kinase class indicates that certain inhibitors of tyrosine kinase growth factors
2139I 05
'O 94/00492 . j ' PCr/US93/06038
-27- .
may also serve as inhibitors of certain angiotensin AIV ligand-receptor system-
medi~ted cellular hy~tlLlophiC processes (e.g., ventricular hy~cl~lophy), and that
nucleotide probes constructed for compl~ y to portions of RNA encoding the
AIV ligand and receptor sequence may be useful in idcllLiryiilg other members of the
- 5 AIV family of growth factors.
The invention also provides diagnostic applications for the AIV peptide
ligands and antibodies. The role of the AT4 receptor-ligand system in cardiovascular
regulation suggests a possible value to diagnostic tests for monitoring the levels of
AIV ligand and AT4 receptor in biological fluids and tissues (i.e., rather than AII or
AIII). Individuals with high renin-sodium profiles are reportedly at five times greater
risk of myocardial infarction than individuals with low renin-sodium profiles despite
adequate control of systemic blood pressure (28).
The AIV peptides, ligands, receptor fr~pmP.ntc, and the like disclosed herein
are useful in diagnostic assays, e.g., immllno~cs~ys~ for the detection of the presence
or ~molmtc of AIV ligands or receptors in tissues, cells, and biological fluids of
patients. The AIV peptides, ligands, analogs, derivatives, or covalently modified AIV
peptides of the invention may be form--l~ted in buffers with stabilizers, e.g., for use as
positive or negative controls in diagnostic assay, or in reagent test kits for receptor-
binding assays.
Those skilled in the art will recognize that the AIV ligands of the invention
may be readily employed using conventional techniques to produce polyclonal or
monoclonal AIV ligand specific antibodies, and that the isolation and purification of
the AT4 receptor provides materials useful for prepal~ion of polypeptide fra~n~.ntc
(e.g., using CNBr and proteolytic enzymes) that can be subjected to automated amino
acid seq~lenring The amino acid sequçnce of the AT4 receptor, in turn, provides the
sequence data neces~,~.y for construction of conserved and degencl~le nucleotideprobes for cDNA or genomic moleclll~r cloning of nucleic acids e,.~lessing the AT4
receptor, mutant AT4 receptor, or fragm~ntc of the AT4 receptor. A convenient
method for molecular cloning of the receptor is provided in Example 7.
EXAMPLE 1
Physical Characterization of the AIV Receptor
Kinetic bindin~ studies: bovine adrenal cortical Illclllbl~es
In kinetic binding studies, con-lucted as set forth in Example 1 Materials and
Me~hods described below, both l25I Sarl,Ile8-AII and 125I-AIV binding were
characterized by slow association rates (kl=1.01+12x10-2 and 5.58+0.64x10-2 nM~lmin~l, le~,e~ ely), very slow dissociation rates (k l=2.36~0.49x10-2 and
WO 94/00492 ~39~5 PCr/US93/0603~
2.57_0.05x10-2 nM~l min~l, respectively), and high affinity binding (calculated
Kd=2.25+0.26xlO-1M and 4.42_0.46xlO-1M, respectively; number of w~ LS
(n) = 4) (Table 2).
TABLE 2
S Kinetic consL~ s for l25I Sarl,Ile8-Ang II and
l25I-AIV binding to bovine ~drenal cortical mtlllbl l1es. *
T ig~nrls kl (nM~l min~l) k l (min~l) K~(M)
5I Sar1,Ile8-Ang II 1.01 + 12x10-2 2.36 + .49x10-2 2.25 + .26xlO-10
l25I-AIV 5.58 + .64x10-2 2.57 + .OSx10-2 4.42 + .46xlO-10
* n = 3, mean + SD
Equilibrium binding studies: bovine adrenal cortical lllelll~ es
Equilibrium binding studies were contl~lcte~ to evaluate the binding of
l25I-AIV to receptors in bovine cortical membrane plep~lions. Comparisons were
made of the binding of both AIV and of AII, i.e., to the c~ ic~l ATl receptor sites
defined by binding of l25I-Sarl,Ile8-AII. Binding studies were carried out in buffer
con~ -g SmM EDTA, lO~lM Bestatin, SO~M Plummer's inhibitor, and lOO~M
PMSF, developed specifically to inhibit metabolism of angiotensin fr~gmPnt~ and
l S receptors during the assay.
Saturation isotherms for 125I-AII and 125I-AIV indicated the presence of two
distinct separable high-affinity binding sites in bovine adrenal cortical me",bl~ne
plep~ions, i.e., one for AII ligand and a second for AIV ligand. The equilibriumconsL~s calculated from this data were as follows: a) for AII receptor-ligand
binding (i.e., 125I-Sar, lIle8-AII) the Kd=0.54_0.14nM, Bmax-1.03_0.26pmol/mg
membrane protein (n=4); and b) for the. AT4 receptor ligand binding (i.e., l25I-AIV)
the Kd=0.74_0.14nM, Bll,ax=3.82_1.12pmol/mg membrane protein (n=4). The
results of the equilibrium binding studies with ,llelllblai1e-bound AT4 receptor are
nn~i edinTable3.
V 94/00492 j ~ 21 39 1 05 PCr/US93/06038
-29-
TABLE 3
Equilibrium Binding ConsL;a,~ls for 125I Sarl, Ile8-Ang and 125I-AIV Binding
to Bovine Adrenal Cortical Mel.,bl~es.
sma~ Hill
Ligand K~l (nM)(pmol/mg prot ) Coeff. r(Scatchard)
125I Sarl, ILe~-Ang II 0.54 _ .141.03 + .26 1.00 + .03 0.91 + .08
125I - AIV 0.74 + .143.82 + 1.12 1.00 + .02 0.93 + .05
n=4, mean + SD
The results of these kinetic and equilibrium binding studies show: (a) two
separable high affinity binding sites, one for AII and a second for AIV; (b) large
di~erences in the ...s.x;...~l binding (BII~ax) per mg ...~-..b-~ e protein, i.e., with more
than three-fold more AT4 receptor in this p~ep~a~ion than AII receptor; and, (c) no
cross-~ pl~c~m~nt of AII binding by AIV or vice versa. The results provide
10 convincing evidence for the existence of two separable receptors; one for AII and a
second for AIV. However, the theoretical possibility existed that a single receptor
might have di~-ing affinities for AII and AIV. Since it was known that ATl and
AT2 are commonly destroyed during extraction from .llelll~ es, and are also heatlabile (i.e., at 60C) a scheme was devised to rule out the latter possibility by testing
15 for AT4 leceplo-~ in solubilized and heat-treated men-b.~le plep~alions The results
of these studies are presented below.
Equilibrium bindin~ studies: solubilized bovine adrenal cortical AT4 receptor
Initial studies, con-lucted as described in Example 1 Materials and Methods,
col~fi....ed that 125I-AIV bound to solubilized receptors in ,-.e...b-~1e p.~p~lions
20 which would not be expected to contain ATl or AT2 receptors. The kinetics of
binding of l25I-AIV to the solubilized bovine adrenocortical receptor, at an AIVligand conce.~ lion equal to 25% of the appa,~,.ll Kd (with 25~g of .llcll.b.~e
protein), in~lic~ted that equilibrium was reached in appro~i-..alely 100 min. at 37C.)
The plateau region of binding to the solubilized receplor for 125I-AII or 125I-AIV,
(after reaching equilibrium), was stable for at least one additional hour. The off-rate
of the AT4 receptor, as de~ . i-.ed following the addition of 1000-fold excess of
unlabeled AIV, was c-ceedil-gl~ slow, with an average tl/2 =292.4 min (n=5).
Equilibrium binding studies were next cond~cted at 37C with a 120-minute
incubation (as in the Materials and Methods) with the solubilized .llell~b-~l1e receptor
preparations. Saturation isotherms for 125I-AIV (Figure 2A) and 125I-AII (not
shown) were developed to cGlllpare the equilibrium binding constants of the
WO 94/00492 ~i r~ 213D 1~ ~ 30 PCI/US93/0603~
solubilized AT4 receptor. A concentration range of about 5 x 10~M to about
5 x 10-12M AIV was employed in a typical cA~Jelilllen~ using 25~g of total protein.
The best fit for the l-ansrulll,ed data using the LIGAND program revealed a single
AIV binding site with no apparel-L cooperactivity. A sumrnary of the binding data for
5 AIV ligand to solubilized receptor is found in Table 4.
TABLE 4
Equilibrium Binding Data for 125I-AIV to Bovine Adrenal Cortical Solubilized
Receptor.
*K~(~ Bm~"~(fmol/mg protein) r(Scatchard Plot) Hill Coefficient
5.06 ~ .57x10-1 87.9~9.7 0.991 ~.009 0.995~.039
*N=4, mean ~ SD
The data presented in Table 4 shows that the solubilized receptor, like the
e.llbl~e receptûr (Table 3), has an extraordinarily high binding affinity for AIV.
Collll)eliLion binding studies: bovine adrenal cortical melll~ es
To establish the specificity of the AT4 receptor, colll~,e~i~ion curves were
developed with several diLrelell~ angiotensin analogs using a concentration range of
15 10~M to lO-llM. Co...p~i~ons were also made of the binding specificity of ~l~c.ci~
AT1 rèceplor binding sites (i.e., 125I-Sarl,Ile8-AII binding sites). Competitionanalysis (the su.. ~i~ed results of which are presented below in Table 5) also clearly
~lictin~liched the eyict~onre of two distinct rèceplol~ based on their specificity for
di~re..L ligand structures in the angiotensin analogs. (The r values for log-logits
tran~r~,-l--alions of the competition data were typically ~0.98.) Binding of 125I-AII
ligand to the AII receptor (as characterized by binding of 125I-Sarl,Ile8-AII) was
effectively cGllll)tlili~rely h~hil,i~ed by Sarl,Ile8-AII, AIII, and DuP 743. In contrast,
AIV ligand, AII(4 8~, and CGP42112A delllol1sll~led very little affinity for the AII
binding site (Table 5.) The pattern of binding at the AII site is consistent with a
Type I classic AII binding site (20,25). (Binding Sarl, Ile8-Ang II, Sarl, Ile8-Ang II,
AII, AIII, and DuP 753 with high affinity is a pattern of binding specificity consistent
with an ATl site.) In contrast to the AII lece~Jtor~ the binding site for 125I-AIV
ligand was effectively cGl~elili~rely inhibited only by AIV ligand and to a lesser
extent by the peptides in the AIII prepa~lion (Table 5).
'~0 94/00492 ~ ~ -31~- 1 0 ~; PCI /US93/06038
TABLE 5
Competition of l25I-Sarl,Ile8-AII and 125I-AIV Binding to
Bovin~ Adrenal Cortical Men~ nes.
l25I-Sarl,IIe8-AII 125I - AIV
Competitor Binding (Kj, M) Binding (Kj, M)
Sarl,Ile~-AII 0.22 + 0.10 x .10-9 >10
AII 2.01 + 0.67 x .10-9 >10~
AIII 1.15 + 0.34 x .10-9 >14.50 + 2.3 x 10-9
AIV >10~ 0.58 + 0.15 x .10-9
AII(4-8) >10~ >10~
DuP743 3.10 + 0.67 x 10-8 >10-4
CGP 42112A >10 1 >10 1
Binding studies: two receptor binding states in rabbit heart melllblanes
S Studies were next conducted to f-;.. ;.. e the kinetic p~n~,Lers of125I-angiotensin IV binding to receptors in P2 mwll~ f prep~lions from rabbit
heart. Col~ ;sons were made of the binding of both AIV and of AII, i.e., to the
classical AT1 recf~or sites defined by binding of 125I-Sarl,Ile8-AII. Binding studies
were carried out in a buffer (below) co~ ",ng an extensive cocktail of inhibitors that
10 was designf,d to minimi7e metabolism of both the receptor and the test ligand, i.e., the
buffer co~ ned 5mM EDTA, 0.2% BSA, lOIlM Bestatin, 5011M Plummer's inhibitor,
and lOO~MPMSF.
Angiotensin peptides (i.e., AI, AII, AIII, or AIV) were stable in this buffer for
4 h at 37C with less than 10% hydrolysis measured by reverse phase HPLC.
The studies were con~lcted as described in the Materials and Methods,
below. The association rate COIlsl~lt (kl) for 125I-AIV was dele"",i1ed to be
3.05 x 108M-l min~1 and the dissociation rate consL~,I (k l) was 0.028 +/ 0.017 min~l
The overall dissociation con~ (Kd) measured under equilibrium binding conditionswas determined to be 9.15 x 10-11M. (The results rep,~st;llt the mean values from the
20 results of 4 expe"",~ con~ucted using clllplicate s~mrles ) Saturation isotherms
and Scatchard analysis produced data best resolved in a two-site model using non-
linear curve fitting n ethod~ (LIGAND p[og,~., curve fitting options). The Kd for
site #1 was determined to be 10.3 +/- 3mM with B~ =1747 +/- 393 fmoVmg; the Kd
for site #2 was 10.1 +/- 5pM with BmaX=l 5 +/- 4 fmoVmg. Binding to the rabbit heart
25 mtlllbl~nes was competitively inhibited in a specific manner by AIV but not AII, AIII,
125I Sarl,Ile8-AII, DuP753, CGP, AII(4 8~, or DAAI (see Table 6).
Wo 94/00492 PCr/US93/06038
2 139 105
TABLE 6
Coll")t;Li~ on of Binding of l2sI-AIV to Rabbit Heart Membrane Receptors
Competitor Kj (M)
AIV 1.4 x 10-9
AII >10~
AIII 2-3 x 10-7
Sarl ,Ilef~-AII >10
DUP753 >10
CGP >10
AII(4-~) >10~
DAAI 90.5 x 10-9
The data in Table 6 was s~lc~ ted from colllp~Lilion displ~cem~.nt curves for binding
of 0.5nM 125I-AIV to ,l,elll~l~l e fractions; lll~;lllbl~u~es were inr,ub~ted for 120 min. at
5 37C in the presellce of lOpM to lmM co,llpeli~or; possible conversion of AIV to
other (smaller) fr~nrntc was evaluated after 120 min at 37C by adding 20% TCA to
stop the inc~lb~tion, and then ev~ ting the pelcell~age of AIV by reverse-phase
HPLC on a C18 column with a 20% ACN/TEAP3 mobile phase; greater than 92% of
the 125I label present at the conclusion of the inc~lb~tion was present as AIV.
10 Structural re4uiltlll~ s for ligand binding to bovine adrenal AT4 receptol~
The results in Table 7, also include a Sullllll~y of studies ~Içcigned to analyze
the structural realules of the N-terminus of an AIV ligand that are required forbinding to an AT4 l~ceptor. The results of these structural studies are also presented
in Figure 2B. The results show that modification of the N-terminal valine residue
15 (i.e., by N-terminal shortening of AIV to AII(4 8)), or ext~n-ling the N-terminus with a
hydrophobic residue such as Sar or GABA, or c.l~ gil-g the stereoisomer of the L-Val
to D-Val, all drastically decrease binding of an AIV ligand to the AT4 receptor
(Table7). The AT4 receptor also failed to bind DuP 743 (DuP, Figure2B) or
CGP 42112A (CGP, Figure 2B) and thus did not exhibit the pharmacological
20 ~rop~,lies of a classic AII binding site (26). As shown in Figure 2B, the ability of the
various compounds to inhibit AIV binding to the solubilized AT4 receptor was tested.
The following compounds are shown in Figure 2B as follows: DAAI1, desAsp
angiotensin I (i.e., idçntic~l at the N-terrninus to AIII; see open squares with a dot,
Figure 2B); AIV, angiotensin IV (closed di~montlc~ Figure 2B); AII (open squares,
25 Figure 2B); SIAII (AII lacking the Ile residue at position 5, Figure 1; open diamonds,
Figure 2B); DuP (Dup 743, an AII analog; open squares, Figure 2B); CGP
(CGP42112A, another AII analog; closed triangles, Figure2B); and, AIII (open
triangles, Figure2B. The results presented in Figure2B and the Ki values
~vo 94/00492 2 1 3 9 1 0 S PCr/US93/06038
-33 . ~
summarized below in Table 7 show that: (a) only AIV, and peptides in the DAAI1
(i.e., AIII N-terminal sequence), and AIII plep~ions effectively competitively
inhibit binding of the 125I-AIV ligand to the AT4 receptor; and (b) the peptides in the
AIII, Sarl-AIII, and DAAI1 preparations are appro~hl~ately 100 times less effective
than AIV in colllpe~illg binding to the AT4 receptor.
WO 94/00492 PCI /US93/06038
~, ~13~0S
TABLE 7
Competition of 125I-AIV Binding to Solubilized Bovine Adrenal Cortical Receptor.
Analog/Competitor Kj (M)
AIV 5.67 + 1.71 x 10-1
AIII 2.28 + 0.17 x 10-8
AII > 10
AII(4~ 10
d-Vall AIV > 10
Sarl, Ile~-AII > 10~
Sarl-AII 2.80 + 0.41 x 10-7
Sarl -AIII 8.25 + 0.52 x 10-8
Sar~-AIV 1.44 ~ 0.47 x 10-7
GABA-Nterrn-AIV > 10
des Phex-AII > 10~
DuP 753 > 10-4
CGP42112A ~ 10~
des Phe,~-AIV 7.45 + 0.96 x 10-8
AI(~-ln) 9.63 + 1.02 x 10-1
Me-Y-I amide 2.06 x 10-9 ~ 1.59 x 10-1
Me-Y-I 8.30 x 10-9 + 1.80 x 10-9
Me-Y-I-G 8.75 x 10-9 + 1.67 x 10-9
Heptanoyl-Y-I 1.96 x 10-8 + 2.42 x 10-9
KYI 3.00x 10-8+9.67x 10-9
Me-F-I 5.17 x 10-8 + 5 90 x 10-9
KallVal~ AIV 1.03 x 10-9 + 2.21 x 10-1
VallVal~ AIV1.29 x 10-9 + 9.10 x 10-11
Kall AIV 2.13 x 10-9 + 8.93 x 10-1
Val~ AIV 1.01 x 10-8 + 5.62 x 10-9
Vall AIV 1.69x 10-8+5.09x 10-9
D-Vl AIV 9.68 x 10-7 + 5.59 x 10-8
D-Y,AIV 4.16x10-7+7.69x10-8
D-I~ AIV 5.82 x 10-7 + 2.60 x 10-7
D-H4 AIV 2.28 x 10-9 + 7.92 x 10-1
D-P~ AIV 2.32 x 10-9 + 7.31 x 10-1
D-F,~ AIV 1.18 x 10-9 + 5.62 x 10-10
Gl AIV 1.68x 10-7+2.19x 10-8
G~ AIV 1.00 x 10~ + 8.39 x 10-9
G~ AIV 1.16 x 10-7 + 1.43 x 10-8
G4 AIV 6.98 x 10-1 + 6.10 x 10-
G~ AIV 2.10x 10-1+2.03 x 10-
G,~ AIV 7.16 x 10-9 + 1.18 x 10-9
*N=4, mean + SD
., .
YVO 94/00492 21 391 o;~ Pcl/US93/06038
-35-
The combined results show the importance of the valine at the 3 position for
binding of an AIV ligand to the solubilized bovine adrenal gland AT4 receptors (i.e.,
D-Vall; des-Vall-AIV are inactive). The results also show the apparell~ in~ignificance
of Pheg for AIV binding to this AT4 receptor (i.e., desPhe6-AIV still retained AIV
binding activity). Thus, a minim~l AIV penta peptide ligand having a sequence
VYIHP retained AT4 receptor binding activity.
For high affinity binding of an AIV ligand to an AT4 receptor, the structure of
the N-terminal neutral polar amino acid (e.g., valine) is most important. N-terminal
extension is inco~ alible with binding, deletion of the terminal valine residue
ç~ es binding (Ki ~10-6), substitution with Sar decreases binding affinity,
substitution with Phe results in a 5-lO-fold decrease in the affinity of binding, Pro-
substituted AIV peptides bind with 100-fold lower affinity, but substitution with Ile
results in equivalent binding, and Lys substitution results in lO-fold higher binding
affinity of the KYIHPF AIV ligand (data not shown).
In the studies presented in Tables 6-7, and Figure 2B (abpve), a minim~l level
of binding of peptides in AIII prep~ions to the AT4 receptor was observed. This
most probably It;plesen~s an artifact in the assay and/or ligand plel)al~ions that is
easily accounted for by a low level of N-terminal hydrolysis of AIII to AIV, e.g., the
appalellL affinity of peptides in Am plt;lJ~alions for the AT4 receptor (i.e., at
22.8nM) can easily be .oYpl~ined by ap~Jlo~ lely 2.5% hydrolysis of AIII to AIV.This notion was supported by the results of studies cond~lcted with density-gradient-
purified heat-treated ll,t;lllbl~-es (i.e., heat-treated and purified to minimi7e en_ymatic
activity in the Ill~lllbl~le plepa.~ions). In these studies the amount of AIII
conversion to AIV was substantially reduced and the apparellL affinity of AIII for the
AT4 fecel"or was also reduced (Ki=29.3~3.3x109M), i.e., from appr~illla~ely
29-fold less active than AIV ligand (above) to about 52-fold less active (here). This
supports that notion that hydrolytic cleavage in the assay (i.e., medi~ted by a specific
cell Illt;llll)l~e AIV angiotç~ e or by another non~ecific peptidases) can convert
an AI, AII, or AIII peptides to an AIV ligand, and that low levels of AIV ligand in
these ple~&la~ions accounts for their appare.l~ binding to the AT4 receptor.
Additional support for this notion is provided by the results of studies (data not
shown) which show that the percellLage hydrolysis of AII or AIII in a preparation
correlates with the effectiveness of a given ple~ Lion as an inhibitor of AIV ligand
binding to the AT4 receptor. In this study pl epal ~Lions of AII or AIII were incubated
at 37C for dif~lenL periods of time and (i.e., x% AIV) the extent of hydrolysis to
AIV was detellllined by reverse phase HPLC (i.e., x% AIV). In every in~t~nce 100%
O 94/00492 PCr/US93/0603
2139105 -36-
of the apparent binding of l25I-AII or l25I-AIII was due to actual binding of
125I AIV.
As further shown in Table 7, the D-substitution and glycine-substitution data
confirms that positions 1-3 of the AIV molecule are critical for determining binding
5 affinity to the receptor. Positions 4-6 are less critical. In fact removal of C-terminal
groups appears to ~nh~nce binding affinity perhaps by redu-~.ing steric constraints.
T.ig~nds co~ g C-N nonpeptide bonds can be produced that possess high afflnity.
In general, highest affinity is obtainable by dual modifications at bonds between amino
acids 1-2 and 3-4. Val(l)Val(3) AIV appears totally resistant to en_ymatic
10 degradation upon exposure to rat kidney homogenates. As further shown in Table 7,
tripeptides CG~ g straight chain aliphatic amino acids in position 1 exhibit high
affinity. To date, the highest affinity is achievable with the Me-Y-I amide, suggesting
that amides are preferable to free acids and that the chain length found in Nle is
optimal (both longer and shorter bind with lower affinity).
Note that a similar approach to that taken with the N-terminus is useful to
characterize the C-terminal. Stereoisomers (e.g., D-Phe), C-terminal extension, and
sequential C-terminal deletion, and other peptide analogs can be synth~ei7ed andtested for their ability to co...~,eli~ ely inhibit with 1251-AIV binding to purified
bovine adrenal melllbl~les. If C-terminal extended peptides are found to bind to the
20 receptor, appropliate C-terminal extended peptides can be constructed for use in the
affinity chlc,llla~ography purification of the receptor. Substitutions and modifications
of internal amino acids can also be ~Y~mined for their effects on binding. Once
physiological or second messçnger systems have been id~ntified (Example 6) as being
AIV-dependent events, then they can be used for drug screelf~ng, and a second round
25 of synthesis can co. "~ nce focusing on the development of receptor antagonists.
The materials and methods and eA~tlh~,tn~al assay conditions employed in
Example 1 are described below:
Materials and Methods:
Peptide Syll~hesis
The angiotensin analogs employed in this study were synth~.ci7ed by the
sl~dald Merrifield method utili7ing t-Boc protected amino acids and
chlolc,lllt;lllylated resins on a Vega250 coupler a~lolllaled synthPci7er Following
synthesis, the crude peptides were purified by prep~a~ e reverse-phase HPLC using
a 1 h gradient for elution at 9ml/min. Initial conditions were 90% H20, 10%
ace~oni~lile, and 0.1% TFA and the final conditions at the top of the gradient were
65% H2O, 35% acetonitrile and 1% TFA. Purified peptides were amino acid
.
~O 94/00492 2 1 3 9 1 0 ~ PCr/US93/06038
-37-
analyzed to determine both peptide and total purity. Typically the peptides produced
were greater than 99% pure and contain 20-25% acetate.
Tissue Plèpal~lion: bovine adrenal cortical tissues
Adrenal cortex was removed from bovine adrenals obtained from a local
5 s~ ghtçrhouse. The minced cortex was then homogenized in a Polytron as a 40:1
suspension in assay buffer at 10 sec/ml. The homogenate was then centrifuged at
500g for 10 min. to remove whole cells and nuclei. After a rehomogenization and
rece~lLlir~lgation the combined supellla~ s were spun at 40,000 x g for 20 min. The
pellet was rehomogenized and respun at 40,000 rpm for 30 min. This final pellet was
10 resuspended in assay buffer and layered on a discontinuous sucrose gradient
(0.8M/1.2M). After a 100,000 x g spin for 90 min. the purified membranes were
located at the density interface and were removed. The sucrose COI~ g Illelllbl~le
suspension was diluted 1:10 in assay buffer and spun a last time at 40,000xg for30min. The pellet was resuspended in assay buffer at a concenLl~Lion of 10mg
protein/ml and heat treated at 60C for 30 min. in the plesellce of 20mM MgCl2. The
llle~llbl~1es~ now devoid of almost all peptidase activity, were ready for use in the
binding assay.
Bindin~ studies: bovine adrenal cortical melllbl~1es
To test the ability of the synthesi7ed analogs to colllpeLiLively inhibit for
20 l25I-AIV binding a tii~pl~r,Pmrnt curve was established using purified bovine adrenal
cortical lllelllbl~es. Binding was carried out in 10-75mm siliconized glass culture
tubes CG..~ g 0.2nM l25I-AIV, 25mg of membrane protein, and the desired analog
over a concentration range of 10-l2M to 10 4M using half-log dilutions. All binding
inr,ub~tions were carried out in duplicate at 37C for 2 h in a buffer co-~ g:
50mM Tris, 150mM NaCl, 5mM EDTA, 10~M bestatin, 50~M Plummer~s ~e~grnt,
lOOIlM PMSF and 0.2% BSA (assay buffer) in a total volume of 0.25ml. After
incub~tion, the inr,ubates were filtered through GF-B filters soaked in 0.3%
polyethyle.~e;...;..e and washed with four~ml washes of PBS. The filters were then
counted in a BeçL-m~n 5500 gamma counter. A typical e~ elilllenL rX~mines 5
analogs simlllt~neously and inr,ludes a positive control curve in which AIV was used
as the displacer. All curves were run in quadruplicate, each with a dirrèlellL tissue
pre~ aLion Nonspecirlc binding was defined as total binding minus binding
observed in the presence of 100mM Sarl,Ile8-AII or 100mM AIV. No cross
displ~crmrnt (i.e., of AII binding by AIV or AIV by AII) was observed. HPLC
analysis of both the bound and free 125I-AII or 125I-AIV ligand intlic~ted that 100%
W O 94/00492 PC~r/US93/0603
2139105 -38-
ofthe "specifically bound" label was either l25I-AII or 125I-AIV, respectively, and the
overall hydrolysis of l25I-AII under conditions of the assay was less than 2%.
Data were analyzed by the LIGAND program (29) from which Ki values can
be obtained.
5 Binding studies: solubilized bovine adrenal cortical receptor
Solubilization and characterization of the receptor from bovine adrenal
Illelllbl~nes was accomplished by homogenizing the ~ lllblanes (above) in hypertonic
buffer followed by fractionation of the melll~ es by sucrose density gradient
centrifugation. The Illelll~l~e pl~p~Lion was then heat treated at 60C in the
10 presence of MgC12 (to inactivate AT1 receptors). The heat LleAtlll~ t also reduced
endogenous peptidase activity in the plepal~ions by 90-95%. The AT4 receptor in
the prep~lions was then solubilized using 1% zwitterionic detergent
3-[(3-cholamidopropyl) dimethyl ~Ill~lolLo]-l-plu~.Anes~lfonic acid (CHAPS).
Binding studies: rabbit heart
P2 nlc;lllbl~1es from rabbit heart were plepared by homoge~ ;on and
di~erellLial centrifugation at 4C. Binding was carried out in the presence of 5mM
(EDTA), 0.2% heat-treated bovine serum albumin (HTBSA), lO,uM Bestatin, 50~1M
Plummer's inhibitor, 100~M phell~ ,eLll~lsulfonylfluoride (PMSF), and 50mM Tris,pH7.4, at 22C. Binding was initiAted by the addition of lOOmg protein and
20 applupliale amounts of labeled ligand. (For kinetic binding studies the samples were
inc~bAted for 10, 20, 30, 40, 50, 60, 90, 120, 150, 180, and 220 mimltes at 37C. For
equilibrium binding studies the same conditions were used and samples were
inr,~lbated for 120 min at 37C.) All ;.~ ul~al;ons were c-)ndllcted at a final volume of
250ml in 12 x 75mm siliconized (SigmaCote) borosilicate tubes, and they were
25 termin~ted by rapid vacuum filtration in a Brandel cell harvester through glass fiber
filters (schlpichp~r and Schuell #32) soaked in 0.3% polyethyl~-~;...;..~. Filters were
immP.di~tely rinsed with 4x4ml 150mM NaCI, 50mM Na2HPO4, pH7.2 at room
telll?elaLl~re~ Filters were allûwed to air dry, placed in fresh tubes and counted in a
gamma counter. Specific binding was defined as the di~rei1ce bt;Lween the absence
30 and presence of l.OmM ~i~pl~in~ ligand.
Dissociation (i.e., of ligand from lecepLol~) e ~ illlellLs were con-l~lcted by
adding lmM unlabeled AIV ligand colllp~tiLor to the assay at 120 mimltes after
iniSi~ting binding (at 37C) with 0.5nM 125I-AIV.
Saturation isotherms for binding were conducted with approx;..,~tely 25~1g of
tissue protein inr,~lbated with various concentrations of l25I-AIV for 120 min. at
~094/00492 21 391 OS Pcr/US93/06038
-3~-
37C; nonspecific binding was defined in the presence of lmM AIV. Three
cA~uelilllents were contlllcted resulting in 36 data points for Scatchard analysis.
EXAMPLE 2
Tissue and Species Distribution of the AIV Receptor
5 Species Distribution:
A second major approach to dçfining separate and distinct binding sites was to
e~mine their relative tissue and species distribution (Table 8). The results presented
in Table8 show the fentamoles of AII or AIV bound per milligram of lll~lllbl~e
protein in extracts plepared under identical conditions from the tissues and species
10 indicated.
TABLE 8
*Dist-ibution of l25I-SI-AII and 12sI-AIV Binding in l~mm~ n Tissues.**
I2sI-AIV
Species Tissue125I sI AII(frnoVmgprot ) (fmol/mg/prot.)
cow adrenal medll l"218.8 + 56.2 397.3 _ 53.6
pig whole adrena ND 70.8 ~ 6.7
horse whole adrena 1.8 + 1.0 72.7 + 13.5
dog whole adrenal 4.6 ~ .7 36.5 ~ 5.4
cat who:e adrenal 3.3 ~ 2.3 199.6 + 19.7
rabbit wnoeadrenal 79.6+21.6 105.3 1 15.6
rat who e adrenal158.2 ~ 21.6 N.Det.
guinea pig wnole adrenal 45.6 + 9.2 101.2 + 26.3
cow coronary venule2.9 + 0.3 85.1 + 3.3
endothelial cells
rabbit heart 10.6 1 3.6 249.9+36.3
*2511g of .. ~ protein was I with 500,000 cpm of label. Specific binding was defined
as total binding no~ ;r.r binding at lOO nM ~ ed peptide.
~"*n = 2~; mean ' SE; l25I-SI-AII=l25I-Sarl,Ile8-AII; fmol/mg ~r ~ ~I~~ (i.e. lo-l5M)
of AII ligand bound per mg of total protein in the pr~p ~r:nion
N.Det. = not d- - ' 'e, i.e., less than 1.8 fmol/mg protein.
The results show that: a) the human AIV ligand binds AT4 receptors in a
wide variety of ".A."".Ali~n species; and b) most --~------~li~n adrenal tissue express an
20 AT4 receptor capable of binding the AIV ~peptide VYI~F.
Tissue Distribution:
In order to begin to assign physiological functions to the AIV ligand-AT4
receptor interaction, plelil.~in&,y tissue distribution studies have been con~lcted in
guinea pigs. Guinea pigs were chosen because their adrenal tissues demonstrated high
25 levels of AIV binding (Table 8). The tissue distribution of AT4 receptors was
WO94/00492 'L39~0~ PCr/US93/0603~
-40-
measured by assaying radioligand binding to ".c"~,~ne plep~ions, i.e., as described
in Example 1, above.
TABLE 9
Distribution of 125I Sarl, Ile8-AII and 125I-AIV Binding sites
5in the Me",b,~l1es of Guin~a Pig Tissues.*
125I Sarl, Ile8AII 125I-AIV
Tissue Binding (fmol/mg prot) Binding (fmol/mg prot)
aorta3.17 ~ 2.2 45.4 ~ 11.0
brain17.70 + 9.5 60.8 ~ 13.5
heart5.70 + 0.6 83.3 ~ 20.8
cidney 8.10 + 1.19 22.7 ~ 12.1
iver22.40 ~ 5.3 28.9 ~ 6.4
lung12.20 ~ 6.4 56.1 ~ 10.1
uterus 4.00 ~ 1.5 87.0 ~ 6.3
*n = 4; Binding was carried out as described in Table 6, above.
The co",bhled results presented in Table 9, above, show that the receptor was
widely distributed in the tissues. The co",bi,led results suggest evolutionary
conservation of both the AT4 receptor and the AIV ligand.
10Additional studies were next cond~lcted to physically co",p~c the receptors inguinea pig brain, rabbit heart, and bovine colon~-y venule endothelial cells with
respect to their binding affinities for AIV ligand. The results of these studies in-lic~te
that cells in these di~lcllL tissues all have AT4 receptors with cGlllp~able binding
affinity for AIV ligand; each cell type has an AT4 receptor with a Kd for 125I-AIV of
15 about O.lnM to about 0.5nM. Next, cG",~eLilion studies similar to those presented
above in Example 1 were con-lucted to evaluate the structural requile",ellL~ forbuilding AIV in di~relll tissues. The results inrlic~te that each AT4 receptor exhibits
the same N-terminal specificity recorded, above, i.e., for V, I, or K. The results of
these co",~ined studies support the notion that a rclll~kable degree of evolutionary
20 conservation has been ~ ;..ed for AIV ligand-AT4 rec~Lor system, and this level
of conservation is cGIll.llonly predictive of i"~pO"~I~, and probably critical,
physiological functions.
Surprisingly, it has not been possible to demonstrate binding of 125I-AIV in rattissues incl~din~ brain, heart, kidney, aorta, lung, liver, or cultured smooth muscle
25 cells (n=5) (Table 8). At this time, the ~i~ific~nce of this finding is uncertain. It is
possible that the in vitro assay conditions may not be optimal for this species and that
the AT4 receptor or AIV ligand may be rapidly inactivated, e.g., by high levels of
peptidases known to be present in rat tissues.
`YO 94/00492 21 ~gl OS PCr/US93/06038
In addition, on a technical note, binding of the AIV ligand to the AT4 receptor
was inhibited in the presence of Bacitracin (i.e., 10mg/ml; at a final calculated
concentration in the assay of 0.07M). Bacitracin is a polypeptide antibiotic with the
sequence ICLEIKOIFHDD (i.e., O is ol.lill~ille), and it is often inr,l~lded in angiotensin
5 binding assays to inhibit the action of nolls~ecific proteases (i.e., as an altemative
substrate for the proteases). The observation of bacitracin il~le~rt;~ t;nce is of potential
significance for at least two reasons: 1) previous investigators who have inrhlded
bacitracin in their assay buffers may have inhibited AIV ligand binding to the AT4
receptor; and, 2) inhibition of AIV binding by this polypeptide (notably at a very high
10 molar concentration) may provide an indication of amino acid sequences that may
contribute to ele-;l,usl~Lic interactions in the AT4 receptor binding site (e.g.,
RlIR2HR2, where Rl is an amino acid with an aromatic side chain such as OH, SH,
or NH, and R2 is a polar amino acid).
The results of the tissue distribution studies, above, inrlic~te that the receptor
is present in the adrenal tissues in most .. ,.. ~ n species, and can be isolated in P2
lllblal1e prep~lions from most m~mm~ n species.
Receptor Autoradio~raphy in Tissues:
Receptor autoradiography is a useful extension of radioligand binding studies
since it provides detailed anatomical hlrul'''alion about the location of receptors in
20 tissues and groups of cells in tissues, and thus it f~rilit~tes underst~n-ling the function
of the AIV ligands and AT4 receptors in those sites. Autoradiographic analyses of
serial sections of guinea pig brain (20mM thirlrness) were pelro,llled. The
autoradiographs showed a pattem of distribution for AII receptors and AT4 receptors
in the H~hemll~ (Figure 3), Hippocampus (data not shown) Cerebellum (data not
25 shown), Plt;fiullli~l Cortex (data not shown), and Th~l~mlls (data not shown). In each
case the receptor distribution in the tissue was detemlined by binding of
125I-Sarl,Ile8-AII, or 125I-AIV, re~,ec~ ely. Specificity of ligand binding in these
autoradiographic studies was d~..oh~ led by co~ Jetil1g the binding of the specific
ligand (i.e., AII or AIV) with l...l~hF.led 100nM Sarl,Ile8-AII, or 100nM AIV,
30 respectively. The data dellloh~lale that while specific AII and AT4 receptors are
located at similar sites in the Habenula, Hippocampus, and Cerebellum of guinea pig
brain, the two l~;c~l"ol~ are distinct with regard to exact groups of cells that express
the two di~lenl receplol~. Major differences were observed in the ~I~;fiolllal Cortex
and Th~l~mlls where AT4 receptors were abundant but AII receptors appear to be
3 5 relatively rare.
WO 94/00492 . ~ PCr/US93/0603~
?~.3g~0~ -42-
In the Hippocampus the AT4 receptor is present in the pyramidal cell layer
CA1, CA2, and CA3 of the Hippoc~mpus and dentate gyrus. Binding of AIV
occurred at a single binding site with high affinity (Kd=1.29_0.18nM, mean + SD, Hill
Coef. = 0.993+0.015) and in a saturable manner (BmaX-449_62 fmol/mg protein).
5 The propel lies of the hippocampal AIV ligand-receptor system as described in greater
detail below (see Fx~mple 7). The fintlines of AT4 receptol~ in the Hippocampus
suggest that the AIV ligand-receptor interactions in the Hippocampus may me~i~teunique angiotensin-dependent functions in~ ing memory ~l~h~l~ce...el~l The AIV
ligand-receptor system may provide a link bt;~wc;en the Hippocampus and memory.
The mutually exclusive cellular distribution of AIV and AII receptors is
de,llon~ ed in the autoradiograph shown in Figure 3. Panel A reveals intense
125I-AIV binding in the habenula, while Panel D inriic~tes that 125I-Sarl,Ile8-AII
binding is localized primarily to fiber tracts inr.lu-ling the visual te~...r...l~l relay zone
and the medial !~...,-i~c~.s. The specificity of ligand binding to receptors in these
15 tissues was illustrated by co...~ g 125I-AIV binding with lOOnM non-labeled AIV
(not AII) [(Figure 3, Panels B and C)]; and, lOOnM non-labeled Sarl,Ile8-AII
displaced only the 125I-Sarl,Ile8-AII binding [(Figure 3, Panels E and F)]. Sometissues, however, may contain both AII and AT4 receplo,~.
Q~ live aspects of binding in brain is presented in Fx~mple 7, and other
20 tissues data is presented in Example 1, above. The results show that all illlpolL~Il
cardiovascular tissues in guinea pigs contain the AT4 receptor. This result is not
surprising in light of the observation (above) that vascular endothelial cells contain
high concentrations of lecep~ol~, but this is not re~,ollsible for tissue binding of AIV
ligand because every vascularized tissue will possess AT4 receptol~, i.e., skin and
25 skeletal muscle has low levels of lecep~or.
Materials and Methods:
Autoradiographic analysis of 125I-AIV and 125I-Sarl,Ile8-AlI binding in
guinea pig tissue was dettlll~ed as follows. Heart, kidney, brain, and other tissues
were cryostat-sectioned into 20mm section~ that were mounted on chrome-alum-
30 gelatin-coated slides in mllltiple sets of seven. The slide-mounted tissue sections were
thawed (35C) and pr~inr,ub~ted in assay buffer (150mM NaCl, 50mM Tris, 5mM
EDTA, 0.1% BSA, lO~M bestatin, 5011M Plummer's inhibitor, lOO~lM PMSF, at
pH7.4) for 30 min. and then inr,ubated for 1 h in the same buffer with the addition of
225-250pM of 125I-Sarl,Ile8-AII (for viell~li7ing AII receptors) or 125I-AIV (for
35 vi~u~li7ing AT4 receptors). To define the specificity of the ligand binding, tissue
sections were incub~ted in the radioligand in the presence and absence of lOOnM
`'VO 94/00492 2 1 3 9 1 0 5 PCr/US93/06038
-43 -
unlabeled AII or AIV peptide. After appropliate washing, autoradiograms were
prepared by apposing the slide-mounted tissue sections to X-ray film (Hyperfilm,Amersham) for an appl Opl iate exposure time. The amount of radioligand binding in a
tissue was quantified using densitometric techniques and 125I standards (Microscales,
5 Amersham, Arlington Hts, IL).
EXAMPLE 3
Receptor Isolation~ Purification. and Pl opel ~ies and Production
of Monoclonal Antibodies
Receptor Isolation and Purification:
10The AT4 reccll~or was solubilized in high yield from purified bovine adrenal
~clllbl~1es using the zwitterionic dclergclll CHAPS (1%) at 4C over 4 h under
conditions where peptidase activity and diffclclllial solubilization of the AT4 receptor
(but not the AII receptors) is pelll,iLled (see also Example 4, Materials and Methods,
below). For L-~...ple, lllcl~ es from a variety of di~cncn~ tissues and cells (e.g.,
1525mg of P2 ",e",~ es, E~,lple 1) were inc~lb~ted for 4 h in Hepes buffer (20mM,
pH7.4) co.~ g 1% CHAPS and a cocktail of protease inhibitors and alternative
protease sub~ es, i.e., lO~lM bestatin; 50~1M Plummers' inhibitor; 0.2% BSA
(bovine serum albumin); and lOOIlM PMSF (phenylmethylsulfonyl fluoride).
A most useful collll)oncllt of any AII receptor purification scheme was
20 in~ (ling a step whcrein the solubilized ~lclll~ e proteins were subjected to a heat
L~ c~ l at 60C, e.g., for 20 min~ltes and in the plescllce of 20mM Mg++. This step
was useful in dcsLluying any residual AII receptor leaving the AT4 receptor intact.
The AT4 receptor was stable to cl-ro"lalofocusing and SDS-PAGE, allowing
isoelectric focusing, or one- or two-dimensional PAGE or SDS-PAGE to be used for25 purification. Due tû the slow-ûff rate of 125I-AIV binding, the receptor was
radiolabeled with l25I-AIV ligand to allow ease of identification during purification.
As an additional aid to purification, the rccepLor was sllccçssfiJlly cross-linked to a
125I-radiolabeled AIV analog ligand having a C-terminal extension, i.e., from
residue 8, with Iysine residues (i.e., l25I-Lysll-AIV). The Lysll-AIV analog binds to
30 the AT4 leceplor with a Kd that is similar to AIV ligand. Using Bis
(sulfos~lcGinimi~lly) ~ubclilllidate (BS3) as the cross-linking agent, the 125I-Lysll-AIV
analog of AIV was bound to the AT4 receptor and then cross-linked to the AT4
eccp~or through the e-amino group of Lys. Purification of the AT4 receptor may
also be achieved, for example, by ion exçh~nge, lectin cl~rolllaLography, hydrophobic
35 chrolllaLography with conventional techniques, HPLC, or FPLC.
- 21391o5 P~t/US93/0603
~44~ 03 Rec'd PCT/PT0 15 JUl t~
SDS-PAGE analysis of isolated and purified receptor indicated a molecular
weight between 130KDa and 150KDa, at about 146KDa for the BS3-cross-linked
AT4 receptor from bovine adrenal tissue. The purified, uncrosslinked receptor
appears to have a significantly smaller molecular weight, on the order of 60KDa.5 Receptor Properties:
Identification of the family to which a receptor belongs commonly permits
predictions to be made about possible improvements in purification, useful methods
for stabilizing the receptor during purification, cellular sources and assays useful for
molecular cloning of the receptor, and identification of novel physiological roles for a
10 receptor. For in~t~nce, neulol~a~ rs and hormones are known to interact with
four types of plasma "lt",b,~1e receptors: 1) mlllti~llbunit receptors that regulate an
intrinsic ion ch~nn~l; 2) G-protein linked receptors that, via the G-protein, can
activate membrane ch~nn~lc and enzymes; 3) guanylate cyclase receptors that possess
intrinsic guanylate cyclase activity in a single membrane spanning polypeptide chain;
15 and, 4) protein tyrosine kinase receptors that have intrinsic tyrosine kinase activity
capable of phosphorylating multiple protein substrates.
Many comm~n ne~,o~ rs like acetylcholine, glycine, glllt~m~te, and
GABA activate receptor-ion çh~nn~lc. The interaction of the neulo~ i...;ller andreceptor results in the opening of an intrinsic ion ~h~nnPl In all cases these receptors
20 are constructed as heteromllltim~rs and are most likely evolutionarily related. Despite
the h"~o"ance of this receptor class, to our knowledge no known peptide transmitter
or hormone acts by such a ~"ech~ m Thus, it is reasoned unlikely that the AT4
receptor is a member of this family of receptors.
Studies have been conducted to determine the receptor family to which the
25 AT4 receptor belongs (see Examples 5 and 7). It has been reported previously that
the AII receptor may be a member of the G-protein-linked family of cellular receptors.
The majority of known peptide receptors belonging to this family are characterized by
seven membrane-spAnl-il-g alpha-helical regions and when stiml~l~ted are capable of
activating membrane bound enzymes like adenylate cyclase, phosphodiesterase, and30 phospholipase C. (30). Additionally, membrane channel or ion transporter plope-lies
can be indirectly modified by the intervening G-protein (31). Although many
strategies have been devised to test a particular receptor's linkage with a G-protein,
three strategies seem to predominate. In one form or another these include the
following approaches: 1) GTP and its analogs are known to alter the binding affinity
35 of agonists to their receptors. Therefore, the ability of GTP or analogs to change
agonist-binding affinity is diagnostic of a G-protein-linked receptor In the presence
~'S'.R~71~251,B DOC
AMENDED ~;HEET
~O 94/00492 2 1 3 9 1 0 5 PCI/US93/06038
-45-
of GTP, dissociation of the G-protein subunits from the receptor results in a lowered
afflnity for agonists. This was PY~mined (see Example 5) by the direct ~esesemçnt of
GTP (of GTP~S) effects on agonist binding via chal1ges in dissociation rates or total
binding over a range of GTP conce~ alions~ or indirectly by monitoring shifts in ICso
values for agonists during competition for antagonist binding. 2) Another indication
of G-protein linkage is the ability of agonists to stim~ te the intrinsic GTPase activity
of the alpha subunit of G-proteins. This GTPase activity is triggered following
receptor occupation and subsequent dissociation of alpha and beta-gamma subunits.
3) A final approach is to determine whether an agonist can f~ t~te nucleotide
cycling. A crucial step in G-protein signal tr~ned~lction is the agonist-stim.ll~te~l
dissociation of GDP from the alpha-subunit and its repl~cPmPnt with GTP. Changesin cycling are often ~esessed by co"")a,ing the binding of radiolabeled irreversibly
bound GTP analogs before and after agonist stiml-l~tiQn.
Studies to date include studies to d~Le""i"e the cellular signal tr~ned-lction
".e~ ."e activated following binding of AIV ligand to the receptor. The data
obtained with ieol~ted AT4 receptor now ~L,ongly suggest that although the AT4
receptor may be G-protein linked in certain cells (see Example 5) the AT4 lecepLor
does not belong to the çl~esic~l family of G-protein-linked receptors for at least three
reasons: namely,
1.) Solubilization and stability characteristics of the AII receptor (i.e.,
binding 125I-Sarl,Ile8 AII) and the AT4 ~cepLo~ (i.e., binding 125I-AIV) are
significantly di~elt;"~ which is consisLe~lL with: a) large structural di~le,lces between
the two leceplo,~, and, b) dirrere"ces in the structural basis of receptor integration
into ~"e~ es. Thus, it is re~eon~ble to assume that if the AIVATl receptor is a
",e",l)el of the G-protein linked family of receptors, then the AT4 receptor probably is
not.
2.) Receplo,~ of the G-protein-linlced family of leCep~Ol~ are reportedly
susceptible to inhibition at micromolar concenL.aLions of GTP~S. Studies were
thelero~t; contl~1cte~ to ~Y~mine 125I-AIV ligand binding to the AT4 receptor in the
presellce of GTPy S. The binding of radiolabeled AIV ligand to AT4 receptors
isolated from bovine adrenal ",e",l"~ es is not altered by adding GTPy S to the assay
buffer at conc~ lions ranging from 10-10M- lO~M. (Under these conditions
binding of control pltpalaLions of AII ligand to AII receptors [i.e. in the sameme",b,~le pre~ Lions] revealed the typical pattern of a G-protein linked receptor
with decreased binding of 125I-Sarl,Ile8 AII at i"creas;,-g conce"L~Lions of GTP~ S;
number of t;AI,t;,i",ents = 5).
WO 94/00492 PCr/US93/0603
2~,3g~1~5 -46-
3.) The AT4 receptor has a demonstrated molecular size of 140 to
150kDa (on SDS-PAGE) for the isolated and purified receptor, and 146KDa for the
BS3 cross-linked bovine adrenal AT4 receptor. These molecular sizes are
significantly di~.t;lll from the molecular weights of 55KDa to 65KDa that are
5 commonly associated with members of the G-protein-linked family of receptors.
If the AIV site is not a classical G-protein-linked receptor, then to what family
of receptors does it belong? Evidence in recent years indicates the presence of
peptide receptors with intrinsic guanylate cyclase activity. These receptors, best
exemplified by the .. ~.. ~li~n ANP receptor, consist of a single polypeptide chain
10 with one melllb~1e-sp~nning region that possesses guanylate cyclase activity that
resides near the intr~cçll~ r C-terminus(32). Since only two such ~.-~---,-.~li~n
receptors have been identified (to date), the ANP and rat ;~le~ enterotoxin
receptor, it is difficult to speculate on the probability that the AT4 lecep~or is a
Illelllber of such a family of receptors. Nevertheless, the similarity in the molecular
15 weights and in ion requirements of the ANP and AT4 receptors neces~ tes the
consideration that the AT4 receptor may be a member of such a family.
The final receptor family to which the AT4 receptor may belong is the
tyrosine-kinase growth factor family of recel~lo,~. These receptors are characterized
by a protein kinase activity which p,e~elellLially phosphorylates tyrosine residues.
20 Among the substrates of phosphorylation are the leceplor itself and phospholipase C,
which when phosphorylated initiates the inositol phosphate c~c~de (33). The tissue
response to prototypical peptides which act as tyrosine kinase receptors in~hldes long-
term alterations that invariably involve chal~ges in the l-~s~ lion rate of selective
mRNAs. Although often accol.lpal~ied by acute effects, these peptides appear to play
25 a role in the adaptation of target tissues to chronic cl~nges in the level of a factor. In
addition, tyrosine kinase receptors often "cross talk" with other cellular receptor
types (34) in lesponse to physiological and cl-~...;c~l stimuli. This type of role is
precisely the function envisaged for the AIV ligand-,c;ce~lor system.
A co.llp& ison of the solubilization, physical p.ope.lies, and functional
30 activities of the AT4 eceptor with the cellular biology of ebe.~ of the tyrosine
kinase family of growth factor recepto. ~ (e.g., fibroblast growth factor . C;Ct;~lo., FGF)
s~l~gestC a closer relationship of the AT4 receptor to this family of c;ce~to~ than to
the guanylate cyclase family of receptors. For ;~ llc~ both the AT4 receptor andthe FGF receptor have related biochemical characteristics, e.g., the FGF receptor has
a molecular weight of about 140-150kDa (35), is relatively heat stable (i.e., at 56C),
and has divalent ion req~ elll~ s (28). Moreover, as described herein, AT4
`~0 94/00492 2 1 3 9 1 0 5 PCI/US93/06038
-
-47-
receptors appear to have growth factor activity on at least endothelial cells and
myocytes. (In the latter case, the tissue distribution and the activities of AT4receptors are also consistent with a role for AT4 receptors in growth regulation. For
;.,~l~nce, as disclosed above, high concentration of AT4 receptors is present in5 cardiovascular tissues where angiotensins are reported to enhance tissue growth.)
At least three observations are significant in ~ccigning the AT4 receptor to a
receptor family. First, the molecular weight of the AT4 receptor is in the range of
.,.~...be. ~ of the tyrosine kinase families of receptors. Second, the AT4 receptor, like
members of both the tyrosine kinase families of receplo-~, is characterized by divalent
10 cation binding sites (i.e., Mg++). And third, the AT4 receptor, like members of the
tyrosine kinase and guanylate cyclase families of receptors, is characterized byrelatively high heat stability (i.e., 60C/20...;....les). (For colllpalisol1, the epidermal
growth factor .ece~lor (EGF) is heat stable at 50C for 30 min., and has specific
binding sites for Mn++ and Mg++ [28]). Thus, by at least these criteria the AT4
15 recep~or appears to be a ...e...bel of the tyrosine kinase farnily of receptors, and not
the G-protein-linked family of leCePLO-~. EA~.;...t;..Lal approaches to validate this
vision are presented below, the e,~t;-i-.-ents ~ e the ability of AIV ligand to
stim~ te phosphorylation of tyrosine residues in ...~ e proteins. In addition, the
focus of the e A~.i.--ents that follow in Example4 (below) was directed toward
20 ~lP.fining the cellular biology of the AIV ligand receptor interaction, and these studies
will also help confirm the c1~c.cific~tion of the AT4 recep~or as a member of the
tyrosine kinase family of receptor (e.g., capable of re~ ting cell growth and intrinsic
tyrosine kinase activity of the AT4 1 eceptor).
Materials and Methods:
25 Cross-linking to the AT4 lece~tor
Cross-linking 125I-AIV to the AT4 receptor can be accomplished with Bis
(sulfosuccinimi~lyl) s~e-ill idate (BS3) as diccussed above. The cross-linked recepLol
(approx. mw of 146,000) can then be electroeluted from PAGE gel slices in a
s~s~ 11y pure form for use as an electrophoretic sL~1da.d.
For cross-linking one milligram of total solubilized .. e.. ~.~le protein
col~ .;..g AT4 recep~or was il.-"~bAIed with 30 x lO~cpm of 125I-AIV in 50mM
Tris, pH7.4 and 150mM NaCI col-lAinil~g a cocktail of pro~casc/peptidase inhibitors
for 2 hr at 37C (final volume 0.5ml). Following inr.~1bation, the in~bate was spun
through two succeccive spin columns packed with 0.8ml of BiogelP-6 t;AL~ne
35 (Biorad) that has been pre-equilibrated with 20mM NaP buffer, pH7.4 co.~l~inil~g
0.01% CHAPS.
WO 94/00492 2~L3910S PCI/US93/0603
-48-
The labeled receptor, now in phosphate buffer, was cross-linked with BS3
(final conc. 9mM; added as 90mM in DMSO). The mixture was incubated 30 min. at
0C. Cross-linking was termin~ted by the addition of lOOml of lM Tris, pH9.0 with
an additional 10 min. inr,~lb~tion at 0C. The mixture was then spun through a final
spin column to remove reactant and free ligand. The centrifugate was now ready for
PAGE.
Production of Monoclonal Antibodies:
Monoclonal antibodies are useful for purification of receptor, and for
ide~lLirying the receptor (and fragmPntc thereof) in tissues, cells, and biological fluids.
Purified or semi-purified AT4 receptor (preferably nontl~n~hlred) can be used as an
in vivo or in vitro immlmogen. (Those skilled artisans will recognize a variety of
options available to them for evoking monoclonal and polyclonal antibodies, e.g., see
Harlow, E. and D. Lane, Eds. "Antibodies: A Laboratory Manual" Cold Spring
Harbor Laboratory, 1988). For in vitro immlmi7~tion antigen can be in~bated in
picogram ql)~ntities with murine, rat, or human lymphocytes. Production of
antibodies can be screened by testing for the ability of l25I-AIV-prelabeled receptor
to bind to antibodies adsorbed on a poly~Lylci-e plastic surface, e.g., in 96 well plates;
or, alternatively, by testing the ability of the antibody to inhibit binding of a purified
labeled receptor to AIV ligand adsorbed to a solid phase. In either case, antibody
producing cells are idçntifiP,(l, cultured, and cloned. The monoclonal antibody
product of the cloned cell lines can bind the AT4 receptor in ligand-binding and non-
binding domains of the AT4 receptor. Non-binding domains can include structural
regions of the molecule as well as enzyme active sites, phosphorylation sites,
glycosylation sites, and the like. The presence of antibodies specific for the ligand-
binding domain can be ~csessed directly via the ability of the mono to competitively
inhibit in binding assays. As mPnfionP,d earlier antibodies are useful for receptor
. purification and immllnohistochP.miG~l studies de-si~ed to Pl~lrid~te the cellular
location of receptors and also in structure/activity studies decigned to map functional
domains in the receptor.
EXAMPLE 4
AIV Receptor Antagonists and Agonists
To test the ability of ~y~ P~;~ed AIV ligands to co,llpcLiLi~ely inhibit for
125I-AIV ligand binding to the AT4 lecep~or, displ~cPmPnt curves were constructed
using heat-treated (60C for 20 min. in 20mM MgC12) purified bovine adrenal cortical
mel"l"anes using Methods desc,ibed in Example 1, above. Effects of AIV analogueson renal blood flow were determined as described in Example 6.
~vo 94/00492 PCl /US93/06038
49~13~1Q5
The design of AIV analogs followed a question based approach. The unifying
question: What are the essçnti~l ligand domains for receptor binding and activation?
Individual chemical modifications were made to ask specific questions about spacial
orientation of molecular surfaces, charge, hydrophobicity and occupancy of space5 (volume occupied at a specific location). A standardized assay of analog competition
was employed to study receptor-ligand binding affinity of the high affinity l25I-AIV-
binding receptor in heat treated, sucrose density gradient purified bovine adrenal
cortical ",tl"b,~les. Energy ..~ ed7 computer generated models ("Macromodel"
program run on a Vax ",a"~fi~le) provided the visual representations of the
10 molecular col~""alion of highest probability.
Agonist versus antagonist activity was ~csesced using a laser doppler to
monitor renal cortical blood flow f~llowing infusion of a test analog into the renal
artery (see F.Yh~llple 6). Maximal response was co",pa,ed to the le~l,ollse (increased
in flow) to AIV. (Note that under these conditions AII produces a decrease in blood
15 flow in this assay.) Inte,,ule~lalion of physiologic and binding data was based on the
precept of a lock and key model of receptor binding and that dynamic change of the
receptor upon interaction of the ligand was required for activation (full agonist
activity; with second m~s~el~eel activation).
The following main assumptions were used:
1.) T.ig~nds with the highest affinity, when modeled in an energy .. ~ ed
co~ru""alion offer a visual ,~ s~ hl;on of the receptor binding site field surface
(i.e., hydrophobic charge interactions) and charge locations in the "pre-binding state"
and "non-activated state" (i.e., just as a clay imprint on a well fitting key represents
the interaction surface of the lock).
2.) Specific ligand domains induce çh~l~g~o~ in the receptor upon binding that
produce cellular responses. T.ig~n~s that fit the "pre-binding" receptor with high
affinity may not activate the receptor, i.e., they may act as antagonists, whilestructures that induce ~h~n~,cs in the collrullllalion ofthe receptor may be cor"palille
with, and part of, the cl-AI-g~s that produce high affinity binding, i.e., they may act as
agonists.
The following is a ~ullllllaly of the questions asked and the compounds
~y..ll.~ci~ed to identify ~n~gonictc and agonists of the AT4 receptor that interfer with
binding of physiological AIV fr~gm~ntc. The inventors believe that mapping the
recel)lor binding site (herein) and underst~n~ling of the structure of the receptor and
35 its signal tr~nc~llction meçh~nicm form the requisite basis for rational design of
WO 94/00492 PCr/US93/06038
2139105 ~50-
pharmacologic therapeutic agents that interact with this receptor system in vivo in
",~""..~
Question #1. What are the absolute AIV ligand amino acid requirements for
binding to the AT4 receptor?
The approach used to answer this question involved deletion of residues from
either the N- or C-termius of AIV (i.e., VYIHPF), or from the larger AI sequence of
which AIV is a part (i.e., Figure 1). For the most part these studies employed the
bovine adrenal cortical AT4 receptor present in melllbl~i1e p-epa-~lions pl~pared as
described above in Example 1. The binding assays were also cond~lcted as described
in Examples 1 and 2, above, and certain of the results summarized here are also
presented above in those E~llples.
Deletion of the N-terminal Vall residue from VYIHPF to produce YI~PF
reduced binding affinity to the bovine adrenal cortical AT4 receptor by 1000-fold.
Addition of d-arginine to the N-terminal, (i.e., a peptidase analogue of AIII), reduced
affinity by 100-fold. Deletion of the C-terminal Phe (i.e., des-Phe6AIV) did not alter
binding signifis~ntly. Further truncation of the C-terminal Pro5, however, produced a
moderate affinity (i.e., 21-fold less than AIV). FragsnP-nt~ co~ g less than
positions #l through #4 (i.e., N-VYIH-C) have Kj's>500nM. Addition of hi~titline to
the C-terminus, (i.e., AI(3 9~, Figure 1), did not alter binding ~ignifir~ntly, and further
addition of leucine (i.e., AI(3 10) ~igure 1) reduced affinity by just 2-fold and resulted
in data best plotted in Scalcha~d analysis to fit a two site binding model.
These results suggest that the binding domain in the AT4 receptor recognizes
the N-terminus of AIV with a high degree of specificity. The receptor appears tointeract less closely with the C-terminal region of AIV, but binding of receptors to this
region of AIV may determine the receptor subtype specificity of AT4 receptors indi~elelll tissues.
Ouestion #2. Does the binding site that interacts with the #1 residue in AIV
(i.e., valine) exhibit any ~leleo~lecilicity for particular orientations of the N-terminal
residue?
Repl~cP-mPnt of the L-valinel in AIV with D-valinel reduced binding affinity by
1000-fold. This in~lic~tçs that the domain in the AT4 receptor binding site thatinteracts with the #1 position amino acid residue in AIV possesses a minimllm of "4
non-planar ligand interacting sub-domains that have a fixed spacial orientation" that
can be dç~ign~ted by the L-cGl~llllaLion of an L-valine amino acid. Examples of the
latter "4 non-planar ligand interacting sub-domains" may be supplied by the side chain
residues of 4 amino acids that appear in a requisite 3-dimensional space within this
'~'0 94/00492 2 1 3 9 1 0 5 PCI/US93/06038
-51 -
subdomain of the receptor binding site. (Results ~iccllcced in response to Question
#4, suggest that one of the 4 non-planar ligand interacting sub-domains interacts with
the 1-amine in the N-terminal amino acid.) Compounds that mimic the space filled
by L-valine in a hydrophobic en~holll"~ may mimic the interactions of L-valine with
5 this subdomain of the receptor.
Question #3 . Is the hydrophobic nature of the Rl-group (i.e., Vall) in AIV a
requirement for receptor binding and agonist activity?
Four analogues were synth~ci7ed and tested. Substitution of Vall with Ilel
produced a slightly more hydrophobic peptide (i.e., IYIHPF) as determined by
10 retention on reverse phase HPLC, and this peptide exhibited a slight increase in
binding to AT4 It;C~;~)L(jl~. Substitution of Vall with Phe greatly increased
hydrophobicity but decreased binding affinity to the AT4 receptor by 4-fold.
SullJIisingly, substitution of Vall with Lys (i.e., KYIHPF) co..lA;..;..g a positively
charged side chain, greatly de~ileased hydrophobicity but hlcleased binding affinity to
15 the receptor by more than 45-fold. Sul.slilulion of Vall with a negatively ch~,ed
side chain (i.e., Asp) resulted in an analogue (i.e., DYI~F) with virtually no affinity
for the AT4 receptor.
These results indiçate that the nature of the Rl-group (i.e., a rigid aromatic
ring versus a flexible ~liph~sic carbon chain having an optional positive charge)
20 dictates the interaction with the binding site in the AT4 receptor, and not the just the
degree of hydrophobicity of the amino acid residue. The results presented in
Figures 5A and 5B also in~icate that Lysl-AIV (i.e., KYI~F) exhibits full (or
increased) agonist activity relative to AIV (i.e., VYIHPF). Figure 5 shows changes in
blood flow that result from binding of agonist Lysl-AIV (i.e., KYIHPF) to AT4
25 receptors in kidney, without ~h~ges in systemic blood p,es~ule. Systemic arterial
pressure and cortical renal blood flow were measured as desc,ibed in Example 3,
above. (No. of e,.~;,i",enls= 10.) Figure 5A shows no ci nific~nt chA.~ges in arterial
blood pressure following a~h~ cllation of KYI~F at lOOpmole/25mVmin (open
circles) or saline control (closed circles). Figure 5B shows çhAl~ges in renal blood
30 flow following a~l...;..~.alion of KYI~F at lOOpmole/25ml/min (open circles) or
saline control (closed circles), with the increased blood flow being equal to 38% of
the ~ x;~ a~ hle with a strong vAco-lilAtory agent (i.e., bradykinin, as
described above).
Question #4. Does the ,ulilll~y (1)amine in the N-terminal amino acid
35 interact specifically with the Vall-binding subdomain in the AT4 receptor binding
site?
WO 94/00492 ~L39~n 52 PCr/US93/0603R
As described above in response to Question#2, IlelYIHPF binds to the
receptor with nearly the same binding affinity as VYIHPF. Methylation of Ilel in the
latter peptide (i.e., to form N-methyl-IlelYIHPF) reduced bindng affinity for the AT4
receptor by 67-fold. Substitution of a secondary amine into the R1 position of AIV
5 (i.e., ProlYIHPF) reduced binding affinity to the AT4 receptor by 8-fold.
Substitution of R1 with benzoic acid (a partial structural analogue of Phe) or with
6-amino hexanoic acid (a structural analog of Lys) produced peptides with Ki's
>lmM. Pl~cçm~nt of GABA (gamma-amino-butyric acid) in the R1 position
decreased binding by 250-fold, i.e., relative to binding with AIV.
This data sllggtoctc that the lece~.lor contains a binding site sub-domain that
closely interacts with the p~hllaly amine function in the R1 residue with respect to
absolute space oci~p~ncy (volume) and probably a eleillosL~Lic charge, i.e., thereceptor non-planar NH3-binding colllpon~llL of the R1-binding sub-domain (the same
non-planar sub-domain component described in response to Question #1 above), most
likely is a negatively charged residue that resides adjacent to the 1-amine when the
Rl group is ç~in~ the receptor sub-domain.
Question #5. Is the positive charge of the e-amine in Lys1 responsible for the
increased binding affinity of KYIHPF to the AT4 receptor, or is this plopt;lLy
attributable to the flexible, linear carbon chain?
Four di~e~ellL Rl positicn AIV analogues were synth~ci7ed to answer this
question: 1) Lysl-substituted AIV (i.e., KYIHPF); 2) norleucine-substituted AIV,(i.e., NLel-YIHPF); 3) ornithine-substituted AIV (Ornl-YIHPF); and, 4) norvaline-
substitubed AIV (i.e., Nval-YIHPF). The çh~mic~l structures of these side chains are
shown in Table 10.
TABLE 10
Chemical Structures of Aliphatic Carbon Side Chains
Lys Nle Orn Nv
CH2 l H2 l 2 l H2
2 1 2 1 2 I H2
l H2 l H2 l 2 CH3
CH2 CH3 NH3
NH3
'~'0 94/00492 21 39 1 05 PCI/US93/06038
-53 -
NVa-substituted AIV had a 4-fold greater afflnity for the AT4 receptor than
Orn-substituted AIV. Nle-substituted AIV had a remarkable binding afflnity 60-fold
higher than Lys-substituted AIV: i.e., NlelYIHPF had a Ki of <1 x 10-12M, a virtually
irreversible binding ligand and indicative of partial-agonist activity. To confirm the
5 agonist activity of Nle-substituted AIV, studies were con~lcted to evaluate the ability
of this analogue to stim~ te m~im~l arterial blood flow in rat renal arteries. The
studies were con.1ucted as described Example 6, above. Infusion of 0.10
picomoles/minute of NlelYIHPF into the rat renal artery produced the effect of
lll~imal blood flow, however, the absolute levels of flow stimlll~ted by this analogue
10 were less than the absolute levels produced by AIV or LyslYIHPF, intlic~ting that
NlelYIHPF is a partial agonist Figures 6A and 6B, described below. Figure 6A
shows ~.h~nges in arterial blood pressure following ad...;l.xLl~Lion of NorLeuYIHPF at
1 OOpmole/25mUmin (open circles), 50pmole/25ml/min (open squares) or saline
- control (closed squares). Figure 6B shows çh~nges in renal blood flow following
a~minetration of NorLeuYIHPF at 100pmole/25ml/min (open circles),
50pmole/25ml/min (open squares) or saline control (closed squares). The infusion of
0.05pmole NorLeulYIHPF had no effect on mean arterial pressure (Figure 6A) but
increased renal blood flow in a dose-dependent lll~mCl. a m~imum of 19% increasein renal blood flow was observed with infusions of 0.05pmole (Figure 6B); 19% also
at 0.1 pmole (Figure 6B); 21% at 10pmole (Figure 6B); and, 100pmole
NorLeulYIHPF increased renal blood flow by 30% (Figure 6B). (Infusion of 0.15M
NaCl in control animals were without any .eignifi~.~nt effect.)
The data in-licates that a flexible, linear carbon chain interacts specifically with
the receptor in a high affinity ",al~nc" chains having a four carbon atoms bind with a
higher affinity than chains with three carbon atoms; a positive charge is deleterious to
binding, but does provide an analogue having full agonist activity (i.e., Nlel-AIV).
Ouestion #6. What is the specificity ofthe lecepLor for the R2 residue?
Analogues were plc~arcd with ~tyrosine s~lbstit~ltion for L-tyrosine in the R2
position of AIV (i.e., D-Tyr2 AIV). The latter analogues exhibited low binding
affinity for the AT4 rcceptor. Reversal of the positions of the Phe and Tyr residues in
AIV (i.e., Phe2Tyr6 AIV; VPIHYF) also resulted in analogues that had very low
binding affinity.
These results suggest strict recognition of the R2 Tyr residue, possibly throughhydrophobic and hydrogen-bonding interactions. Substitution with Phe, Ala, and
beta-alanine is useful to map the nature of the interactions with this sub-domain of the
AT4 receptor binding site.
WO 94/00492 PCI/US93/0603X
2139~5 ~54~
Question #7. Will the receptor tolerate the introduction of non-peptide
bonds?
Compounds were synthP~i7ed with methylene bond isosteres (i.e., (-CH2-NH-)
to answer this question. The synthesis was accomplished using the r~cem~te free
amino aldehyde synthesis, Schiffs base formation, and reduction with sodium
cyanoborohydride. Specifically, synthesis of +H3N-Val(CH2NH)Tyr-Val(CH2NH)-
His-Pro-Phe-COO~ (design~ted divalinal AIV) was accomplished ~1tili7:ing standard
solid phase protocols with t-Boc protected amino acids and amino aldehydes. The
same general protocol is used to produce other AIV ligands with methylene bonds
between desired amino acid residues using the applopliate amino acid aldehyde as a
reagent. R-group protection was: Tosyl for His and 2,6-dichlorobenzyl for Tyr.
Synthesis occurred on a t-Boc-Phe s~sLiLuLed resin (0.76mmol/gram of 1% cross-
linked divinyl ben,.ene resin from P~ninc~
For amino acid coupling the following protocol was used: methylene chloride
wash: lX1 min; 45% w/v trifluoroacetic acid and 0.08% indole in methylene chloride
deprotection: lX3 min and lX30 min; methylene chloride wash: 5X1 min;
isopropanol wash: 3Xl min; methylene chloride wash: 3Xl min; 10% v/v
triethylamine in methylene chloride neutralization: lXl min and lX5 min; methylene
chloride wash: 2Xl min; isoplopanol wash: 2X1 min; methylene chloride wash:
2X1 min; isopropanol wash: 2X1 min; methylene chloride wash: 3X1 min; amino
acid coupling with a 2.5 or 5-fold excess of amino acid and EDC in methylene
chloride: reaction times of 1.5 to 3.5 hours; methylene chloride wash: 3X1 min;
isopropal1ol wash: 3Xl min; methylene chloride wash: 3X1 min. The above
protocol was repeated for each cycle. Re-links of amino acids repeated all stepsbegil-l-;l-g with the neutralization. All linkages and deprotections were monitored
with the Kaiser ninhydrin test. Acylations less than 94% were repeated.
Valinal (N-t-Boc-L valine aldehyde from p~.nin~ ) was linked to the free
amino-tenninal of the growing peptide by formation of a Schiffs base intermedi~te
with subsequent bond reduction. For this reaction the above protocol was utilized
with the following alterations: prior to coupling, the resin was washed with dimethyl
Çc,l.."....;de 3Xl min; a 5-fold excess of valinal was added in 1% acetic acid/dimethyl
ru,...~...;~e; a 10-fold mole ratio excess of sodium cynoborohydride (Sigma) wasdissolved in 3ml 1% acetic acid/dhll~Lhyl rol...i~..;de and added in equal aliquots at
0,3,5,10,15,20,25,30,40 and 50 min with concurrent nitrogen purge; the coupling was
35 allowed to continue for 70 additional min; the resin was washed with dimethyl
wo 94/00492 2 1 3 9 1 0 5 PCI/US93/06038
_
-55-
follllalllide 3X1 min. Linkage was ~csessed with the Kaiser test and revealed a
slightly reddish color of the beads when greater than 94%.
The fini~hrd N-terminal deprotected resin-linked peptide was cleaved from the
resin and side chain deprotected with anhydrous HF co,~ g 10% anisole at 0C
for 40 min. The HF and anisole were removed under vacuum and the peptide washed
with anhydrous ether. The peptide was extracted with 20% glacial acetic acid andIyophilized. The crude peptide was then purified by prepal~ e reversed phase HPLC
in two steps, the first an isocratic method using acetonitrile:triethylamine-phosphate,
pH3 followed by a second gradient method using acr~ollillile:water (0.1% TFA). The
purified product was analyzed by analytical reversed phase HPLC
(acetonitrile:triethylamine-phosphate, pH3) gradient method (12-18% over 60 min at
2ml/min).
Repl~c~m~nt of the Rl-R2 peptide bond with the methylene bond reduced
affinity of binding to the AT4 receptor by 5-fold. Double repl~cçm~.nt of both the
Rl-R2 and the R3-R4 peptide bonds and substitution of the R3 Val with Ile produced
the peptide: N-Vl-CH2-NH-Y2V3-CH2-NH-H4P5F6-C (Divalinal AIV) that had
equal or better affinity than AIV for the AT4 receptor. In addition, divalinal AIV has
been shown to exhibit çnh~nced metabolic stability and to be a potent antagonist of
AT4 receptor activity. Figure 11 illustrates the colllp~ e stability of 125I-AIV and
125I-Dival AIV following exposure to a lllt;~ e fraction prepaled from rat kidney.
Kidney was chosen as the tissue of study because of its well-known degradative
capacity. The metabolish of l25I-AIV and 125I-Dival AIV by rat kidney ,llelll~ es
was deLellllined as follows: Rat lllclllbl~es (25~g protein) were inr,ub~ted with .6nM
125I-peptide at room telll~lt;lalult; in a buffer co~ l-g Tris, 50mM, pH7.4; NaCl,
150mM; BSA, 0.1%; EDTA, 5mM; bestatin, 20~1M; and Plullllllrl's inhibitor, 50~M.Metabolism was stopped by the addition of acetonitrile (final concenLlalion 50%), and
the s~mples were analyzed by reverse phase (Cl8) HPLC. As can be seen in
Figure 11, AIV is rapidly degraded while Dival AIV remains 100% intact after 4 hr of
inr,llb~tion.
In addition, following the procedures of E~l,ples 4 and 6, it has been found
that preinfusion with divalinal AIV co...i.letkly blocks LyslAIV-in~uced increases in
blood flow, and preinfusion with divalinal AIV actually ll~lSr~llll5 AIV's effects on
blood flow from an increase to a decrease. This effect of divalinal AIV on AIV
sll~ests that AIV also acts at AII receptors, the effects of which are normally m~c~ed
35 by AIV's action on AT4 receptors. Divalinal AIV llr.~l,.lk,.l by itself did not alter
W094/00492 21391S PCr/US93/0603
blood pressure or renal blood flow (Figure 12A). Additionally, it had no effect on
AIV-ind~lced decreases in blood flow (Figure 12B).
It has been further found that AIV potenti~tes the pclroll,lance of rats in a
passive avoidance task in a dose-dependent manner while AII exhibited no specific
effect. In this experiment, the mean latency (see + SEM) for independent groups of
rats to reenter the dark co~"?a"",ent following passive avoidance conditioning on
Day 1. On Day 1 (5 min prior to testing for retention) the Control Group received
2~11 aCSF, angiotensinII (AII), AIV, or divalinal AIV. Each group except the
divalinal AIV revealed ci nifiç~nt elevations in latency to reenter the dark
colll?~",cll~ - co",?~hlg Days 1 and2. In addition, the groups that received
100pmole or lnmole of AIV indicated a signifiç~nt elevation in latency to reenter
com?ared with those groups that received aCSF and AII, while these latter groups did
not differ from each other. Rats treated with divalinal AIV were not st~tictic.~lly
dirrclclll from preshock controls. I~llerc~lingly, Ire~l",~ of rats with divalinol AIV
blocked the typical increase in latency seen in control rats. Re~,onses by rats treated
with divalinal AIV were not st~tictic~lly dirrc,c"~ than preshock controls. These data
indicate that while AIV potently çnh~nced cognitive function, divalinal AIV acting as
an AIV antagonist completely blocks the learning and/or retrieval of the passiveavoidance task. Fu~Lhc~ ole, these data suggest that endogenous AIV must play a
critical role in cognitive function.
These results intlic.~te that the AT4 receptor binding site domain binds
analogues in which the peptide bond has been replaced with a non-carbonyl (non-
peptidase sensitive) bond that has a similar bond length, and that is non-planar and has
a non-rigid carbon-nitrogen bond. Non-peptide bonds offer pharmacological
advantages for a therapeutic composition, i.e., prolonged half-life. `
Ouestion #8. What delclll,ines agonist versus antgonist activity?
Both AIV (i.e., VYI~F) and Lysl-AIV (i.e., KYI~F) exhibit full agnoistic
activity, while Mel-AIV (i.e., NleYI~F) is only a partial ~gonict The model
capable of ~Ypl~ining this behavior has the following co",?onc"l parts:
a) The ,ecc~Lor binding site sub-domain interactions with the
side groups (i.e., of Rl) determines receptor activation;
b) The interaction at the Rl-sub-domain binding site involves a
hydrophobic pocket;
c) The space in the latter Lydlo?hobic pocket col~lllls very
closely with the 4 carbon side chain of norleucine;
~'O 94/00492 2 1 3 9 1 0 ~ PCI/US93/06038
-57-~ ~
d) Mel (i.e., in NlelYI~F) interacts with the hydrophobic
pocket without çh~nging the co~lll.alion of the pocket;
e) Vall (i.e., in VYI~F) must occupy an "e~p~nded"
hydrophobic pocket, i.e., where the receptor hydrophobic pocket is
displaced laterally to accomodate the branched carbon side chain in
these residues. Lysl (i.e., in KYIE~F) must similarly occupy an
"exr~nded" hydrophobic pocket because of the charge repulsion from
the hydrophobic "walls" of the pocket; and,
f) The process of "c~ n~ g" the hydrophobic pocket
con~titute~ a molecular trigger for the process transitioning the receptor
from the "pre-binding state" to the "binding state".
To study the plopclLies of the "hydrophobic pocket" subdomain of the AT4
receptor binding site it is useful to prepare derivatives of Ornl (i.e., Ornl YI~F) at
the delta amino group to: a) the charge of the group; b) place a planar,
15 col~llllaLionally-fixed bond in the 4 carbon side-chain group that will inhibit binding
in the hydrophobic pocket if the "walls" of the pocket are unable to move to
accomodate the space required by the col~lll.aLion; and, c) synthesi7e
col~,...alionally-fixed bonds in carbon side-chains of di~.cnl length (e.g., 3-5carbons) to explore the optimal longihl-lin~l dimensions of the flexible wall space in
20 the receptor pocket. Suitable N-delta groups for this exploration are acetate,
propionate, benzoic acid, isobutyric acid, and LlilllcLllyl acetic acid.
Question #9. Can the shorter peptide AIV(~ 1) analogues (e.g., VYIH) be
converted to high affinity ligand by norleucine substitution at position Rl?
Answers to this question provide tetrapeptides agonists and antagonists whose
25 interactions with the AT4 receptor are easier to molecularly model, and mimic. The
peptides Nlel-AIV(1 5) (i.e., MeYI~), Nlel-AIV(1 4) (i.e., NleYIH), and
Nlel-AIV(1 3)(i.e., MeYI) may be useful for testing space-filling modifications that
can be made to alter binding in the receptor binding site sub-dom~in~. It is considered
highly likely that independent mo~lifir.~tions that can be made to alter the binding of
30 the latter small Nlel peptides into the AT4 cceptor binding site sub-domains will be
paralleled when the modifir~tion are inco.l.o.~Led into larger AIV ligands.
Question #10. Will ~ub~LiLuLion of Ilel at position R6 (e.g., to form VYIHPI,
KYIHPI, or NleYIHPI) create antagonist activity?
Three Ile6 substituted AIV analogues were synth~i7ed (VallIle6-AIV,
35 LyslIle6-AIV and NlelIle6-AIV). When tested for in vitro receptor binding activity
VallIle6-AIV had a hi~her binding affinity for the AT4 receptor than AIV (i.e.,
WO 94/00492 PCr/US93/0603
2~.'3g~'j -58-
VYIHPI >VYIHPF); and LyslIle6-AIV had a lower affinity than Lysl-AIV (i.e.,
K~IHPI <KYIHPF).
The results suggest that the AT4 receptor binding site is a multi-domain
binding site with interactions such that binding in one sub-domain (e.g., within the
S hydrophobic pocket of the Rl sub-domain) can be excluded by high affinity binding at
a distant sub-domain site (e.g., within the subdomain with specificity for the
C-terminal Ile6 or Pro5 residues; i.e., at the R6 subdomain binding site in the
receptor). The in~luced-fit model supplied above in response to Question #8 is
coll,palible with the observed exclusionary binding properties: i.e., binding of Rl
10 hydrophobic pocket that con~tit ltes the Rl-binding subdomain requires flexibility of
expansion in the pocket, and binding of R6 in the R6 sub-domain binding site confers a
rigidity to the ,eceplor that inhibits flexibility in the Rl-binding subdomain.
Materials and Methods: -
Binding was carried out as described in Example 1, above, in siliconized glass
culture tubes co.. ~ ing 0.2nM 125I-AIV, 25~1g of melllbl~le protein, and the desired
analogue over a concellL-~Iion range of 10-12 to 10 4M using half-log dilutions. All
binding inr,ub~tion~ were carried out in duplicate at 37C for 2 h in a buffer
co~ g: 50mM Tris, lSOmM NaCl, 5mM EDTA, lO~,lM bestatin, 5011M
Plummer's l?e~gf-nt, 100~,1M PMSF and 2% BSA (Assay buffer) in a total volume of20 0.25ml. After incub~tion, the incub~tiQn ~ lures were filtered through glass fiber
(GF-B) filters soaked in 0.3% polyethylf ~ -e and washed with 4-4ml washes of
PBS. The filters were then counted on a Bec.~m~n 5500 gamma counter. A typical
CA~I~lilllelll f .~..,;ned 5 analogues simlllt~neously and inrluded a positive control
curve in which non-radiolabeled AIV ligand was used as the displacer to inhibit
25 binding of l25I-AIV to the AT4 receptor. All s~mrlf s were run in quadruplicate, each
with a di~lenl tissue ~,e~)a,alion. Data was analyzed by the LIGAND program (29)from which Ki values were obtained. AIV analogues that are peptides were
sy.lll-f~;~ed by the standard Merrifield method utili7.ing t-Boc protected amino acids
and chloro",t;ll,ylated resins on a Vega 250 coupler automated Sy~ f"~ (as
30 des~i,il,ed in E~"ple 1, above). Following sy"lllesis, the crude peptides were
purified by l)rel~ali~le reverse-phase HPLC. The amino acid co",;)osilion of thepurified peptides was dete"llll ed with respect to both co~ )osilion and total purity.
Typically the peptides used in these studies were greater than 99% pure and colllail ed
about 20-25% acetate.
~ !0 94/00492 2 1 3 9 1 0 5 PCI /US93/06038
-59-
EXAMPLE 5
Vascular Effects of the AIV Ligand-AIV Receptor Interactions
In endothelial cells (such as bovine colon~y venular endothelial cells), it has
been reported previously that these cells may play a critical role in angiogenesis
5 (review, 21). In one study by others angiotensins were reported to be capable of
sfim~ ting angiogenesis (22). However, studies in the inventors' laboratory over the
past ten years have failed no less than six times to de,llollsllale detect~ble levels of
AII receptors in prepal~lions of endothelial cells that were free of smooth muscle
co..l;....;l~AI;on (a finding contradictory to one report that AII receptors may be
present on endothelial cells, (23). In addition, AII and Sarl,Ile8-AII have beenreported to stim~ te bovine endothelial cell proliferation (24), but the possible
mecl~ were not clear, especially in light of other studies reportedly showillg that
AII and Sarl,Ile8-AlI were rapidly metabolized in tissues and biological fluids to
smaller metabolites. In light of the present disclosure it is now clear, in hintl~ight that
hydrolysis of AII or Am to AIV can result in binding of AIV to AT4 l~cep~ol~ on
endothelial cells with triggering of cell proliferation, and may possibly be involved in
the initiation of hyperplastic growth of endothelial cells or vascular smooth muscle
cells.
AT4 l~ce~lolY, in vascular cells
The following study describes the characteristics of a new class of angiotensin
binding sites in vascular endothelium that exhibit high spe~.ifi~ity and affinity for
h~;A~pe~ide AIV. Analysis of 125I-AIV binding was pelrolllled in melllbl~e
fractions of two endothelial cell lines, bovine corol1~y venular endothelial cells
(CVEC) and bovine aortic endothelial cells (BAEC). Kinetic analysis of binding
25 indicated that equilibrium was reached in 60 min. at 37C, le~Ai.-~d stable for at least
4 h, and produced a ç~lcl-lPted kinetic Kd Of 0.3nM. Saturation equilibrium binding
studies analyzed by non-linear curve fitting s~lgge.~ted the following two site models
(mean +/- SEM): CVEC Kdl=14.6 +/- 26.5pM, BmaXl = 6 +/- lfmol/mg protein,
Kd2=4.4 +/- 0.8nM, BmaX2 = 434 +/- 51 fmol/mg protein. Compteition binding
curves from CVEC d~.lol~l-a~ed high spe~.ifi~.ity of the recel)lor for for AIV. The
colnl)t;lili~e binding affinities of analogues to the receptor showed ~ffinites that (in
decreasing order) were AIV >AII(3 7~ >AIII >AII ~SarlIle8-AlI, or AII(4 8~
>>DUP 753, or CGP42112A. The AT4 receptor in endothelial cells may not be
G-protein linked because the non-hydrolyzable GTP analog GTP~S had no effect on
l25I-AIV binding to receptors in BAEC cells. These data indicate that AIV binds to a
WO 94/00492 2~39~05 PCI/US93/0603
-60-
site in vascular tissues that is distinct and separate from the classic ATl or AT2
angiotensin receptors.
Kinetic binding studies
Kinetic analysis of 125I-AIV binding to AT4 receptols in CVEC membrane
5 revealed that equilibirum was reached in approAhllalely 60 min. and rem~in~d stable
for at least 4 h. at 37C (Figure 7A). The kinetic propclLies of 125I-AIV binding to
AT4 receptors in Illt~ lane fractions of bovine colonaly venular endothelial cells
(CVEC) at 37C. are shown in Figures 7A and 7B. The association (Figure 7A) and
dissociation (Figure 7B) rate cons~ s for binding of 0.6nM l25I-AIV were 9.3 x 107
10 M~lmin~l and 0.028 min~l, respe~ ely. The kinetic Kd calculated from these rate
con.cl~..ls is 0.3nM. C~lc~ tions were pelrulllled by the LIGAND program from a
mean of four cAI,elilllenls with d~lp!icate samples. Data presented here represent the
results from a single eApelill~ L.
Analysis of a~soci~tion data under pseudo first order rate conditions resulted
in an observed association rat COIIS~ (kobs)= 0.084 +/- 0.013. The dissociation rate
constatnt (k 1)= 0.028 +/- 0.005 min~1 was estim~ted by the addition of lmM
undlabeled ligand following inc~lb~tion of 0.6nM 125I-AIV for 120 min at 37
(Figure7B) and the actual association rate constant (k1) was calculated to be
9.3 x 107 M~lmin~l. Based on these rate COllS~all~S, the appa~en~ kinetic Kd value is
0.3nM. Less than 10% degradation of the ligand occurred under the binding
conditions used here.
Equilibrium bindin~ studies
As shown in Figures 7A and 7B, quilibirum binding of l25I-AIV to AT4
receptors in CVEC and BAEC Ill~lllbl~es reached saturation at 37C in 120 min.
Equilibrium saturation binding and ScaLcllald ~l~lsrulllla~ion analysis (insert) for
125I-AIV binding to AT4 recel)~olS in CVEC is shown in Figure 7A, and BAEC in
Figure 7B, ll~cllll~l~le fractions after 12û min. at 37C. The data were best fit by a
two site model ~ltili~in~ the non-linear curve fitting program LIGAND (No. of
expts.=4, each with ~lllpli~te salllple~).
Sca~cllald ~l~lsrullllalion of these data sl~g~ested the presence of multiple
binding sites in endothelial cell Ill~,.ll~l~le-associated AT4 receptors. The data were
best resolved into two collll)oneellls collt;~l,ol1ding to a high and a low affinity
binding site. The final Kd and BmaX values were as follows:
In CVEV (Figure 7A): site #1 14.6 +/- 26.5pM with 6 +/- lfmoVmg protein;
site #2 1.4 +/- 0.2nM with 594 +/- 4fmollmg protein; and,
"'O 94/00492 2 13 9 1 0 5 PCl/US93/06038
-61-
In BAEC (Figure 7B): site #1 26.9 +/- 9pM with 10 +/- 2fmol/mg protein;
site #2 4.4 +/- 0.8nM with 434 +/- 51fmol/mg protein.
Values obtained when fitting the data to a single receptor affinity site model
were: in CVEC 0.7 +/- O.lnM Kd with BmaX=lo +/- 2fmol/mg protein; and in BAEC
1.0 +/- 0.2nM with Bma,C-260 +/- 38fmol/mg protein. The two site model produced a
significantly better fit for both cell types as compared with the single site model (i.e.,
F-test, p<0.001).
Co~llpclilion bindin~ studies
C~n")elilion studies were con~lcted to rlicFl~ce 125I-AIV binding to AT4
receptors in CVEC membrane plepalalions with specific ligand, i.e., AIV, and other
related angiotensin fr~grnP.ntc. Figure 8 shows co",pclilion displ~m~nt curves
dPli~ ;ng the ability of angiotensin fr~gmPntc to inhibit specific binding of 0.5nM
125I-AIV to AT4 receptors in "lel,.b.~e prcpa.~lions of bovine coronaly venular
endothelial cells (CVEC). (No. of exper. =2; each con~llcted with duplicate samples.)
The rank order affinity of co.. pt;lili~e analogues con.l)clili~/ely displacing
bound AIV from its receptor were as follows: AIV >AII(3 7) > AIII >AII
>SarlIle8-AII, or AII(48~ >>DUP753, or CGP42112A. (For sequences of AII
analogues see Figure 1). The results of these studies are su------a,i~ed in Table 11.
TABLE 11
Co.-.l)elilion of 12sI-AIV binding to AT4 receplo.
in CVEC membrane prel~a ~lions
Fragment Sequence Kj
AIV VYIHPV 1.1 +/- 0.2nM
AII(~-7) VYIHP 7.3 +/- 1.2nM
Am RVYIHPF 23.3 +/- 3.4nM
AII(, X) DRVYIHPF 193.8 +/- 44.5nM
AII(4-~) YIHPF 252.6 +/- 89. lnM
Sarl,Ile~-AII SRVYIHPI 261.0 +/- 89.1nM
DUP 753 --- >10~
CGP 42112A --- >10 4
*AII values lc~resenl mean +/- SEM of two e~el..ll~llLs with duplicate samples; K
determined by LIGAND.
G-protein linka~e of the AT4 receptor in vascular cells
G-protein interactions with vascular angiotensin receplo,~ are shown in
Figure 9, where ".~;...I,.ane fractions from rat vascular smooth muscle cells (RVSMC)
or bovine aortic endothelial cells (BAEC) were preinc~lb~ted in various conce~ aLions
of a non-hydrolyzable GTP analogue (i.e., GTP~S) for 60 mimltes at 22C prior to
WO 94/00492 PCr/US93/0603
21391~ -62-
use in equilibrium binding assays with 0.5nM 125I-AII (RVSMC) or 0.6nM l25I-AIV
(BAEC). (No.l of exper. =3; each with duplicate samples.) Data presented here
represent results from a single experiment.
Addition of non-hydrolyzable GTP (i.e., GTP~S) to the binding assays did not
5 inhibit (or alter) binding of l25I-AIV to AT4 receptors in BAEC lllelllbl ~1e
prep~aLions (Figure 9). In constrast, in a positive control GTP~S inhibited 125I-AII
binding to AT1 receptors in rat vascular smooth muscle cell (RVSMC) lllelllbl~ eple~ ions in a dose-dependent manner (Figure 9); in a~,lt;~lllenl with observations
reported previously by others. (This property flietin~ hes AT4 receptors of the
10 invention from ATl and AT2 receptors reported by others previously in vascular
tissues.)
Discussion
This study is the first to describe a novel angiotensin binding site in vascularendothelium that exhibits high affinity and specificity for the h~ apeplide AIV
15 fragment of angiotensin AII. The AT4 receptor is distinct from the AT1 or AT2receptors in vascular tissue. Analysis of the binding characteristics intli~ctes that the
AT4 receptor binds AIV in a saturable and reversible manner, and that l25I-AIV
reaches equilibrium in binding to the AT4 receptor in lllellll,.~e plep~lions inapploAilllalely 60 min. at 37C. Binding of AIV to its receptor lelllaills stable for at
20 least 4 h (Figure 7A) ~,vith less than 10% degradation of the ligand under these binding
conditions. Scatchard analysis of the AT4 receptor binding site by the non-linear
curve fitting program LIGAND reveals two collll)onellls to the binding data. The first
colllpol1elll is a high affinity component that e-Ahibits Kds of 14 and 27pM with BmaX~s
of 6 and lOfmol/mg protein for receptors in CVEC and BAEC Illt;lllbl~-e
25 prep~lions, le~ecLi~ely. (Because of the t;A~l~lllely low number of these high
affinity sites it is unclear at present whether this is a physiologically important state of
the receptor; or, is a result of modification of AT4 rec~ol~ in the nle~ ne
prep~lions, or ch~npes in receptor binding affinity reslllting from co-operativebinding of AIV; or alternatively, that this site is an artifact created in the melll~ e
30 prep~alions or assay con~ition~-) The second binding collli)ontilll is a lower affinity
cGlllpol1elll with Kds of 1.4 and 4.4nM (i.e., in CVEC and BAEC, resl,e~ ely). The
second corlll)on~.ll d;s~ a high concellllalion of ligand binding co, .""e~ lrate with
large numbers of such receptor sites in the Ill~ e prep~lions: i.e., these sitesbind 594 and 434fmol/mg protein in CVEC and BAEC lllenlbl~-e plel)a,aLions,
35 respectively.
~0 94/00492 2 1 ~ 9 1 0 5 PCI/US93/06038
-63~
The overall binding affinity (i.e., Kd, single or composite site fit produced byLIGAND) was calculated to be 0.7nM for CVEC and 1.0nM for BAEC. These
results are in good agleelllt;llL with the Kd calculated from the results of kinetic
binding studies (0.3nM).
The pharmacological profile derived from co~ ;LiLion displ~cem~nt of 125I-
ATV bound to these AT4 receptors in vascular tissues is presented in Figure 8 and
Table 11, above. This profile reveals a strict structural requilt;lllelll for the
N-terminus of the AIV ligand, i.e., removal of the N-terminus (Vall) of the AIV
ligand results in a 200-fold decrease in affinity of the AIV ligand for the AT4 receptor
in vascular tissues (i.e., an increase in the Ki). In addition, N-terminal extension, i.e.,
beyond Vall, is d~llh,~ l to the binding of AIV ligands to the vascular AT4
l~cel,lor as inr1ir~ted by the inability of AII and Sarl,Ile8-AII to co---l.eLiLi~ely inhibit
binding of AIV to the AT4 t;cel.lor, (i.e., note the 200-fold increase in Ki seen with
AII and Sarl,Ile8-AII, when co..l~aled with AIV in Table 11). (This property
15 ~iictin~liches AT4 receplGls of the invention from AT1 and AT2 receptors.) The
app~c;.-l affinity of Am for the vascular AT4 receptor (i.e., 20-fold higher Ki than
AIV, Table 11) may be an artifact of N-terminal metabolism of AIII to form AIV in
these --t;---b-~le prep&,~lions. (In previous studies, above, 125I-AIII binding to
bovine adrenal AT4 receptors was directly plopo-lional to the amount of Am
20 hydrolyzed to AIV.)
The vascular AT4 receplor appears to exhibit less sper.ifi~.ity for the
C-terminus than exhibited for the N-terminus: i.e., the AIV(1 7~ fragment (with the
C-terminal Phe8 deleted still bound with re~oll~ble affinity to the lt;ce~llor (i.e., only a
7-fold increase in Ki over AIV). (These r~ ii"gs are in ag-ee...t..l with the fintling~
25 above in Example 1 using AT4 receptors in bovine adrenal cortical tissues.)
The vascular AT4 r~ce~lo.~ do not apparelllly bind either DUP753 or
CGP 42112A (i.e., Ki >10 ~), but ATl or AT2 receptors are well-known to do so
(Ti.. t.. ~1s, P. et al. 17P5 12:55-62, 1991; Whilel~.~zd, S. et al. Biochem. Biophys.
Res. Comm. 163:284-291, 1989). (This propc;.ly offailure to bind either DUP 753 or
CGP42112A .li~tin~ hes AT4 rece~ol~ of the invention from AT1 and AT2
receptors.)
Bil-di-1g of 125I-AIV to vasular endothelial AT4 rect;plo-~ was not sensitive toinhibition by f~l~nine nucleotides. In contrast, binding of AII to AT1 and AT2
receptors in .n~;.nb.~le prep~lions of rat vascular smooth muscle cells (RVSMC,
Figure 9) was sensitive to inhibition by guanine nucleotides in a dose-dependentmanner, i.e., the affinity of the AT1 receptor for AII was shifted to a lower value
Wo 94/00492 PCr/US93/06038
-64-
2~3g~0S
when the receptor was uncoupled from G-proteins by the presence of the GTP
analogue GTPyS (Figure 9). This shift in binding affinity in response to gunainenucleotides is a characteristic of the high affinity form of the AT1 receptor
(Gloccm~nn H. et al. J. Biol. Chem. 249:664-666, 1974). The in~n~itivity of the
5 AT4 receptor to G-protein uncoupling agents was also observed with AT4 receptors
in membrane p,t;pal~lions of bovine adrenal cortex. (This plopel ly of imçn~ifivity to
G-protein uncoupling agents (lictin~ hes AT4 lecep~ol~ of the invention from AT1and AT2 receptors.)
Despite the inability of AIV to bind to AII receptors, several recent studies
10 have sllg~ested that that AIV-like fr~m~nt~ of AII may have unique biologicalattributes. In cultured chick myocytes, AIV-like fr~ ntc of AII have been reported
to antagonize the effects of AII-indllced increases in cytosolic free c~l~.illm protein
synthesis, and hy~elLlopl~ic cell growth while being unable to cc,lllp~liLi~ely inhibit for
125I-AII binding (Baker, K.M. et al. Am. J. Physiol. 259:H610-H618, 1990). Topical
15 application of both AII and AIV-like fra~m~nte of AII have been reported to me~i~te
endothelium-dependent vasodilation in rabbit brain arterioles. However, in the
presence of the amino peptidase inhibitor ~m~ct~tin the vascular response to AII, but
not AIVlike fr~gmPntc, was reportedly blocked (Haberl, R.L. et al. Circ. Res.
68:1621-1627, 1991). AIV-like AII fr~gmPntc and AII have also been reportedly
20 applied intracerel)lo~entricularly in the rat where they reportedly are equipotent in
enh~nt~.ing memory and learning (Braszko et al. Brain Res. 542:49-54, 1991). Given
the low affinity of AIV for ATl and AT2, disclosed herein, it is most likely that the
latter activities previously attributed to binding of AII and/or AIV-like fr~gm~ntc at
AT1 and AT2 sites are, in fact, the result of binding of AIV at the AT4 receptor sites
25 of the invention.
It is likely that the actions evoked by AIV binding to its specific AIV recepotrs
may act collll~y to the actions ofthe AII and AT1 and AT2 receptors. For example,
infusion of AIV into rat kidney, as shown above, to stimlll~te a significant increase in
blood flow in the renal cortex, while AII binding to AT1 and AT2 recep~ol~ in these
30 tissues produces the converse effect - a cignific~nt decrease in blood flow.
Effects on Vascular Tissues:
~ seC~ of AIV effects on the contractile plopelLies of aorta and inferior
vena cava was delllol1s~la~ed using tissues from rabbits. The presence of numerous
AT4 receptors in aortic tissue suggest a possible action of AIV ligand on cerebral
35 vessels. The routine use of rabbit aortic strips or rings in cardiovascular
pharmacology dictate that rabbits are suitable for use in such studies.
~VO 94/00492 2 1 3 9 1 0 5 PCI/US93/06038
-65-
The following protocols are useful for: 1) co"r",l.ing the vasodilating
potential of an AIV ligand, delllo~ laLi,lg that ligand action is dependent on an AT4
receptor, and showing that the action is independent of AI or AII receptors;
2)establishing that any observed vasodilation is endothelium dependent;
5 3) dete",~ g whether the me~.h~ ", of vasodilation involves prost~gl~n~in.c,
EDRF, or other factors like EDHF as second ...ess~l-g~.~; and 4) determining thefunctionality of the many AIV analogues (i.e., such as those synthesi7ed in
Example 4) as either AIV ligands or as agonists, antagonists, inhibitors, or promoters
of the AIV ligand-receptor interaction.
AIV and AII ligands and various analogues (Example 4) in the presence or
absence of angiotensin inhibitors (e.g., Sarl,Ilex-AlI, DUP 753, and CGP42112A)
were screened for the vasodilating activity using rabbit aorta and inferior vena cava
rings or spiral strips suspended in 20ml organ baths co..~ ,;..g Krebs solution at 37C
and continuously gassed with 5% C02 in oxygen. After a 1 h equilibration period,c.-mul~tive dose-re~onse curves were constructed for the analogues over a
concentration range of 10-10M to 10-sM. In relaxation studies, aortic strips were pre-
contracted to 70% of m~im~-m di~met~r with phenylephrine, and then the test ligand
is added and relaxation of the vessel is qu~ntifiçd Changes in contractile or relaxant
response may be c~lcul~ted for each dose of each di~erell~ ligand or analogue and
subsequently analyzed by analysis of variance.
Effects on Endothelial Cells:
The effect of AIV ligand on endothelial cells was ~ .";.~ed by measuring
growth of bovine endothelial cells. Cells were grown at 37C in 35mm culture plates
C02/air under 5% C02/95% air in Dulbecco's Modified Eagle's Medium (DMEM)
suppl~mented with 511g/ml insulin and 10% (v/v) newbornbovine serum (NBBS).
The test metlil-m was suppl~m~nted with 3H-thymidine and either AII ligand (50nM)
or AIV ligand (SOnM) or lOng/ml acidic or basic FGF (as a positive control).
Negative controls were also inc1uded using ethi~1i--m bromide (lmM). The cells were
harvested at various times, and cellular lysates were prepaled for sçintill~tion counting
by lysing and waslllllg the cells on glass fiber filters.
Materials and Methods:
ntS
AIV (VYIHPF), AII(3 7~ (VYIHP), and AII(4 8~ (YIHPF) were synth~si7ed as
described in Example 1, above. All reagents and other peptides were obtained from
Sigma Chemical Co., with the exception of: Plummer's inihibitor (Calbiochem);
bestatin (P~ninc..l~ Biochem); DUP 753 was a gift from Dr. Ron Smith of
WO 94/00492 -1 0 ~ -66- PCI/US93/06038
Dupont/Merck and CGP42112A was a gift from Dr.Marc deGasparo of Ciba-
Geigy. Angiotensin fragmPnt~ numbering was based on the sequence of AII
(Figure 1).
Cell Culture
Bovine coronary venular endothelial cells (CVEC) were isolated by a bead-
perfusion technique and characterized as described previously (SçhPllinE M.E. et al.
Am. J. Physiol. 254:H1211-H1217, 1988). Bovine aortic endothelial cells (BAEC)
were a gift from Dr. Stephen Schwartz (University of Wa~hinEton). Cells were
grown in 100mm tissue culture plates (Falcon, Becton Dickinson Co.) coated with
1.5% gelatin in PBS (per liter of distilled water: 8.12g NaCL, 1.14g Na2HPO4, 0.28g
NaH2P04) in Dulbecco's modified Eagle's mç~ lm (DMEM; FLOW Labs)
s.lppl~ ed with 2mM sodium pyruvate, 2mM L-El-~t~mine., 100mglml heparin,
100mg/ml Penicillin-G, 50mg/ml SlreplolllyGin, 44mM NaHC03, and 10% fetal
bovine serum (GIBCO). Cells were passaged 1 :3 by tryptic digestion (0.05% trypsin,
0.025% EDTA in Ca++/Mg~-free PBS, pH7.4 at 37C). All data collected in this
study was from cell lines p~s~Eed between passage 5 and passage 9.
Tissue pl t;?~u ~lion
Cells were grown to confl~çnce in 100mm culture dishes. Dishes were
washed once in Ca++/Mg~-free PBS, pH7.4 at 37C follwed by the addition of 2ml
of cold isotonic assay buffer (150mM NaCI, 50mM Tris, lmM PMSF, 1011M bestatin,
50~M Plummer's inhibitor, pH7.4 at 4C). Cells were then removed from the plateswith a rubber policeman and homogenized in 5ml assay buffer for applo~illlalely
10 sec (Polytron, Brinkman Inst. Co.). Cell extracts were centrifuged at 40,000 x g
for 20 min at 4C, the ~upelllal~ll was discarded and the pellet was rehomogenized in
assay buffer and centrifugation was repealed for a total of two high speed
centrifugation steps. The final pellet was resuspended in assay buffer to a working
col~ce~ a~ion of appr~.,.;...~P.Iy Smg/ml as delelll~ed by the method of Lowry (J.
Biol. Chem. 193:265-267, 1951).
Iodination of AIV
AIV (and other peptides) were io-lin~ted using an immobilized
lactoperoxidase-glucose oxidase system (Enzymobeads, Biorad Laboratories) to a
specific activity of 2176Ci/mmole. l25I-AIV was separated from unlabeled peptide by
HPLC (Beç~ n) using a reverse phase Cl8 column (5mm x 250mm; Adsorbosphere,
Alltech, Associates).
WO 94/00492 2 I 3 91 0 5 PCI/US93/06038
-67-
Receptor binding assays
Binding assays were performed at 37C in a total volume of 250ml (isotonic
buffer, pH7.4 at 37C). Bound and free ligand were separated at the conclusion of
each eAI~e~il.lent by the addition of ice-cold PBS (pH7.4), and separation of bound
5 from free was achieved by 4 vacuum filtration washes with 4ml of this buffer
(Schleicher and Schuell #32, Brandel Cell Harvester). Radioactivity retained by the
filters was determined using a Tracor Analytic gamma counter, model #1185 having68% counting efficiency. Nonsl,e~iific binding was ascellailled in the presence of
lmM unlabeled AIV.
Kinetic binding ~iAI~elilll~llLs (N=3) were pe,f~""ed at 37C over a time courseof 240 min with 11 time points and duplicate s~ p!es. The appale..l pseudo-firstorder association rate consl~.~ kob5 was detc.;..,ined by the non-linear curve fitting
program LIGAND. Dissociation eA~e i..-e.lLs (N=4) were conrhlcted at 37C by
pr~incub~ting cell eAtracts for 120 min with 0.5nM radiolabeled ligand followed by
15 the addition of lmM unlabeled ligand (final conc.). Binding was de~e-....lled for
duplicate samples represe..l;ng 10 time points over 180 min. The appale..L
dissociation rate consl~-L, k l, was dett-.. ined by LIGAND. The appare..L
association rate consL~.L, kl, was then c~lcul~ted from the equation
k1= (kobs - k 1)/[L], where [L] is the radioligand concellLlaLion~ and the apparenL kinetic
20 equilibrium dissociation consL~u.L~ Kd, was derived from the equation Kd = k l/kl.
Saturation equilibrium binding and col..peLiLion diepl~cemrnt studies (with
CVEC, N=4 expts., 46 total data points; BAEC, N=3, 34 data points) were
conrlucted over 120 min. of inr,ub~tion in the presence of incl~,asing conce--L-~lions of
radioligand or co...~,elh~g ligands, ~e~;Li~ely. Saturation data were analyzed by
LIGAND for the dt;Le.. linaLions of .. ~ ;.. number of binding sites (Bma,~) and Kd.
For delt",--l~,g the linkage of G-proteins to the AT4 receptor, l~l~lllblane
- prepa~aLions were first pr~inruh~ted in GTP assay buffer (50mM Tris, 150mM NaCI,
5mM MgCi2 lmM EGTA, lmM PMSF, SO~M Plun~ner's inhibitor, lO~M bestatin,
p~I7.4) at 22C for 60 min in solutions of GTPyS ç~ ted to produce a final
concenLlaLion in the assay of lOOmM, lOmM, lOnM and 0 GTPrS. The rat vascular
smooth muscle cell line WKY IV passage #17, was inr~ 1ed as a positive control for
G-protein linkage to ATl lecel)lo.~. All data are plesenled as the mean +/- SEM,standard error of the mean.
Endothelial cell growth and the effects of AIV ligand on EDRF production
Bovine aortic endothelial cells were grown at 37C in 35mm culture plates
under 5% CO2 in air in Dulbecco's Modified Eagle's Medium (DMEM) supplrmented
WO 94/00492 2~39~Q PCI/US93/06038
-68-
with 5~1g/ml insulin and 10% (v/v) newborn bovine serum (NBBS). The m~ m was
aspirated 10-12 hours after seeding and replaced with serum-free medillm The
me.1illm was again aspirated 10-12 hours later and replaced with either test or control
metlillm Control medillm was DMEM with 5g/ml insulin and 2%, 5%, or 10% (v/v)
NBBS as indicated. The test medillm was supplçm~nted with either AII or AIV
ligand ~ the antagonist Sarl,Ile8-AII at various concentrations. The merlillm was
çhAI~ged every 48 h (i.e., with supportive DMEM metlillm for the r~mAinrler of the
~e-illlent).
For measurements to determine the effects of AIV in stiml~lA~ting an increase inendothelial cell numbers, cells can be harvested on various days during the culture
period by washing the plates with calcium free mç-lillm (CMF) two times for 5 min.
followed by in-,ub~tion in 0.1% trypsin in CMF for 5 min. The cells can then be
washed free from the plate and as~ ed by Pasteur pipet into 15ml centrifuge tubes
cG,~I~;n;,~g 3ml DMEM with 20% (v/v) NBBS. The plates can be washed with an
additional lml DMEM 20% NBBS which was Ll~lsr~lled to the appropl;ate
centrifuge tube and spun at 300 x g for 10 min. Excess metlillm was aspirated and the
pellet resuspended in a final volume of lml of the control metlillm Aliquots can then
be counted using a hemocytometer and cell number eA~,lessed as cells/plate.
As an adjunct to the detellllllld~ion of cell llulllbel~, thymidine incorporation
was measured. For qu~ntit~tion of DNA synthesis [methyl-3Hlthymidine (60Ci/mmol,1 OmCi per plate) was added to cultures 12 h after addition of the AII or AIV. Twelve
h later, medillm was removed and lml of a 1% aqueous solution of Triton X-100 was
added. The cells were inr,~lb~ted with this solution for 5 min. and the entire contents
of the plate Ll~l~r~ d to lOml of absolute ethanol. This material was then filtered
under vacuum through 2.4cm glass fiber filters (GF/A~ Whatman), and the filters were
washed twice with lOml of absolute ethanol and assayed for radioactivity by
sçintill~tion counting.
EXAMPLE 6
Physiological Function of Angiotensin IV Receptor and Ligand
Angio~ensins AI, AII, and Am are reported to have a wide variety of effects
on target issues, some of which are acute while others appear more long-term. AII
reportedly has a cellular effect of incleasing c-fos levels in cultured vascular smooth
muscle cells (17), and c-fos is reported to be one common pathway for triggering cell
growth. Considering the widespread distribution of AT4 receptors in many organs
and tissues (EXAMPLES 1 and 2, above), it is likely that AIV has multiple functions,
~0 94/00492 ~ . PCr/US93/06038
-69 2139105
inrl~1ing long-term effects on cells by triggering increased ~A~uression of c-fos, i.e.,
activities previously miet~k~nly attributed to AII and AIII.
The following studies focus on the role that the AIV ligand-receptor system
may play in three organs enriched in AT4 rece,ulola: blood vessels, kidney~ and
adrenal glands. (Other organs such as brain or heart which also possess high levels of
specifically localized AT4 receptors can be studied in a similar manner.)
Renal Blood Flow: The AIV Receptor and AII Receptor Have Physiolo~ically
Distinct and Opposin~ Activities:
Physiological studies, des~il;bed below, investig~ted the involvement of AIV
ligand in the regulation of renal blood flow. The rationale for initially choosing to
f~x~mine the kidney was at least two-fold. First, the AT4 receplor is found in high
conce~ ions in kidney and endothelial cells (Example 1 and 2, above). Second,
vascular endothelial cells are reported to regulate vascular tone and to play a role in
the control of renal blood flow.
Superficial blood flow in the rat kidney was ~sç~ed using laser doppler
methods in ~nestheti7ed rats following direct infusion of a test substance into the renal
artery. The results are presented in Figure 4 which depicts the pel ce--lage change in
cortical renal blood flow following infusion into the renal artery of 25~1Vmin of a
0.15M NaCI solution co..l~ g 100pmoV25111 AIV (closed circles; number of
eA~e in-e-"s (n)=13); 0.15M saline (open circles; n=9); 100pmoV25111 of AIV lacking
the N-terminal Vall residue (i.e., YIHPF; D-Vall; closed squares; n=9); and
100pmoV25~1 of AII (open squares; n=8). The infusion of eAI elillltillLal compounds
and saline had no effect on systemic arterial blood pressure (see results in Example 4).
The infusion of AIV (closed circles) show that AIV ligand infused at 100pmoVmin.25 stiml~tes a ploround and long-lasting increase in blood flow. In contrast, infusion of
AII (also at 100pmoVmin.; open squares Figure 4) produced a dramatic decrease inrenal blood flow. The AIV analogue d-Vall-AIV (i.e., lacking the N-terminal valine
and lacking binding activity for the AT4 receptor, see F.y~mple 1, above) had noeffect on renal blood flow (closed squares; Figure 4).
The eA~ enlal protocols employed in these studies is det~iled in the
Materials and Methods, below.
Materials and Methods:
EAIJel;ll1elllal Protocol #1:
For co---p~;son of AII, AIV, d-Vall AIV and saline infusion on renal blood
flow, the rta~ue~ re agents were infused into the renal artery at 100pmoVmin. for
10 min. at 25~Vmin. Saline and the AIV analogue d-Vall AIV were inr.l~lded as
WO 94/00492 ~39~oS PCr/US93/06038
controls, i.e., the number of cA~ illlel~ 8; average standard error of the mean
(SE) = + 3% change blood in flow. As expected, saline and d-Vall AIV had no effect
on renal blood flow. Also, as expected, AII produced a dramatic decrease in flow
followed by an autoregulatory return toward baseline. AIV produced an equally
5 dramatic increase in flow that showed little autoregulation.
Consistent with the involvement of di~renl receptors in the mediation of AII
and AIV effects, the specific AII antagonist Sarl,Ile8-AII (lnmol/min - lOmin.
p~Lle~,.,P.nt) completely blocked the AII effect while having no effect on AIV. The
decrease in blood flow w;~ ed with AIV was dose dependent and was not
10 accG..~p~Ilied by alterations in mean arterial pres~ure, sllg,~e~ g that the effects of the
AIV ligand-receptor system may be limited to selective vascular beds or that
co~ .s~lory ~.h~ng~ in cardiac output occurred during AIV infusion.
EA~;,i".e."al Protocol #2:
The AIV-in-luced increase in renal blood flow was not blocked pr~infi.~ing
15 AII: Sarl,Ile8-AlI was infused over the 10 min. immedi~t~ly prior to infusion at
lnmol/min., and a COIllp~iSOll was made with the change in blood flow that occurred
when AIV ligand was infused will~oul the AII preinfusion. In 8 expe~i",~"~s an
average change in AIV-in~uced blood flow of <3% was recorded with the AII
preinfusion, which was within the ~L~ da,d error of the cA~,i",ellls, i.e., SE = _ 3%.
20 Thus, as predicted from the co""~liLion binding studies con~1cted above
(Example 1), Sarl,Ile8-AlI was unable to block the vasodilatory effect of AIV ligand.
When tested in control cA~t;,i",ents for the ability of AIV ligand to block AII-medi~ted decrease in blood flow (i.e., in the same type of preinfusion eAI,e~i",e,lL, but
using AIV preinfil~ion instead of AII). AIV ligand completely blocked the
25 constrictive action of AII. The,eru,c;, the results support the notion that AIV may
antagonize certain of the actions of AII.
Effects of AIV-Ligand-Receptor Interactions on Renal Functions
Results presented above dç~..ol.~l.a~e that intravenous application of AIV
ligand can dr~m~tic~lly inclease renal blood flow and urine flow in a dose-dependent
30 fashion. This effect appears to be metli~ted by the AT4 receptor and not by
nollspeciflc, nol~rece~"or-dependent processes. Neither AII nor d-Vall AIV (a
nonbillding AIV analogue) could reproduce the effects of AIV ligand, and the specific
AII antagonist Sarl,Ile8-AlI was unable to block the action of AIV ligand.
Another ~çc~ ..l of the AIV ligand-receptor effects on renal functions was
35 provided by analyzing distribution of radio-labeled insulin and p-~minohirpicuric acid;
in coll,bh-a~ion with ,lleasw~",e"~s of urine flow, urine osmolality, urine Na+ and K+,
~vo 94/00492 2 1 3 9 1 0 5 PCI/US93/06038
-
-71- - ~.
- and hematocrit. The effects of AIV ligand, AII, and other AIV analogues were
determined, i.e., a) on renal blood flow, b) glomerular filtration rate, c) osmolal
clearance, d) filtration fraction, and e) tubular function. Dose-response curves for
AIV ligand and AII ligand were constructed in the presence and absence of the AII
5 antagonist Sarl,Ile8-AII. In addition, AIV analogues with special in vitro properties
(e.g., AIV antagonists, AIV superagonists, or metabolically IGs;sL~lL analogues of
AIV) were tested in a similar manner (above) to determine their effects on renalfunction. Studies were carried out as acute prepal~Lions in ~nestheti7ed rabbits and
using jugular and urethral c~thetçrs.
EXAMPLE 7
Neurological Effects of the AIV-AIV Ligand-Receptor Interaction
Local Effects:
Given the plGsellce of AT4 receptors in the brain (Example2, above;
Figures 6-10) and most likely in cognitive and motor memory and learning centers(i.e., hippocampus, frontal cortex, cerebellum, and th~l~ml-s), and in areas within the
hindbrain cardiovascular nuclei involving the tractus solitarious, it is reasonable to
suspect that at least in some tissues AIV ligand is produced locally in neural tissues,
i.e., by synthesis of Al and conversion to AIV. Two scenarios of local production can
be envisioned. In the first, AIV ligand is produced locally from precursors
synthP~si7ed in the tissue. In the second, circ~ ting AIV precursors (e.g., Al, AII or
Am) are converted locally to AIV ligand. Whether the first or second scenario is an
operative ...e~.h~ni.cm in a particular tissue can be d~LG.I..~lled by introducing
radiolabeled precursors (i.e., l25I-AI) into the bodily fluid bathing the tissue (e.g.,
plasma or CNS fluid), and by then collecting samples of the fluid at di~erG..L times and
25 assayil~g by reverse-phase HPLC to d~Le~ e if the AIV precursor has been
converted to AIV ligand in the fluid. If it has been converted, the second scenario is
operative; if it has not been converted a second series of ~ e-h.ltllLs is cond~lcted In
the second series of e.~GlilllGnls bios-ynthesis of AIV precursors is evaluated (i.e.,
with radiolabeled amino acids) and conversion of the precursor into AIV ligand is
30 e~ ed in pulse-chase type ~ G,hllellLs. If biosynthp~ti~lly radiolabeled AIV
precursor chases into AIV ligand, then the first scenario is operative in the tissue.
Changes in the AIV-Ligand-Receptor System in Response to Neurological Effects:
A represGllLaLi~te e,~l~tl;lllGIlLal protocols for showing çl~ngP~S in the AIV-
ligand-receptor system in re~uonse to neurological and physiological effects is
35 described in the Materials and Methods, below.
W O 94/00492 PC~r/US93/06038
? 13 g 1~5 -72-
AT4 receptors in brain:
A co"l?~ison was made of the binding affinities (under equilibrium binding
conditions) of AT4 receptors in dirrelell~ regions of guinea pig brain. The results of
Scatchard analysis of binding data (con~ucted in the manner described above in
5 Example 1) are sullllllali~ed in Table 12, below.
TABLE 12
Binding of AIV in Regions of Bra na
Brain Region Kf~ (nM) Bm~X (finol/mg)
HSTAb 0.11 +/- 0.051 168 +/- 52.7
Hippocampus 0.10 +/- 0.073 306 +/- 95.1
Cerebellum 0.21 +/- 0.237 232 +/- 93.2
Brain stem 0.09 +/- 0.054 197 +/- 63.9
a.) mean +/- SD; no. of c,.~ ls =4
b.) HSTA= hypoth~l~mlls, th~l~mllc, septum, antereoventral third ventricular area.
10 The Hippocampal AIV Ligand-Receptor System:
Hippocampal AT4 receptors identified in tissues by receptor autoradiography
in Example 2, above, were evaluated further by isolating hippocampal Illt;lll~ es
(i.e., inr.l~ltling hypoth~l~m..s, th~l~m..c, septum, anteroventral third ventricular area,
HSTA, above) and then solubilizing the receptor. (A similar approach may be
15 employed with AT4 receptors in other tissues.) The results presented below show
that the guinea pig hippocampal AT4 receptor binds AIV ligand with a high affinity
(Kd= 1.29+0.18nM, mean +SD, Hill Coeff. = 0.993+0.015) and in a saturable
manner (Bmax=449+62fmol/mg protein). (It is not~wolllly that the guinea pig
hippocampal AT4 receptor binds AIV ligand with applo~ill-a~ely the sarne binding20 affinity as the bovine adrenal AT4 receptor desc,il,ed in F.Y~mple 1, above.) The
density of the AT4 receptors in hippocampàl cells and tissues was considerably higher
than l~l,olled in brain for AII receptors(43,44). In the present studies no AII
receptors could be detected in Hippocampus by binding of 12sI-Sarl,Ile8-AII (data
not shown). The N-terminal structure of the binding AIV ligand is pal~l,olmt in
25 dt;~t;l,l~ling the binding affinity. The C-terminal requilel~lenls seem less ~lh~gt;ll~ as
evidPnr,ed by the binding affinity of AII(3 7~ (Kd = 20.9 + 2. lnM). Neither AII, AIII,
Sarl,Ile8-AII, Dup 753 nor CGP42112A appear to bind intlic~tinp that this binding
site is neither the ATl nor AT2 sites described for AII/AIII. Autoradiographic
analysis of Hippocampus binding COI~lllS the inability of Sarl,Ile8-AII to
30 colllpt;~ ely inhibit for l25I-AIV binding. Conversely AIV was unable to displace
`~0 94/00492 2 1 3 9 1 0 5 PCI/US93/06038
-7~ -
l25I-Sar1,Ile8-AII binding at this site. The finding of AT4 receptors in the
Hippocampus s~1ggestc that AIV ligand-receptor interactions may merli~te unique
central angiotensin-dependent functions inr~ ling memory rnh~nr~em~nt and provide a
link between the Hippocampus and memory.
Saturation isotherms and corresponding Rosenthal plot for l25I-AIV binding
to AT4 receptors in guinea pig hippocampal ~ lllbl~nes show specific binding of
125I-AIV ligand to isolated hippocampal lllt;lllblane AT4 receptors purified from
guinea pig brain. Nonsl)ecirlc binding was defined in the presence of non-labeled
competitor, i.e., lOOnM AIV. The eA~ lenl was carried out 5 times (n=5);
125I-AIV bound saturably and ligand analysis of the binding data indicated the
presence of a single high af~inity binding site (Kd = 1.29 + 0.18nM), Bll~aX = 449 ~ 62
femtomol/mg protein; Hill Coef= .993 + .015; mean + SD.
Structural characteristics of AIV ligands that d~elll~ine binding to the
hippocampal AT4 receptor were det~...;.ed in colll~,~Lilion binding studies, i.e.,
15 similar to those described above in Example 1. The results of these competition
studies are presented in Table 13.
TABLE 13
Colllpe~ilion of 125I-AIV Binding -o Guinea Pig Hippocampus Melllbl~les*
Compound Kj (M)
AII <10~
Am 1.60 + .09 x 10-7
AIV 4.28 + .51 x 10-9
AII(~-7) 2.09 + .45 x 10-8
AII(4 ~ >10
AII(~ ~ >10
Sarl,Ile8-AII >10
Dup 753 >10-4-
CGP42112A >10-4-
*n = 2, mean_ SD; 25mg of total lll~e protein was inr,~lb~ted with 0.6nM
20 125I-AIV plus a variable concellLI~lion of unlabeled angiotensin as a colllpc;~ilor
The results of these studies confirm those pre3ellled above in Example 1 with bovine
adrenal AT4 rece~lol~. The N-terminal of the AIV ligand (e.g., valine) is a major
detellllil~ of binding affinity. In agreement with the saturation isotherm data, AIV
exhibited a high specificity for AIV (Table 13). N-terminal extended peptides
25 inrl~ltling Sarl, Ile8-AII, AII, and Am had significantly reduced affinities for the AT4
WO 94/00492 2~39~QS PCI/US93/0603
receptor while AII(4 8), which has the N-terminal L-Val removed, did not bind. (The
low, but apparenl ability of AIII to bind, may (as above) be due to conversion of AIII
to AIV. The C-terminal specificity of the hippocampal AT4 receptor appears less.Removal of Phe from the C-terminal of AIV ligand diminieh~e, but does not ~ e
5 binding (Table 13), while removal of Phe, Pro, and Ile ~limin~tes binding. As seen in
Table7 neither Dup753 nor CGP42112A colllp~ ely inhibited for the binding of
125I-AIV to the AT4 receptor. In addition, the peptides listed in Table 14, failed to
bind to the HIV rece~lor in guinea pig brain as evidenced by their inability to
significantly alter binding of AIV to receptors in this tissue.
TABLE 14
Nonbinding Peptides (K,l >lO~M)a
~pGlu,Cyt,~l-AVP(4 9)
pmp, O-Me-Tyr2-Argx
Argx -Vasopl essi
Ne:urolellsin
Oxytocin
Substances P
VIP
Neuropeptide Y
Atriopeptin
TRH
Tetradecape~Lide
Met-Enk
Leu-Enk
Gly-Phe-Ala
Bradykinin
a.) Peptides that fail to bind to guinea pig brain tissues as evidenced by Kd >10~M.
This study demonstrates the exiet~once of a unique angiotensin binding site in
guinea pig Hippocampus which is specific for the N-terminal deleted AII hc~eptide,
15 AIV. The location of this specific binding site in the Hippocampus supports the
hyl~olllesis that the AT4 leceplor is the receptor that metli~t~s angiotensin-dependent
cognitive effects in the brain. It is clear from the autoradiographic sections shown in
Figures 6-10, above, that the l25I-AT4 receptor is not restricted to the Hippoc~mpus.
The localization of 125I-AIV binding sites in other brain regions det~iled in Table 15
20 presents an OppOI lullily to expand the realm of angiotensin AIV-related actions.
``'O 94/00492 2 1 3 9 1 0 S PCr/US93/06038
-- -75 -
TABLE 15
Autoradiographic Qu~ntit~tion of AIV Receptors in Brain
AIV Displaced by AII
Regiona AIV Bound (fmol/gm) (fmol/gm)b
Cerebellum 6950.7 +/- 1675.7 6122.2 +/- 1496.3
Hippoc~mpus 5059 +/- 1963.7 4501.8 +/- 223.0
P;lir~llll Cortex2771 +/- 954.7 2605.6 +/- 789.2
Par 1/2 1644.3 +/- 343.5 1710.9 +/- 369.8
Fr 1/2 1551.9 +/- 604.2 1446.2 +/- 453.6
Ca~ te Putamen1755.1 +/- 622.1 1663.3 +/- 654.1
HDB 2082.3 +/- 702.2 1985.6 +/- 621.1
Th~l~ml.s 2077.9 +/- 390.9 1904.4 +/- 646.5
Inferior Colliculus 2432.1 +/- 871.49 2235.0 +/- 663.8
SOL** 2446.3 +/- 881 2053.6 +/- 714.9
ION** 3323.1 +/- 136.3 3267.7 +/- 461.0
a.) n=4 t:AI~Clilll~ S; *n=3 t~pelhll~llls; **n=2 e"~.efil~ s,
b.) displ~cem~nt of 125I-AIV by Sar,Ile-AII
5 Cognitive effects of the AIV ligand-receptor system
Lea~ g. The results presented in Figure 10A show the mean latency
(sec +/- SEM) for indepelldellL groups of rats to re-enter the dark colll~ lllell~ on
Days 2-4 following passive avoidance conditioning on Day 1. One minute prior to the
shock trial on Day 1, lllelllbel~ of each group received aCSF (2ml), or 100pmol in a
10 total volume of 2ml aCSF of AII or AIV. On subsequent test days each animal was
placed back into the lighted CGlllp~h llllelll and latency to enter the dark COlll~ lllent
was measured. Members of the group that received AIV on Day 1 showed
.~iEnific~ntly elevated latency times to re-enter the dark side on Day 2, as colll~ ed
with the mean results from animals in the aCSF and AII test groups. On day 1
15 artificial cel ~rospinal fluid (aCSF), AII, or AIV was ~11mini~tered by
intracel~broventribular (icv) injection into rat brains one minute prior to training.
Training was con~litiQned (as desribed above) to avoid a dark CCilll~ llllenl. On Days
2,3, and 4 of the c,~l~c;lilllc;lll the animals were tested for the latency of time before
they would re-enter the dark colllp~lmelll. F.nll~nc~ of memory retrieval was
20 observed on days 2 and 3 after learning of the reponse (Figure 10B). As can be seen
WO 94/00492 ` PCr/US93/0603
213910~ -76-
from the results presented in Figure 10A the effect diminiched with time after the
learning of the response.
Memory Retrieval: The effects of AIV ligand on learning and memory were
tested in rats by measuring the passive avoidance response, i.e., the mean latency
period (time in seconds) for which the animal avoided a dark COlllp~ l",ent. Training
was conditioned to avoid the dark Colllp~Llllt;ll~ by a(lminictrring a 0.25mA foot
shock over a period of 2 seconds with the door to a lighted COIlllJal Lll1~11L closed. On
day 2 retrieval of the cognitive memory was tested 5 minllteS after
intracel~l~ entribular (icv) injection of AII or AIV. The results presented in
Figure 12A show that AIV has a positive effect on memory retrieval at lnmol and
100pmol, i.e., the AIV test animals avoided the dark side for a longer latency period
than AII-injected ~nim~l~, or CSF-injected control ~nim~lc
Materials and Methods:
Hippocampal AT4 receptor studies:
Hippocampus was from 4-month old male guinea pigs following decapiLaLion.
The tissue was homogenized in 40 volumes of hypotonic buffer co~ g 50mM
Tris, pH7.4 and 5mM EDTA, and spun at 1000g for 10 min. The supernatant was
removed and recel~Llir~lged at 40,000g for 30 min. The pellet was rehomogenized in
hypotonic buffer and lt;cenL~iruged. The 40,000g pellet was homogeni7ed in isotonic
buffer (50mM Tris, pH7.4, 5mM EDTA, 150mM NaCl, 20mM bestatin, 50mM
Plummer's inhibitor, 100mM PMSF, and 0.1% heat treated BSA) and ~ect"L~i~uged a
final time at 40,000 x g. The pellet was resuspended at a concellLl~lion of 2.5mg
protein/ml as determined by the Lowry protein assay. Binding assays, which totaled
250ml, co"lailled 10ml l25I-AIV ligand (sp. act-2176 Ci/mmol), 10ml tissue
homogenate, 10ml unlabeled peptide (if employed), and the rçm~indçr isotonic buffer.
Tnr.ub~tions were carried out for 2 h at 37C. Plelilllill~y c,.~ "ents demonstrated
that ;l.r..lbAlion for 1 h at 37C was n~cç.cc,..y for equilibrium to be reached and that
binding was stable for at least 4 h. At that time less than 10% of the l25I-AIV was
shown to be by HPLC analysis. Saturation isoLl,~""s were developed using 12
concc;nl,~lions of 125I-AIV in duplir~te and inrl-lded total and nonspecir,c binding
[+lOOnM AIVl. Co~.pet;l;on curves were developed using 500,000cpm/tube
(0.6nM) of 125I-AIV and varying llnl~beled peptide (10~M to 10-1lM~ in half-log
dilutions (Dup 753), CGP42112A: 10~Mto 10-11M).
Autoradiographic studies:
Autoradiographic analysis of Hippocampus binding was carried out using
20mM tissue sections mounted on slides. Slices were initially prPincub~ted in isotonic
~vo 94/00492 2 1 3 9 1 0 5 Pcr/us93/06038
-77-
buffer for 30 min at room temperature, then incllbated in labeled ligand (0.6nM) for
2 h, rinsed, dried, and exposed to X-ray film as previously described.
EXAMPLE 8
Isolation~ Purification. and Characterization of the AIV An~iotçn~in~e Enz,vme
AIV An~iotPn.~in~e:
The results of studies conduced in Examples 1-3, above, with bovine adrenal
cortex inrlic~te that a high affinity peptidase (Km=3nM) is present in these
plepal~ions that is capable of catalyzing hydrolysis of AII or AIII to AIV.
Hydrolytic conversion of AII (or AIII) to AIV may result from the action of an AIV-
specific aminoendopeptidase, capable of hydrolyzing an arginyl-valinyl peptide bond
(between positions #2 and #3 in AII; Figure 1) in an angiotensin termed herein
AIV-angiotçn~in~e. (Alternatively, the conversion of AIII to AIV may result fromthe action of no~ ,ec;r.c proteases but these en~.yllles may also cleave all angiotensins
at sites other than the AII R2-V3, and are not termed herein AIV angiot-Pn~in~e.). In
either case, cleavage of the AII Arg2-Val3 peptide bond in AI, AII, or AIII generates
AIV.
Considering the important evolutionary conservation of the AT4 receptor and
AIV ligand, and their most signifir~nt physiological roles, it is most likely that certain
tissues and cells possess a specific AIV angiol~ e el.~yllle(s), i.e., that cleaves
AI, AII, and Am in an çffit iPnt manner to permit re~ t~ble formation of AIV. The
AIV angiotçn~in~e enzyme may be id~ntifietl isolated, and purified using the
experimental approaches described below, in the Materials and Methods, in
colllbination with the assays described in the Examples above (see Example 1). Data
pl~;sellled herein indicate that AII and AIII are excellent and specific inhibitors of
125I AIV formation from 125I-AI.
Materials and Methods:
Ex~o~lilll~llL #1. Formation of AIV Ligand From AIV Precursors in Circulation.
12sI-labeled angiotens~s (107 dpm) - AI, AII, or Am, and tetr~dec~replide
can be injected into the carotid artery of a guinea pig and blood s~mrlçs (50~11) can be
collected at 30-sec or 1-min. intervals from a second cannula in the femoral artery into
100~11 of 20% TCA for 10 min. Samples may be analyzed by reverse-phase HPLC
ili7.ing methods that have been reported previously (47). The data are analyzed to
deterrnine the rate of formation of AIV ligand from potential AIV precursors.
Ex~elilllt;llL #2: Formation of AIV from Precursors via Action of Adrenal Enzymes.
Guinea pig adrenals were excised and homogenized in a Krebs-Ringer buffer
co.... ...l;.il~ g the full comrlçm-Pnt of ions (as above in Example 1). After a low speed
WO 94/00492 PCI /US93/0603~
21391û~
spin at 500g for 10min. to remove whole cells and nuclei, the supellla~ is
centrifuged at 40,000g for 30 min. The supernatant is recellllirllged at lOO,OOOg for
90 min. yielding both a soluble (lOO,OOOg sup~llla~ ) and a microsomal (lOO,OOOgpellet) fraction. The 40,000xg pellet is rehomogenized and fractionated on a
discontinuous sucrose fractionated gradient (0.4M-1.2M sucrose, in 0.2M steps).
The lll~lllbl~1es at the 0.8M to 1.M and lM to 1.2M interfaces can be collected and
cGllll,hled, resuspended in a 10X excess of Krebs buffer. The lllelll~lanes were then
centrifuged at 40,000 x g for 30 min. After a final resuspension in Krebs buffer and
centrifugation at 40,000 x g for 30 min., the final plasma lll~;lllbl~e fraction is ready
for the assay. Soluble, lllelllbl~ e, and microsomal fractions may be in~ub~ted at
various protein concc;llLl~lions and times at 37C with 106 cpm of 125I-AI, AII, AIII,
and tetradecapeptide. Conditions were chosen (as above) to yield less than 10% total
pre~iul~or hydrolysis thus assuring that Colllp~isons of conversion rates is carried out
under initial rate conrii~ionc The reaction is termin~ted with 20% TCA and the
products were evaluated by reverse-phase HPLC. The assay may also be useful for
identifying AIV angiotencin~ce enzyme in clrollla~ographic and other SDS-PAGE
fractions isolated from adrenal, plasma, neural, and other tissues and bodily fluids.
E~ c;lhllt;ll~ #3: Characterization of AIV-Specific Angiotçncin~ce.
If guinea pig adrenal tissue (as expected) possesses an AIV angiot~ncin~c~ the
specificity of the el~yllle(s), its activity on various substrates, and metal ion
requilt;lll~;llL~ can be established by inr.ubating plt;p~uaLions of the icol~ted enzyme
with angiotensins (e.g., in the presence of ;l~ Ol~ of nons~,ec;rlc proteases), and
followed by ~...;n~lion of the hydrolytic products on reverse-phase HPLC. The
sequence of the hydrolytic products may be de~ellllined by automated amino acid
25 sequencing. Tncubation conditions with varying concellLl~;ons of the angiotensin
substrate were used to develop data for double reciprocal plots thus allowing the
affinity of el~yllle(s) for the dirrel~ angiotensins to be detellll.ned. Next,
colllpeL;l;on studies can be undertaken using various angiotensin analogues and
ul~ela~ed peptides in order to establish the structural lt;~lu;lt;lll~ s of the AIV
30 angiotenc;~ce el~yllle(s). Finally, the ability of numerous divalent ions to activate
AIV angiote-ncin~ce can be moni~ored. These c,~elilll~llLs can be carried out with
AIV angiotçncin~ce el~yllles that have been EDTA-~Ll;pped and the EDTA/Me++
removed by dialysis.
-`'094/00492 2139105 PCI/US93/06038
-79-
CITATIONS
1. M.J. Peach, Physio. Rev. 57, 313 (1977)
2. C.I. Johnston, Drugs 39 (Suppl. 1), 21 (1990
3. J.R. Blair-West et al., J. Clin. Endocrinol. Metab. 32, 575 (1971) [see
also #9, #10]
4. J.W. Harding and D. Felix, Brain Res. 410, 130 (1987)
5. D. Regoli, B. Riniker, and H. Brunner, Biochem. Pharmacol. 12, 637-646
(1963) [see also #2, #9, #10]
6. F.M. Bumpus, P.A. Khairallah, K. Arakawo, I.H. Page and R.R. Smeby,
Biochem. Biophys. Acta 46, 38-44 (1961)
7. D. Regoli, W.K. Park and F. Rioux, Pharmacol. Reviews 26, 69-123 (1974)
[see also #6, #10, #3]
8. Bennett, J.P. and Snyder, S.H., Angiotensin II binding to .. A.. ~ n braines, J. Biol. Chem. 251, 7423-7430, (1976). Colossman, H.,
Bankal A., and Catt K.J. Plopcllies of angiotensin II receptors in the bovine
and rat adrenal cortex. J. BioL Chem. 249, 825-834 (1974)
9. Fitsimons, J.T. J. Physiol Lond. 214, 295-303 (1971).
10. Tonnaer, J.A., Weigant, V.M., Degong, W. and DeWeid, D., Brain Res. 236,
417-428 (1982).
11. Siemens, I.R., Swanson, O.N., Flaharty, S.J., and Harding, J.W., J.
Neurochem 57, 690-700 (1991)
12. T. Kono, F. Ikeda, F. Oseko, Y. Ohmori, R. Nakano, H. Muranaka, A.
T~ni~1chi H. Imura, M.C. Khosla and F.M. Bumpus, Acta endocr. 99,
577-584 (1982).
13. Kono, T. et al., Acta Endocr. 109, 249-253 (1985)
14. R.L. Haberl, P.J. Decker and K.M. Ejnhaupl, Circ. Res. 68, 1621-1627
(1991)
15. J.J. Brazko, J. Wlasienko, W. Koziolkiewicz, A. Janecka and K. Wisniewski,
Brain Res. 542, 49-54 (1991)
16. J.J. Brazko, G. Kuply~,t;w~ki, B. Witczuk and K. Wisniewski, Neurosci 27,
777-783 (1988)
17. J.J. Brazko, K. Wisniewski, G. Kllply~c;wski and B. Witczuk, Beh~v. Brain
Res. 25, 195-203 (1987)
18. P.F. Semple, A.S. Boyd, P.M. Dawes and J.J. Morton, Circ. Res. 39,
671-678 (1976).
19. B. Blumberg, A.L., et al., (1977) Circ. Res 41, 154-158 (1977).
WO 94/00492 39~5 -80- PCI`/US93/0603
20. J.P. Bennett and S.H. Snyder, Eur. J. Pharmacol. 67, 11 (1980).
21. Kumar, S. Keegen, A., Erroi, A., West, D. Kumar P., and Gaffney, J., Prog.
App. Microcirc. 4, 54-75 (1984).
22. Fernandez, L.A., Twickler, J. and Mead, A. Lab. Clin Med. 105, 141-145
(1985)
23. Patel, J.W. et al., Amer. J. Physiol. 256, 987-993 (1989).
24. King, S.J., Beck, J.C., Harding, J.W., and Hosick, H.L., Abstract Amer. Soc.
Cell Biol. (1986)
25. Baker, K.M. and Aceto, J.F., Am J. Physiol. 259, H610-H618 (1990).
10 26. Baker, K.M., Chernim, M.I., Wixson, S.K., and Aceto, J.F., Am. J. Physiol.,
259, H324-H332 (1990).
27. Y~m~lr.hi, T., Naito, Z., Stoner, G.D., Franco-Saanz, R. and Mulrow, P.J.,
Hypertension 16, 635-641 (1990).
28. Carpenter, G., King, L. Jr., and Cohen, S., J. Biol. Chem. 254, 4884-4891
(1979).
29. Munson, P.J., and Rodbard, D., Anal. Biochem. 107, 220-239 (1980).
30. FrPi~sm-lth~ M., Casey, P.J., and Gilman, A.G. FASEB J. 3, 2125-2131
(1989).
31. Brown, A.M. and Birnbauer, L., Am. J. Physiol. 254, H401-H410 (1988).
32. Schulz, S., Chinkers, M., and Garbers, D.L., FASEB J. 3, 2026-2035 (1989).
33. Nishibe, S. Wahl, M.I., Hernandez-Sotomayor, S.M.T., Tonks, N.K.,
Rhee, S.G., and C~l,t;.,ler, G., Science 250, 1253-1256 (1990).
34. P~n-liPII~ A., Bequinot, L., Vin~.çn~ini, L.M., and Meldotesi, J., TIPS 10,
411-414 (1989).
35. Cohen, S., Carpenter, G., and King, L. Jr., J. Biol. Chem., 255, 4834-4842
(1980).
36. Pang, T.P., Wang, J.K.T., Valtork, F., RP.ntPn~ti F., and Coreengard, P.,
PNAS 85, 762-766 (1988)
37. Dean, N.M. and Moyer, J.D., J. Biol. Chem. 250, 493-500 (1988).
38. Wright, J.W., Jensen, L.L., Roberts, K.A., Sardinia, M.F., and Harding, J.W,
Am. J. P~ysiol. 257, R1551-R1557 (1989).
39. Gill, G.N., Ill, C.R., and Simonian, M.H., Proc. Nat. Aca~ Sci. 74,
5569-5573 (1977).
40. Livett, B.G., Mit~`hpllhill K.I., and Dean, D.M., "In vitro methods for studying secretion", 171-176 (1987b).
2139105
'`'O 94/00492 , , PCr/US93/06038
-81-
41. Livett, B.G., Marley, P.D., ~it~ llhill, K.I., Wan, D.C.C., and White, T.D.,
"In vitro methods for studying secretion", 177-204 (1987a).
42. Aceto, J.F. and Baker, K.H., Am J. Physiol. 258, H806-H813 (1990).
43. Men~lel~ohn, F.A.D. et al., Proc. Natl. Acad. Sci. USA 81, 1575-1579
(1984).
44. Speth, R.C. et al. In: J.W. Harding et al. (Eds) "Angiotensin and Blood
Pressure Re~ tion", Acad. Press, S.D., CA. p. 1-3 (1988).
45. Paul, A.K. Marada, R.B., Jaiswal, R.K., and Sharma, R.K., Science 235,
1224-1226 (1987).
46. Glaring et al. 1989
47. Abhold, R.H. and Harding, J.W., J Pharmacol Enp. Ther. 245, 171-177
(1988).
WO 94/00492 -82- PCT/US93/06038
SEQUENCE LISTING
(l)GENERAL INFORMATION:
(i) APPLICANT:Harding, J.W.
(ii)TITLE OF INVENTION:"~n~int~ncin IV Peptides and Receptor"
(iii)NUMBER OF SEQUENCES:6
(iv)CORRESPONDENCE ADDRESS:
(A)ADDRESSEE:Ch. .~ .., O'Connor, Johnson and Kinrln.o.cc
(B)STREET:2800 Pacific First Center, 1420 Fifth Avenue
(C)CITY: Seattle
(D)STATE W~
(E)COUNTRY:USA
(F)ZIP:98101-2347
(v)COMPUTER READABLE FORM:
(A)MEDIUM mE:Diskette-5.25 inch, 1.2Mb storage
1 5 (B)COMPUTER:IBM PC/386 C~
(C)OPERATING SYSTEM:MS-DOS 4.01
(D)SOFTWARE:Word for Windows-t
(vi)CURRENT APPLICATION DATA:
(A)APPLICATION NUMBER:
(B)FLING DATE:
(C)CLASSIFICATION:
(vii)PRIOR APPLICATION DATA:
(A)APPLICATION NUMBER:none
(B)FILING DATE:none
(viii)ATTORNEY/AGENT INFORMATION:
(A)NAME:S~ln~lCn n,John,S.
(B)REGISTRATION NUMBER:34,446
(C)REFERENCE/DOCKET NUMBER:WSUR-1-6263
(ix)TELECOMMUNICATION INFORMATION
(A)TELEPHONE:1-206-682-8100, 1-206-224-0727 (direct)
(B)TELEFAX: 1-206-224-0779
(C)TELEX:4938023
(2)INFORMATION FOR SEQ ID NO: 1:
(i)SEQUENCE CHARACTERISTICS:
(A)LENGTH:14 amino acids
""O 94/00492 2 1 3 9 1 0 5 PCr/US93/06038
-83 -
(13)TYPE:amino acid
(C)STRANDEDNESS:single
(D)TOPOLOGY:linear
(ii)MOLECULE TYPE:peptide
- 5 (A)DESCRlPTION ~n~;ot~ ~r,~
(ix)SEQUENCE DESCRIPTION: SEQ ID NO: 1:
Asp Arg Val Tyr Ile His Pro Phe His Leu Val Ile His Ser
(3)INFORMATION FOR SEQ ID NO:2:
(i)SEQUENCE CHARACTERISTICS:
(A)LENGTH: 10 amino acids
(B)TYPE:amino acid
(C)STRANDEDNESS:single
1 5 (D)TOPOLOGY:linear
(ii)MOLECULE TYPF pepti-l~
(A)DESCRIPTION:~ngi~t.oncin I
(ix)SEQUENCE DESCRIPTION: SEQ ID NO:2:
Asp Arg Val Tyr Ile His Pro Phe His Leu
1 10
(4)INFORMATION FOR SEQ ID NO:3:
(i)SEQUENCE CHARACTERISTICS:
(A)LENGTH:9 amino acids
(B)mE:amino acid
(C)STRANDEDNESS:single
(D)TOPOLOGY:linear
(ii)MOLECULE TYPF ~pti.l.~
(A)DESCRIPTION: [des-Asp] ~- jr: I
(ix)SEQUENCE DESCRIPTION: SEQ ID NO:3:
Arg Val Tyr Ile His Pro Phe His Leu
(5)1NFORMATION FOR SEQ ID NO:4:
(i)SEQUENCE CHARACTERISTICS:
(A)LENGTH:8 amino acids
WO 94/00492 - PCT/US93/0603~
213910S -84-
(B)TYPE:amino acid
(C)STRANDEDNESS:single
(D)TOPOLOGY:linear
(ii)MOLECULE TYPE:peptide
(A)DESCRI~ION: An~
(ix)SEQUENCE DESCRIPTION: SEQ ID NO:4:
Asp Arg Val Tyr Ile His Pro Phe
(6)rNFORMATION FOR SEQ ID NO:5:
(i)SEQUENCE CHARACTERISTICS:
(A)LENGTH:7 amino acids
(B)TYPE:amino acid
(C)STRANDEDNESS:single
1 5 (D)TOPOLOGY:linear
(ii)MOLECULE TYPE:peptide
(A)DESCRI~I ION:An~int~ncin nI
(ix)SEQUENCE DESCRIPTION: SEQ ID NO:5:
Arg Val Tyr Ile His Pro Phe
1 7
(7)INFORMATION FOR SEQ ID NO:6:
(i)SEQUENCE CHARACTERISTICS:
(A)LENGTH:6 amino acids
(B)TYPE:amino acid
(C)STRANDEDNESS:single
(D)TOPOLOGY:linear
(ii)MOLECULE TYPF.-p~tid~
(A)DESCRIPIlON:A~ IV
(ix)SEQUENCE DESCRIPTION: SEQ ID NO:6:
Val Tyr Ile His Pro Phe