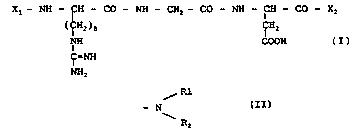Note: Descriptions are shown in the official language in which they were submitted.
- ~l3942g
WO 94/01456 - 1 - PCT/FR93/00670
OLIGOPEPTIDE COLLAGEN DERIVATIVES AND THEIR USE IN
SECONDARY CATAR~CT PREVENTION
The present invention aims to provide new means
for secondary cataract prevention.
A secondary cataract i~ the opacifying which
occurs in a large number of cases after extracapsular
extraction of cataracted lenses.
To prevent secondary cataract, it has already
been proposed in EP-A-0 299 467 to instil into the
anterior or posterior chamber of the eye, during or after
extraction of the lens, a conjugate of an antibody, such
as an anticollagen, and a cytotoxin capable of inhibiting
the growth of epithelial cells.
It has also been proposed, in WO-A- 89/12093, to
prevent secondary cataract by the administration of
specific monoclonal antibodies which are possibly conju-
gated with cytotoxins.
The present invention aims to provide new means
for secondary cataract prevention.
The present invention relates thus to a
process for secondary cataract prevention, which consists
in administering a compound of formula:
Xl- NH - CH - CO - N~ - C~2- CO - NH - CH - CO - X2
(7H~)3 C~H2
NH COOH (I)
~-N~
NH,
in which:
Xl represents a hydrogen atom, a Gly residue, an
N-protecting group, in particular a group
- COR3 or - CO - OR3
R3 being a C,-Cl8 alkyl group, a C3-C8 cycloalkyl group, an
aryl and in particular a phenyl group, an aryl, in
particular a phenyl, group bearing one or more
substituents selected from a Cl-C6 alkyl group, a hydroxy
group, a Cl-C6 alkoxy group, a mono- or polyhydroxylated
Cl-C6 alkyl group, a mono- or poly(Cl-C6 alkoxy) (Cl-C6
2139~29
.
-- 2 --
alkyl) group, an amino group, a mono- or di(Cl-C6
alkyl)amino group, an acetamido group and a sulphonamido
group, or a (Cl-C4 alkyl)aryl group, in particular a (Cl-C~
alkyl)phenyl group, and
X2 represents a hydroxyl group, a Ser residue, a Ser-Pro
or Ser-Pro-Cys group,
or a group of formula:
~ R~
- N
~R2
Rl and R2 representing, independently of each other, a
C1-Cl8 alkyl group, a C3-C8 cycloalkyl group, a (C1-C4
alkyl)aryl group, in particular a ( Cl-C4 alkyl)phenyl
group, or
Rl and R2 form, together and with the nitrogen atom to
which they are attached, a pyrrolidino~ piperidino or
piperazinyl group which is optionally substituted with a
C1-C4 alkyl radical or a 4-hydroxypiperidino group,
or one of its addition salts with a pharmaceutically
acceptable acid.
The present invention relates also to
ophthalmic compositions cont~in;ng a compound of formula
I as defined above or one of its addition salts with a
pharmaceutically acceptable acid, dissolved in an oph-
thalmic solvent.
The present invention relates also to
implants and ansae in which are incorporated, or on which
are deposited, a compound of formula I as defined above
or one of its addition salts with a pharmaceutically
acceptable acid.
The present invention relates also to
compounds for the implementation of the invention. These
compounds are compounds of formula I in which:
X1 represents an N-protecting group as defined above and
REPLACEMENT SHEET
2139~29
-- 3 --
X2 represents a group of formula
- N R2
as defined above,
and their addition salts with pharmaceutically acceptable
acids.
"Addition salts with pharmaceutically acceptable
acids" is understood to refer to the salts which give the
biological properties of the free bases, without having
any undesirable effect. These salts may in particular be
those formed with inorganic acids, such as hydrochloric
acid, hydrobromic acid, sulphuric acid, nitric acid and
phosphoric acid; acidic metal salts, such as disodium
orthophosphate and monopotasgium sulphate, and organic
acids, such as formic acid, acetic acid, propionic acid,
glycolic acid, oxalic acid, fumaric acid, lactic acid,
succinic acid, tartaric acid and pamoic acid.
The compounds of formula I may be prepared in a
conventional manner by peptide synthesis in a liquid or
solid phase, by successive couplings of the various
amino acid residues to be incorporated, from the
C-terminal end to the N-terminal end, and the N-terminal
ends and reactive side chains of which residues are
blocked beforehand with groups such as those mentioned
below:
- N-terminal end blocked by BOC, Fmoc and Acyl groups
- C-terminal end blocked by ester and amide groups
- side ends
of aspartyl: blocked by an ester group such as a benzyl
ester,
of arginyl: blocked by an H' (protonation) or tosyl
group.
It is also possible to use various coupling
methods:
1) coupling of the residues by carbodiimides
(example: DCC, EDC) with or without catalysis (HOBT), or
REPLACEMENT SHEET
2139429
-- 4 --
other coupling agent (EEDQ);
2) use of symmetrical anhydrides of the amino
acids used;
3) use of activated esters of the amino acids
employed (example: HOBT, ester, p-nitrophenyl ester).
The liquid phase synthesis of a compound of
formula I may be carried out in six steps, eight steps if
the final amino acid, N-substituted arginine, is not
obtained commercially.
The solvents employed are most often dimethyl-
formamide (DMF), tetrahydrofuran (THF) and 1,4-dioxane;
the coupling reagents dicyclohexylcarho~iimide (DCC) and
the catalyst hydroxybenzotriazole (HOBT).
An example of a synthesis scheme (Scheme I) in
which GP" GP2, GP3 and GP4 denote N-protecting groups will
be given below.
2139~29
-- 5 --
- SCHEME I
H ~1--N H C R
GP,--N~ f;2 GP,--N~ , '
2~ o=~ `F'2
O - GP2 Coupling agent o -GP2
Catalyst
Solvent
Step A Deprotection 1
~ Step B
H ~ N $1 , ~ GP~--N ~`OH R
o - GP2 Coupling agent o - GP2
Catalyst
Solvent
Step C
Deprotection 4
Step D ~ O
GP 3--N~ -
NH
H R ~(~N--~
H2~ H ~ $1 N~
Coupling agent
Catalyst NH O - G~2
Solvent ~zN
Step E
Deprotection 2
Step F
o ~H O ~-- R2
o-t~
Ni(
H2N =~
NH2 ( Ia )
~139429
-
-- 6 --
The first step (step A) consists firstly in
preparing the Ca-amide analogues of an Na- and
C~-protected aspartic acid by reacting with the latter an
amine HNR,R2 in the presence of a coupling agent and a
catalyst, in a suitable solvent. After purification and
control of its purity, the product is deprotected (step
B) by a deprotecting reagent which is characteristic for
the protecting group GP1.
Once purified, this amino acid derivative is
converted into a dipeptide (step C) by reaction with a
glycine residue which is protected on its amino group
(protecting group GP4) in the presence of a coupling
reagent and a catalyst, in a suitable solvent. The
addition of one equivalent of triethylamine (or of a
sterically hindered base) is necessary if the aspartyl
derivative is obtained in protonated form. The protected
dipeptide thus obtained is purified.
Step D is a deprotection step specific for the
protecting group GP4. The dipeptide thus obtained, after
purification, is reacted with an Na- and NG(H+)-protected
arginine residue (step E) in the presence of a coupling
agent and a catalyst, in a suitable solvent. The addition
of one equivalent of triethylamine is carried out when
the deprotected dipeptide is obtained in protonated form.
The tripeptide product obtained is purified and then
treated with a deprotecting reagent for the protecting
group GP2, thus liberating the carboxylate function of
the aspartic residue (step F) in order to give the end
tripeptide of formula I.
The examples which follow illustrate the prepara-
tion of the compounds of formula I.
In these examples, the characterization methods
employed are as follows:
1) Thin-layer chromatography:
- Merck 5719 silica support with UV and I2 detection
- migration solvents:
- CH2Cl2:CH30H (16/1 to 16/4) for the synthesis inter-
mediates
- CH30H:AcOH for the end products (Rf between 0.3 and
- 2139429
._
-- 7 --
0.5).
2) lH NMR:
- Brucker 250 MHz.
Control of the purity to within 1-2% by spectro-
scopic study of all the intermediates and finishedproducts.
3) Nass spectrG~
Each end tripeptide is studied in Positive Fast
Atom Bombardment (FAB+) mode in a glycerol or thio-
glycerol matrix.
EXAMPLE 1
Preparation of [Na-acetyl-NG(H~)-arginyl]-glycyl-
[C~(H)-C~-benzyl]-aspartamide chloride (Xl = acetyl,
X2
a) Preparation of tN-(t-butoxycarbonyl)-C~-
benzylester]-benzyl a~partamide.
[Na-(t-Boc)-C~-(benzylester)]aspartic acid (8 g;
0.025 mol) and hydroxybenzotriazole (3.37 g; 0.025 mol)
are dissolved, at 0-5C, in 160 ml of THF. A solution of
DCC (5.15 g; 0.025 mol) in a minimum of THF is added to
this mixture and the solution is left stirring. At the
start of formation of the DCU precipitate, benzylamine
(2.68 g; 0.025 mol) is added. The mixture is then allowed
to warm to room temperature with stirring for 24 hours.
The DCU is finally filtered off and the THF is evaporated
off. The residue is taken up in an excess of ethyl ether
and the insoluble material is filtered off. The ether is
evaporated off and the residue is chromatographed (SiO2;
CH2Cl2:CH3OH 90/10) to give the desired amide (9 g : 87%).
1H NMR:(CDCl3). 1.42 (sing; 9H; BOC); 2.93 (resolved AB;
2H; CH2 asp.); 4.39-4.70 (resolved q. + unresolved multi-
plet; 3H : H asp + CH2 benzylamide); 5.13 (s; 2H; CH2
benzyl ester); 5.69 (d broad; lH: NH carbamate); 6.80 (d
broad; lH; NH benzylamide); 7.19-7.5 (unresolved
multiplet; 10 H; H ar.) TLC:SiO2; CH2Cl2:CH3OH 90/10;
Rf = 0.8-0.9;
~39429
.
-- 8 --
b) Preparation of tC~-benzyl ester-Ca-benzyl]-
aspartamide hydrochloride.
The above aspartic derivative (9 g; 0.0218 mol)
is dissolved in ethyl acetate (200 ml) at 0-5C. gaseous
5 HCl is subsequently passed through the solution at this
temperature and it is left stirring for 4 hours. The
precipitate formed is then filtered off and dried, to
give the desired hydrochloride in the form of a white
powder (7 g; 0.02 mol; 92%).
lH NM~:(DMSO-d6). 2.98 (t; 2H; CH2 asp.); 4.12 (t; lH; H
asp.); 4.30 (d; 2H; CH2 benzylamide); 5.14 (s; 2H; CH2
benzyl ester); 7.10-7.15 (unresolved multiplet; 10H; H
ar.); 8.28 (s. broad; 3H; H ammonium); 8.95 (t; lH; H
amide).
TLC:SiO2; does not migrate.
c) Pr~paration of [Na-(t-butyloxyr~rhQr~yl)
glycyl]-[C~-benzyl ester-Ca-benzyl]-aspart~mide
N-(t-Boc)-glycine (3.5 g; 0.02 mol), HOBT
(2.7 g; 0.02 mol) and then DCC (4.12 g; 0.02 mol) are
dissolved, at 0-5C, in THF (40 ml). The mixture is
stirred for 20 minutes at this temperature, followed by
addition of a mixture of the product obtained in b) and
triethylamine (equimolar; 7 g of product obtained in b)
(0.02 mol); 2 g of NEt3 (0.02 mol)) in the minimum amount
of THF (# 10 ml).
The mixture is stirred for 24 hours (TLC monitor-
ing) and the DCU formed is then filtered off. The THF is
evaporated off and the residue is taken up in ethyl
ether. The precipitate which again forms (DCU) is removed
by filtration and the solvent is evaporated off. This
residue is dissolved in CH2C12, filtered again and chroma-
tographed (SiO2; CH2C12:CH3OH 9/1) to give the desired
product (8 g; 0.017 mol; 8596) in the form of an oil which
crystallizes by trituration with petroleum ether.
lH NMR;(CDC13).1.39 (s.; 9H; BOC); 2.94 (resolved disym-
metric AB; 2H; CH2 asp.); 3.72 (t; 2H; CH2 glycyl); 4.42
(resolved d.; 2H; CH2 benzylamide); 4.88 (m; lH; H asp);
5.11 (d; 3H; CH2 benzyl ester + NH BOC); 7.0 - 7.50
(unresolved multiplet; 12 H; H ar + 2 NH).
2139429
_
_ 9 _
- TLC: SiO2; CH2Cl2:CH3OH 9S/5; Rf = 0.5-0.6.
d) Preparation of (glycyl)-tC~-benzyl ester-Ca-
benzyl]-aspartamide ~ oehloride.
The dipeptide derivative I,c (8 g; 0.017 mol) is
dissolved, at 0C, in ethyl acetate (300 ml). The
solution is treated at this temperature by sparging with
gaseous HCl. The deprotection reaction is monitored by
TLC over 2 hours. After degassing using nitrogen, the
ethyl acetate is evaporated to half its volume, under
vacuum at room temperature. The crystals formed are
recovered by filtration, washed with ethyl ether and
dried under vacuum (6.7 g; 0.0165 mol; 97%).
H NMR:(DMSO-d6). 2.80 (resolved AB; 2H; CH2 asp.); 3.61
(q; 2H; CH2 glycyl); 4.27 (d; 2H; CH2 benzylamide); 4.76
(q; lH; H asp.); 5.10 (s; 2~; CH2 benzyl ester); 7.0-7.50
(unresolved multiplet; 10H; H ar); 8.20 (s broad; 3H;
ammonium); 8.70 (t; lH; NH benzylamide); 8.89 (d; lH; NH
amide).
TLC:SiO2; does not migrate.
e) Preparation of tNa-acetyl-NG(E+)-arginyl]-
glycyl-tC~-benzyl e~ter-Ca-benzyl]-a~partamide chloride
Finely pulverized Na-acetylarginine dihydrate
(1.86 g; 0.00738 mol) and the compound obtained in d)
(3 g; 0.00738 mol) are dissolved in DMF (200 ml) at room
temperature. After stirring for 20 minutes, DCC (1.52 g;
0.00738 mol) and HOBT (0.998 g; 0.00738 mol) are added.
The mixture is stirred for 3 days.
The insoluble material is filtered off and the
DMF is evaporated off under vacuum. The residue is taken
up in the minimum amount of dichloromethane and the
insoluble material is filtered off. The solvent is
evaporated off and the residue is triturated with ethyl
acetate, and then with ethyl ether. The solid thus
obtained is filtered off and dried to give the desired
compound (3 g; 0.005 mol; 67%).
lH NMR:(DMSO-d6). 1.30-1.80 (unresolved multiplet; 4H;
2CH2 Arg.); 1.85 (s; CH3 acetyl); 2.79 (resolved AB; 2H;
CH2 asp.); 3.08 (d. broad; 2H; CH2 Arg.); 3.75 (resolved
q.; 2H; CH2 glycyl); 4.10-4.40 (d+m; 3H; CH2 benzylamide
2139429
-- 10 --
+ H arg.); 4.72 (m; lH; H asp.); 5.08 (s, 2H; CH2 benzyl
ester); 6.7-7.5 (m; 15 H; H ar. + guanidinium); 7.76
(broad t; lH; NH); 8.80-8.70 (m; 3H; 3 NH).
TLC:SiO2;CH2Cl2:CH3OH 80/20; Rf = 0.1
f) Preparation of [N~-acetyl-NG(E~)-arginyll-
glycyl-[C~(~)-Ca-benzyl]-aspartamide chloride
The protected tripeptide obtained in e) (3 g;
0.005 mol) is reduced in methanol (100 ml) by hydrogen in
the presence of 5% Pd/charcoal catalyst (0.3 g) at room
temperature overnight. The mixture is then filtered over
Celite and the methanol is evaporated off to give the
desired compound in the form of a hydroscopic white
powder (2.3 g; 0.0045 mol; 90%).
lH NMR:(DMSO-d6). 1.30-1.80 (unresolved multiplet; 4H;
2CH2 arg.); 1.86 (s; 3H; CH3 acetyl); 2.65 (resolved AB;
2H; CH2 Asp.); 3.09 (d + m; 2H; CH2 Arg.); 3.72 (resolved
q; 2H; CH2 glycyl); 4.10-4.50 (d+m; 3H; CH2 benzylamlde +
H arg.); 4.61 (q; lH; H Asp.); 7.24 (unresolved
multiplet; 10 H; H ar. + guanidinium); 7.80 (unresolved
multiplet; lH; NH); 8.10-8.60 (unresolved multiplet; 3H;
3 NH); 12.38 (s broad; lH; COOH).
FAB+: for [C2,H32N,O6]+, Cl-: [M]+ = 478;
TLC:SiO2; CH30H; AcOH 99.75/0.25 Rf = 0.5-0.6.
BXAMPL~ 2
Preparation of tNa-acetyl-Nc(E~)-arginyl]-glycyl-
[C~-(H)-Ca-isopropyl]aspartamide chloride
(Xl = acetyl, X2 = -NH-C~( C~3 ) 2 )
This i8 obtained as in Example 1. Its character-
istics are as follows:
lH NMR
1.00-1.20 (2 doublets, 2 CH3 isopropyl); 1.45-1.80 (unre-
solved multiplet, 2 CH2 arg.); 1.90 (s, CH3 acetyl); 2.60
(resolved AB, CH2 Asp.); 3.10 (poorly defined q, CH2
Arg.); 3.70 (q, CH2 Gly.); 3.85 (m, H isopropyl); 4.20
(poorly defined q, CH Arg.); 4.50 (poorly defined q, CH
Asp); 6.80-7.60 (unresolved multiplet + d, 4H
gllAn; ~; ne+N-H); 7.80 (poorly defined t, N-H); 8.05 (d, N-
H); 8.25: (d,N-H); 8.35: (t, N-H).
TLC: SiO2 , MeOH-AcOH (20:0.05); Rf=0.45.
2139429
-- 11 --
FAB+: for [Cl7H32N,O6], Cl: [M] 430
EX~aMPLE 3
Preparation of tNa-acetyl-NG(H~)-arginyl]-glycyl-
tC~ )-Ca-cyclohexyl)a~partamide chloride
S (X, = acetyl, = X2 = -NR-O )
This is obtained as in Example 1. Its character-
istics are as follows:
H NMR
1.00-1.80 (unresolved multiplet, 5 CH2 cyclohexyl + 2 CH2
Arg.); 1.88 (s, CH3 acetyl); 2.50 (unresolved multiplet,
DMSO + CH2 Asp.); 3.10 (poorly defined q, CH2 Arg.); 3.25-
3.55 (s + unresolved multiplet, H2O + H cyclohexyl); 3.70
(d, CH2 Gly.); 4.00-4.30 (m, CH Arg.); 4.50 (m, CH Asp.);
6.80-7.50 (unresolved multiplet + d, N-H+4H guanidine);
8.00-8.20 (2 doublets, 2N-H); 8.40 (t, N-H).
TLC: SiO2, MeOH:AcOH (20:0.05); Rf = 0.5
FAB+: for [C20H36N,O6]+, CL-[M]+ = 470.
EXANPLl~ 4
Preparation of tNa-acetyl-NG( ~)-arginyl]-glycyl-
tc~-(El)-Ca-diethyl]aspartamide chloride
(X, = acetyl, X2 = -N~_ )
This is obtained as in Example 1. Its character-
istics are as follows:
lH NMR
0.99 (t, CH3 ethyl); 1.14 (t, CH3 ethyl); 1.35-1.80
(unresolved multiplet, 2CH2Arg.); 1.89 (s, CH3, acetyl);
2.22-2.80 (resolved disymmetric AB centred on 2.50, CH2
Asp.); 2.95-3.50 (unresolved multiplet, H2O + 2 CH2 ethyl
+ CH2 Arg.); 3.69 (d, CH2 Gly.); 4.25 (unresolved
multiplet, CH Arg.); 4.95 (q, CH Asp.); 6.70-7.60 (unre-
solved multiplet broad, 4H guanidine); 7.95 (s broad, H+
guanidinium); 8.10 (d, N-H Asp. or Arg.); 8.22 (t, N-H
Gly); 8.32 (d, N-H Arg. or Asp.).
TLC: SiO2, MeOH:AcOH (20:0.05); Rf = 0.3.
FAB for [Cl8H34N7O6], Cl-:tM]+ = 444 + dimer at 887 (5%).
2139429
.
-- 12 --
- EXA~D?L13 5
Preparation of [N,-benzoyl-NG(EI~)-arginyl]-glycyl-
[C~-(H)-Ca-benzyl]aspartamide chloride
(Xl = acetyl, X2 = -NH- ~\
5 1H NMR
1.40-2.00 (unresolved multiplet, 2 CH2 Arg.); 2.79 (unre-
solved multiplet, CH2 Asp.); 3.12 (q, CH2 Arg.); 3.79 (m,
CH2Gly.); 4.29 (d, CH2phenyl); 4.44 (m, CH, Arg.); 4.60
(q, CH, Asp.); 6.75-8.12 (unresolved multiplet, 10 H ar.
+ 5 H gll~n;rlinium); 8.29 (unresolved multiplet, 2N-H);
8.40 (poorly defined t, N-H Gly); 8.64 (d, N,H).
TLC: SiO2, MeC)H:AcOH (20:0.05); Rf = 0.75.
FAB+: for [C26H34N,O6] , Cl tM] 540
EXA~LE 6
Preparation of [N-Boc-Nc( ~ )-arginyl]-glycyl-tC-
(EI)-C-benzyl]aspartamide chloride
(Xl = Boc, Xz = -NH-CH2~))
H NMR
1.20-1.80 (unresolved multiplet + s, 3 CH3Boc + 2CH2
Arg.); 2.55-3.20 (unresolved multiplet, CH2 Arg. + CH2
Asp.); 3.75 (poorly defined d, CH2 Gly.); 3.92 (m, CH
Arg.); 4.20-4.70 (d+m, CH2-phenyl + CH Asp.); 6.70-7.60
(unresolved multiplet, 5H Ar. + N-H Boc+4H guanidine);
7.70 (poorly defined t, N-H); 8.05-8.40 (unresolved
multiplet, 2N-H).
TLC:S io2, MeOH:AcOH (20:0.05); Rf=0.8 (decomposes).
FAB+: for [C24H38N7O,]+, Cl : [M~ = 436.
Test results demonstrating the value of the
compounds of formula I in secondary cataract prevention
will be given ~elow.
Test of inhibition of the adhesion of epithelial
cells to lens capsules
a) Preparation of bovine lens capsules
The lens is extracted from the eye of an ox via
the posterior face. The lens is stored in calcium- and
magnesium-free phosphate buffer (Phosphate Buffer
Saline). The lenses are frozen at -20C in this step. The
2~39429
anterior capsule is cut up using small scissors and
deposited at the bottom of a culture dish. Incubation
with 1% Triton X100 for 30 minutes enables the lens
epithelial cells adhering to the capsule to be detached.
The capsules are subsequently rinsed three times with
PBS. Before use, each capsule is studied under an optical
microscope in order to verify the total absence of cells
from the capsule.
b) TAhellin~ of bovine lens epi~heli~l cells with
3~-leucine
The solution of D.L[(4,5)-3H]-leucine is obtained
from Amersham, as an aqueous 2% ethanol solution. The
specific activity is 25-50 Ci/mmol.
The solution of 3H-leucine used is diluted immed-
iately before use in DMEM culture medium (Dulbeccomodified essential medium).
For the cell labelling, 40 ~Ci are used per 106
cells. Incubation for 18 to 20 hours at 37C in an oven
with CO2 allows the leucine to be incorporated into the
cellular proteins. After 3 washes with DMEM, the cells
are detached with 0.05% Trypsin-EDTA, and the cells in
suspension in the DMEM are stored on a waterbath at 37C
with stirring for 1 hour.
This procedure allows cells to be labelled with
a specific activity of 2-10 dpm/cell.
c) Adhesion of bovine lens epi~h~ l cells to
capsules
The capsules are cut up with a No. 9 diameter
(18 mm) cork borer and spread at the bottom of a well of
a multiwell culture dish, cont~;n;ng 24 wells of 20 mm
diameter each. A rubber O-ring seal i8 affixed to the
bottom of the well in order to correctly attach the
capsule. the capsules are incubated in PBS+ (10 mM CaCl2,
5 mM MgCl2) until their use.
Adhesion medium = DMEM culture medium + 2 mg/ml
bovine serum albumin (DMEM + 0.2% BSA).
Concentrated solutions of test compounds of
formula I are prepared immediately before use, they are
diluted to the desired concentration in the adhesion
21~9~29
- 14 -
- medium.
150 ~l of total volume are deposited per well,
~ they contain 10,000 cells in the presence of increasing
concentrations of test compounds.
After incubation for 1 h at 37C in the oven with
CO2, the wells are washed three times with calcium,
magnesium PBS. Solubilization overnight with 1.5 ml of
0.2 N NaOH and sodium dodecyl sulphate of 1% sodium
enables counting on a scintillation counter.
d) Results
For each test compound, adhesion curves as a
function of the concentration of compound are plotted.
The dose at which a 50% inhibition of the adhesion (IC50)
is observed is calculated.
The following table summarizes the results:
COMPOUND IC 50
Ex. 1 1 mM
Ex. 2 5 mM
Ex. 3 1 mM
20 Ex. 4 10 mM
Ex. 6 3 mM
Arg-Gly-Asp 10 mM
Arg-Gly-Asp-Ser 2 mM
Arg-Gly-Asp-Ser-Pro 1 mM
25Gly-Arg-Gly-Asp-Ser-Pro-Cys l mM
The compounds of formula I may be A~i ni stered in
particular in the form:
- of sterile solutions packaged in syringes, injected
into the open eye during surgical intervention,
- lens implants, into the matrix of which these compounds
have previously been incorporated or whose surface is
impregnated before installation,
2139~29
- 15 -
- - sterile eyedrops packaged in single-dose or multiple-
dose bottles and instilled during the post-operative
period.
The abovementioned ophthalmic compositions may be
provided in the form of ready-to-use solutions or solu-
tions which are made up immediately before use (lyophi-
lysate and solvent) depending on the stability in aqueous
medium of the compound of formula I used.
They comply with the sterility, neutrality and
isotonicity criteria imposed by ophthalmic use.
Examples of ophthalmic solutions will be given
below.
Composition 1
Compound of formula I . . . . . . . . . . . 1.00 g
Anhydrous calcium chloride . . . . . . . . 0.01 g
Potassium chloride . . . . . . . . . . . . 0.02 g
Potassium dihydrogenophosphate . . . . . . 0.02 g
Magnesium chloride hexahydrate . . . . . . 0.01 g
Sodium chloride . . . . . . . . . . . . . 0.80 g
Sodium hydrogenophosphate heptahydrate . . 0.22 g
Purified water . . . . . . qs . . . . . . 100.00 ml
Ophthalmic solution the composition of which corresponds
to phosphate buffered saline.
Composition 2
Compound of formula I . . . . . . . . . . . 1.00 g
Sodium hydrogenophosphate . . . . . . . . 1.04 g
Sodium dihydrogenophosphate . . . . . . . . 0.24 g
Chlorobutanol . . . . . . . . . . . . . . . 0.50 g
Purified water . . . . . . qs . . . . . . 100.00 ml
Multiple-dose ready-to-use eyedrops, the conservation of
which is ensured by chlorobutanol or any other
antibacterial agent.
Composition 3
Compound of formula I . . . . . . . . . . . 1.00 g
Sodium hydrogenophosphate . . . . . . . . . 1.04 g
Sodium dihydrogenophosphate . . . . . . . 0.24 g
Hydroxypropylmethyl cellulose . . . . . . . 0.50 g
Purified water . . . . . . qs . . . . . . 100.00 ml
Single-dose ready-to-use eyedrops, the viscosity of which
2139429
- 16 -
is obtained by hydroxypropylmethyl cellulose or any other
thickening agent.
Composition 4
Lyophilysate
Compound of formula I . . . . . . . . . . . 2.500 g
Dextran 70.000 . . . . . . . . . . . . . . 2.500 g
Anhydrous calcium chloride . . . . . . . . 0.025 g
Potassium chloride . . . . . . . . . . . . 0.050 g
Potassium dihydrogenophosphate . . . . . . 0.050 g
Magnesium chloride hexahydrate . . . . . . 0.025 g
Sodium hydrogenophosphate heptahydrate . . 0.550 g
Purified water . . . . . . qs . . . . . . 100.00 ml
Solvent
Sodium chloride . . . . . . . . . . . . . . 0.80 g
Benzalkonium chloride . . . . . . . . . . . 0.01 g
Purified water . . . . . . qs . . . . . . 100.00 ml
Eyedrops made up immediately before use, consisting:
of a lyophilysate contAining the active principle and
a charge of dextran 70.000 or any other texture agent
of a solvent contAining an antibacterial agent, benz-
alkonium chloride, and which enables rehydration of the
lyophilysate before use.
In addition, the novel compounds according to the
present invention have turned out to display an interest-
ing activity of inhibition of platelet aggregation.
This activity was demonstrated in the following
test:
Test for inhibition of platelet ay~ ~tion:
a) Preparation of the platelets.
The platelets are isolated from rat arterial
blood withdrawn from the abdominal aorta. Platelet-rich
plasma (PRP) and then platelet-poor plasma (PPP) are
prepared by centrifugation (120 g x 10 min and then
2000 g x 15 min). The PRP and PPP are diluted in
MICHAELIS buffer (Diagnostica Stago) in order to obtain
with the PRP a solution contAining 3.105 platelets per
microlitre.
b) Inhibition of platelet aggregation.
300 ~l of PRP are incubated with 10 ~l of the
2139429
._
- 17 -
various peptides to be tested in the aggregometer micro-
cuvette; one minute later, the platelets are stimulated
with 10 ~l of 6.25 ~M ADP.
The results showing the anti-aggregating effect
of the compounds are indicated in the table below. The
dose at which a 50% inhibition of platelet aggregation is
observed (IC50) has been calculated.
Compound IC50
Arg-Gly-Asp 2.6 . 10-3M
Ex. 1 1.5 . 10-3M