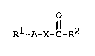Note: Descriptions are shown in the official language in which they were submitted.
21~0~!~7
T 5647
SUBSTITUTED POLYOXYALKYLENE COMPOUNDS
This invention relates to substituted polyoxyalkylene
compounds and particularly, although not exclusively, to the use
of such compounds as rust inhibitors in, for example, lubricating
oil compositions.
Polyoxyalkylene compounds have been relatively widely used
as additives in lubricating oil compositions. For example,
European Patent Application No. 0 309 105 (Exxon) discloses non-
ionic surfactants such as polyoxyalkylene polyols and esters
thereof which are stated to be useful as ashless rust inhibitors
in lubricating oils. Preferred polyols are prepared as block
copolymers so that the compounds have both hydrophobic and
hydrophilic portions. Pluronic (Trade Mark) polyols are stated to
be particularly well suited as rust inhibitors.
U.S. Patent No. 3 509 052 (Lubrizol) discloses lubricating
compositions which include a polyoxyalkylene polyol emulsifier
(or an ester thereof). Preferred polyols have hydrophobic and
hydrophilic portions. Pluronic (Trade Mark) polyols are stated to
be preferred.
U.S. Patent No. 3 784 474 (Chevron) discloses a lubricating
oil composition including a rust inhibitor in the form of a
polyoxyalkylene polyol, glycol, ester or urethane. Preferred
polyoxyalkylene glycols include propylene oxide polymers or block
copolymers. Pluronic (Trade Mark) polyols are disclosed as
commercially available examples of suitable polyols. The
specific examples in the patent describe rust inhibitors prepared
using a polyoxyalkylene glycol block copolymer of oxypropylene
and oxyethylene units.
U.S. Patent No. 3 933 663 (Chevron) discloses a lubricating
composition including an acid neutralisation accelerating
compound in the form of a polyalkoxylated compound. Suitable
compounds are stated to include certain block copolymers of
propylene oxide and ethylene oxide such as Pluronics (Trade
21~0~9~
Mark). The structure of the compounds is stated to be
amphipathic.
U.S. Patent No. 3 957 854 (Lubrizol) discloses oil soluble
carboxylic esters prepared using a polycarboxylic acid acylating
agent and at least one polyoxyalkylene alcohol which is a block
copolymer which includes hydrophobic and hydrophilic portions.
Suitable polyoxyalkylene alcohols are stated to be the Pluronic
(Trade Mark) polyols.
U.S. Patent No. 4 493 776 (Shell) discloses a lubricating
oil composition including a supplemental rust inhibitor which
comprises a mixture of at least one compound selected from alkyl,
aryl, alkaryl or arylalkyl-substituted oxyethylene ethanol or
oxypropylene propanol; and one compound selected from alkyl,
aryl, alkaryl or arylalkyl-substituted copolymers of ethylene
oxide and propylene oxide.
U.S. Patent No. 5 204 012 (Ethyl Corportion) discloses a
lubricating oil composition which includes an esterification
product having a total acid number (TAN) in the range about lO to
about 40 prepared by reacting ethylene oxide/propylene oxide
block copolymer with a long-chain monocarboxylic acid.
This invention is based upon the discovery of a class of
substituted polyoxyalkylene compounds which have unexpectedly
advantageous properties when used in, for example, lubricating
oil compositions.
According to the invention, there is provided a
polyoxyalkylene compound of general formula
Rl-A-X-C-R2
wherein
Rl represents a hydrogen atom or an optionally substituted
alkyl, alkenyl, alkynyl, aryl, arylalkyl, cycloalkyl, alkylaryl
or heterocyclyl group;
R2 represents an optionally substituted alkyl, alkenyl,
alkynyl, aryl, arylalkyl, cycloalkyl, alkylaryl, heterocyclyl,
alkoxy, aryloxy, alkylamino, dialkylamino or arylamino group;
2 1 ~
A represents an oxygen atom (-O-) or a group -NH-; and
X represents a polyoxypropylene chain or a random copolymer
of ethylene oxide/propylene oxide, and an oxygen atom of group X
is bonded directly to the R2CO- moiety.
It has been found unexpectedly that the polyoxyalkylene
compounds of general formula I are advantageous rust inhibitors
in lubricating oil compositions in comparison to other
polyoxyalkylene rust inhibitors which include, for example, a
block copolymeric polyoxyalkylene chain prepared using a Pluronic
(Trade Mark) polyol. In particular, polyoxyalkylene compounds of
general formula I may exhibit improved package stability and/or
have low acid values, whilst still having satisfactory rust
inhibition properties.
Generally, when any moieties described herein comprise an
alkyl, alkenyl or alkynyl group, the group may be linear or
branched and preferably contains l to 50, more preferably l to 25
and, still more preferably, 2 to 20 carbon atoms. When any
moieties described herein comprise an aryl group, the group may
be an optionally substituted phenyl group. When any of the
moieties comprises an arylalkyl group, the group may be an
optionally substituted benzyl group. Preferably, phenyl or
benzyl groups are unsubstituted or substituted by an alkyl group.
When any moieties described herein comprise a cycloalkyl group,
the group may contain from 3 to 8, preferaby 3 to 6, carbon
atoms, e.g. a cyclopentyl or cyclohexyl group. When any of the
moieties comprises a heterocyclyl group, the group may be any
saturated or unsaturated ring system, e.g. a C5-C7 ring system,
containing at least one heteroatom selected from oxygen, nitrogen
and sulphur, 5- and 6-membered rings being especially preferred,
e.g. a tetrahydrofuranyl, furanyl, piperidinyl, pyridinyl,
tetrahydrothiophenyl or thiophenyl (thienyl) group.
Examples of substituent groups include halogen atoms (e.g.
chlorine atoms), nitro, hydroxyl, carboxyl, amino, cyano, formyl,
alkoxycarbonyl, alkanoyl, alkylthio, alkylsulphinyl,
alkylsulphonyl, carbamoyl, alkylamido and polyoxyalkylene groups.
When any of the foregoing substituents contain an alkyl or
2 i ~
alkylene moiety, this may be linear or branched and may contain
up to 12, preferably up to 6, and especially up to 4, carbon
atoms.
Preferably, Rl represents a hydrogen atom or an optionally
S substituted alkyl group.
When A represents a group -NH-, Rl preferably represents a
substituted alkyl group of the general formula
H2N-(CHR3)r-CH2-[o-CH2(CHR )r]s II
wherein each R3 independently represents a hydrogen atom or a
methyl group, r is in the range from 1 to 3 and s is in the range
from 1 to 200.
It is preferred that in formula II, r is 1, each R3
represents a methyl group and s is in the range from 4 to 40.
When A represents an oxygen atom, Rl preferably represents
an unsubstituted alkyl group, particularly a C4-C16 alkyl group.
Preferably, Rl represents a linear alkyl group.
Preferably, R2 represents an optionally substituted alkyl,
phenyl or benzyl group.
Where R2 represents an optionally substituted alkyl group,
the group preferably has at least 4 carbon atoms. Examples of
optionally substituted alkyl groups include -(CH2)4COOH, t-butyl,
nonyl, l-ethylpentyl, linear alkyl groups of general formula
CH3(CH2)n- wherein n represents 8 to 20 and alkyl groups of
general formula
R4
R5-C- III
R6
wherein R4 represents a group of formula CaH2a+l - ; R5
represents a group of formula CbH2b+l - i R6 represents a group
of general formula CCH2c+l - ; and the total of a + b + c is in
the range from 3 to 20, preferably 3 to 8.
Where R2 represents a substituted alkyl group, the alkyl
group may be substituted by a group of general formula
- 21~0 l~2
R7-A'-Y-C- IV
wherein R7 represents a hydrogen atom or an optionally
substituted alkyl, alkenyl, alkynyl, aryl, arylalkyl, cycloalkyl,
alkylaryl or heterocyclyl group; A' represents an oxygen atom
(-O-) or a group -NH-; and Y represents a polyoxypropylene chain
or a random copolymer of ethylene oxide/propylene oxide, and an
oxygen atom of group Y is bonded directly to the carbonyl group.
Preferably, R7 and Rl represent the same atom or group.
Preferably, Y and X represent the same moiety. Preferably A and
A' both represent an oxygen atom or a group -NH-.
The number average molecular weight (Mn) of group X or Y is
preferably in the range 300 to 5000, more preferably in the range
300 to 3500, and especially in the range 500 to 2500. Where
group X or Y represents a polyoxypropylene chain, the number
average molecular weight of said chain may be in the range 300 to
3000. Where group X or Y represents a random copolymer of
ethylene oxide/propylene oxide, the number average molecular
weight may be in the range lO00 to 3000.
Group X or Y preferably comprises ~ wt~ of ethylene oxide
and (lO0 - ~) wt~ of propylene oxide where ~ is in the range 0 to
60. More preferably, ~ is in the range 0 to 30.
The total acid number (TAN) of a material is the quantity of
base, expressed in milligrams of potassium hydroxide, that is
required to neutralise all acidic constituents present in l gram
of the material. Preferably, the TAN of said compound of general
formula I is less than 5 and, more preferably, less than 2.
Especially preferred is the case where the TAN is less than 0.5.
The invention extends to a process for the preparation of a
compound of general formula I, the process comprising reacting a
compound of general formula
Rl-A-X-OH V
wherein Rl, A and X are as defined above, with a compound of
general formula
21~0~
.
R2-C-L1 VI
wherein R2 is as defined above and L1 is a leaving group.
The reaction is preferably carried out in the presence of an
aprotic solvent and optionally in the presence of an acid, e.g.
para-toluene sulphonic acid, or a base, e.g. an alkali metal
carbonate or bicarbonate, or an amine, e.g. a trialkyl amine or
pyridine. The reaction is conveniently carried out at a
temperature in the range from 40~C to 180~C. The reaction is
preferably carried out under reflux.
L1 is suitably selected so that compounds of general
formulas V and VI react together to form an ester. L1 may
represent a halogen, especially a chlorine atom, or a hydroxy
group. Alternatively, said compound of general formula VI may
represent an anhydride, in which case L1 represents a group
R2COO- where R2 is as defined above.
Where, in the compound of general formula I, R2 represents
an alkyl group substituted by a group of general formula IV as
described above, said compound of general formula I may be
prepared by reacting at least one compound of general formula V p
O O
L2-C-z_c_L3 VII
wherein L2 and L3 may be the same or different and represent
leaving groups; and Z represents a divalent linear or branched
hydrocarbon moiety of general formula, -(CR8R9)m- wherein m is an
integer, each R8 independently represents a hydrogen atom or an
alkyl group, and each R9 independently represents a hydrogen atom
or an alkyl group.
L2 and L3 are suitably selected and the conditions for the
reaction are such that compounds of general formulas V and VII
react together to produce a compound of general formula I having
at least two ester groups. L2 and L3 may independently represent
a halogen, especially a chlorine atom, or a hydroxy group.
Preferably, L2 and L3 both represent a hydroxy group. In this
case, said compound of general formula V represents a
21~4~
dicarboxylic acid, e.g. adipic acid.
The compounds of general formulae V, VI and VII are known
compounds or may be prepared by processes analogous to known
processes.
For example, compounds of general formula V in which A
represents an oxygen atom and Rl represents an alkyl group, or in
which A represents a group -NH- and Rl represents a substituted
alkyl group of formula II above, may conveniently be prepared by
polymerising propylene oxide, and optionally ethylene oxide, in
the presence of an alcohol initiator such as those sold by member
companies of the Royal Dutch/Shell Group under the trade mark
"DOBANOL", in particular "DOBANOL" 25, or an amine initiator of
the general formula
H2N-(CHR3)r-CH2-[o-CH2-(CHR3)r]s-NH2 (II')
wherein R3, r and s are as defined above, which is commercially
available from the Texaco Chemical Company, Bellaire, Texas,
U.S.A.
Compounds of general formula VI in which R2 represents a
branched alkyl group, e.g. an alkyl group of formula III above,
and Ll represents a hydroxy group are commercially available from
Exxon under the trade mark "CEKANOIC", e.g. "CEKANOIC" l0 acid,
and from member companies of the Royal Dutch/Shell Group under
the trade mark "VERSATIC", e.g. "VERSATIC" 5 acid and "VERSATIC"
l0 acid.
The invention extends to a compound prepared by reacting
compounds of general formulas V and VI together.
The invention extends to a lubricating oil composition
comprising a major amount (more than 50wt% based on total
composition) of a lubricating oil and a minor amount of a
compound of general formula I.
The lubricating oil used in the present composition can be
natural, mineral or synthetic in origin. Natural lubricating
oils include animal and vegetable oils, such as castor oil.
Mineral oils comprise the lubricating oil fractions derived from
crude oils, coal or shale, which fractions may have been
2 1 ~
subjected to certain treatments such as clay-, acid-, solvent- or
hydrogenation treatments. Synthetic lubricating oils include
synthetic polymers or hydrocarbons, modified alkylene oxide
polymers, and ester lubricants, which are known in the art.
These lubricating oils are preferably crankcase lubricating oils
for spark-ignition and compression-ignition engines, but include
also hydraulic lubricants, metal-working fluids, automatic
transmission fluids and the like.
The lubricating oil composition preferably contains the
compound of general formula I in an amount from 0.05 to l0wt%,
more preferably from 0.l to Swt%, and especially from 0.l to
lwt~, based on the total composition.
The lubricating oil composition may contain various other
additives known in the art, such as viscosity index improvers,
e.g. linear or star-shaped polymers of a diene such as isoprene
or butadiene, or a copolymer of such a diene with optionally
substituted styrene. These copolymers are suitably block
copolymers and are preferably hydrogenated to such an extent as
to saturate most of the olefinic unsaturation.
The lubricating oil composition may further contain an
ashless dispersant selected from oil soluble salts, amides,
imides, oxazolines and esters, or mixtures thereof, of long chain
hydrocarbon substituted mono and dicarboxylic acids or their
anhydrides; and long chain aliphatic hydrocarbons having a
polyamine attached directly thereto. Especially preferred ashless
dispersants include polyolefin-substituted succinimides.
Other suitable additives that may be present in the
lubricating oil composition include extreme pressure/anti-wear
additives such as zinc or sodium dithiophosphates, anti-oxidants,
friction modifiers or metal-containing detergents such as
phenates, sulphonates, alkylsalicylates or naphthenates, all of
which detergents may be overbased.
The lubricating oil composition according to the present
invention is suitably prepared by blending an additive
concentrate into a lubricating base oil. Such a concentrate
generally comprises a lubricating oil as solvent/diluent and one
- 21~0~
or more additives in a concentrated form. Hence the present
invention further provides a lubricating oil concentrate
comprising a diluent and a compound of general formula I as
described above, in an amount of l to 80wt~ based on the total
concentrate.
The invention extends to the use of a compound of general
formula I as a rust inhibitor.
The invention further extends to a method of operating an
internal combustion engine to inhibit rust formation, the method
comprising lubricating said engine with a lubricating oil
composition as described above.
The compound of general formula I may also be used in fuels,
for example gasoline, diesel fuel, kerosine and fuel oils.
Suitably, the compound of general formula I can be used as
carrier fluid for gasoline and diesel fuel additive packages.
Accordingly, the invention extends to a fuel composition
comprising a major amount (more than 50wt~ based on total
composition) of a fuel and a minor amount of a compound of
general formula I.
The fuel compositions of the present invention desirably
also contain a minor amount of at least one hydrocarbon-soluble
ashless dispersant. The compounds useful as ashless dispersants
generally are characterised by a "polar" group attached to a
relatively high molecular weight hydrocarbon chain. The "polar"
group generally contains one or more of the elements nitrogen,
oxygen and phosphorus. The solubilising chains are generally
higher in molecular weight than those employed with the metallic
types, but in some instances they may be quite similar.
In general, any of the ashless dispersants which are known
in the art for use in lubricants and fuels can be utilised in the
fuel compositions of the present invention.
In one em~odiment of the present invention, the dispersant
is selected from the group consisting of
(i) at least one hydrocarbyl-substituted amine wherein the
hydrocarbyl substituent is substantially aliphatic and contains
at least 8 carbon atoms;
21~49~
-- 10 --
(ii) at least one acylated, nitrogen-containing compound
having a hydrocarbon-based substituent of at least lO aliphatic
carbon atoms made by reacting a carboxylic acid acylating agent
with at least one amino compound containing at least one
-NH-
group, said acylating agent being linked to said amino compound
through an imido, amido, amidine, or acyloxy ammonium linkage;
(iii) at least one nitrogen-containing condensate of a
phenol, aldehyde and amino compound having at least one
-NH-
group;
(iv) at least one ester of a substituted carboxylic acid;
(v) at least one polymeric dispersant;
(vi) at least one hydrocarbon-substituted phenolic
dispersant; and
(vii) at least one fuel soluble alkoxylated derivative of an
alcohol, phenol or amine.
The hydrocarbyl-substituted amines useful in the fuel
compositions of this invention are well known to those skilled in
the art and they are described in a number of patents. Among
these are U.S. Patents Nos. 3,275,554, 3,438,757, 3,454,555,
3,565,804, 3,755,433 and 3,822,209. These patents disclose
suitable hydrocarbyl-substituted amines for use in the present
invention including their method of preparation.
A typical hydrocarbyl-substituted amine has the general
formula:
[RlORllN]x[-N([-uN-]d[-uQ]e)]yRl2fHl+2y+dy-f VIII
wherein RlO is hydrogen, a hydrocarbyl group of from l to lO
carbon atoms, or hydroxyhydrocarbyl group of from l to lO carbon
atoms; Rll is hydrogen, a hydrocarbyl group of from l to lO
carbon atoms, or hydroxyhydrocarbyl group of from l to lO carbon
atoms, and may be taken together with RlO and N to form a ring of
from 5 to 6 annular members and up to 12 carbon atoms; U is an
alkylene group of from 2 to lO carbon atoms, any necessary
- 2 ~
hydrocarbons to accommodate the trivalent nitrogens are implied
herein, R12 is an aliphatic hydrocarbon of from 30 to 400 carbon
atoms; Q is a piperazine structure; d is an integer of from 0 to
lO; e is an integer of from 0 to l; d+2e is an integer of from l
S to lO; f is an integer of from l to 5 and is an average in the
range of l to 4, and equal to or less than the number of nitrogen
atoms in the molecule; x is an integer of from 0 to l; y is an
integer of from 0 to l; and x+y is equal to l.
In interpreting this formula, it is to be understood that
the Rl2 and H atoms are attached to the unsatisfied nitrogen
valences within the brackets of the formula. Thus, for example,
the formula includes sub-generic formulae wherein the Rl2 is
attached to terminal nitrogens and isomeric subgeneric formulae
wherein it is attached to non-terminal nitrogen atoms. Nitrogen
atoms not attached to an Rl2 may bear a hydrogen or an RlORllN
substituent.
The hydrocarbyl-substituted amines useful in this invention
and embraced by formula VIII above include monoamines such as
poly(propylene)amine, N,N-dimethyl-n-poly(ethylene/propylene)-
amine (50:50 mole ratio of monomers), poly(isobutene)amine,
N,N-di(hydroxyethyl)-N-poly(isobutene)amine, poly(isobutene/-
l-butene/2-butene)-amine (50:25:25 mole ratio of monomers),
N-(2-hydroxy-ethyl)-N-poly(isobutene)-amine, N-(2-hydroxypropyl)-
N-poly(isobutene)amine, N-poly(l-buten-e)-aniline, and N-poly-
(isobutene)-morpholine; and polyamines such as N-poly(isobutene)
ethylene diamine, N-poly(propylene) trimethylene diamine, N-
poly(l-butene) diethylene triamine, N',N'-poly(isobutene)
tetraethylene pentamine, N,N-dimethyl-N'-poly(propylene), and
l,3-propylene diamine.
The hydrocarbyl-substituted amines useful in the fuel
compositions of the invention also include certain N-amino-
hydrocarbyl morpholines of the general formula:
Rl2N(Rl0)UM IX
wherein Rl2 is an aliphatic hydrocarbon group of from 30 to 400
carbons, RlO is hydrogen, a hydrocarbyl group of from l to lO
21~1)49~
- 12 -
carbon atoms or hydroxyhydrocarbyl group of from 1 to 10 carbon
atoms, U is an alkylene group of from 2 to 10 carbon atoms, and M
is a morpholine structure. These hydrocarbyl-substituted
aminohydrocarbyl morpholines as well as the polyamines described
by formula VIII are among the typical hydrocarbyl-substituted
amines used in preparing compositions of this invention.
A number of acylated, nitrogen-containing compounds having a
hydrocarbon-based substituent of at least 10 aliphatic carbon
atoms and made by reacting a carboxylic acid acylating agent with
an amino compound are known to those skilled in the art. The
acylating agent is linked to the amino compound through an imido,
amido, amidine or acyloxy ammonium linkage. The hydrocarbon-
based substituent of at least 10 aliphatic carbon atoms may be in
either the carboxylic acid acylating agent derived portion of the
molecule or in the amino compound derived portion of the
molecule. Preferably, however, it is in the acylating agent
portion. The acylating agent can vary from formic acid and its
acylating derivatives to acylating agents having high molecular
weight aliphatic substituents of up to 5,000, 10,000 or 20,000
carbon atoms. The amino compounds can vary from ammonia itself
to amines having aliphatic substituents of up to 30 carbon atoms.
A typical class of acylated, nitrogen-containing compounds
useful in the compositions of this invention are those made by
reacting an acylating agent having an aliphatic substituent of at
least 10 carbon atoms and a nitrogen compound characterised by
the presence of at least one -NH- group. Typically, the
acylating agent will be a mono- or polycarboxylic acid (or
reactive equivalent thereof) such as a substituted succinic or
propionic acid and the amino compound will be a polyamine or
mixture of polyamines, most typically, a mixture of ethylene
polyamines. The amine may also be a hydroxyalkyl-substituted
polyamine. The aliphatic substituent in such acylating agents
preferably averages at least 30 or 50 and up to 400 carbon atoms.
Illustrative hydrocarbon-based substituent groups containing
at least ten aliphatic carbon atoms are n-decyl, n-dodecyl,
tetrapropenyl, n-octadecyl, oleyl, chlorooctadecyl and
2i4~9~
- 13 -
triicontanyl. Generally, the hydrocarbon-based substituents are
made from homo- or interpolymers (e.g., copolymers, terpolymers)
of mono- and diolefins having 2 to 10 carbon atoms, such as
ethylene, propylene, butene-1, isobutene, butadiene, isoprene, 1-
hexene and 1-octene. Typically, these olefins are 1-monoolefins.
The substituent can also be derived from the halogenated (e.g.,
chlorinated or brominated) analogues of such homo- or
interpolymers. The substituent can, however, be made from other
sources, such as monomeric high molecular weight alkenes (e.g.,
1-tetracontene) and chlorinated analogues and hydrochlorinated
analogues thereof, aliphatic petroleum fractions, particularly
paraffin waxes and cracked and chlorinated analogues and
hydrochlorinated analogues thereof, white oils, synthetic alkenes
such as those produced by the Ziegler-Natta process (e.g.,
poly(ethylene) greases) and other sources known to those skilled
in the art. Any unsaturation in the substituent may be reduced
or eliminated by hydrogenation according to procedures known in
the art.
As used in this specification, the term "hydrocarbon-based"
denotes a group having a carbon atom directly attached to the
remainder of the molecule and having a predominantly hydrocarbon
character within the context of this invention. Therefore,
hydrocarbon-based groups can contain up to one non-hydrocarbon
group for every ten carbon atoms provided this non-hydrocarbon
group does not significantly alter the predominantly hydrocarbon
character of the group. Those skilled in the art will be aware
of such groups, which include, for example, hydroxyl, halo
(especially chloro and fluoro), alkoxyl, alkyl mercapto and alkyl
sulphoxy groups. Usually, however, the hydrocarbon-based
substituents are purely hydrocarbyl and contain no such non-
hydrocarbyl groups.
The hydrocarbon-based substituents are substantially
saturated, that is, they contain no more than one carbon-to-
carbon unsaturated bond for every ten carbon-to-carbon single
bonds present. Usually, they contain no more than one carbon-to-
carbon non-aromatic unsaturated bond for every 50 carbon-to-
- 21~92
carbon bonds present.
The hydrocarbon-based substituents are also substantially
aliphatic in nature, that is, they contain no more than one non-
aliphatic moiety (cycloalkyl, cycloalkenyl or aromatic) group of
six or less carbon atoms for every ten carbon atoms in the
substituent. Usually, however, the substituents contain no more
than one such non-aliphatic group for every fifty carbon atoms,
and in many cases, they contain no such non-aliphatic groups at
all; that is, the typical substituents are purely aliphatic.
Typically, these purely aliphatic substituents are alkyl or
alkenyl groups.
Specific examples of the substantially saturated
hydrocarbon-based substituents containing an average of more than
30 carbon atoms are the following: a mixture of
poly(ethylene/propylene) groups of 35 to 70 carbon atoms, a
mixture of oxidatively or mechanically degraded
poly(ethylene/propylene) groups of 35 to 70 carbon atoms, a
mixture of poly(proplene/1-hexene) groups of 80 to 150 carbon
atoms, and a mixture of polyisobùtene groups having an average of
50 to 75 carbon atoms.
A preferred source of the substituents are polyisobutenes
obtained by polymerisation of a C4 refinery stream having a
butene content of 35 to 75 weight per cent and isobutene content
of 30 to 60 weight per cent in the presence of a Lewis acid
catalyst such as aluminium trichloride or boron trifluoride.
These polyisobutenes contain predominantly (greater than 80~ of
total repeating units) isobutene repeating units of the
configuration:
-C(CH3)2CH2-
Exemplary of amino compounds useful in making the acylated
compounds are the following: (1) polyalkylene polyamines of the
general formula:
(R14)2N[p-N(R14)]tR14 X
wherein each R14 is independently a hydrogen atom, a hydrocarbyl
21~0~
- 15 -
group or a hydroxy-substituted hydrocarbyl group containing up to
30 carbon atoms, with the proviso that at least one R14 is a
hydrogen atom, t is a whole number of 1 to 10 and P is a C1_18
alkylene group;
(2) hydroxyalkyl-substituted polyamines wherein the polyamines
are as described above;
(3) heterocyclic-substituted polyamines wherein the polyamines
are as described above and the heterocyclic substituent is
derived from, for example, piperazine, imidazoline, pyrimidine or
morpholine; and
(4) aromatic polyamines of the general formula:
Ar(NR142~z XI
wherein Ar is an aromatic nucleus of 6 to 20 carbon atoms, each
R14 is as defined above and z is 2 to 8.
Specific examples of polyalkylene polyamines of formula X
are ethylene diamine, tetra(ethylene)pentamine, tri-
(trimethylene)-tetramine and 1,2-propylene diamine.
Specific examples of hydroxyalkyl-substituted polyamines
include N-(2-hydroxyethyl) ethylene diamine, N,N'-bis-(2-hydroxy-
ethyl) ethylene diamine and N-(3-hydroxybutyl) tetramethylene
diamine.
Specific examples of heterocyclic-substituted polyamines are
N-2-aminoethyl piperazine, N-2- and N-3-amino propyl morpholine,
N-3-(dimethyl amino) propyl piperazine, 2-heptyl-3-(2-
aminopropyl) imidazoline, 1,4-bis (2-aminoethyl) piperazine, 1-
(2-hydroxy ethyl) piperazine, and 2-heptadecyl-l-(2-
hydroxyethyl)-imidazoline.
Specific examples of aromatic polyamines are the various
isomeric phenylene diamines and the various isomeric naphthalene
diamines.
Many patents have described useful acylated nitrogen
compounds including U.S. Patents Nos. 3,172,892, 3,219,666,
3,272,746, 3,310,492, 3,341,542, 3,444,170, 3,455,831, 3,455,832,
3,576,743, 3,630,904, 3,632,511, 3,804,763 and 4,234,435. A
typical acylated nitrogen-containing compound of this class is
21~0~
- 16 -
that made by reacting a polyisobutene-substituted succinic
anhydride acylating agent wherein the polyisobutene substituent
has from 50 to 400 carbon atoms with a mixture of ethylene
polyamines having 3 to 7 amino nitrogen atoms per ethylene
polyamine.
Another type of acylated nitrogen compound belonging to this
class is that made by reacting the aforementioned alkylene amines
with the aforementioned substituted succinic acids or anhydrides
and aliphatic monocarboxylic acids having from 2 to 22 carbon
atoms. In these types of acylated nitrogen compounds, the mole
ratio of succinic acid to monocarboxylic acid is in the range
from 1:0.1 to 1:1. Typical of the monocarboxylic acids are
formic acid, acetic acid, dodecanoic acid, butanoic acid, oleic
acid, stearic acid, the commercial mixture of stearic acid
isomers known as isostearic acid and tolyl acid. Such materials
are more fully described in U.S. Patents Nos. 3,216,936 and
3,250,715.
Still another type of acylated nitrogen compound useful in
the fuel compositions of the invention is the product of the
reaction of a fatty monocarboxylic acid of 12 to 30 carbon atoms
and the aforementioned alkylene amines, typically, ethylene,
propylene or trimethylene polyamines containing 2 to 8 amino
groups and mixtures thereof. The fatty monocarboxylic acids are
generally mixtures of straight and branched chain fatty
carboxylic acids containing 12 to 30 carbon atoms. A widely used
type of acylated nitrogen compound is made by reacting the
aforementioned alkylene polyamines with a mixture of fatty acids
having from 5 to 30 mole per cent straight chain acid and 70 to
95 mole per cent branched chain fatty acids. Among the
commercially available mixtures are those known widely in the
trade as isostearic acid. These mixtures are produced as a by-
product from the dimerisation of unsaturated fatty acids as
described in U.S. Patents Nos. 2,812,342 and 3,260,671.
The branched chain fatty acids can also include those in
which the branch is not alkyl in nature, such as found in phenyl
and cyclohexyl stearic acid and the chloro-stearic acids.
2140~9~
Branched chain fatty carboxylic acid/alkylene polyamine products
have been described, for example, in U.S. Patents Nos. 3,110,673,
3,251,853, 3,326,801, 3,337,459, 3,405,064, 3,429,674, 3,468,639
and 3,857,791.
The phenol/aldehyde/amino compound condensates useful as
dispersants in the fuel compositions of the invention include
those generically referred to as Mannich condensates. Generally,
they are made by reacting simultaneously or sequentially at least
one active hydrogen compound such as a hydrocarbon-substituted
phenol (e.g., an alkyl phenol wherein the alkyl group has at
least an average of 12 to 400, preferably 30 to 400, carbon
atoms), having at least one hydrogen atom bonded to an aromatic
carbon, with at least one aldehyde or aldehyde-producing material
(typically formaldehyde precursor) and at least one amino or
polyamino compound having at least one NH group. The amino
compounds include primary or secondary monoamines having
hydrocarbon substituents of 1 to 30 carbon atoms or hydroxyl-
substituted hydrocarbon substituents of 1 to 30 carbon atoms.
Another type of typical amino compound are the polyamines
described during the discussion of the acylated nitrogen-
containing compounds.
Exemplary monoamines include methyl ethyl amine, methyl
octadecyl amines, aniline, diethyl amine, diethanol amine and
dipropyl amine. The following patents contain extensive
descriptions of Mannich condensates: U.S. Patents Nos.
2,459,112, 3,413,347, 3,558,743, 2,962,442, 3,442,808, 3,586,629,
2,984,550, 3,448,047, 3,591,598, 3,036,003, 3,454,497, 3,600,372,
3,166,516, 3,459,661, 3,634,515, 3,236,770, 3,461,172, 3,649,229,
3,355,270, 3,493,520, 3,697,574, 3,368,972 and 3,539,633.
Condensates made from sulphur-containing reactants can also
be used in the fuel compositions of the present invention. Such
sulphur-containing condensates are described in U.S. Patents Nos.
3,368,972, 3,649,229, 3,600,372, 3,649,659 and 3,741,896. These
patents also disclose sulphur-containing Mannich condensates.
Generally the condensates used in making compositions of this
invention are made from a phenol bearing an alkyl substituent of
214~49~
- 18 -
6 to 400 carbon atoms, more typically, 30 to 250 carbon atoms.
These typical condensates are made from formaldehyde or C2_7
aliphatic aldehyde and an amino compound such as those used in
making the acylated nitrogen-containing compounds described
above.
These preferred condensates are prepared by reacting one
molar portion of phenolic compound with 1 to 2 molar portions of
aldehyde and 1 to 5 equivalent portions of amino compound (an
equivalent of amino compound is its molecular weight divided by
the number of -NH- groups present). The conditions under which
such condensation reactions are carried out are well known to
those skilled in the art.
A particularly preferred class of nitrogen-containing
condensation products for use in the fuel compositions of the
present invention are those made by (1) reacting at least one
hydroxy aromatic compound containing an aliphatic-based or
cycloaliphatic-based substituent which has at least 30 carbon
atoms and up to 400 carbon atoms with a lower aliphatic Cl_7
aldehyde or reversible polymer thereof in the presence of an
alkaline reagent, such as an alkali metal hydroxide, at a
temperature up to 150~C; (2) substantially neutralising the
intermediate reaction mixture thus formed; and (3) reacting the
neutralised intermediate with at least one compound which
contains an amino group having at least one -NH- group.
More preferably, these condensates are made from (a) phenols
bearing a hydrocarbon-based substituent having 30 to 250 carbon
atoms, said substituent being derived from a polymer of
propylene, l-butene, 2-butene, or isobutene and (b) formaldehyde,
or reversible polymer thereof, (e.g., trioxane, paraformaldehyde)
or functional equivalent thereof, (e.g., methylol) and (c) an
alkylene polyamine such as ethylene polyamines having from 2 to
10 nitrogen atoms.
The esters useful as dispersants in the fuel compositions of
the invention are derivatives of substituted carboxylic acids in
which the substituent is a substantially aliphatic, substantially
saturated hydrocarbon-based group containing at least 30,
- 2 1 ~
-- 19 -
preferably at least 50, up to 750 aliphatic carbon atoms. As
used herein, the term "hydrocarbon-based group" denotes a group
having a carbon atom directly attached to the remainder of the
molecule and having predominantly hydrocarbon character within
the context of this invention. Such groups include the
following:
(l) Hydrocarbon groups; that is, aliphatic groups, aromatic-
andalicyclic-substituted aliphatic groups, and the like, of the
type known to those skilled in the art.
(2) Substituted hydrocarbon groups; that is, groups containing
non-hydrocarbon substituents which, in the context of this
invention, do not alter the predominantly hydrocarbon character
of the group. Those skilled in the art will be aware of suitable
substituents; examples are halo, nitro, hydroxy, alkoxy,
carbalkoxy and alkylthio.
(3) Hetero groups; that is, groups which, while predominantly
hydrocarbon in character within the context of this invention,
contain atoms other than carbon present in a chain or ring
otherwise composed of carbon atoms. Suitable hetero atoms will be
apparent to those skilled in the art and include, for example,
nitrogen, oxygen and sulphur.
In general, no more than about three substituents or hetero
atoms, and preferably no more than one, will be present for each
lO carbon atoms in the hydrocarbon-based group.
The substituted carboxylic acids are normally prepared by
the alkylation of an unsaturated acid, or a derivative thereof
such as an anhydride, with a source of the desired hydrocarbon-
based group. Suitable unsaturated acids and derivatives thereof
include acrylic acid, methacrylic acid, maleic acid, maleic
anhydride, fumaric acid, itaconic acid, itaconic anhydride,
citraconic acid, citraconic anhydride, mesaconic acid, glutaconic
acid, chloromaleic acid, aconitic acid, crotonic acid,
methylcrotonic acid, sorbic acid, 3-hexenoic acid, lO-decenoic
acid and 2-pentene-l,3,5-tri-carboxylic acid. Particularly
preferred are the unsaturated dicarboxylic acids and their
derivatives, especially maleic acid, fumaric acid and maleic
21~0~Z
- 20 -
anhydride.
Suitable alkylating agents include homopolymers and inter-
polymers of polymerisable olefin monomers containing from 2 to 10
and usually from 2 to 6 carbon atoms, and polar substituent-
containing derivatives thereof. Such polymers are substantiallysaturated (i.e., they contain no more than about 5% olefinic
linkages) and substantially aliphatic (i.e., they contain at
least 80% and preferably at least 95% by weight of units derived
from aliphatic monoolefins). Illustrative monomers which may be
used to produce such polymers are ethylene, propylene, 1-butene,
2-butene, isobutene, 1-octene and 1-decene. Any unsaturated
units may be derived from conjugated dienes such as 1,3-butadiene
and isoprene; non-conjugated dienes such as l,4-hexadiene, 1,4-
cyclohexadiene, 5-ethylidene-2-norbornene and 1,6-octadiene; and
trienes such as 1-isopropylidene-3a,4,7,7a-tetrahydroindene, 1-
isopropylidene-dicyclopentadiene and 2-(2-methylene-4-methyl-3-
pentenyl)[2.2.1]-bicyclo-5-heptene.
A first preferred class of polymers comprises those of
terminal olefins such as propylene, 1-butene, isobutene and 1-
hexene. Especially preferred within this class are polybutenescomprising predominantly isobutene units. A second preferred
class comprises terpolymers of ethylene, a C3-8 alpha-monoolefin
and a polyene selected from the group consisting of non-
conjugated dienes (which are especially preferred) and trienes.
Illustrative of these terpolymers is "Ortholeum 2052"
manufactured by E.I. duPont de Nemours & Company, which is a
terpolymer containing about 48 mole per cent ethylene groups, 48
mole per cent propylene groups and 4 mole per cent 1,4-hexadiene
groups and having an inherent viscosity of 1.35 (8.2 grams of
polymer in 10 ml of carbon tetrachloride at 30~C).
Methods for the preparation of the substituted carboxylic
acids and derivatives thereof are well known in the art and need
not be described in detail. Reference is made, for example, to
U.S. Patents Nos. 3,272,746, 3,522,179 and 4,234,435. The mole
ratio of the polymer to the unsaturated acid or derivative
thereof may be equal to, greater than or less than 1, depending
- 2l~n4$,~
- 21 -
on the type of product desired.
The esters are those of the above-described substituted
carboxylic acids with hydroxy compounds which may be aliphatic
compounds such as monohydric and polyhydric alcohols or aromatic
compounds such as phenols and naphthols. Examples of aromatic
hydroxy compounds include phenol, beta-naphthol, alpha-naphthol,
cresol, resorcinol, catechol, p,p'-dihydroxybiphenyl, 2-
chlorophenol, 2,4-dibutylphenol, propene tetramer-substituted
phenol, didodecylphenol, 4,4'-methylene-bis-phenol, alpha-decyl-
beta-naphthol, polyisobutene (molecular weight of 1000)-
substituted phenol, the condensation product of heptylphenol with
formaldehyde, the condensation product of octyl-phenol with
acetone, di(hydroxyphenyl)-oxide, di(hydroxyphenyl)sulphide,
di(hydroxyphenyl)disulphide, and 4-cyclo-hexylphenol. Phenol and
alkylated phenols having up to three alkyl substituents are
preferred. Each of the alkyl substituents may contain 100 or
more carbon atoms.
The aliphatic alcohols from which the esters may be derived
preferably contain up to 40 aliphatic carbon atoms. They may be
monohydric alcohols such as methanol, ethanol, isooctanol,
dodecanol, cyclohexanol, cyclopentanol, behenyl alcohol,
hexatriacontanol, neopentyl alcohol, isobutyl alcohol, benzyl
alcohol, beta-phenylethyl alcohol, 2-methylcyclohexanol, beta-
chloroethanol, monomethyl ether of ethylene glycol, monobutyl
ether of ethylene glycol, monopropyl ether of diethylene glycol,
monododecyl ether of triethylene glycol, monooleate of ethylene
glycol, monostearate of diethylene glycol, secpentyl alcohol,
tertbutyl alcohol, 5-bromo-dodecanol, nitro-octadecanol and
dioleate of glycerol. The polyhydric alcohols preferably contain
from 2 to 10 hydroxy radicals. They are illustrated by, for
example, ethylene glycol, diethylene glycol, triethylene glycol,
tetraethylene glycol, dipropylene glycol, tripropylene glycol,
dibutylene glycol, tri-butylene glycol, and other alkylene
glycols in which the alkylene radical contains from 2 to 8 carbon
atoms. Other useful polyhydric alcohols include glycerol,
monooleate of glycerol, monostearate of glycerol, monomethyl
21~0~
- 22 -
ether of glycerol, pentaerythritol, 9,10-dihydroxy stearic acid,
methyl ester of 9,10-dihydroxy stearic acid, 1,2-butanediol, 2,3-
hexanediol, 2,4-hexanediol, penacol, erythritol, arabitol,
sorbitol, mannitol, 1,2-cyclohexanediol, and xylene glycol.
Carbohydrates such as sugars, starches and cellulose may also
yield esters useful in this invention. The carbohydrates may be
exemplified by glucose, fructose, sucrose, rhamnose, mannose,
glyceraldehyde and galactose.
An especially preferred class of polyhydric alcohols are
those having at least three hydroxy radicals, some of which have
been esterified with a monocarboxylic acid having from 8 to 30
carbon atoms, such as octanoic acid, oleic acid, stearic acid,
linoleic acid, dodecanoic acid, or tall oil acid. Examples of
such partially esterified polyhydric alcohols are the monooleate
of sorbitol, distearate of sorbitol, monooleate of glycerol,
monostearate of glycerol, di-dodecanoate of erythritol.
The esters may also be derived from unsaturated alcohols
such as allyl alcohol, cinnamyl alcohol, propargyl alcohol, 1-
cyclohexene-3-ol and oleyl alcohol. Still another class of the
alcohols capable of yielding the esters useful in this invention
comprise the ether-alcohols and amino-alcohols including, for
example, the oxyalkylene-, oxyarylene-, amino-alkylene- and
amino-arylene-substituted alcohols having one or more
oxyalkylene, oxyarylene, amino-alkylene or amino-arylene
radicals. They are exemplified by Cellosolve, carbitol,
phenoxyethanol, heptylphenyl-(oxypropylene)6-H, octyl-
(oxyethylene)30-H, phenyl-~oxyoctylene)2-H, mono(heptylphenyl-
oxypropylene)-substituted glycerol, poly(styrene oxide), amino-
ethanol, 3-amino ethyl-pentanol, di(hydroxyethyl) amine, p-amino-
phenol, tri(hydroxypropyl)amine, N-hydroxyethyl ethylene diamine
and N,N,N',N'-tetrahydroxy-trimethylene diamine. For the most
part, the ether-alcohols having up to about 150 oxyalkylene
radicals in which the alkylene radical contains from 1 to 8
carbon atoms are preferred.
The esters may be diesters of succinic acids or acidic
esters, i.e., partially esterified polyhydric alcohols or
2l~n~s~
phenols, i.e., esters having free alcoholic or phenolic hydroxyl
radicals. Mixtures of the above-illustrated esters likewise are
contemplated within the scope of the invention.
The succinic acid esters may be prepared by one of several
methods. The method which is preferred because of convenience
and superior properties of the esters it produces, involves the
reaction of a suitable alcohol or phenol with a substantially
hydrocarbon-substituted succinic anhydride. The esterification
is usually carried out at a temperature above about 100~C,
preferably between 150~C and 300~C.
The water formed as a by-product is removed by distillation
as the esterification proceeds. A solvent may be used in the
esterification to facilitate mixing and temperature control. It
also facilitates the removal of water from the reaction mixture.
The useful solvents include xylene, toluene, diphenyl ether,
chlorobenzene and mineral oil.
A modification of the above process involves the replacement
of the substituted succinic anhydride with the corresponding
succinic acid. However, succinic acids readily undergo
dehydration at temperatures above about 100oC and are thus
converted to their anhydrides which are then esterified by the
reaction with the alcohol reactant. In this regard, succinic
acids appear to be the substantial equivalent of their anhydrides
in the process.
The relative proportions of the succinic reactant and the
hydroxy reactant which are to be used depend to a large measure
upon the type of the product desired and the number of hydroxyl
groups present in the molecule of the hydroxy reactant. For
instance, the formation of a half ester of a succinic acid, i.e.,
one in which only one of the two acid radicals is esterified,
involves the use of one mole of a monohydric alcohol for each
mole of the substituted succinic acid reactant, whereas the
formation of a diester of a succinic acid involves the use of two
moles of the alcohol for each mole of the acid. On the other
hand, one mole of a hexahydric alcohol may combine with as many
as six moles of a succinic acid to form an ester in which each of
- 21404~2
- 24 -
the six hydroxyl radicals of the alcohol is esterified with one
of the two acid radicals of the succinic acid. Thus, the m~ximl~m
proportion of the succinic acid to be used with a polyhydric
alcohol is determined by the number of hydroxyl groups present in
the molecule of the hydroxy reactant. For the purposes of this
invention, it has been found that esters obtained by the
reaction of equimolar amounts of the succinic acid reactant and
hydroxy reactant have superior properties and are therefore
preferred.
In some instances, it is advantageous to carry out the
esterification in the presence of a catalyst such as sulphuric
acid, pyridine hydrochloride, hydrochloric acid, benzenesulphonic
acid, p-toluenesulphonic acid, phosphoric acid, or any other
known esterification catalyst. The amount of the catalyst in the
reaction may be as little as 0.01% (by weight of the reaction
mixture), more often from 0.1% to 5%.
The succinic acid esters may alternatively be obtained by
the reaction of a substituted succinic acid or anhydride with an
epoxide or a mixture of an epoxide and water. Such reaction is
similar to one involving the acid or anhydride with a glycol.
For instance, the product may be prepared by the reaction of a
substituted succinic acid with one mole of ethylene oxide.
Similarly, the product may be obtained by the reaction of a
substituted succinic acid with two moles of ethylene oxide.
Other epoxides which are commonly available for use in such
reaction include, for example, propylene oxide, styrene oxide,
l,2-butylene oxide, 2,3-butylene oxide, epichlorohydrin,
cyclohexene oxide, l,2-octylene oxide, epoxidised soya bean oil,
methyl ester of 9,l0-epoxy-stearic acid and butadiene mono-
epoxide. For the most part, the epoxides are the alkylene oxides
in which the alkylene radical has from 2 to 8 carbon atoms; or
the epoxidised fatty acid esters in which the fatty acid radical
has up to 30 carbon atoms and the ester radical is derived from a
lower alcohol having up to 8 carbon atoms.
In lieu of the succinic acid or anhydride, a lactone acid or
a substituted succinic acid halide may be used in the processes
- 21~g~
illustrated above. Such acid halides may be acid dibromides,
acid dichlorides, acid monochlorides, and acid monobromides. The
substituted succinic anhydrides and acids can be prepared by, for
example, the reaction of maleic anhydride with a high molecular
weight olefin or a halogenated hydrocarbon such as is obtained by
the chlorination of an olefin polymer described previously. The
reaction involves merely heating the reactants at a temperature
preferably from 100~C to 250~C. The product from such a reaction
is an alkenyl succinic anhydride. The alkenyl group may be
hydrogenated to an alkyl group. The anhydride may be hydrolysed
by treatment with water or steam to the corresponding acid.
Another method useful for preparing the succinic acids or
anhydrides involves the reaction of itaconic acid or anhydride
with an olefin or a chlorinated hydrocarbon at a temperature
usually within the range from 100~C to 250~C. The succinic acid
halides can be prepared by the reaction of the acids or their
anhydrides with a halogenation agent such as phosphorous
tribromide, phosphorus pentachloride, or thionyl chloride. These
and other methods of preparing the succinic compounds are well
known in the art and need not be illustrated in further detail
here.
Still further methods of preparing esters useful in the fuel
compositions of the present invention are available. For
instance, the esters may be obtained by the reaction of maleic
acid or anhydride with an alcohol such as is illustrated above to
form a mono- or diester of maleic acid and then the reaction of
this ester with an olefin or a chlorinated hydrocarbon such as is
illustrated above. They may also be obtained by first
esterifying itaconic anhydride or acid and subsequently reacting
the ester intermediate with an olefin or a chlorinated
hydrocarbon under conditions similar to those described
hereinabove.
A large number of different types of polymeric dispersants
have been suggested as useful in lubricating oil formulations,
and such polymeric dispersants are useful in the fuel
compositions of the present invention. Often, such additives have
21~0~
- 26 -
been described as being useful in lubricating formulations as
viscosity index improvers with dispersing characteristics. The
polymeric dispersants generally are polymers or copolymers having
a long carbon chain and containing "polar" groups to impart the
dispersancy characteristics. Examples of polar groups include
amino, amido, imino, imido, hydroxyl and ether groups. For
example, the polymeric dispersants may be copolymers of
methacrylates or acrylates containing additional polar groups,
ethylene/propylene copolymers containing polar groups or vinyl
acetate/fumaric acid ester copolymers.
Many such polymeric dispersants have been described in the
prior art, for example in U.S. Patents Nos. 4,402,844, 3,356,763
and 3,891,721.
A number of the polymeric dispersants may be prepared by
grafting polar monomers on to polyolefinic backbones. For
example, U.S. Patents Nos. 3,687,849 and 3,687,905 describe the
use of maleic anhydride as a graft monomer to a polyolefinic
backbone. Maleic acid or anhydride is particularly desirable as
a graft monomer because this monomer is relatively inexpensive,
provides an economical route to the incorporation of dispersant
nitrogen compounds into polymers by further reaction of the
carboxyl groups of the maleic acid or anhydride with, for
example, nitrogen compounds or hydroxy compounds. U.S. Patent
No. 4,160,739 describes graft copolymers obtained by the grafting
of a monomer system comprising maleic acid or anhydride and at
least one other different monomer which is addition
copolymerisable therewith; the grafted monomer system then being
post-reacted with a polyamine. The monomers which are
copolymerisable with maleic acid or anhydride are any alpha,
beta-monoethylenically unsaturated monomers which are
sufficiently soluble in the reaction medium and reactive towards
maleic acid or anhydride so that substantially larger amounts of
maleic acid or anhydride can be incorporated into the grafted
polymeric product. Accordingly, suitable monomers include the
esters, amides and nitriles of acrylic and methacrylic acid, and
monomers containing no free acid groups. The incorporation of
21~0~92
heterocyclic monomers into graft polymers is described by a
process which comprises a first step of graft polymerising an
alkyl ester of acrylic acid or methacrylic acid, alone or in
combination with styrene, onto a backbone copolymer which is a
hydrogenated block copolymer of styrene and a conjugated diene
having 4 to 6 carbon atoms to form a first graft polymer. In the
second step, a polymerisable heterocyclic monomer, alone or in
combination with a hydrophobising vinyl ester is co-polymerised
onto the first graft copolymer to form a second graft copolymer.
Other patents describing graft polymers useful as
dispersants in the fuel compositions of this invention include
U.S. Patents Nos. 3,243,481, 3,475,514, 3,723,575, 4,026,167,
4,085,055, 4,181,618 and 4,476,283.
Another class of polymeric dispersant useful in the fuel
compositions of the invention are the so-called "star" polymers
and copolymers. Such polymers are described in, for example U.S.
Patents Nos. 4,346,193, 4,141,847, 4,358,565, 4,409,120 and
4,077,893.
The hydrocarbon-substituted phenolic dispersants useful in
the fuel compositions of the present invention include the
hydrocarbon-substituted phenolic compounds wherein the
hydrocarbon substituents have a molecular weight which is
sufficient to render the phenolic compound fuel soluble.
Generally, the hydrocarbon substituent will be a substantially
saturated, hydrocarbon-based group of at least 30 carbon atoms.
The phenolic compounds may be represented generally by the
following formula:
(R15) -Ar1-(OH) XII
wherein R15 is a substantially saturated hydrocarbon-based
substituent having an average of from 30 to 400 aliphatic carbon
atoms, and g and h are each 1, 2 or 3. Arl is an aromatic moiety
such as a benzene nucleus, naphthalene nucleus or linked benzene
nuclei. optionally, the above phenates as represented by formula
XII may contain other substituents such as lower alkyl, lower
alkoxy, nitro, amino and halo groups. Preferred examples of
21~04 ~ ~
optional substituents are the nitro and amino groups.
The substantially saturated hydrocarbon-based group Rl5 in
formula XII may contain up to 750 aliphatic carbon atoms although
it usually has a m~x;m1,m of an average of 400 carbon atoms. In
some instances Rl5 has a mi ni mllm of 50 carbon atoms. As noted,
the phenolic compounds may contain more than one Rl5 group for
each aromatic nucleus in the aromatic moiety Arl.
Generally, the hydrocarbon-based groups Rl5 are derived from
homo- or interpolymers (e.g., copolymers, terpolymers) of mono-
and diolefins having 2 to lO carbon atoms, such as ethylene,propylene, butene-l, isobutene, butadiene, isoprene, l-hexene and
l-octene. Typically, these olefins are l-monoolefins. The Rl5
groups can also be derived from the halogenated (e.g.,
chlorinated or brominated) analogues of such homo- or
interpolymers. The Rl5 groups can, however, be made from other
sources, such as monomeric high molecular weight alkenes (e.g. l-
tetracontene) and chlorinated analogues and hydrochlorinated
analogues thereof, aliphatic petroleum fractions, particularly
paraffin waxes and cracked and chlorinated analogues and
hydrochlorinated analogues thereof, white oils, synthetic alkenes
such as those produced by the Ziegler-Natta process (e.g.,
poly(ethylene) greases) and other sources known to those skilled
in the art. Any unsaturation in the Rl5 groups may be reduced or
eliminated by hydrogenation according to procedures known in the
art before the nitration step described hereafter.
Specific examples of the substantially saturated
hydrocarbon-based Rl5 groups are the following: a tetracontanyl
group, a henpentacontanyl group, a mixture of
poly(ethylene/propylene) groups of 35 to 70 carbon atoms, a
mixture of oxidatively or mechanically degraded
poly(ethylene/propylene) groups of 35 to 70 carbon atoms, a
mixture of poly(propylene/l-hexene) groups of 80 to 150 carbon
atoms, a mixture of polyisobutene groups having 20 to 32 carbon
atoms, and a mixture of polyisobutene groups having an average of
50 to 75 carbon atoms.
A preferred source of the group Rl5 are polyisobutenes
21~049~
- 29 -
obtained by polymerlsation of a C4 refinery stream having a
butene content of 35 to 75 weight per cent and isobutene content
of 30 to 60 weight per cent in the presence of a Lewis acid
catalyst such as aluminium trichloride or boron trifluoride.
These polyisobutenes contain predominatly (greater than 80~ of
total repeat units) isobutene repeating units of the
configuration.
-C(CH3)2CH2
The attachment of the hydrocarbon-based group R15 to the
aromatic moiety Ar1 can be accomplished by a number of techniques
well known to those skilled in the art.
In one preferred embodiment, the phenolic dispersants useful
in the fuel compositions of the present invention are
hydrocarbon-substituted nitro phenols as represented by formula
XII wherein the optional substituent is one or more nitro groups.
The nitro phenols can be conveniently prepared by nitrating
appropriate phenols, and typically, the nitro phenols are formed
by nitration of alkyl phenols having an alkyl group of at least
30 and preferably at least S0 carbon atoms. The preparation of a
number of hydrocarbon-substituted nitro phenols useful in the
fuel compositions of the present invention is described in U.S.
Patent No. 4,347,148.
In another preferred embodiment, the hydrocarbon-substituted
phenol dispersants useful in the present invention are
hydrocarbon-substituted amino phenols such as represented by
formula XII wherein the optional substituent is one or more amino
groups. These amino phenols can conveniently be prepared by
nitrating an appropriate hydroxy aromatic compound as described
above and thereafter reducing the nitro groups to amino groups.
Typically, the useful amino phenols are formed by nitration and
reduction of alkyl phenols having an alkyl or alkenyl group of at
least 30 and preferably at least 50 carbon atoms. The
preparation of a large number of hydrocarbon-substituted amino
phenols useful as dispersants in the present invention is
described in U.S. Patent No. 4,320,021.
2140~
- 30 -
Also useful as dispersants in the fuel compositions of the
present invention are fuel-soluble alkoxylated derivatives of
alcohols, phenols and amines. A wide variety of such derivatives
can be utilised as long as the derivatives are fuel-soluble.
More preferably, the derivatives in addition to being fuel-
soluble should be water-insoluble. Accordingly, in a preferred
embodiment, the fuel-soluble alkoxylated derivatives useful as
the dispersants are characterised as having an HLB of from l to
13.
As is well known to those skilled in the art, the fuel-
solubility and water-insolubility characteristics of the
alkoxylated derivatives can be controlled by selection of the
alcohol, phenol or amine, selection of the particular alkoxy
reactant, and by selection of the amount of alkoxy reactant which
is reacted with the alcohol, phenol or amine. Accordingly, the
alcohols which are utilised to prepare the alkoxylated
derivatives are hydrocarbon-based alcohols while the amines are
hydrocarbyl-substituted amines as described above. The phenols
may be phenols or hydrocarbon-substituted phenols and the
hydrocarbon substituent may contain as few as 1 carbon atom.
The alkoxylated derivatives are obtained by reacting the
alcohol, phenol or amine with an epoxide or a mixture of an
epoxide and water. For example, the derivative may be prepared
by the reaction of the alcohol, phenol or amine with an equal
molar amount or an excess of ethylene oxide. Other epoxides
which can be reacted with the alcohol, phenol or amine include,
for example, propylene oxide, styrene oxide, 1,2-butylene oxide,
2,3-butylene oxide, epichlorohydrin, cyclohexene oxide and 1,2-
octylene oxide. Preferably, the epoxides are the alkylene oxides
in which the alkylene group has from 2 to 8 carbon atoms. As
mentioned above, it is desirable and preferred that the amount of
alkylene oxide reacted with the alcohol, phenol or amine be
insufficient to render the derivative water-soluble.
The following are examples of commercially available
alkylene oxide derivatives which may be utilised as dispersants
in the fuel compositions of the present invention: Ethomeen
-- 21~!)4~
S/12, tertiary amines ethylene oxide condensation products of the
primary fatty amines (HLB, 4.15; Armak Industries); and Plurafac
A-24, an oxyethylated straight-chain alcohol available from BASF
Wyandotte Industries (H~B 5.0). Other suitable fuel-soluble
alkoxylated derivatives of alcohols, phenols and amines will be
readily apparent to those skilled in the art.
In a particularly preferred embodiment, further to the
compound of formula I, the fuel composition of the invention may
additionally contain, as ashless dispersant, a minor amount of a
polyolefin-substituted succinimide derivative wherein the
polyolefin has number average molecular weight (Mn) in the range
800 to 5000, preferably 1000 to 5000, more preferably at least
1750, 1800 or 1850 and at most 4000, 3500, 3000 or 2500. The
amine from which the succinimide is formed is preferably a C1_30
amine, especially a C4_12 amine containing 3 to 7 nitrogen atoms,
e.g. diethylene triamine, triethylene tetramine, tetramethylene
pentamine, pentaethylene hexamine, hexaethylene heptamine,
tripropylene tetramine and mixtures of any 2 or more thereof.
Preferably the hydrocarbon-soluble ashless dispersant is
present in an amount in the range 30 to 500 ppmw, more preferably
100 to 300 ppmw, based on total composition.
The fuel composition may additionally include (e.g. as an
alternative to inclusion of succinimide derivative) an oil
soluble polyamine as described in EP-A-290 088 or an N-
substituted carbamate as described in EP-A-414 963, in each case
in similar quantities to those described therein.
The fuel composition may further include, as flame-speed
improver, an alkali metal or alkaline earth metal salt of a
succinic acid derivative as described in EP-A-290 088, in similar
quantities to those described therein.
Apart from components already described above, the fuel
composition may also contain other additives. Thus, it can
contain a lead compound as an anti-knock additive, and
accordingly the fuel composition according to the invention
includes both leaded and unleaded gasoline. The fuel composition
can also contain antioxidants such as phenolics, e.g. 2,6-di-
2140~2
- 32 -
tert-butyl-phenol, or phenylenediamines, e.g. N,N'-di-sec-butyl-
p-phenylene-diamine, or antiknock additives other than lead
compounds, or polyether amino additives, e.g. as described in
U.S. Patent No. 4,477,261 and EP-A-151 621.
In the fuel composition according to the invention, the fuel
used is preferably gasoline, i.e. a hydrocarbon base fuel boiling
essentially in the gasoline boiling range from 30 to 230~C. Such
a base fuel may comprise mixtures of saturated, olefinic and
aromatic hydrocarbons. It can be derived from straight-run
gasoline, synthetically produced aromatic hydrocarbon mixtures,
thermally or catalytically cracked hydrocarbon feedstocks,
hydrocracked petroleum fractions or catalytically reformed
hydrocarbons. The octane number of the base fuel is not critical
and will generally be above 65. In the gasoline, hydrocarbons
can be replaced up to substantial amounts by alcohols, ethers,
ketones, or esters. Naturally, the base fuel is desirably
substantially free of water, since water may impede a smooth
combustion.
This invention is illustrated by the following examples, in
which the number average molecular weights (Mn) quoted were,
unless otherwise indicated, determined by gel permeation
chromatography (GPC) against polystyrene and polypropylene glycol
standards.
The following terms used in the Examples are explained
below.
Polyglycol I - a C12 - C15 linear alcohol initiated
propylene oxide homopolymer of number average molecular weight
(Mn) 1500;
Polyglycol II - a C14 - C15 linear alcohol initiated
propylene oxide homopolymer of number average molecular weight
(~n) 1500;
Polyglycol III - a butanol initiated 85 wt% propylene
oxide/15 wt~ ethylene oxide random copolymer of number average
molecular weight (Mn) 2000;
Polyglycol IV - a butanol initiated 85 wt~ propylene
oxide/15 wt~ ethylene oxide random copolymer of number average
~ 1 4 ~
- 33 -
molecular weight (Mn) 2500;
Polyglycol V - a polyethylene glycol initiated 50 wt%
propylene oxide/50 wt~ ethylene oxide partially random/partially
block copolymer of number average molecular weight (Mn~ 2000;
Polyglycol VI - a C12 - C15 linear alcohol initiated
propylene oxide homopolymer of number average molecular weight
(Mn) 500.
"VERSATIC" 5 (trade mark) acid (2,2-dimethylpropanoic acid)
which is commercially available from member companies of the
Royal Dutch/Shell Group.
"VERSATIC" 10 (trade mark) acid - a mixture of branched
carboxylic acids of general formula
R5-C-Co2H (III')
R6
where R4 represents a group of formula CaH2a- ; R5
represents a group of formula CbH2b+1- ; R6 represents a group of
general formula CCH2c+l-; and a + b + c = 8, which is
commercially available from member companies of the Royal
Dutch/Shell Group.
Polyglycol PE6100 - an ethylene oxide/propylene
oxide/ethylene oxide block copolymer of number average molecular
weight (Mn) 2000 (method of Mn determination unknown). This
material corresponds to "PLURONIC" ~trade mark) PL-61 polyol
available from Wyandotte Chemical Company.
Polyglycol PE8100 - an ethylene oxide/propylene
oxide/ethylene oxide block copolymer of number average molecular
weight (Mn) 2600 (method of Mn determination unknown). This
material corresponds to "PLURONIC" (trade mark) PL-81 polyol
available from Wyandotte Chemical Company.
4 ~ 2
- 34 -
r ,le 1
Preparation of ester of Polyglycol I and "VERSATIC" 10 (trade
mark) acid (a : _-u,.d of formula I in which R1 i~ C12-C15 alkyl,
A i8 -O-, X is a polyoxypropylene chain and R2 i8 a group of
f_ 1 A III in which a + b + c = 8)
To a solution of the Polyglycol I (300g, 0.21M) in toluene
(150ml) in a round-bottomed 3-necked flask was added anhydrous
potassium carbonate (34.5g, 0.25M) followed by the acid chloride
derivative of "VERSATIC" 10 (trade mark) acid (47.6g, 0.25M) with
stirring at ambient temperature (20~C). The flask was fitted with
an overhead stirrer, a thermometer and a condenser and immersed
in an oil bath. The mixture was refluxed at a temperature of
about 120 - 130~C for 16 hours. The crude reaction mixture was
then allowed to cool to ambient temperature and filtered through
a sintered glass funnel. The excess solvent was then removed in
vacuo. The crude product was then stirred with sodium carbonate
(50g, 0.3M) and water (5ml) for 8 hours. The mixture was then
diluted with toluene (150ml) and filtered. The resultant
solution was dried over anhydrous sodium sulphate (50g), filtered
and the solution concentrated in vacuo to afford the required
product (315g, 95~ yield).
r-- ,le 2 to 19
By processes analogous to the process of Example 1, the
polyglycols and carboxylic acid derivatives noted in Table 1 were
reacted to prepare further compounds of general formula I
according to the invention.
Example 20
Preparation of Adipate ester of Polyglycol I (a -~ _ ' of
formula I in which R1 i8 C12-C15 alkyl, A i8 -O-, X i~ a
polyoxypropylene chain and R2 i~ a group -(CH2)4C(o)-Y-A'-R7
where R7, A' and Y have the ~ame definitions respectively as R',
A and X)
To a mixture of the Polyglycol I (300g, 0.2M) and Adipic
acid (15.5g, O.lM) in a round-bottomed 3-necked flask fitted with
a Dean and Stark trap was added, with stirring, under nitrogen,
toluene (150ml) and para-Toluene sulphonic acid (9.5g, 0.05M).
2140~9~
The resulting mixture was stirred under nitrogen for 16 hours at
130~C, before being cooled to ambient temperature (20~C), washed
with lM aqueous potassium hydroxide solution (3 x 200ml), washed
with lO~w/v aqueous sodium sulphate solution (5 x 200ml), dried
S over anhydrous sodium sulphate (Na2SO4), filtered and evaporated
under reduced pressure. The crude product was then stirred with
sodium carbonate (50g, 0.3M) and water (5ml) for 8 hours. The
mixture was then diluted with toluene (150ml) and filtered. The
resultant solution was dried over anhydrous sodium sulphate
(50g), filtered and the solution concentrated in vacuo to afford
the required product (248g, 80% yield).
1_ 21
Preparation of ester of Polyglycol II and "VERSATIC" 5 (trade
mark) acid (a c -u..d of formula I in which R1 is C14-C15 alkyl,
A i~ -O-, X i~ a polyoxypropylene chain and R2 is t-butyl)
To a mixture of the Polyglycol II (150g, O.lM) and pyridine
(150ml) in a round bottomed flask was added the anhydride
derivative of "VERSATIC" 5 (trade mark) acid (46.5g, 0.25M). The
resulting mixture was refluxed for 14 hours, before being cooled
to ambient temperature, poured into water (400ml) and extracted
into 60-80 petroleum spirit (3 x 200ml). The organic phase was
washed with water (3 x 200ml), dried over anhydrous sodium
sulphate (Na2SO4), filtered and evaporated under reduced
pressure. The crude product was then stirred with sodium
carbonate (50g, 0.3M) and water (5ml) for 8 hours. The mixture
was then diluted with toluene (150ml) and filtered. The
resultant solution was dried over anhydrous sodium sulphate
(50g), filtered and the solution concentrated in vacuo to afford
the required product (119g, 75% yield).
r 1~ 22
Preparation of ester of Polyglycol VI and "VERSATIC" 10 ~trade
mark) acid ~a ~ of formula I in which R1 i~ C12-C15 alkyl,
A is -O-, X is a polyoxypropylene chain and R2 i5 a group of
formula III in which a + b + c = 8)
To a solution of the Polyglycol VI (150g, 0.25M) in toluene
(150ml) in a round-bottomed 3-necked flask was added
2141)~
- 36 -
triethylamine (35.4g, 0.35M) followed by the acid chloride
derivative of "VERSATIC" 10 (trade mark) acid (66.7g, 0.35M) with
stirring at ambient temperature. The flask was fitted with an
overhead stirrer, a thermometer and a condenser and immersed in
an oil bath. The mixture was refluxed at a temperature of about
120 - 130~C for 7 hours. The crude reaction mixture was then
allowed to cool at ambient temperature, washed with water (3 x
200ml), dried over anhydrous sodium sulphate (Na2SO4), filtered
and the solvent evaporated under reduced pressure. The crude
product was then stirred with sodium carbonate (50g, 0.3M) and
water (5ml) for 8 hours. The mixture was then diluted with
toluene (150ml) and filtered. The resultant solution was dried
over anhydrous sodium sulphate (50g), filtered and the solution
concentrated in vacuo to afford the required product (150g, 80
yield).
Comparative r le~ C1 to C4
By processes analogous to the process of Example 1, the
polyglycols and carboxylic acid derivatives noted in Table 2 were
reacted to prepare other substituted polyoxyalkylene compounds
for comparison with the compounds of the invention.
C~ ative r le C5
Preparation of bis - oleic acid ester of Polyglycol PE6100
To a solution of the Polyglycol PE6100 (300g, 0.15M) in
toluene (200ml) in a round-bottomed 3-necked flask was added
para-toluene sulphonic acid (0.6g) and Oleic acid (120g, 3 equiv)
with stirring under nitrogen. The flask was fitted with an
overhead stirrer and a Dean and Stark trap. The mixture was
heated to reflux at a temperature of 119~C for 8 hours. The
toluene was removed by heating the flask contents to a
temperature of 150~C and passing a stream of nitrogen therethrough
at a rate of 1,500cc/min.
C~ --ative r ~le C6
Preparation of bis - oleic acid ester of Polyglycol PE8100
To a solution of Polyglycol PE8100 (300g, 0.108M) in toluene
(400 ml) in a round-bottomed 3-necked flask was added para-
toluene sulphonic acid (0.3g) and Oleic acid (87g, 3 equiv) with
-- 21~ 9 h
stirring under nitrogen. The flask was fitted with an overhead
stirrer and a Dean and Stark trap. The mixture was heated to
reflux at a temperature of 118~C for 7 hours. The toluene was
removed by heating the flask contents to a temperature of 150~C
and passing a stream of nitrogen therethrough at a rate of
l,500cc/min.
~s~_ t of Polyoxyalkylene ~ ..d~
The following assessments of the compounds of Examples 1 to
22 and Comparative Examples C1 to C6 were undertaken. Results
are provided in Tables 3, 4 and 5.
(i) Total Acid Number (TAN)
The TAN of a material is the quantity of base, expressed in
milligrams of potassium hydroxide (mg KOH), that is required to
neutralise all acidic constituents present in 1 gram of the
material. The TAN is obtained by standard test method ASTM
D664-81.
(ii) Kinematic viscosity at 40~C and at 100~C was determined
according to standard test method ASTM D445.
(iii) Viscosity Index was calculated according to standard test
method ASTM D2270-86.
(iv) Package Stability Test Background
Standard lubricant additive packages contain inter alia, one
or more metal detergents. The packages fall into two categories:
single metal (e.g. calcium only) formulations and mixed metal
(e.g. calcium and magnesium formulations. Ideally, any potential
rust inhibitor should be capable of working in both single metal
and mixed metal formulations.
Procedure
Test samples were prepared by incorporating each of the
compounds of Examples 1 to 16, 18 to 22 and Comparative Examples
Cl to C5 into one or both of two commercially available lubricant
additive packages containing metal detergents, at a concentration
of 3wt~. One of the additive packages was of the single metal
type (calcium only), whilst the other was of the mixed metal type
(calcium and magnesium). The test samples were stored at 60~C for
up to three months and the relative stability of the samples
- 2l~n4~
- 38 -
measured.
Tables 3 and 4 list the results in terms of either deposit
(D) or haze (H) of the samples. The terms "TD" and "VSH" used in
the Tables mean "trace deposit" and "very slight haze". The
Tables also note the "storage time" after which measurements were
taken.
(v) Neutralisation Rate Test Background
U.S. Patent No. 3,933,662 (Chevron) describes a method for
ranking candidate rust fix additives by means of a neutralisation
rate test which it suggests can be correlated to results from
rust engine tests, e.g. Sequence IIB engine test. The method
involves neutralisation of an acidic aqueous phase with a basic
oil phase and monitoring the progress of neutralisation using a
pH meter.
Procedure
Each of the compounds of Examples 1 to 22 and Comparative
Examples C1 to C6 was incorporated into samples of the two
lubricant additive packages described above for the Package
Stability Test, at a concentration of 3wt%. The resulting
concentrates were then blended at a concentration of lOwt% with a
base oil being a mixture of "HVI" 60 (trade mark) base oil (a
bright and clear high viscosity index base oil having kinematic
viscosity at 100~C of 4.4 to 4.9 mm2/s (ASTM D445) and min;mllm
flash point of 204~C (ASTM D92)) and "HVI" 115 (trade mark) base
oil (a bright and clear high viscosity index base oil having
kinematic viscosity at 100~C of ~.2 to 9.2 mm2/s (ASTM D445) and
minimum flash point of 232~C (ASTM D92)), the weight ratio of
"HVI" 60 (trade mark) base oil to "HVI" 115 base oil in the
mixture being 5:1. The resulting oils were then tested as
follows.
A 250ml 3-necked round-bottomed flask was fitted with a
combined pH electrode and an overhead stirrer. The flask was
immersed to a set level in a constant temperature oil bath at
50~C. Hydrochloric acid (O.OlM; 30ml) was charged to the flask
and allowed to reach constant temperature (30 minutes). The test
oil (20ml) was then introduced to the flask using a syringe and
- 2140~2
the pH measured after ten minutes.
Tables 3 and 4 list the pH values obtained.
It will be seen from Tables 3 and 4 that, whereas the
neutralisation rate test results indicate that the compounds of
Examples 1 to 22 (compounds of formula I according to the
invention) would possess comparable rust fix properties to the
compounds of Comparative Examples Cl to C6, the package stability
test results clearly show that the additive concentrates
(packages) of the invention containing the compounds of formula I
had good storage stability, whilst those containing the
comparative compounds were insufficiently stable.
(vi) Sequence IID Engine Test Procedure
A series of oils prepared as described above for the
neutralisation rate test containing the compounds of Examples 1,
4, 7 and 14 were tested in the Sequence IID Engine Test to
evaluate the rust fix properties of these compounds. In this
test, a figure of 8.5 or above represents a pass. The results
obtained are shown in Table 5 below.
TABLE 1
RATIO OF MOLES OF REACTANTS
EXAMPLE NO. POLYGLYCOL TYPE CARBOXYLIC ACID DERIVATIVE (ACID DERIVATIVE TO
POLYGLYCOL)
1 I "VERSATIC" 10 [(R4)(R5)(R6)CCOCl]1.2
2 II "VERSATIC" 10 [(R4)(R5)(R6)CCOCl]1.2
3 III "VERSATIC" 10 [(R4)(R5)(R6)CCOCl] 2
4 I (CH3)~CCOCl 1.2
III (CH~)~CCOCl 1.2
6 IV (CH~)~CCOCl 1.2
7 VI (CH~)3CCOCl 1.2
8 I CH3(CH~)~COCl 1.1
9 III CH~(CH?)~COCl 1.1
I CH~(CH?)~CH(C?H~)COCl 1.2 t--~
11 III CH~(CH?)~CH(C~H~)COCl 1.1 O
12 I CH~(CH?)14COC1 1.2
13 III CH~(CH?)14COCl 1.2
14 I CH3(CH~)~6COCl 1.3
III CH~(CH~)16COCl 1.1
16 I C~H~CH?COCl 1.3
17 III C~H~CH~COCl 1.2
TABLE 1 ~cont'd)
RATIO OF MOLES OF REACTANTS
EXAMPLE NO. POLYGLYCOL TYPE CARBOXYLIC ACID DERIVATIVE(ACID DERIVATIVE TO
POLYGLYCOL)
18 I C6H5COCl 1.3
19 III C~H~COCl 1.2
I HOOC(CH~)4COOH 0.5
21 II "VERSATIC" 5 [(CH~)~CCo]~O 2.5
22 VI "VERSATIC" 10 [(R4)(R5)(R6)CCOCl] 1.4
TABLE 2
RATIO OF MOLES OF REACTANTS
EXAMPLE NO. POLYGLYCOL TYPE CARBOXYLIC ACID DERIVATIVE (ACID DERIVATIVE TO
POLYGLYCOL)
Cl V "VERSATIC" 10 [(R4)(R5)(R6)CCOCl] 2
C2 V "VERSATIC" 10 [(R4)(R5)(R6)CCOCl] 4
C3 V (CH3)~CCOCl
C4 V (CH~)~CCOCl 2
C5 PE6100 CH~(CH~)7CH=CH(CH~)7CO~H 3
C6 PE8100 CH~(CH~)7CH=CH(CH~)7Co?H 3
TABLE 3
EXAMPLE TAN KINEMATIC KINEMATIC VISCOSITY STORAGE
NUMBER mgKOH/g VISCOSITY VISCOSITY INDEX pH PACKAGE TIMEAT 40~CAT 100~C NEUTRALISATION STABILITY TEST (MONTHS) cSt cSt ALL MIXED ALL MIXED
(mm2/S)(mm2/S) CALCIUM METAL CALCIUM METAL
1 0.0491.8 17.0 202 6.4 7.1 3~H TD 3
2 0.0593.0 16.8 196 5.6 6.9 3~H 3
3 0.10159.3 28.1 216 6.8 7.215~H 12~H 3
4 0.0675.9 14.8 206 6.3 7.015~H 15~H 3
0.04142.8 27.1 228 6.6 7.0 l~H 10~H 3
6 0.03216.2 43.8 258 6.2 7.0 6%H 10~H 3
7 0.0518.9 4.9 197 5.0 5.4 4~H TD 3 ~_~
8 0.0572.2 15.0 219 6.0 7.110~H l~H 3 C~
9 0.05128.9 25.5 233 6.7 7.2 3~H 10~H 3
0.0582.9 16.0 208 6.3 6.9 VSH 3 i"~
11 0.10135.7 25.5 224 6.7 7.1 15~H 3
12 0.0590.3 17.6 214 6.2 6.8 VSH 3
13 0.10135.0 26.2 231 6.7 7.2 15~H 3
14 0.1092.8 18.1 215 5.9 7.0 TD 3
TABLE 3 (cont'd
EXAMPLE TAN KINEMATIC KINEMATIC VISCOSITY STORAGE
NUMBER mgKOH/g VISCOSITY VISCOSITY INDEX pH PACKAGE TIME
AT 40~CAT 100~C NEUTRALISATION STABILITY TEST (MONTHS)
cSt cSt ALL MIXED ALL MIXED
(mm2/S)(mm2/S) CALCIUM METAL CALCIUM METAL
0.10 140.1 26.8 229 6.6 7.0 15~H 3
16 0.05 86.0 16.1 201 6.4 6.7 TD 3
17 0.05 139.q 25.5 218 6.6 7.2 3
18 0.09 95.0 16.6 190 6.1 6.9 TD 3
19 0.10 160.5 27.3 209 6.5 7.2 15~H 3
0.10 200.6 34.5 220 6.3 6.9 TD 3
21 0.07 81.5 15.5 203 6.6 7.1 4~H 3 ~_,
22 0.10 23.6 5.2 163 5.5 5.2 8~H 3 ~a~
o
C~
~ _
TABLE 4
EXAMPLE TAN KINEMATIC KINEMATIC VISCOSITY STORAGE
NUMBER mgKOH/g VISCOSITY VISCOSITY INDEX pH PACKAGE TIME
AT 40~C AT 100~C NEUTRALISATION STABILITY TEST (MONTHS) cSt cSt ALL MIXED ALL MIXED
(mm2/s) (mm2/5) CALCIUM METAL CALCIUM METAL
Cl 0.10218.9 36.5 217 6.7 7.2 20~H 3
C2 0.10152.0 27.7 221 6.8 7.2 20~H 3
C3 0.10199.2 34.7 223 6.7 7.2 20~H 3
C4 0.10175.8 31.9 226 6.7 7.3 20~H 3
C5 16.24118.9 23.6 231 6.8 6.9 10~H 15~H
C6 38.00176.7 30.5 216 6.5 7.1
o
'- 2140~2
-- 46 --
rL r~
-
u
H
H
0l H
t,)
E~ g ,,,