Note: Descriptions are shown in the official language in which they were submitted.
,- , ` 21gO92~
Beiersdorf Aktiengesellschaft
Description
Germicidal active compound combinations
The present invention relates to active compound
combinations for protection against microorganisms. It
furthermore relates to formulations, preferably cosmetic
and dermatological formulations, in particular cosmetic
deodorants, comprising such active compound combinations.
It also relates to processes for stabilizing formula-
tions, in particular cosmetic and dermatological formula-
tions, against attack by microorganisms.
Active compound combinations for protection
against microorganisms are used, for example, in formula-
tions which are applied to the skin or mucous membrane of
a person or an An; ~1 in order to reduce or eliminate
microorganism attack on or in the skin or to prevent
attack by microorganisms. Such compositions include the
terms topical dermatic agents and cosmetics.
However, protection against microorganism attack
on formulations themselves, in particular cosmetic and
dermatological formulations, is also regarded as protec-
tion against microorganisms in the context of the present
invention. In technical terms, this protection is called
preservation. Preservation in itself is not limited to
cosmetic or dermatological formulations. Rather, it
relates to protection of all organic materials against
microbial degradation.
Cosmetic formulations for protection against
microorganisms are primarily deodorants, that is to say
formulations which are intended to eliminate body odour.
Body odour is formed when fresh perspiration, which is in
itself odourless, is decomposed by microorganisms. During
this operation, a large number of highly volatile sub-
stances are formed, malodorous isovaleric acid being
mentioned only as an example.
- 2140921~
Deodorants:
Cosmetic deodorants of the prior art are based on
various action principles.
The formation of perspiration can be suppressed
by astringents - aluminium salts, such as aluminium
hydroxychloride, are chiefly used. Apart from the fact
that the associated denaturing of skin proteins is an
undesirable secondary reaction, the substances used for
this purpose furthermore intervene in the heat balance of
the skin and should at best be used in exceptional cases.
The bacterial flora on the skin may be reduced by
antimicrobial substances. In the ideal case, only the
microorganisms causing an odour should be destroyed here.
In practice, however, it has been found that the entire
microflora of the skin is harmed to the same extent. The
microorganisms which cause no odour are occasionally even
harmed more severely.
Finally, body odour can also be masked by fra-
grances, the classical method which, however, meets the
aesthetic requirements of the consumer the least, since
the mixture of body odour and perfume fragrance smells
rather unpleasant.
Deodorants should meet the following conditions:
(1) The biological processes of the skin should not be
impaired.
(2) The deodorants should not have a pronounced intrin-
sic smell.
(3) They must be harmless in the event of an overdose or
other use not as specified.
(4) They should not become concentrated on the skin
after repeated use.
(5) They should be easy to incorporate into commercially
available cosmetic formulations.
Both liquid deodorants, for example aerosol
sprays, roll-ons and the like, and solid formulations,
for example deo sticks, powders, powder sprays, intimate
cleansing compositions and the like, are known and
customary.
An object of the present invention was thus to
214092 0
develop cosmetic deodorants which do not have the disad-
vantages of the prior art. In particular, the deodorants
should largely preserve the microflora of the skin, but
selectively reduce the microorganisms which are respon-
sible for body odour.
Topical dermatic aqents:
Some of the unpleasant or pathogenic germs attack
the various layers of the skin, including the acne
pathogen Propionibacterium acnes. P. acnes preferentially
populates the hair follicles and therefore usually breaks
out during puberty of the persons affected. The often
considerable skin lesions associated with it are at best
unattractive, but may also considerably trouble the
patient emotionally.
Lantibiotics have also already been proposed as
therapeutics against skin complaints caused microbially,
namely acne. A disadvantage is that these formulations
are stable for only a short time.
Another object of the present invention was thus
to provide formulations and processes which remedy this
shortcoming.
Stabilization of perfumed cosmetic formulations:
Cosmetic formulations, that is to say deodorants,
having an active content of lantibiotics are known from
DE-A 39 38 140. The compositions described therein are
distinguished by an outstanding action and tolerability.
However, the cosmetic deodorants described
therein have the disadvantage that an addition of perfume
constituents reduces the deodorizing activity of the
lantibiotics in an undesirable manner in a way which has
not yet been clarified exhaustively. Since almost all
cosmetics, whether deodorizing or not, comprise perfume
constituents, this reduction in action represents a
considerable problem.
Another object of the invention was thus to
suppress the harmful action of perfume constituents on
active compound components in cosmetic formulations.
Description of the invention:
It was astonishing and not foreseeable to the
21~0~20
-- 4
expert, and herein lies the achievement of all these
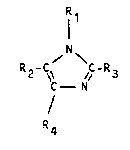
objects, that combinations of imidazole and/or one or
more derivatives of imidazole which can have the follow-
ing general structural formula
Rl
/~\
R2-c\ /~-R~
f N
R4
- wherein the radicals can have the following
meaning:
R1 = H or C(1 25) - alkyl,
R2 = ~ or C~1 25) - alkyl,
R3 = H or C~1 25) - alkyl,
R4 = H or C~1 25) - alkyl,
- wherein R4 can also, and preferably if R1, R2 and
R3 are a hydrogen atom, be the radicals
-CH2 -cH2 -NRsR6
and -CH2-CH(NRsR6)-COOR"
wherein
Rs = H or C~1 25) - alkyl,
R6 = H or C~l 25) - alkyl,
R, = H or C~1 25 ) - alkyl,
and a lantibiotic or a mixture of two or more
lantibiotics
- would be stable during storage,
- would have a sufficiently high half-life on the
skin,
- would be suitable for use as a cosmetic,
- do not allow interactions between lantibiotics
and perfume constituents,
- would have a suitable stability when used in
cosmetics,
- would be active selectively against odour-gener-
ating microorganisms,
- 2140920
-- 5 --
- would preserve the symbiotic microflora of the
skin,
- would also be fully active when perfume compo-
nents are used,
- would achieve a significant improvement in the
deodorizing action compared with imidazole-free
compositions,
- would have a suitable stability when used as
preservatives,
- would have a suitable stability when used in
foodstuffs.
In particular, it was astonishing that the
compositions according to the invention not only are
suitable for cosmetic purposes but moreover are more
effective and gentler than the compositions of the prior
art.
The invention also relates to the use of one or
more derivatives of imidazole which have the following
general structural formula
1 1
R C/ \C R
C N
R4
- wherein the radicals can have the following
meaning:
Rl = H or C~l 25) - alkyl,
R2 = H or C(l 25) - alkyl,
R3 = H or C~l 25 ) - alkyl,
R4 = H or C~l 25) - alkyl,
- wherein R4 can also, and preferably if Rl, R2 and
R3 are a hydrogen atom, be the radicals
-CH2 -cH2 -NR5R6
and -CH2-CH(NRsR6)-COOR"
wherein
2140920
- 6 -
Rs = H or C(l_ 25) - alkyl,
R6 = H or C(l 25~ - alkyl,
R, = H or C(l 25) - alkyl,
as the active principle in cosmetic deodorants.
Lantibiotics per se have been known for many
years. They are polypeptides which are synthesized by
microorganisms and are distinguished by representatives
of the amino acid group of lanthionines as a structural
element in the peptide sequence.
The lanthionines have the following structures:
HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH
also written as:
H2N-ala-S-ala-COOH
l l (meso-lanthionine)
COOH NH2
and
- 15 HOOC-CH(NH2)-CH2-S-CH(CH3)-CH(NH2)-COOH
also written as:
H2N-ala-S-aba-COOH
l l (threo-methyl-lanthionine)
COOH NH2
Ring structures are formed in the lantibiotics
with these lanthionines.
Examples of lantibiotics are nisin, epidermin,
subtilin, cinramycin, duramycin, ancovenin, gallidermin
and Pep 5.
The abovementioned substances are known per se
and can be found under the Chemical Abstracts registry
numbers:
Nisin :1414-45-5
Epidermin :99165-17-0
Subtilin :1393-38-0
Pep 5 :110655-58-8
Duramycin :1391-36-2
Ancovenin :88201-41-6
21~092~
Gallidermin : 117978-77-5
Nisin, for example, is a peptide of 34 amino
acids synthesized by Streptococcus lactis.
Nisin has the following amino acid sequence
(primary structure):
., . I S
H2N-ile-dhb-ala-ile-dha-leu-ala-aba-pro-gly-ala-lys-
S
-aba-gly-ala-leu-met-gly-ala-asn^
S
, S
I I
-met-lys-aba-ala-aba-ala-his-ala-
S
-ser-ile-his-val-dha-lys-COOH
wherein dhb = dehydrobutyrine
dha = dehydroalanine
aba = aminobutyric acid
E. Gross, J.L. Morell, "The Structure of Nisin", J. Amer.
Chem. Soc. 93, pages 4634-4637 (1971).
The action mechanism of nisin and the lantibiot-
ics related to nisin which have essentially the sameaction can be explained as follows: cell walls are
destroyed by release of autolysines. Since channels are
also formed in the cytoplasm membrane, low molecular
weight cell constituents can diffuse out, whereby the
(prokaryotic) cell is destroyed.
The accuracy of this explanation, however, is of
no relevance to the invention. An attempt is merely being
made to explain the microbiological processes.
Eukaryotic cells, that is to say skin cells,
- 2140920
- 8
fungi and the like, are resistant to nisin and other
lantibiotics.
Lantibiotics are practically non-toxic to
warm-blooded animals. The LD50 for nisin, for example, is
greater than 7 g/kg (determined for rats and cats). It is
known that nisin and the other lantibiotics chiefly act
against micrococci and coryneform bacteria. In some
countries, chiefly in Eastern Europe, it is a substance
which is approved as a foodstuffs preservative (not in
Germany).
For traditional reasons, lantibiotics are often
counted among the so-called "peptide antibiotics".
However, for various reasons they are not to be inter-
preted as conventional antibiotics, at least not all
their representatives, least of all nisin. Everything
suggests, rather, that they should be given the designa-
tion "bacteriocins". Bacteriocins are proteins which are
produced by bacteria and kill or inhibit the growth of
related bacteria species or strains. They are usually
coded by plasmids, the so-called bacteriocin factors.
Epidermin is a lantibiotic which has been iso-
lated from microorganisms occurring on the human skin.
The use of epidermin as an antibiotic/therapeutic having
a topical action is described in EP-A-0 181 578. It is
used for combating infectious diseases.
Gallidermin is a lantibiotic which differs from
epidermin by only one amino acid. EP-A-0 342 486 cites a
cosmetic agent comprising gallidermin, but without
cosmetics being disclosed and without the advantageous
combination of lantibiotics and imidazole or derivatives
thereof being obvious.
International Patent Application W0 89/12399
furthermore discloses combinations of nisin and complex-
ing agents and/or emulsifiers for use as preservatives.
Imidazole is characterized by the structural
formula
- 21~0g20
N j
/,
C - N
Imidazole is likewise practically non-toxic to warm-
blooded An;~-ls, but acts as an antimetabolite against
histamine and nicotinic acid in lower Ani~-ls. Certain
derivatives of imidazole are used as antimycotics.
Derivatives of imidazole which can likewise be
used according to the invention are those of the general
structural formula
R2-c\\ ,/-R3
C N
/
R4
wherein the radicals can have the following meaning:
Rl = H or C(1 2s) - alkyl,
10 R2 = H or C~l 25) - alkyl,
R3 = H or C~l 25) - alkyl,
R4 = H or C~l 25) - alkyl,
wherein R4 can also, and preferably if Rl, R2 and R3 are a
hydrogen atom, be the radicals
-CH2-CH2-NR5R6
and -CH2-CH(NR5R6)-COOR"
wherein
R5 = H or C(l 25) - alkyl,
R6 = H or C(l 25) - alkyl,
20 R, = H or C(l 25) - alkyl.
Among these derivatives of imidazole, those which
are water- and/or alcohol-soluble are preferred. Those
derivatives in which Rl = H are particularly preferred.
21~092(1
--- - 10 --
It is particularly advantageous to use the non-
derivatized parent substance (R, - R4 = H) of imidazole.
The derivatives histidine
/ N \
H-C\ /C-H
C N
HOOC-CH-CH2
NH2
and histamine
H
I
~N ~
C N
H2N-cH2-cH2
are also advantageous.
The activity of selected compounds according to
the invention decreases in the following sequence:
Imidazole (unsubstituted) > histamine > histidine
It is of course clear to the expert that the use
of histamine in products which come into direct contact
with the human or animal organism, that is to say, for
example, cosmetics and foodstuffs, would not be accept-
able, since histamine, as a tissue hormone, can bring
about undesirable reactions: histamine occurs in an
increased amount with allergies and anaphylaxes.
The use of the combination according to the
invention of histamine and lantibiotics should therefore
be subject to medical supervision.
The combinations according to the invention are
advantageously characterized by a content of
0.01 - 99.99 % by weight of an imidazole component
- 2140~2~
99.99 - 0.01 % by weight of an individual substance
or a mixture from the group
of lantibiotics,
based on the total weight of the combination.
The combinations according to the invention are
particularly advantageously characterized by a content of
0.1 - 99.9 % by weight of an imidazole component
and
99.9 - 0.1 % by weight of an individual substance
or a mixture from the group
of lantibiotics,
based on the total weight of the combination.
The combinations according to the invention are
especially advantageously characterized by a content of
1.0 - 99.0 % by weight of an imidazole component
and
99.0 - 1.0 % by weight of an individual substance
or a mixture from the group
of lantibiotics,
based on the total weight of the combination.
The lantibiotics are preferably present in the
final formulations in concentrations of 0.1 - 10000 ppm.
The lantibiotics are particularly preferably present in
the final formulations in concentrations of 0.1
750 ppm, especially preferably in concentrations of 0.5 -
400 ppm. The concentration data in each case relate to
the content of pure active compound and to the total
weight of the composition.
Formulations having an active content of nisin,
epidermin and/or gallidermin have proved to be particu-
larly advantageous. However, the other lantibiotics
mentioned are also particularly suitable for the use
according to the invention.
It is especially advantageous to use nisin.
It is possible and, where appropriate,
advantageous to employ a mixture of lantibiotics as the
active principle of the compositions according to the
invention.
2140920
- - 12 -
It is particularly advantageous to buffer the
compositions according to the invention to a pH range of
2.5 - 6.5. It is particularly favourable to choose the pH
in the range of 3.5 - 4.8.
The concentrationæ of imidazole are advantageous-
ly 0.01 - 5.00% by weight, based on the total weight of
the composition, preferably 0.10 - 2.00% by weight,
especially preferably 0.25 - 1.00% by weight.
Compositions which are particularly advantageous
are those which comprise combinations having a content of
0.1 - 10000ppm of lantibiotics, in
particular nisin, and
0.01 - 5.00% by weight of imidazole,
preferably
2.0 - 750ppm of lantibiotics, in particu-
lar nisin, and
0.10 - 2.00% by weight of imidazole,
particularly preferably
5.0 - 400 ppm of lantibiotics, in particu-
lar nisin, and
0.25 - 1.00 % by weight of imidazole,
in each case based on the total weight of the
formulation.
Those formulations which comprise 0.4 - 0.6% by
weight of imidazole or derivatives thereof are particu-
larly preferred.
Perfume oils which cause lasting deactivation of
lantibiotics in particular are listed in Table 1. How-
ever, if lantibiotics are present in combination with
imidazole or derivatives thereof, the destabilization is
compensated.
Table 1
Aldehyde C12 Hydroxycitronellol
Allyl amyl glycolate Isoeugenol
35 Ambrettolide Isoraldein 70
Amyl-cinnAm~ldehyde Jonol
Anisaldehyde Lilial (Firmenich)
Aurantesin Lyral
Benzyl acetate Methyl dihydrojasmonate
214092~
- 13 -
Benzyl salicylate Musk ketone
Castoreum p-Hydroxybenzylacetone
Cedryl ketone Patchouli oil
Eugenol Phenylethyl alcohol
5 Florol (Firmenich) Phenylethyl phenylacetate
Gamma decalactone Phenylethyl pivalate
Geraniol Tagetes oil
Geranium oil Bourbon Terpentine oil
Geranyl acetate Vertosine
10 Helosine Ylang-ylang-oil
Hexenyl salicylate Zibeth
Hydrocarboresin C;nnA~-ldehyde
Hydroxycitronellal (Firmenich) Cinnamyl alcohol
Apart from the perfume oil labelled otherwise,
the perfume oils listed in Table 1 are marketed by the
company Haarmann & Reimer.
Stabilization with respect to the substances
mentioned in Table 1 is claimed according to the inven-
tion, but is not to be limited thereto.
The invention thus also relates to the use of one
or more derivatives of imidazole which have the following
general structural formula
~N1
R 2 - C, ,C - R 3
R4
- wherein the radicals can have the following
meaning:
Rl = H or C(l 25~ - alkyl,
R2 = H or C(l 25) - alkyl,
R3 = H or C(l 25~ - alkyl,
R4 = H or C(l 25) - alkyl,
- wherein R, can also, and preferably if Rl, R2 and
21gO92~ .
_ - 14
R3 are a hydrogen atom, be the radicals
-CH2 -CH2 -NR5R6
and -CH2-CH(NR5R6)-COOR"
wherein
R5 = H or C(l 25~ - alkyl,
R6 = H or C(l 25, - alkyl,
R7 = H or C(l 25~ - alkyl,
for stabilizing lantibiotics.
The deodorizing cosmetic compositions according
to the invention can be in the form of a preparation
which can be sprayed from aerosol containers, squeeze
bottles or by a pump device, or in the form of a liquid
composition which can be applied by means of roll-on
devices, but also in the form of a water-in-oil or oil-
in-water emulsion, for example a cream or lotion, which
can be applied from normal bottles and containers. Other
cosmetic deodorants can be in the form of deodorizing
tinctures, deodorizing intimate cleansing compositions,
deodorizing shampoos, deodorant soap, deodorizing shower
or bath formulations, deodorizing powders or deodorizing
powder sprays. However, customary deo stick bases can
also serve as carriers for solid formulations and sticks.
Customary cosmetic carrier substances which can
be employed for preparation of the deodorizing composi-
tions according to the invention are, in addition towater, ethanol, isopropanol, glycerol and propylene
glycol, skincare fatty or fat-like substances, such as
partial glycerides of fatty acid mixtures, oleic acid
decyl ester, cetyl alcohol, cetyl stearyl alcohol and 2-
octyldodecanol, in the ratios of amounts customary forsuch preparations, as well as mucilaginous substances and
thickeners, for example methylcellulose, polyacrylic acid
and polyvinylpyrrolidone, and in addition also small
amounts of cyclic silicone oils (polydimethylsiloxanes),
as well as liquid polymethylphenylsiloxanes of low
ViSCOS ity .
Suitable propellants for deodorizing cosmetic
compositions according to the invention which can be
sprayed from aerosol containers in the form of a spray
- 2140~
- - 15 -
jet on actuation of the valve are the customary known
highly volatile liquefied propellants, for example
hydrocarbons (propane, butane and isobutane~, which can
be employed by themselves or as a mixture with one
another. Compressed air can also advantageously be used,
where appropriate.
Emulsifiers which have proved suitable for the
preparation of the deodorizing cosmetic compositions
according to the invention, which are preferably to be
applied to the desired areas of skin as liquid formula-
tions by means of a roll-on device, and can be used in
the compositions in a small amount tfor example) of 2 to
5% by weight, based on the total composition, are
nonionic types, such as polyoxyethylene fatty alcohol
ethers, for example cetostearyl alcohol polyethylene
glycol ether having 12 or 20 added-on ethylene oxide
units per molecule of cetostearyl alcohol, and sorbitan
esters and sorbitan ester-ethylene oxide compounds (for
example sorbitan monostearate and polyoxyethylene
sorbitan monostearate), as well as long-chain higher
molecular weight waxy polyglycol ethers.
In addition to the constituents mentioned, it is
possible to admix to the deodorizing cosmetic composi-
tions according to the invention - the pH of which is
preferably brought to 2.5 to 6.5, in particular 3.5
to 4.8, for example, by customary buffer mixtures -
perfume, dyestuffs, antioxidants (for example alpha-
tocopherol or butylated hydroxytoluene (= 2,6-di-tert-
butyl-4-methylphenol in amounts of 0.01 to 0.03%, based
on the total composition), suspending agents, buffer
mixtures or other customary cosmetic base substances,
such as triethanolamine or urea.
Those substances and perfume oils which are
stable, do not irritate the skin and already have
antibacterial (bacteriostatic) properties as such are
also suitable, where appropriate, for perfuming.
In an individual case, however, it is to be
weighed up whether the advantage which the highly selec-
tive antimicrobial action of the lantibiotics and, in
21~û~2~
- - 16 -
particular, of the combinations according to the inven-
tion offers is to be reduced or not by other substances
which, under certain circumstances, have a less selective
action.
However, the invention is not limited to the
formulations and compositions, auxiliaries and carrier
substances mentioned.
The particular amounts of cosmetic carrier
substances and perfume to be employed can easily be
determined by the expert by simple trials, as a function
of the nature of the particular product.
Apart from specific formulations which are noted
in each case separately in the examples, the cosmetic
compositions are prepared in the customary manner,
usually by simple mixing while stirring, if appropriate
with gentle heating. The preparation presents no diffi-
culties. For emulsions, the fat phase and the aqueous
phase, for example, are prepared separately, if necessary
with heating, and then emulsified.
Otherwise, the customary rules for composing
cosmetic formulations, with which the expert is familiar,
are to be observed.
The combinations according to the invention can
be incorporated into the compositions according to the
invention in a simple manner. They are preferably added
in dissolved form (for example as an aqueous, alcoholic
or alcoholic-aqueous solution) to the other consituents
of the formulations. However, it is especially advantage-
ous to incorporate lantibiotics into so-called powder
sprays. It is also advantageous to avoid additives which
can harm the natural microflora, since the combinations
according to the invention in themselves selectively
reduce the odour-generating microorganisms.
If the combinations according to the invention
are to be incorporated into powder sprays, the suspension
bases for this can advantageously be chosen from the
group consisting of
aerosil, kieselguhr, kaolin, talc, modified starch,
titanium dioxide, zinc oxide, silk powder, nylon powder,
- 2140920
- 17 -
polyethylene powder and related substances.
The following examples serve to describe the
invention, without the intention being to l;~it the
invention to these examples.
Perfume compositions:
Perfume No. 1
Benzyl acetate 4.0% by weight
Geraniol 5.0% by weight
Phenylethyl alcohol 9.0% by weight
Various other odoriferous substances to 100.0% by weight
Perfume No. 2
Benzyl acetate 5.4% by weight
Geraniol 8.0% by weight
Ylang-ylang oil 8.4% by weight
Various other odoriferous substances to 100.0% by weight
Perfume No. 3
Benzyl acetate 5.0% by weight
Geraniol 12.0% by weight
Hydroxycitronellal 7.0% by weight
Various other odoriferous substances to 100.0% by weight
Example 1
Pump spray
Nisin (pure substance) 0.025 g
Imidazole 5-000 g
Ethanol pharm. (96%) 354.875 g
Perfume 1 10.000 g
Dyestuff as desired
Water to1,000.000 g
ExamPle 2
Pump spray
Epidermin 0.010 g
Imidazole 3.500 g
Ethanol pharm. (96%) 354.875 g
Perfume 1 10.000 g
35 Dyestuff as desired
Water -to 1,000.000 g
214û920
- - 18 -
ExamPle 3
Deodorant roller (roll-on)
0.150 g
Nisin
' 8.000 g
Imldazole
5 Hydroxyethylcellulose 5.000 g
Propylene glycol 5.000 g
Ethanol pharm. (96%) 355.850 g
10.000 g
Perfume 2
as desired
Dyestuff
10 Water to 1,000.000 g
Example 4
Deodorant roller (roll-on)
Epidermin 0.120 g
Imidazole 6.500 g
15 Hydroxyethylcellulose 5.000 g
Propylene glycol 5-000 g
Ethanol pharm. (96%) 355.850 g
lO.ooo g
- Perfume 2
as desired
Dyestuff
20 Water to 1,000.000 g
Example 5
Spray
0.200 g
Nisin
Imidazole 1.500 g
25 Ethanol pharm. (96%) 150.000 g
50.000 g
Propylene glycol
300.000 g
Dimethyl ether
10.000 g
Perfume 3
to 1,000.000 g
Water
Example 6
Spray
Epidermin 0.150 g
Imidazole 2.000 g
Ethanol pharm. (96%) 150.000 g
35 Propylene glycol 50-000 g
300.000 g
Dimethyl ether
21~0920
- 19
10.000 g
Perfume 3
Water to 1,000.000 g
ExamPle 7
Spray
0.180 g
Nisin
17.000 g
Imldazole
Ethanol pharm. (96%) 497.160 g
2-Octyldodecanol 2.660 g
10.000 g
Perfume 1
Dimethyl ether to 1,000.000 g
ExamPle 8
Spray
Epidermin 0.150 g
Imidazole 13.800 g
Ethanol pharm. (96%) 497.160 g
2-Octyldodecanol 2.660 g
10.000 g
Perfume 1
Dimethyl ether to 1,000.000 g
Example 9
Nisin 1.200 g
Imidazole 32.000 g
Cetylstearyl alcohol 20.000 g
2-Octyldodecanol 20.000 g
' 200.200 g
Kaolln
200.200 g
25 Talc
48.200 g
Aerosll
10.000 g
Perfume 3
to 1,000.000 g
Rice starch
Example 10
30 Epidermin 1.000 g
27.000 g
Imidazole
Cetylstearyl alcohol 20.000 g
2-Octyldodecanol 20.000 g
200.200 g
Kaolin
200.200 g
35 Talc
21~092~
- - 20 -
Aerosil 48.200 g
Perfume 3 10.000 g
Rice starch to 1,000.000 g
ExamPle 1 1
5 Washing gel concentrate
Nisin 9.000 g
Imidazole 17.000 g
Cocoamidopropylbetaine 613.300 g
Tipa-lauryl ether sulphate 306.700 g
10 Sodium chloride as desired
Perfume 2 40.000 g
Dyestuff as desired
Citric acid 1.000 g
Glycerol 10.000 g
Water to 1,000.000 g
Example 12
Washing gel concentrate
Epidermin 7-000 g
Imidazole 12.000 g
Cocoamidopropylbetaine 613.300 g
Tipa-lauryl ether sulphate 306.700 g
Sodium chloride as desired
Perfume 12 40.000 g
Dyestuff as desired
25 Citric acid 1.000 g
Glycerol 10.000 g
Water to 1,000.000 g
Example 13
Powder spray I
30 a) Suspension base
Polymethylsiloxane
(Cyclomethicone) 72.000 g
Talc 24.000 g
Bentonite gel IPM 3-000 g
Nisin - 1.000 g
Imidazole 5-000 g
21~û92~
- - - 21 -
Perfume 1 2.500 g
b) Finished spray
Suspension base I 20.000 g
Propane/butane 80.000 g
Example 14
Powder spray II
a) Suspension base
Polymethylsiloxane
(Cyclomethicone) 72.000 g
Titanium dioxide 24.000 g
Bentonite gel IPM 3.000 g
Nisin 1.000 g
Imidazole 8.000 g
Perfume 3 2.000 g
b) Finished spray
Suspension base I 20.000 g
Propane/butane 80.000 g
ExamPle 15
Powder spray III
a) Suspension base
Polymethylsiloxane
(Cyclomethicone) 72.000 g
Silk powder 24.000 g
Bentonite gel IPM 3.000 g
Nisin 1.000 g
Imidazole 6.000 g
Perfume 2 2.000 g
b) Finished spray
Suspension base I 20.000 g
Propane/butane 80.000 g
The superiority of the present invention is
demonstrated with the aid of the following experiments.
The compositions according to the examples were evaluated
092~
-- - 22 -
with the aid of the so-called "sniff test".
Experiment 1:
Compositions according to the invention from
Example 1 were tested against a placebo, that is to say
a composition which is identical apart from the content
of active substance.
A group of-30 subjects were told to treat in each
case one armpit with composition according to the inven-
tion and the other with placebo. The subjects then wore
a shirt with slip inserts under the shoulders for three
hours. After this period of time, the slip inserts were
transferred to separate bottles. The smell of the inserts
was evaluated by three test persons. The experiment was
carried out as a double-blind experiment, so that neither
the subjects nor the test persons knew which armpit had
been treated with which composition.
It was found that the formulations cont~;n;ng
active compound had a better action when evaluated
sensorially than the corresponding placebos in each case
in 29 out of 30 cases. In 1 out of 30 cases the test
person stated that the treated and untreated samples did
not differ from one another.
ExPeriment 2:
Compositions from Example 4 (comprising
imidazole, formulations C) according to the invention
were tested against compositions identical to these
without imidazole (formulations D).
A group of 30 subjects were told to treat in each
case one armpit with formulation C and the other with
formulation D. The subjects then wore a shirt with slip
inserts under the shoulders for three hours. After this
period of time, the slip inserts were transferred to
separate bottles. The smell of the inserts was evaluated
by five test persons. The experiment was carried out as
a double-blind experiment, so that neither the subjects
nor the test persons knew which armpit had been treated
with which composition.
It was found that the formulations C had a better
action when evaluated sensorially than formulations D in
2141~92~
- 23 -
each case in 25 out of 30 cases.
In each case in 4 out of 30 cases, formulations D
were evaluated as being better sensorially than formula-
tions C.
5In 1 out of 30 cases, the test person stated that
the treated and untreated samples did not differ from one
another. - -