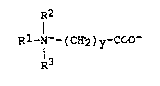Note: Descriptions are shown in the official language in which they were submitted.
21~259
-
HOECHST AKTIENGESELLSCHAFT HOE 94/F 902J Dr.GL-nu
Werk Gendorf
Process for the preparation of betaines
Description
The invention relates to a process for the preparation of
betaines of the formula 1
R2
Rl-N+- ( CH2 ) y~COO~ ( 1 )
R3
in which Rl is an alkyl radical having at least 8
carbon atoms or is the radical R4CoNH(CH2)X- in
which R4Co is an acyl radical derived from a
carboxylic acid having from 6 to 18 carbon atoms and
x is 2, 3 or 4, R2 and R3 are identical or different
and are an alkyl radical having from 1 to 4 carbon
atoms or are the radical -(CH2)zOH where z = 1, 2 or
3 and y is 1, 2 or 3
by quaternization of a tertiary amine of the formula 2
R2
Rl-N (2)
R3
in which Rl, R2 and R3 are as defined
with an ~-haloalkanecarboxylic acid of the formula 3
X-(CH2)y~COOH (3)
21~1259
-
- 2 -
in which X is a halogen and y is as defined
or a salt thereof, in the liquid phase.
A procesæ of this kind is described in DE-A 42 05 880,
where the quaternization is carried out in the aqueous
phase at a temperature of from 115 to 180C. Using this
relatively high reaction temperature, which leads to
breakdown of the ~-haloalkanecarboxylic acid employed and
of the ~-dihaloalkanecarboxylic acid present therein as
an impurity, ensures that the betaine solutions obtained
are virtually free from compounds contAin;ng organically
bonded halogen (chlorine) such as sodium mono- and
-dichloroacetate. However, the breakdown of haloalkane-
carboxylic acid, i.e. one of the two reaction components,
also meanæ that the betaine solution obtained contains a
more or less substantial quantity of unreacted starting
amine. In other words, the advantage of obtA;n;ng betaine
solutions which are free from organically bonded halogen
is countered by the disadvantage that these solutions are
contAm;nAted with the amine compound employed.
In order to obtain betaine solutions which are free from
starting amine and from organically bonded halogen,
US-A 4 497 825 advocates carrying out the quaternization
reaction at a pH of from 7.5 to 10.5. Although these
betaine solutions contain virtually no residual amine
compound, they are likely to contain an undesirably high
residual quantity of organically bonded halogen in the
form of the ~ono~A1ocarboxylic acid employed and/or of
its impurity, namely dihalocarboxylic acid, as also
referred to in the abovementioned DE-A 42 05 880.
The object of the invention, accordingly, is to provide
a process for the preparation of betaine solutions which
possess the desired purity with regard both to organi-
cally bonded halogen and to amine compounds; in other
words, the content of tertiary starting amine should be
~ 0.5% by weight and that of halocarboxylic acid
compounds should be ~ 10 ppm each.
'~ 2 5 9
-- 3
The process according to the invention comprises
a) in a first reaction step, in which the tertiary
amine and the ~-haloalkanecarboxylic acid or salt
thereof are employed in a molar ratio of 1: from 1
to 1.5, preferably 1: from 1.03 to 1.3, carrying out
quaternization at a temperature of from 60 to 98C,
preferably from 70 to 95C, and at a pH of from 7 to
11, preferably from 8 to 10, to obtain a betaine
solution having the desired purity with regard to
starting amine, and
b) in a second reaction step, adjusting the betaine
solution obtained in the first step to a pH of from
1 to 14, preferably from 5 to 12, and maintaining it
at a temperature of from 95 to 170C, preferably
from 100 to 150C, until the betaine solution also
has the desired purity with regard to organically
bonded halogen.
In the process according to the invention the reaction
between the tertiary amine and the haloalkanecarboxylic
acid or the haloalkanecarboxylate salt, which is prefer-
ably an alkali metal salt (for the sake of simplicity
this description only uses the terms haloalkanecarboxylic
acid or halocarboxylic acid) is carried out such that,
first of all, a betaine solution is prepared which
contains less than 0.5% by weight of starting amine,
percentages by weight being based on the solution. This
is achieved by a combination of selected values for the
molar ratio of reaction components, the reaction tempera-
ture and the pH of the reaction solution during the
quaternization. Thus from 1 to 1.5 mol, preferably from
1.03 to 1.3 mol, of haloalkanecarboxylic acid are
employed per mole of tertiary amine compound. The pH of
the initial mixture is adjusted to from 7 to 11, prefer-
ably from 8 to 10, and is maintained until the end of the
quaternization. The temperature at which the quaterniza-
tion reaction is carried out is from 60 to 98C, prefer-
ably from 70 to 95C. The adjustment and maintenance of
the specified pH of the mixture is effected (insofar as
2 ~ 9
-- 4
this pH is not already present, as is the case, for
example, when using haloalkanecarboxylate salts) by
addition of a preferably aqueous alkali metal hydroxide
solution, prior to and/or during the quaternization
reaction. The resulting betaine solution (the reaction
time for the quaternization is from about 6 to 20 hours)
is pure with regard to starting amine, but not with
regard to the monohalocarboxylic acid employed and the
dihalocarboxylic acid.
This betaine solution is then, in a second step, brought
to a temperature of from 95 to 170C, preferably from 100
to 150C, and maintained at this temperature until the
halocarboxylic acid compounds under discussion have been
broken down, in other words until virtually no further
lS organically bonded halogen is present. Furthermore, in
the betaine solution from the first step a pH of from 1
to 14, preferably from 5 to 12, is adjusted and main-
tained, preferably with the aid of an aqueous mineral or
carboxylic acid or of an aqueous alkali metal hydroxide
solution. The addition of the acid solution or hydroxide
solution to the betaine solution from the first step can
be carried out before and/or while the latter solution is
heated to the specified temperature of from 95 to 170C,
preferably from 100 to 150C. The betaine solution is
maintained at this temperature and at the specified pH
from 1 to 14, preferably from 5 to 12, until the content
of organically bonded hydrogen is < 10 ppm (the reaction
time for the breakdown of organically bonded halogen
depends on temperature and pH which is in general from 3
to 50 hours). The resulting betaine solution now has the
required purity with regard to amine and halocarboxylic
acid compounds. Its betaine content (content of active
substance) is in general from 20 to 55% by weight,
preferably from 25 to 50% by weight; in other words, the
solvent (the liquid phase) is employed in a quantity such
that betaine solutions having this content of active
substance are obtained. The solvent may be water, a lower
alcohol such as methanol, ethanol, propanol, isopropanol
'~14~25g
-- 5 --
and/or propylene glycol, or a mixture of water and
alcohol, preference being given to water and to mixtures
of water and alcohol. The water and also the other
solvents may be employed as such or in the form of
solutions of alkali metal hydroxide, amine compound
and/or halocarboxylic acid compound. The process accord-
ing to the invention may be carried out batchwise or
continuously, for example in one or more stirred vessels
which are arranged in series or in cascade formation. The
betaine solutions as obtained already constitute valuable
products. 8etaines, indeed, are surface-active compounds
with a wide variety of possible applications. Because of
their good skin compatibility they are employed princi-
pally in bodycare.
With regard to the starting compounds - tertiary amine,
~-monohalocarboxylic acid or a salt thereof, preferably
an alkali metal salt, and if desired alkali metal
hydroxide - the following comments apply: the tertiary
starting amines are of the formula 2 given at the
beginning. The long alkyl radical R1 may also contain
double bonds, preferably from 1 to 3. Preferred starting
amines are those of the formula 2 in which Rl is an alkyl
radical having from 8 to 18 carbon atoms or is a radical
of the formula R4CoNH(cH2)x- in which R4Co is an acyl
radical which is derived from a carboxylic acid having
from 6 to 18 carbon atoms and x is 2, 3 or 4, and R2 and
R3 are each methyl. Examples are dimethyloctylamine,
dimethyllaurylamine, dimethylstearylamine, dimethylcoco-
alkylamine, dimethyltallow-alkylamine and the like, and
also lauroylaminopropyldimethylamine, stearoylamino-
propyldimethylamine,cocoacylaminopropyldimethylamineand
the like. The ~-halocarboxylic acid is preferably mono-
chloroacetic acid or, respectively, sodium monochloro-
acetate. The alkali metal hydroxide is preferably sodium
hydroxide or potassium hydroxide.
The invention is now illustrated in more detail with
reference to examples according to the invention and
214~259
-- 6
comparative examples.
Examples according to the invention
Examples 1 to 3 relate to the first step of the process
according to the invention:
Example 1
188 g (0.587 mol) of cocamidopropyl-N,N-dimethylamine
(ami~o~;ne) and 345 g of water are introduced as initial
charge into a 1 1 glass flask equipped with stirrer,
thermometer, reflux co~n~er and dropping funnel. The
mixture is heated to about 82C with ætirring. While
maint~;n;ng this temperature of about 82C, 72.8 g
(0.616 mol) of an 80% strength by weight aqueous solution
of monochloroacetic acid are added dropwise to this
suspension, slowly and continuously, over 5.5 hours, and
also 53.7 g (0.671 mol) of a 50% strength by weight
aqueous solution of NaOH, in order to establish a pH of
from 8 to 9 (the molar ratio of amidoamine to monochloro-
acetic acid is 1:1.05). After addition is complete the
mixture is left to continue reacting at about 80C for 9
hours. The 30% strength by weight aqueous betaine
solution obtained, with regard to cocamidoamine, mono-
chloroacetic acid (MCA) and dichloroacetic acid (DCA),
has the following contents in percent by weight or ppm,
based on the solution:
Ami~o~m;ne: 0.14%
MCA: 0.1%
DCA: 110 ppm
Example 2
Example 1 is repeated but with the following changes:
Molar ratio of amidoamine: MCA = 1:1.25
Temperature: 95C
21~259
-- 7
pH: from 8 to 9
Time of continued reaction: 7 hours
Results:
Amidoamine: 0.11%.
5 MCA: 0.13%
DCA: 100 ppm
Example 3
Example 1 is repeated but with the following changes:
Molar ratio of amidoamine: MCA = 1:1.05
Temperature: 70C
pH: from 8 to 9
Time of continued reaction: 12 hours
Results:
Amidoamine: 0.25%
MCA: 0.09%
DCA: 115 ppm
Therefore the betaine solutions have the low value
desired with regard to amidoamine but not with regard to
MCA and DCA.
Examples 4 to 17 relate to the second step of the process
according to the invention:
In the second step the MCA and DCA content of the betaine
solution from the first step is reduced down to the ppm
range while ret~; n; ng the low values for amidoamine. For
convenience, only the betaine solution of Example 1 is
employed.
Example 4
The betaine solution of Example 1 is adjusted to a pH of
12 using 50% strength by weight aqueous sodium hydroxide
2141259
-- 8
and is then stirred at a temperature of 105C for
48 hours in a stirred autoclave. The betaine solution
obtained then has an MCA and DCA content of ~ 10 ppm
each.
Examples 5 to 18
The betaine solution of Example 1 is adjusted to a
defined pH with 50% strength by weight aqueous sodium
hydroxide (Examples 5 to 15) or with from 30 to 36%
strength by weight aqueous hydrochloric acid (Examples 16
to 18), and is then stirred at a defined temperature for
a greater or lesser period (reaction time) in a stirred
autoclave. These reaction conditions and the result with
regard to MCA and DCA content are compiled in the table
below, together with the values for Example 4.
Using the process according to the invention, therefore,
betaine solutions are obtained which have the low content
required in each case with regard both to starting amine
and to halogenated organic compounds (MCA and DCA).
Table
Example No. 4 S 6 7 8 9 10 11 12 13 14 15 16 17 18
p~ 12 12 12 14 14 8 10 12 14 14 14 14 6 4.5 2
~emperature (C~ 105 110 115 115 120 125 125 135 95 llo 105 115 135 135 135
Reaction time 48 20 20 16 3 24 16 3 50 16 20 6 5 4 5(h)
MCA content ~ 10 ~ 10 c 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10
(ppm)
DCA content ~ 10 ~ 10 ~ 10 c 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~ 10 ~_
(ppm) ~_~
~'
'~41259
- 10 -
Comparative Examples (Reworking of Examples 2.1 and 2.3
from DE-A 42 05 880)
Comparative Example 1
59.3 g (0.51 mol) of sodium monochloroacetate, 154.3 g
(0.5 mol) of cocamidopropyl-N,N-dimethylamine (cocamido-
amine) and 355.7 g of water are introduced as initial
charge into a 1 l autoclave and are heated to 120C,
during which a pressure of 2.6 bar i8 established. After
a reaction time of 8 hours the reaction solution is
cooled. The product is characterized by the following
data:
Sodium chloride content: 5.2%
Cocamidoamine content: 2.4%
Glycolic acid content: 0.37%
15 Sodium monochloroacetate content: ~ 20 ppm
Sodium dichloroacetate content: ~ 10 ppm
Comparative Example 2
Comparative Example 1 is repeated at a reaction tempera-
ture of 140C. A pressure of 3.2 bar is established. The
reaction time is again 8 hours. The product is character-
ized by the following data:
Sodium chloride content: 5.2%
Cocamidoamine content: 2.5%
Glycolic acid content: 0.42%
25 Sodium monochloroacetate content: ~ 20 ppm
Sodium dichloroacetate content: ~ 10 ppm