Note: Descriptions are shown in the official language in which they were submitted.
` :
w094/0373t 21~13~ 7 Pcr/US93/07110
I^ !:
CENTR~FUGAL BLOOD PUMP
BACKGROUN~ QETHE ~Q~
The present invention rela~es to pumps and more particularly to
centrifugal blood pumps without the requirement of a rotating seal to pro~ect the
S pump beanngs from the pumped blood.
Delicate surgical procedures require that the site of surgery
remain motionless during the surgical process. This rnade early heart surgery
difficult to impossible as interruption of the heart's pumping ac~on for the
required length of time would be invanably fatal.
During the 1960s, prolonged and non-fatal stoppage of the heart
became possible by use of newly developed "heart-lung" machines. These
machines consisted of a mechanical blood pump combined with a blood
oxygenator. They were capable of ta~ng over the function of the natural he~
and lungs for periods of up to several hours, enabling the development of
15 techniques leading to today's extensive practice of open-heart surgery.
The first practical mechanical b~ood pumps used were peristaltic
or "soller" pumps. The pumping ac~on of a roller pump derives from the
compression of a section of the flexible plastic tubing which ca~ies the blood
through the heart-lung machine. A moving roller presses the tubing against a
20 semicircular platen, moving the blood forward in the tubing. The speed of themo~ing roller and the diameter of the tubing control the rate of blood flow.
Although the roller pump was and is simple and reliable, it has
two characteristics which can endanger the patient undergoing surgery. First,
if flow is inadvertently obstructed, the pressure produced by a roller pump may
25 exceed the bursting strength of the tubing circuit. Second, if air is accidentally
introduced into the tubing circuit, it will be pumped to the patient along with the
blood. Either of these conditions may result in serious or fatal consequences tOthe patient.
In 1976, centrifugal blood pumps began to replace the roller
W O 94103731 PC~r/US93/07110
21~i~27
j :
-2-
pump as the ~heart" of the heart-lung machine. The pumping action of a
centrifugal pump derives from the rotation of an impeller within a pumping
chamber. Pump pressure is controlled by the rotational speed of the impeller.
At operational speeds, excessive pressure cannot be produced. Additionally, the
centrifugal forces in the pump form a natural air trap and, with massive
introduction of air, deprime and discontinue pumping altogether. These two
safety features, and the lower blood damage caused by these pumps, is now
widely recogniæd, and has led to their extensive use for open heart surgery.
In the early 1980s it was demonstrated that a mechanîcal blood
10 pump could be used as a heart-assist pump for patients who could not be
separated from the heart-lung machine following surgery. The readily available
centrifugal blood pumps were quickly applied to this situation as well as to themore routine use during heart surgery.
The fragility of the blood presents several problems for the design
15 of mechanical blood pumps. Excessive shear forces cause rupture of the red
blood cells (hemolysis). High flow velocity rates are needed over local areas
of friction (such as seals) to prevent points of high temperature which cause
blood damage and the accumulation of clot deposits.
Application of rotational impeller motion by conventional shaft
20 drives has not been practical due to the need for a sterile baITier between the
pumped blood and the pump drive mechanism. For this reason, centrifugal
blood pumps commonly utilize a magnetic coupling between the pump impeller
(or impeller shaft) and the drive motor.
Previous centrifugal blood pumps have relied on conventional ball
25 bea;rings to support the impeller shaft. A rotating seal was used to protect the
bea;rings ~rom contamination by the pumped blood. Some centrifugal blood
pumps utilized magnets carried by the impeller, which was supported by
bearings mounted on a stationary shaft. A shaft seal was also required to
protect the bearings from ~ontamination by the pumped blood.
WO 94/03731 P~lUS93tO7110
2~`~I3~7
Due to the corrosive nature of blood, shaft seals usually fail after
a relatively short time, exposing the bearings to cont~mination. If the failure
is not detected, bearings may overheat, causing damage to the blood. Blood
damage can lead to hemolysis, clot formation and s~oke. The short useful life
of current cen~ifugal blood pumps mandates their frequent re~lacement and is
the single most important problem yet to be solved with these devices.
SUMMARY QF l~ INVENTI~
The present invention is a sealless centrifugal pump for pumping
biological fluids such as blood. The pump has a housing which defines a
pumping chamber. An impeller which rotates about an ax~s is disposed within
the pumping chamber. l~e pumping chamber has an inlet provided at the
impeller axis of rota~on and an outlet provided along ~e periphery of the
impeller. An ex~ernal source of ro~on is disposed outside the pumping
chamber which causes the impeller to rotate. The hous~ng has constraining
mechanisms (preferably journal bearings) which constrain both the inlet side andthe base side of the impeller from movement m both axial and lateral direc~ons.
Because there are no moving parts which extend through a wall
of the housing, and particuhrly because there is no torque-providing shaft whichextends through a housing wall, the present invention has no seal around a
moving part and no opporhlnity for seal failure. Because the source of rotation
does not contact the blood, sterility can be ensured. Because the impeller is
constrained both on its inlet and base side from movement in both the axial and
lateral directions, there is no opportunity for dislocation or misalignment of the
Impeller.
BRIEF DFSCRIP'llON QF THE I)RAWl~GS
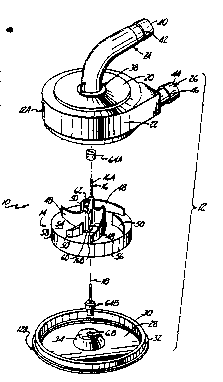
FIG. 1 is an exploded perspective view of ~e present invention.
FIG. 2 is a top plan view of the present invention.
FIGS. 3A and 3B are top and bottom plan views of the impeller
of the present invention.
w094/03731 PCr/US93/07110
21~3~
....
~- .
FIG. 4 is a cross-se~tional side view of the present invention as ,,,
seen from section line 4~ of FIG. 2. .
FIG. S is an cross-sectional side view of an alternate embodiment
of the inlet bearing surface of the present invention.
FIG. 6 is a cross-sectional top view of the alternate embodiment -~
of the inlet beanng surface as seen from section line ~6 of FIG. 5. - `'
DETAILED DE~I~LQF l~, P~EFERREl;) EMBODIMENTS
A Fi~s Embodiment ~ GS. 1~ ,,~--
A preferred embodiment of a centrifugal blood pump 10 of the
present invention is shown in FIGS. 1-4. Blood pump 10 includes housing 12 '
which encloses impella 14 and support shaft or spindle 16. Impeller 14 and i '
spindle 16 rotate about axis of rotation 18. Housing 12 has separate parts for ~ '-
ease of assembly, including upper enclosure 12A and base 12B, which are ' '
connected and sealed together, such as by ultr,asonic welding. '
I5 Upper enclosure 12A includes inlet wall 20, circumfe~ential wall ~ ,'
22, inlet 24, and outlet 26. Base 12~ includestottom waIl 28, cylindrical side ~'
wall 30, mounting flange 32, and pedestal 34. Pumping chamber 36 (FIG. 4) ,,'
is defined as the volume enclosed by inlet wall 20, circumferential wall 22 and i ~
bottom wall 28. ''
Inlet 24 is a J-shaped tubular member which has one end 38
attached to inlet wall 20 and an opposite free end 40. Ridge 42 on an outer
surface of inlet 24 facilitates attachment of inlet tubing (not shown) from a
reservoir/oxygenator or from the patient to free end 40 of inlet 24.
Outlet 26 is a tubular member which extends fro~n circumferential
wall 22 to free end 44. Ridge 46, adjacent free end 44, facilitates attachment
to outlet 26. of outlet tubing (not shown) which leads to the patient.
As shown in FIG. 3A, impeller 14 rotates about a~is of rotation `'
18 with a direction of rotation indicated by arrow R. Impeller 14 has full
impeller blades 48 and short impeller blades 50 which are attached to platform
wos4/n373l Pcr/us93/o7llo ¦ ~
~ ~ ~ 3 ~ 7
-s-
section 52 of impeller 14. Platforsn section 52 is disk-sh~ped and includes top
surface 54, bottom sur,face 56, outer cylindrical surface 58 and central
circulation hole 60. Full blades 48 extend from hub 62 across central
circulation hole 60 to platform section 52, while short blades 50 extend only
S along top surface 54 of platform section 52.
As shown in FIG. 3B, bottom surface 56 of platform section 52
contains a plurality of r,adial grooves 63. The purpose of grooves 63 is to
counteract a tendency of impeller 14 to shift in the a~cial direction toward inlet
24 as impeller 14 rotates.
FIG. 4 shows impeller 14 attached to spindle 16, so that impeller
14 and spindle 16 rotate together about axis of rotation 18. Inlet end 16A of
spindle 16 extends into and rotates within journal bearing 64A. Base end 16B
of spindle 16 extends into and rotates within journal bearing 64B.
The constraining mechanism of the present invention is provided
by journal bearings 64A and 64B, which capture and support spindle 16 while
permitting spindle 16 to rotate. Journal bearings 64A,64B are disposed
coaxially with and circumferentially around axis of rotation 18. Journal be~ring64A is press fit into recess 66 in the interior side wall of inlet 24. Journal
beanng 64B is press fit into recess 68 in pedestal 34.
The inner diameters of the recesses of journal bearings 64A and
64B are slightly greater than the outer diameters of the respective ends 16A,16Bof spindle 16~ so that a small lateral clearance is defined. The distance between
the inner end surfaces of journal bearings 64A,64B is slightly greater than the
length of spindle 16~ defining a small axial clearance. '
~5 Platforrn section 52 of impeller 14 contains magnets 70, whichcouple wi,th magnets 72 carried by rotor 74, to rotate impeller 14 and spindle
16 about axis of rotation 18. Electric motor drive shaft 76 is cor~nected to rotor
74 and provides torque to rotate magnets 72 and rotor 74 about axis of rotation
18. Magnets 70 and 72 couple together so that impeller 14 rotates at the same
W O 94J03731 PC~r/US93/07110 ~ ~
2l4~3Z l I
-6-
speed as rotor 74. The speed of drive shaft 76, therefore, deterrnines the speed ,
of impeller 14. `
Impeller 14 is attached to spindle I6 such that bottom surface 56
of platform section 52 is a small distance above bottom wall 28. Impeller 14
fits within pumping chamber 36 to leave clea~ance between the top and sides of ~
impeller 14 and upper enclosure member 12A. G,''
Housing 12 is shown in ~:IG. 4 adjacent thin mounting surface 78. !:
Housing 12 includes mounting flange 32, which may facilitate attachment of the
housing 12 to the mounting surface 78. Housing 12 may be attached to the
mounting surface 78 by an attachment mechanism (not shown) so as to provide
correct positioning of the blood pump 10 with respect to the external source of
rotation (i.e., rotor 74 and magnets 72~.
eration of the Firs~ ~m~diment -
Blood from the patient enters pumping chamber 36 through inlet
24. As it enters pumping chamber 36, inlet flow is in the axial direction at axis ~-
of rotation 18. This orientation and location of inlet flow allows the blood to
make a gentle directional t~ansition without placing excess forces on the blood.The blood contacts rotating impeller blades 48 and 50, and is propelled to and
through outlet 26 and back to the patient.
Blood pump 10 of the present invention is magneticaIly driven by
a source of rotation which is external to pumping chamber 36. Therefore, blood
pump 10 of the present invention does not have a torque-providing shaft or otherpart extending through a wall of housing 12. This eliminates the need for a sealand the possibility of seal wear and leakage.
The possibility of dislocation or misalignment of impeller 14 is
prevented by spindle 16 being constrained in the axial and the lateral directions
by journal bearings 64A and 64B. Bottom surface 56 of impeller 14 does not
contact bottom wall 28, preventing any friction between these surfaces. The
clearance between the top and sides of impeller 14 and enclosure 12A likewise
W O 94/03731 P~r/~S93/07110 1 ~
- 21~1327 ~ ~
prevents any friction bet veen these surfa~es.
l~e structure of tbe constraining mechanism reduces the amount
of fri~on between the rotating spindle 16 and the housing 12. The small lateral
clearance between the ends 16A,16B of spindle 16 and jourI~al bearings
564A,64B allows for slight lateral movement of spindle 16 and ensures minimal
pressure between parts. This allows minimal f~ic~on and minimal heat buildup
between ends 16A,16B of spindle 16 and journal beanngs 64A,64B. l~e small
axial cl~nce between ends 16A,16B of the spindle 16 and the inner end
surfaces of journal bearings 64A,64B ensures minimal pressure between parts,
10again reducing friction and heat buildup.
Bearings 64~ ~4B of the present invention are lo~ated in areas of
high blood flow velocity. Base journal bearing 64B is located by pedestal 34
in the center of cen~al circula~on hole ~0. Inlet joumal bea~ing 64A is located
in inlet 24, and is exposed to the inlet flow of blood int~ pumping chamber 36
15The location of bearings 64A,64B ensures rapid dissipation of any frictional heat
that is created.
Mounting flange 32 allows blood pump 10 to be quicldy and
easily removed, and a new pump can be quickly and easily attached. Quiclc and
easy removal and attachment of the pump is useful for the frequent replacement
20necessary to ensure sterility. Quick replacement also aids in the event of a
pump malfunction. Because the source of rotation does not have to be replaced
with replacement of the rest of the pump, the cost of replacement is lowered.
An impor~ant feature of blood pump 10 is the èliminahon of the
25requir~ment for a shaft seal. It was described previously that early failure of
the shaft seal is the primary reason for the reladvely short operational life ofcurrently available centnfugal blood pumps.
Elimination of the shaft seal requires that bearings be designed
which can operate effectively in blood. By using spindle 16 with a minimum
~ 94/03731 Pcr/US93/071 10
t~
3~ ~
--8
diameter consistent with the required shaft strength, surface velocity is
minimized. Minimization of surface velocity also minirnizes friction, frictionalheat and shear forces, all of which can cause blood damage and clot formation.
While a small diameter for spindle 16 is beneficial in reducing
S blood damage, it also reduces the areas of spindle 16 which senre as axial thrust
bearings, namely at both ends 16A and 16B of spindle 16. Since pump 10 is
driven by magnetic coupling, there is an axial load in the direction of the
magnetic coupling (i.e., toward rotor 74) when pump 10 is at rest or operating
at low speeds. The use of a magnetic coupling requires a close proximity of
impeller magnet 70 and drive magnet 72. Therefore, the preferred design is to
have impeller blades 48 and 50 only on the side of impeller 14 which faces the
pump inlet 24. This causes an asymmetrical axial flow across the two faces of
impeller 14 and results in an increasing axial foree toward pump inlet 24 as flow
increases (the "lifting forcen). The required area of the asial thrust bearing and
hence, the minimum diameter of spindle 16 is determined by this maximum
axial load.
As shown in FIG. 3B, the lifting force is preferably counteracted
by placing radial grooves 63 in the base side of impeller 14 to increase the axial
flow across this surface. The use of grooves 63, rather than blades, does not
require increased space between the driven and drive magnets 70 and 72 (which
would require more or stronger magnets to maintain the same coupling
strength). By proper selection of the number and depth of grooves 63, pump
10 can be designed such that the lifting force "floats" impeller 14 such that,
within the operating rpm of pump lO, the axial load on both bearings 64A and
64B and both spindle ends 16A and 16B is minimal. This reduces frictional
heat, blood damage and bearing wear.
In a preferred embodiment, a groove 63 extends radially between
adjacent magnets 70 of opposite polarity. This is illustrated in FIG. 3B by the
polarity symbols "N" and "S".
wo94/03731 PCr/US93/07110
~ ~ 4 ~ ~3.2 7
A Se~ond Em~imçnt ~ 5-6
An alt~rnative embodiment of the present invention is shown in
FI&S. S and 6. This embodiment has journal bearing 64A dispos~d in the
cente~ of st~aight inlet tube 26'. Jours~al bearing 64A is supported in cup 90 by
S struts 92, whieh extend from cup 90 to the inner wall of inlet 26'. This allows
inlet 26' to remain straight ~rather than J-shaped as in FIG. l) while still
providing inlet flow in the a7cial direction at a~is of rotation 18.
Although the present invention has been described with reference
to preferred embodiments, worlcers skilled in the art will recognize that changes
10 may be made in form and detail without depar~ng from the spirit and scope of
the invention. For instance, a stationary pin going through a central hole in hub
62 of impeller 14 may be substituted for spindle 16, such that the top side and
the bottom side of impeller 14 are constrained from movement in the a7cial and
lateral directions by the stationary pin, while impeller 14 remains ~ree to rotate
15 around the pin. Also, in some embodiments grooves 63 are not required and
therefore are omitted.
.' q ';
~ '
.`~