Note: Descriptions are shown in the official language in which they were submitted.
2141400
ELASTOMERIC LAMINATES CONTAINING A
SOLVENTLESS ELASTOMERIC ADHESIVE COMPOSITION
Field of the Invention
The present invention relates to elastomeric
laminates cont~;n;ng an adhesive composition or cushion
- layer which has excellent blowout protection. More
specifically, the adhesive cushion comprises a blend of
bis-imides, elastomeric compounds, and tackifiers.
Background of the Invention
Heretofore, adhesive compositions commercially
utilized for adhering various elastomer components to
one another have generally contained solvents therein.
Although some formulations may not contain any solvents,
they nevertheless had poor blowout resistance when
cured.
Summary of the Invention
The invention relates to a solventless elastomeric
adhesive composition or cushion material useful for
adhering or binding various elastomeric layers such as
in tire retreading, splicing and the like. The
solventless adhesive composition includes a mixture of
at least one elastomer, at least one tackifier, and at
least one bis-imide compound of the general formula:
C C\ /C C R3
N X N
C C C
2/ ~\ // \R4
~141400
where R1, R2 , R3, and R4, independently, are hydrogen,
an alkyl group having from 1 to 5 carbon atoms, a phenyl
group, an alkylphenyl group having 7 to 10 carbon atoms
or a halogen substituted alkyl group having from 1 to 5
carbon atoms, a halogen substituted phenyl group, or a
halogen substituted alkylphenyl group having a total of
from 7 to 10 carbon atoms, and where X is an alkylene
group having from 1 to 5 carbon atoms, a phenylene
group, an alkylphenylene or alkylenephenyl group having
7 to 10 carbon atoms or a halogen substituted alkylene
having from 1 to 5 carbon atoms, a halogen substituted
phenylene group, or a halogen substituted alkylphenylene
or alkylenephenyl group having a total of from 7 to 10
carbon atoms.
The present invention advantageously provides an
elastomeric adhesive composition which is free of
solvent before its application and which has improved
blowout protection.
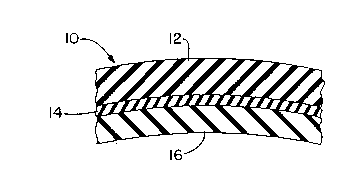
Brief Description of the Drawinq
Fig. 1 is a partial side elevation of a schematic
cross section of a laminate in the form of a retreaded
tire prepared according to the present invention, and
Fig. 2 is a schematic cross section of a tire tread
spliced together using the elastomeric adhesive
composition of the present invention.
Detailed Description of the Invention
According to an embodiment of the present
invention, a laminate in the form of a retreaded tire 10
comprises a cured or uncured tread of retread stock 12
adhered directly to a cured tire carcass 16 by a
solventless elastomeric adhesive composition in ~the
form of an adhesive cushion layer 14.
The retread stock or tread stock 12 is well known
to the art and can be any cured or uncured conventional
2141400
rubber such as rubbers made from conjugated dienes
having from 4 to 10 carbon atoms (e.g. polybutadiene,
polyisoprene, natural rubber, etc.), rubbers made from
conjugated diene monomers having from 4 to 10 carbon
atoms with vinyl substituted aromatic monomers having
from 8 to 12 carbon atoms (e.g., styrene-butadiene
rubber), and the like, as well as blends thereof. Such
rubbers generally contain various antioxidants, fillers
such as carbon black, oils, sulfur, accelerators,
antioxidants, stearic acid, antiozonants and the like in
conventional amounts.
The cured carcass compounds are well known to the
art and literature and generally include a variety of
synthetic rubbers, natural rubber or blends thereof.
Illustrative examples include rubbers made from
conjugated dienes, such as polyisoprene or natural
rubber, rubbers made from conjugated dienes having from
4 to 12 carbon atoms and vinyl substituted aromatics
having from 8 to 12 carbon atoms, such as styrene-
butadiene rubber, and the like, as well as blends
thereof. Such rubbers are generally compounded with
oil, fillers such as carbon black, processing aids, zinc
oxide, stearic acid, sulfur, accelerators, antioxidants,
antiozonants and the like in conventional amounts.
The adhesive composition or cushion layer is
prepared from a solventless adhesive composition which
comprises at least one elastomer and at least a compound
of the general formula:
R \ // ~ /R3
C C --C
N- X N
R2 ~ O \R4
21~1~00
-- 4
where Rl, R2 , R3, and R4, independently, and X is as
defined hereinabove. For the sake of brevity such
compounds will hereafter be referred to as bis-imides.
An example of a bis-imide that is particularly
preferred for use in the invention is N,N'-m-xylylene
- bis-citraconic imide (Perkalink~ 900 manufactured by
Akzo), which has the following structural formula:
Cl H3
jN- H2C- ~ - CH2 N\
H/c ~,o o~C_f
Another suitable bis-imide compound is N,N'-m-phenylene
bismaleimide (HVA-2~ manufactured by DuPont) which has
the structural formula:
H fl ~ H
C -C\ /C C
~N ~ N
/C 11 ~;; C\
H 0 H
Still another suitable bis-imide compound is
1,l'(methylenedi-4,1-phenylene) bismaleimide (BM,
available from Aldrich) which has the structural
formula:
2141~00
-- 5
H~ /0 0
C C
~ ~ CH2 ~
H ~ O
The bis-imide compounds are used in an amount which
is effective to achieve improved blowout resistance,
reduced heat generation, improved durability, or reduced
reversion or to achieve a combination of enhanced
properties. Suitable amounts of a bis-imide compound
are generally at least 0.1 or 0.2, desirably from about
0.3 to about 5 or 10, and preferably from about 0.5 to
about 2 parts by weight per 100 parts by weight of the
elastomer (phr) in the elastomeric adhesive composition.
Suitable elastomers which can be used in preparing
the solventless elastomeric adhesive composition of the
present invention include natural rubber and various
synthetic elastomers as well as various blends thereof.
Examples of synthetic rubber which can be used in the
solventless adhesive composition or cushion layer
include copolymers of conjugated dienes having from 4 to
about 7 carbon atoms and vinyl substituted aromatic
compounds having from 8 to about 12 carbon atoms, such
as styrene-butadiene rubbers; polymers and copolymers of
conjugated dienes having from 4 to 7 carbon atoms, such
as natural rubber (i.e., cis-1,4-polyisoprene),
polybutadiene and polyisoprene; polychloroprene
(neoprene rubber); and the like; as well as various
blends thereof. Especially preferred are blends
containing from about 20 to about 80 percent by weight
of natural rubber and from about 80 to about 20 percent
by weight of polybutadiene based upon the total weight
of the elastomers in the adhesive composition.
2141400
In addition to the elastomeric component and the
bis-imide compound, the adhesive compositions of the
invention can include one or more compatible tackifying
agents which are utilized in an effective amount to
promote good adhesion with both vulcanized elastomeric
substrates and with uncured, vulcanizable elastomeric
substrates (i.e. non-vulcanized or at least
substantially non-vulcanized). Various tackifying
resins can be utilized which are generally well known to
the art and to the literature. These resins generally
include rosin and its derivatives and various
hydrocarbon resins. The rosin group comprises rosins,
modified rosins and their various derivatives such as
esters. The hydrocarbon resin group comprises
polyterpines, synthetic hydrocarbon resins, and various
modified or special resins which are primarily
phenolics. Examples of specific rosin tackifiers
include gum rosin, wood rosin, tall oil rosin, and the
like. Such rosins are generally a mixture of organic
acids called rosin acids. Minor components in the rosin
resin include rosin esters and anhydrides,
unsaponifiable matter, and fatty acids. The rosin acids
can be divided into two different groups, abietic acid
type and primaric acid type. The various rosin acids
can be reacted with a variety of alcohol to form esters.
Examples of specific rosin resin tackifiers include
glycerine rosin ester, e.g., Floral 85, manufactured by
Hercules, Inc.; hydrogenated pentaerythritol ester,
e.g., Pentalyn H, manufactured by Hercules, Inc.;
hydrogenated glycerine ester, e.g., Staybelite Ester 10,
manufactured by Hercules, Inc.; modified tall oil rosin,
e.g., Sylvatac RX, manufactured by Sylvachem Corp.;
polymerized rosin such as Sylvatac 95, manufactured by
Sylvachem Corp., and rosin ester such as Zonester 85,
manufactured by Arizona Chemical Co.
2141400
Hydrocarbon tackifier resins are low molecular
weight polymers derived from crude monomer streams.
Steams can be obtained from wood, coal, or petroleum
sources. Hydrocarbon resin streams can be classified as
containing primarily aromatic, aliphatic, and diene
(cyclic olefin) monomers. Polymerization of such
streams is generally carried out using a Lewis acid
catalyst or by a free-radical process using heat and
pressure. The aromatic hydrocarbon resins generally
contain aromatic petroleum resins and resins from coal
tar, commonly called coumarone-indene resins. The
various aliphatic hydrocarbon resins are produced from
light, so called carbon-5 petroleum fractions wherein
the principal monom~rs are cis and trans-piperylene.
Other hydrocarbon resins include mixed aliphatic-
aromatic resins as well as terpene resins.
The above tackifier resins are described in more
detail in the Handbook of Pressure-Sensitive Adhesive
Technology, edited by Donatas Satas, Van Nostrand
Reinhold Company, 1982, Chapter 16, pages 353-369, which
is hereby fully incorporated by reference.
Another and preferred type of tackifier are the
various phenol-formaldehyde resins. Such resins
generally have a number average molecular weight of
2,000 or less. Typically, alkyl phenols are used rather
than phenol itself since the alkyl group improves the
miscibility of the resin with the rubber. Thus, alkyl
groups having from 1 to 15 carbon atoms such as butyl,
octyl, and nonyl, have been attached to the phenolic
nucleus. The manufacture of phenolic resins generally
include the condensation of the alkyl phenol with
formaldehyde to produce the phenolic resins. Since the
phenol has three reactive positions, it will form
insoluble resins when more than one mole of formaldehyde
is used per mole of phenol. When low ratios of
formaldehyde are used, tackifiers are formed. The
2141400
- 8
existence of phenol-formaldehyde tackifiers are well
known to the art and to the literature, e.g., "Resins
Used in Rubbers" by Paul O. Powers, Rubber Chemistry
and Technology, Vol. 36, pages 1542-1562, (1963), and
"Role of Phenolic Tackifiers in Polyisoprene Rubber," by
F. L. Mangus and G. R. Hamed, Rubber Chemistry and
Technology, vol. 64, pages 65-73 (1991). The amount of
tackifying agent is typically in the range from about 1
to about 30, desirably from about 2 to about 15, and
preferably from about 6 to 10 phr.
The elastomeric adhesive compositions or cushions
of the present invention can include one or more
reinforcing agents or fillers in an amount of from about
5 to 100 phr. Examples of such materials include carbon
black, silica in combination with an appropriate
coupling agent, and the like. Carbon black is preferred
and is desirably used in an amount from about 20 to
about 70 and preferably from about 40 to about 55 phr.
Generally, any conventional carbon black is suitable for
use in the practice of this invention. Preferably, the
carbon black has an average mean particle diameter less
than 285 nm, and preferably less than 60 nm such as in
grades N550, N330, and the like (ASTM-D-3849).
The present invention relates to sulfur cure
systems and include one or more cure accelerators.
Suitable amounts of sulfur and/or sulfur donor-type
compounds generally range from about 1 to about 6 and
preferably from about 2 to about 4 phr. The amounts of
sulfur vulcanization accelerator generally range from
about 0.2 to about 4 and preferably from about 0.5 to
about 2.0 phr. Various sulfur accelerators can be used
such as aldehyde-amine accelerators, e.g., the reaction
product of butyraldehyde and aniline, amines such as
hexamethylene tetramine, guanidines such as diphenyl
guanidine, thioureas, sulfenamides, and the like.
Activators such as zinc oxide, stearic acid, litharge,
2141~0
magnesia and amines can be also be used in conventional
amounts to attain good crosslinking efficiency, such as
in amounts of from about 0.5 to about 12 and preferably
from about 1 to about 5 or 10 phr. Various oils such as
napthenic oils are commonly utilized in suitable amounts
such as from about 1 to about 30 and desirably from
about 4 to about 20 phr.
The solventless adhesive compositions of the
invention can also include conventional amounts of
various known rubber compounding ingredients such as
processing aids, stabilizers, antidegradants, and the
like. Suitable antioxidants include hindered phenols,
amines, amino phenols, hydroquinones, alkyldiamines,
amine condensation products and the like.
An important aspect of the present invention is
that the elastomeric adhesive composition has a built-in
or an inherent adhesive property and thus can be applied
without the need of any solvent or other adhesive
composition. That is, the elastomeric adhesive
compositions of the present invention are free of
solvent, although they can be applied with solvent
followed by evaporation of the solvent. However, for
economic or environmental factors, solvents are not
recommended. Another important aspect is that the
solventless elastomeric adhesive composition in the
uncured state has excellent pressure sensitive tack to
both uncured and to cured rubber. Hence, it can be
applied wrinkle-free to a buffed carcass, etc.
The uncured elastomeric adhesive composition of the
present invention can be vulcanized by heat or radiation
according to any conventional vulcanization process.
Typically, the vulcanization is conducted at a
temperature ranging from about 100C to about 250C or
preferably from about 120C to about 170C for a time
period ranging from about 1 to about 300 minutes. The
carcass (also precured tread if used) being retreaded,
2141~00
- 10 -
having been previously been w lcanized, requires no
additional w lcanization.
The present invention can be utilized to form a
laminated retreaded tire for various types of vehicle
tires such as passenger car tires, light and medium
truck tires, off the road tires, and the like, and
preferably is utilized in forming retreaded laminates
for aircraft and medium truck tires.
Suitable tire tread compositions can be prepared by
using conventional mixing techniques including, e.g.,
kneading, roller milling, extruder mixing, internal
mixing (such as with a Banbury~ mixing), etc. The
sequence of mixing and temperatures employed are well
known to skilled rubber compounders, the objective being
the dispersion of fillers, activators, curatives in the
polymer without excessive heat buildup.
The adhesive composition or cushion of the present
invention in addition to forming retreaded tires, can
generally be utilized whenever a good heat resistant,
good blowout protection adhesive layer is required to
bond two or more elastomeric layers together. The
various layers can be uncured, cured, or combinations
thereof. The various one or more elastomeric layers can
generally be any elastomeric rubber composition such as
those set forth with regard to the retreaded tires.
However, in addition to the above noted rubbers, the
various elastomeric layers can include various
conventional rubbers know to the art and literature such
as various nitrile rubbers, rubbers made from ethylene
and propylene monomers, i.e., EPM rubber, various
rubbers made from monomers of ethylene, propylene, and
diene monomers, i.e., EPDM rubber, butyl rubber,
neoprene rubber, and the like. Examples of other
laminates which can utilized the adhesive cushion layer
of the present invention include as an adhesive layer
for a tire bead layer or an apex component, for conveyor
2141400
belts, for lapping rubber layers together, and the like.
Another suitable end use is as a tread splice
adhesive or joint for adhering the ends of a tire tread
to itself since it provides in the uncured stage
excellent pressure sensitive building tack as well as
excellent adhesion after cure. Thus, as shown in Fig.
2, the spliced tire tread, e.g., uncured or cured, is
generally indicated by the numeral 20 contains two ends
of the tire tread 22a and 22b adhered to itself through
tire splice material 26 which is the elastomeric
adhesive composition of the present invention described
herein above. The splice tread portion resides upon a
tire ply or carcass substrate 28. Although not shown,
carcass substrate 28 can be bonded, adhered, or joined
to tire tread 22a or 22b through the use of a
conventional adhesive or through the use of the
elastomeric cushion adhesive of the present invention as
discussed hereinabove and as illustrated in Fig. 1.
The elastomeric adhesive compositions of the
present invention have superior blowout resistance when
utilized as an adhesive tire cushion, as an adhesive
tire splice, etc. In this regard, a suitable blowout
test consists of subjecting a rubber specimen of
suitable size and shape to rapidly oscillating compres-
sive stresses under controlled conditions. The tempera-
ture of the sample is measured versus a set time
required for fatigue failure of the sample by internal
rupture or blowout. The specific blowout test utilized
in the examples of the present invention is ASTM D-623.
Another unexpected property of the solventless adhesive
composition or cushion of the present invention is that
it has long shelf life such as at least three months at
ambient temperature or at cool temperatures of about
5C.
The following examples serve to illustrate the
invention in detail but do not limit the same thereto.
2141400
EXAMPLES
Two solventless adhesive compositions, Samples 1
through 4 were prepared by mixing the ingredients listed
in Table I in a Banbury~ mixer. In addition to the
ingredients listed in Table I, Sample 2 also included 2
parts by weight of N,N'-m-xylylene biscitraconic imide
(Perkalink~ 900). Sample 3 in addition to the
ingredients listed in Table I also contain 1 part by
weight of N,N'-m-phenylene bismaleimide (HVA-2~).
Similarly, sample 4 in addition the ingredients of Table
I also include a one part by weight of 1,1'-
(methylenedi-4,1-phenylene) bismaleimide (BM). After
the samples were mixed in the Banbury~ mixer, they were
passed through a two-roll mill and subsequently
calendered into 40 mil sheets at 82C in a production
four-roll gum calender.
In a typical procedure, the carcass of the used
tire is buffed to provide a surface on which the cushion
compound can be disposed. The cushion layer is directly
applied thereto, that is, there is no intermediate layer
and hence the present invention is free of any layer
between the buff carcass and the cushion layer such as
an adhesive layer, a rubber layer, or the like.
Thereafter the uncured tread compound is applied onto
the outer layer of the cushion compound. The completed
tire assembly is subsequently inserted into a mold where
the tread is embossed in the tread stock and the tread
stock and cushion are vulcanized with heat and pressure.
2141400
- 13 -
Table I
C~SHION LAYER FORMmATION (PARTS BY WEIGHT):
60 polybutadiene rubber
40 natural rubber
50 carbon black
6.5 oil
3 . O zinc oxide
2.S stabilizers
1.5 phenylenediamine type
1.0 octadecanoic acid
4.5 accelerators and curatives
0.8 sulfenamide type
0.2 diphenylguanidine
3 . 5 sulfur
8 phenolic resin as tackifier (reaction of
formaldehyde with nonyl phenol)
Table -I
Rubber (Bis-imide) Blowout or Final Blowout
Composition Parts by Weiqht TemPerature (C) Time
(minutes)
Sample 1 0 218 10.5
(control)
Sample 2 2 (Perkalink~ 900) 171 ~ 60.0
Sample 3 1 (HVA-2T) 163 > 60.0
Sample 4 1 (BM) 143 > 60.0
From Table II it can be seen that with the addition
of only 2 phr of the Perkalink~ 900 in a 60/40
polybutadiene/natural rubber cushion, the blowout time
was increased from 10.5 minutes to greater than 60
minutes. In addition, less heat is generated as the
final temperature reached during the blowout experiment
is reduced from 218C to 174C. Similar improvements
were obtained in samples 3 and 4.
214I400
- 14 -
A still further advantage realized through use of
the 60/40 polybutadiene/natural rubber based elastomeric
adhesive composition (cushion) of the invention is the
fact that there is a significant improvement in the
adhesion produced between the carcass and the tread
stock compared to the 100 natural rubber based control
cushion (see Table IV).
In an adhesion test, the buffed side of a cured
carcass sheet, was wiped with solvent to remove powdered
deposits and then dried. The cushion was applied to the
carcass. To the cushion was applied as a 3x6 inch
Mylar~ separation sheet, followed by a 6x6x0.15 inch
uncured tread. The sample was cured for 60 minutes at
150C and at 100 psi pressure in a bladder cure press.
Strips of lx6 inch were then cut and pulled apart
parallel to the grain of the fabric at room temperature
using an Instron tester at a cross-head speed of 2
inches per minute. The results are listed below in
Table III.
Table III
Adhesive Material Cured Adhesion
Values
(lbs/inch)
100% natural rubber based control 96
cushion
60/40 polybutadiene/natural rubber 421
based cushion plus Perkalink~ 900
60/40 polybutadiene/natural rubber 165
based cushion plus HVA-2~
The elastomeric adhesive cushion of the present
invention has superior cured adhesion values compared to
the control cushion. The control cushion had to be used
in combination with another solvent-based adhesive
cement in order to improve adhesion values to acceptable
standard values (150 lbs/in) as shown in Table IV.
- 15 - 21~14~0
Table IV
Tvpe of TireCured Adhesion
(lbstinch)
Control (100~ Natural Rubber-Based Cushion 195
plus cement)
60/40 Polybutadiene/Natural Rubber-Based 421
Cushion with Perkalink~ 900 and without
cement
An additional advantage of the invention is that
during the vulcanization procedure, the presence of the
bis-imide appears to reduce the tendency of the cushion
material to cause reversion of vulcanization in the
cushion compound.
Table V relates to a comparison of a control using
100 percent natural rubber to a cushion of the present
invention containing a bis-imide.
TABLE V
Comparison of 100% Natural Rubber Based
Control Cushion
vs.
60/40 Polybutadiene/Natural Rubber ~ased Cushion
Containing Perkalink~ 900
2 5 Property Control 60/40 Polybutadiene/
100~ Natural Natural Rubber Plus
Rubber Perkalink~ 900
Blowout (mins.) 18 ~60
Final Temp.(CC) 147 171
A cushion of the composition set forth in Table I
was calendered into a smooth poly liner to a gauge of
O.050" and a width of a 9" in order to accommodate a
8.5" precured tread. A medium radial truck tire was
built where this cushion was applied to the buffed
carcass directly out of roll under tension followed by
precured tread application. The tire was cured for 180
minutes at 127C. Then the tire was cut and adhesion
21~1900
- 16 -
tests were performed using the Scott~ tester. Average
adhesion value was 141 lbs. per inch and it easily
surpasses the m;nlm~lm specification of 100 lbs. per
inch.
Adhesion tests were repeated after the adhesive
cushion in the roll was aged for three months at room
temperature and in the refrigerator at 5C.
As apparent from Table VII, the drop off in
adhesion in room temperature cushion is considered to be
very little and was even less in a refrigerator held
constantly at 5C. Such adhesion values were at least
twice the specification even after three months, thus
indicating its long shelf life.
Table VI
SHELI~ LIFE OF ADHESIVE CIJSHION
(~ased on Adhesion Value~)
Aginq of Adhesive CushionAdhesion*
(lbs/inch)
Fresh 421
3 months at room temperature354
3 months in refrigerator 391
* Military & civilian airplane tire spec: 150 lbs/inch
While in accordance with the Patent Statutes, a
preferred embodiment and best mode has been presented,
the scope of the invention is not limited thereto, but
rather is measured by the scope of the attached claims.