Note: Descriptions are shown in the official language in which they were submitted.
-
~ 094/04636 _1_ 2 1 ~ 2 9 9 1 PCT/US93/07962
UNLEADED MMT FUEL COMPOSITION
~ACKGROUND OF THE INVENTION
Field of the Invention
This invention relates generally to novel unleaded
fuel compositions for spark ignition internal combustion
engines. More particularly, it relates to organomanganese
and unleaded fuel combinations, and a mechanical and/or
chemical means capable of reducing the formation of heavy
manganese oxides (e.g. Mn3O4) during combustion, such that
resultant hydrocarbon emissions can meet minimal legal
environmental standards.
DescriPtion of the Prior Art
The incorporation of various organo-metallic compounds
as antiknock agents in fuels for high compression, spark
ignited, internal combustion engines has been practiced for
some time. The common organo-metallic compound used for
this purpose has been tetraethyl lead (TEL). Generally,
these organo-metallic compounds have served well as
antiknock agents. However, certain environmental hazards
have been associated with the alkyl lead components of
these compounds. This circumstance has precipitated a
series of Environmental Protection Agency (EPA) mandates
which has essentially phased out leaded gasolines.
Many alternatives to tetraethyl lead compounds have
been proposed and/or used. For example, organomanganese
compounds such as cyclomatic manganese tricarbonyls (CMT),
particularly methylcyclopentadienyl manganese tricarbonyl
214~991
W O 94/04636 PC~r/US93/0796 ~
. .
(MMT), were once accepted alternatives to TEL. However,
these compounds produced another set of environmental
problems. Namely, their use steadily increased the amount
of unoxidized and/or partially oxidized hydrocarbon
emissions. Fuels cont~;ning such organomanganese compounds
gradually cause substantially higher levels of hazardous
hydrocarbon emissions than are permitted under law.
Further, industry experience shows that organomanganese
concentrations greater than l/16 gram Mn/per gallon
10(0.0165212 grams/liter) are directly responsible for
catalytic converter plugging. Accordingly, U.S. federal
law bans the use of manganese (i.e. MMT) in all unleaded
gasolines absent an EPA 211(f)(4) Waiver. Thus far every
attempt to obtain such a waiver has failed.
15It is well known in the art that many types of
oxygenated components can be included with unleaded bases
as a means of improving antiknock properties and/or
reducing the carbon monoxide emissions of a fuel. These
oxygenated components include lower molecular weight
alcohols, various ethers, esters, oxides, phenols, ketones
and the like. Several of these components have also been
used as neat motor fuels in their own right, i.e. methanol.
Several of these oxygenated components can be used in
combination with each other. See, for example, "Low-Lead
Fuel with MTBE and C4 alcohols," Csikos, Pallay, Laky, et
al, "Hydrocarbon Processing," July 1976, and U.S. Patents
4,207,077 and 4,207,976.
2142991
0 94/04636 PC~r/US93/07962
-3-
Numerous studies have been conducted on the use of
ethers, particularly methyl tertiary butyl ether and
tertiary amyl methyl ether in gasoline bases, see for
example "Ether Ups Antiknock of Gasoline," Pecci and
Floris, HYDROCARBON PROCESSING, December 1977. The U.S.
Clean Air Act as amended (42 USC 7445) ("CAA"), governs
the usage and introduction of all additives in unleaded
gasolines in the United States. The CAA under Section
211(f)(4) permits the EPA ~;n;~trator to waive the
prohibition on new fuel additives in unleaded gasolines
("Waiver"). The CAA specifically bans manganese ("Mn")
additives in unleaded fuels, absent a waiver. However,
prior to granting a waiver, the A~m;n;~trator must
determine applicant's waiver re~uest meets the burden of
demonstrating that the new fuel or fuel additive will not
cause or contribute to the failure of an emission control
system or regulated emission stan~Ard(s). Under this
section of the CAA, the Administrator has both denied and
granted numerous waiver requests.
However, as noted in the case of organomanganese
compounds (i.e. MMT), the EPA has denied each and every one
of four separate Waiver applications since 1978. Denial was
based upon the failure to demonstrate at l/8, l/16, 1/32,
and 1/64 grams of manganese per gallon (3.785 liters) that
MMT would not cause long term hydrocarbon emission
degradation or emission system failure (e.g. catalyst
plugging). See generally Environmental Protection Agency
RE Applications for MMT Waiver, Federal Register, Vol. 43,
W094/04636 2 i 4 2 9 9 1 PCT/US93/07 ~
No. 181, Monday, September 18, 1978, and Ethyl Corp.;
Denial of Application for Fuel Waiver; Summary of Decision,
Federal Register, Vol. 46, No. 230, Tuesday, Dec. 1, 1981.
In view of the U.S. federally r~n~Ated ban on
manganese, namely, methyl cyclopentadienyl manganese
tricarbonyls (MMT) in unleaded gasoline, the inability of
industry in spite of repeated attempts to obtain a 211(f)
waiver for MMT, and in view of overwhelming industry need,
there exists a very pressing requirement to find a way in
which organomanganese compounds can qualify use in unleaded
gasolines on an environmentally acceptable basis,
particularly in meaningful concentrations.
SUMMARY OF THE lNV~'l'lON
As presented herein, Applicant's discovery resides in
the source of the problem that causes organomanganese
compounds to aggravate hydrocarbon emissions over time.
Applicant has discovered that the formation of heavy
manganese oxides during combustion (e.g. Mn304 and Mn203) are
associated with the build up of engine deposits, catalyst
plugging, and aggravated hydrocarbon emissions, which are
the features responsible for the failure of cyclomatic
manganese tricarbonyl compounds to meet CAA regulated
emission st~ rds. Applicant has discovered that it is the
management of these heavy manganese oxides during
combustion that represents the long eluded solution to the
problem of organomanganese compounds.
O 94/04636 ~ 1 42 ~ 9 ~ PC~r/US93/07962
Applicant's invention is distinguished from the prior
art in that Applicant has discovered the source of the
problem together with the means to solve it.
Applicant has discovered that the adverse hydrocarbon
(HC) emission degradation over time and the other pollution
problems associated with cyclomatic manganese tricarbonyls
are directly traceable to first, the formation of heavy
manganese oxides, namely, Mn304 and Mn2O3, and second, to
their build-up into particles of sufficient size and mass
to become problematic. This particulate build up and engine
deposit process is instantaneous and in combination with
the complex hydrocarbon combustion reactions that occur
during combustion and shortly thereafter. In other words,
these Mn oxides combine with each other, and the other
organic and inorganic material found in gasolines and air
during the rapid combustion and exhaust process.
Those skilled in the art have generally accepted
heavier manganese oxides (i.e. Mn3O4 and Mn2O3) as a given,
i.e. that they are the primary and/or only manganese ("Mn")
oxidation product formed during combustion.
Applicant has discovered that it is the formation of
these heavier manganese oxides during [a less than optimum]
combustion process that ultimately causes spark plug and
catalyst fouling, combustion chamber deposits and the like.
This in turn leads to adverse hydrocarbon emission
problems, preventing organomanganese compounds from
qualifying for 211 (f) EPA waivers, and from complying
with appropriate environmental standards.
W094/04636 ~ 2 1 ~ 2 9 ~1 PCT/US93/079
Applicant has unexpectedly discovered that by
accelerating and improving combustion to an optimum state,
thereby controlling and/or eliminating the formation of
heavier manganese oxides during combustion, that hazardous
combustion emissions (chiefly hydrocarbon emissions),
catalyst plugging, and the like, can be controlled and even
reduced. Such control will permit Applicant's fuels to meet
required environmental st~n~rds and to qualify for 211
(f) EPA waivers.
Applicant has unexpectedly discovered that the control
of these heavier manganese oxides during combustion is
primarily a function of 1) increasing combustion burning
velocity (flame speed) and 2) reducing combustion
temperatures.
By increasing the burning velocity (flame speed, fuel
economy, etc), i.e. shortening the interval of combustion,
the hazardous emission causing heavier manganese oxides are
not readily formed. By increasing burning velocity, when
employing an organomanganese compound, combustion is
efficient and cleaner. Applicant has unexpectedly
discovered that improvements in burning velocity reduces
both hazardous HC and NOx emissions.
Applicant has also unexpectedly discovered that by
reducing combustion temperatures, hazardous combustion
emissions can be additionally controlled. The reduction of
combustion temperatures helps reduce the formation of
problematic heavier manganese oxides during combustion.
~ 0 94/04636 2 I 4 2 9 91 P~r/US93/07962
Applicant has unexpectedly discovered that reductions in
combustion temperatures reduces both HC and NOx emissions.
In the practice of this invention, compounds/
components and/or means that reduce combustion temperatures
are particularly preferred. The greater the temperature
reductions, the better.
Fuel compositions, that as a consequence of their pre-
ignition vaporization into the combustion chamber that
reduce the vapor fraction's temperature, are also
preferred.
Naturally, compounds/components and/or means that both
increase burning velocity and reduce combustion
temperatures are particularly preferred.
By increasing burning velocity (e.g. flame speed,
laminar or turbulent burning velocity, fuel economy,
combustion efficiency, etc.) while simultaneously reducing
combustion temperatures, substantial reductions in heavy
manganese oxide formation can be achieved, hence
controlling hazardous emissions.
It appears that the phenomena of accelerating burning
velocity and/or reducing combustion temperatures also helps
non-manganese fuels. In other words, fuels that do not
contain Mn, but which due to their chemistry, operating
conditions, and the like, have hazardous exhaust emissions,
can be made cleaner by Applicant's invention.
~ It has been unexpectedly discovered that use the of
methylene di methyl ether, carbonic acid dimethyl ester,
methyl tertiary butyl ether, in defined concentrations,
21~2991
W O 94/04636 PC~r/US93/079
together with defined concentrations of Mn in unleaded
gasoline, both increase burning velocities and reduce
temperature of fuels combusted in engines specially
designed for unleaded gasoline usage, resulting in a
significant reduction of hazardous hydrocarbon and NOx
emissions.
Therefore, an integral element of the claimed
invention is the engine, itself, which contributes to the
beneficial results. This is unexpected because the use of
Mn in fuels combusted in such engines in the past has
caused an increase in hazardous hydrocarbon and NOx
emissions.
Therefore, the practice of this invention includes the
utilization of an engine and exhaust system, specially
designed for unleaded fuel usage.
It is now anticipated that due to the discovery of the
source of the problem, that other compounds, which increase
burning velocity, will be identified by routine research.
It is known in the art that several mech~nis-~ may
increase burning velocity. Therefore, in the practice of
this invention, compounds/components and/or means that
increase burning velocity are desired. For example, the
increase of the partial vapor pressure of the vaporized
fraction prior to combustion, resulting in increased
burning velocity, would be desirable. Those
compounds/components and/or means that increase Reid Vapor
Pressure (RVP) of the finished gasoline, which in turn
~ 094/04636 ~I ~ 2 9 g 1 PC~/Us93,0,96~
operates to increase burning velocity, would also be
desirable.
In the practice of this invention, compound/components
and/or means, be they chemical or m~chAn;cal, including
exhaust oxygen sensing systems, which increase the fuel-air
equivalency ratio, resulting in increased burning velocity,
are desirable.
Those means which operate to increase combustion
pressures and/or compression, which in turn increase
burning velocity, are desirable.
In the practice of this invention, combustion
catalysts that operate to improve combustion, and
combustion efficiency, fuel economy, particularity those
also reducing combustion temperatures and/ or increasing
burning velocities are desirable.
Certain molecular features have been identified by
Applicant that reduce heavy manganese oxide formation
during combustion. They include H, H2, CO, and/or OCH3
(methoxy radicals), and/or OH (hydroxyl radicals). Those
compound/components wherein H, H2 CO, OCH3, and/or OH
radicals exist, in high relative concentrations and/or
become intermediate combustion products are preferred. The
higher the relative weight percentage of these structural
components in the component/compound and/or that otherwise
occur/result during combustion, the better. Such features
may be individual or collective. Again, the object is to
reduce adverse emissions by increasing burning velocity
and/or by reducing combustion temperatures.
~1~2g91
W O 94/04636 7' ' ' t PC~r/US93/079 ~
--10--
Those compounds that applicant has thus far identified
that are effective in accomplishing this object, include:
carbon monoxide, methylene di methyl ether (also known as
methylal, di-methoxy methane), carbonic acid dimethyl ester
(also known as dimethyl carbonate), and methyl tertiary
butyl ether (MTBE), C1 to C6 lower molecular weight
alcohols, particularly methanol and ethanol. Applicant
believes many others also exist.
Applicant notes that the desirable OCH3 (methoxy
radical) structure is common to methanol, methylene di
methyl ether (methylal), and carbonic acid dimethyl ester
(dimethyl carbonate). Applicant believes these latter
compounds to be among the best in accomplishing Applicant's
object.
Accordingly, those oxygenates which employ methanol
and ethanol in their manufacture are likely to be effective
and are contemplated within the scope of this invention. It
is believed that such a compound's intermediate combustion
and other features will positively effect Applicant's
object.
~MPLE TEST FUELS
Applicant tested several example fuels, including:
A. MEOH FUEL 0.033025 grams manganese of MMT per
liter of composition, 5% methanol and 5% ethanol by volume,
and unleaded gasoline base.
~ O 94/04636 21 ~ 2g 9 1 PC~r/US93/07962
--11--
B. IS0/HEX FUEL 0.033025 grams manganese of MMT per
liter, 10% isopropanol and 10% hexanol by volume, and
unleaded gasoline base.
C. MTBE FUEL 0.033025 grams manganese of MMT per
liter, 14.6% MTBE by volume, and unleaded gasoline base.
D. DMC FUEL 0.033025 grams manganese of MMT per
liter, 4.6% Dimethyl Carbonate (DMC) by volume, and
unleaded gasoline base.
E. M~lnY~AL FUEL 0.033025 grams manganese of MMT per
liter, 7.2% Methylal by volume, and unleaded gasoline base.
F. THF FUEL 0.033025 grams manganese of MMT per
liter, 1.6% Tetrahydrofuran by volume, and unleaded
gasoline base.
G. HIGH MANGANESE (Mn) FUEL 0.5284 grams manganese of
MMT per liter, 5% Methanol by volume, and unleaded gasoline
base.
H. MANGANESE FUEL 0.033025 grams manganese of MMT per
liter, and unleaded gasoline base.
I. BASE FUEL Unleaded gasoline base (Clear fuel).
Test MethodologY
The above example fuels were tested in a 1988
Chevrolet C1500 pickup truck with a 350 CID V-8 engine,
having a throttle-body fuel injection system and oxygen
sensor-closed loop fuel control. The inclusion of this
oxygen sensing system provided a means of adjusting the
stoichiometry to compensate for variations in the oxygen
content of the various test fuels. This feature eliminated
W O 94/04636 -12- PC~r/US93/079
bias in HC and NOx emissions due to oxygen content
differences. In other words, a test fuel with a high oxygen
content, t~n~ing to enlean combustion, would not
necessarily show better or worse emissions than the fuel
containing little or no oxygen.
Furthermore, this is an integral feature of the
unleaded fuel engine and exhaust systems, and particularly,
the regulated emission control systems of the instant
invention.
10The engine heads and valves were cleaned before each
fuel was tested. A new oxygen sensor and new spark plugs
were also installed.
Each fuel was subjected to two (2) principal tests.
The First Test ("Test One") measured HC and NOx emissions
15from the engine over a forty (40) hour, steady state, 1000
rpm, no load cycle. The purpose of this no load, steady
state test was to elicit worse case hydrocarbon (HC)
emissions over time. This test condition accelerated HC
emission degradation from what would have been expected had
a much longer durability type test been conducted.
Prior to introducing each new test fuel, the clean
engine was cured on the BASE fuel ("clear fuel") until
exhaust emissions stabilized. This generally required
approximately 3 to 6 hours of steady state operation.
Hydrocarbon and NOx emissions were measured periodically
utilizing Beckman exhaust emission analyzers. See TABLE 1
for a summary of data.
094/04636 ~ PCT/US93/07962
-13-
The Second Test ("Test Two") was conducted immediately
after the First Test using the same fuel with the same
engine still warm. The Second Test was conducted with the
vehicle on a stationary chassis dynamometer. Test
measurements were made at 50 mph (80.5 kilometers per hour
"kph") under varying load conditions, e.g. from 15 to 24
indicated horse power ("ihp"). This test measured
differences in combustion temperatures, HC and NOx
emissions, and fuel economy. See Figures 1 through 6 for
a summary of results.
The test results for Test One and Test Two are set
forth as follows:
A. MEOH FUEL 0.033025 grams Manganese of MMT per
liter of composition, 5% methanol and 5% ethanol by volume,
15 and unleaded gasoline base.
-Test One-
ACCUMULATIVE HC NOx
TEST TIME (hrs) ~mC EE_
0.8 2490 95
5.3 2310 89
16.0 2520 80
18.5 2680 93
19.5 2690 91
24.0 2840 78
25.5 2940 83
37.5 3080 74
-Test Two-
ENGINE GAS HC NOx FUEL ECON
TEMP F/C opm P~m(MPG/KPH)
COMMENTS
722(383C) 1.33 4.23 18.0/30.0 50 MPH (80.5kph)
@ 15 ihp
785(418C) 1.87 6.18 14.2/22.8 50 MPH (80.5kph)
Q 24 ihp
21~2~1
W094/04636 PCT/US93/079~2
B. ISO/HEX FUEL 0.033025 grams Manganese of MMT per
liter, 10% isopropanol and 10% hexanol by volume, and
unleaded gasoline base.
-Test One-
ACCUMULATIVE HC NOx
TEST TIME (hrs) ~pmC ~m
0.5 1870 89
4.2 1840 96
7.S 1800 96
11.2 1780 113
20.7 1640 113
24.4 2064 87
34.9 1960 g6
39.8 2050 77
-Test Two-
Not conducted
C. MTBE FUEL 0.033025 grams Manganese of MMT per
liter, 14.6% MTBE by volume, and unleaded gasoline base.
-Test One-
ACCUMULATIVE HC NOx
TEST TIME (hrs) PPmc ~m
1.0 2490 98
4.0 2290 98
10.0 2300 91
20.0 2100 103
24.0 2220 108
26.0 2260 109
28.0 2250 104
34.0 2350 97
44.0 2600 81
-Test Two-
ENGINE GAS HC NOx FUEL ECON
TEMP F/C qPm pm (mpg/kpl) COMMENTS
749(398C) 2.06 4.31 19.3/8.2 50 MPH (80.5 kph) @
16 ihp
778(414C) 2.33 5.34 16.2/6.9 50 MPH (80.5 kph) @
22 ihp
21~299~
094/04636 PC~r/US93/07962
-15-
D. DMC FUEL 0. 033025 grams Manganese of MMT per
liter, 4.6% Dimethyl Carbonate (DMC) by volume, and
unleaded gasoline base.
-Test One-
ACCUMULATIVE HC NOx
TEST TIME (hrs) EE~ EE_
0.0 2860 63
2.0 2720 69
4.0 2900 62
10.0 3000 59
20.0 3180 55
22.0 3360 55
24.0 2400 76
26.0 2440 78
28.0 2490 76
34.0 2370 81
44.0 2390 78
-Test Two-
ENGINE GAS HC NOx FUEL ECON
TEMP F/C apm ~m (m~q/k~l) COMMENTS
717 (381C) 2.23 3.78 18.7/7.96 50 mph (80.5 kph)
Q 15 ihp
798(426C) 2.61 5.02 14.7/6.26 50 mph (80.5 kph)
@ 24 ihp
E. M~;l~YLAL E'UEL O .033025 grams Manganese of MMT per
liter, 7.2% Methylal by volume, and unleaded gasoline base.
-Test one-
ACCUMUL~TIVE HC NOx
TEST TIME (hrs) EE~ EE_
1.0 2930 83
3.0 2860 81
8.0 2870 75
18.0 2670 75
20.0 2750 83
22.0 2800 86
26.0 2790 80
32.0 2760 66
42.0 2490 74
-Test Two-
ENGINE GAS HC NOx FUEL ECON
TEMP F/C a~m E ~ (mpg/kpl) COMMENTS
724 (384C) 2.04 4.70 18.0/7.66 50 mph (80.5 kph)
795(424C) 2.44 6.29 13.8/5.87 50 mph (80.5 kph)
@ 24 ihp
W O 94/04636 i ~ ~ 1 4 2 9 9 1 PC~r/US93/079
F. THF FUEL 0.033025 grams Manganese of MMT per
liter, 1.6% Tetrahydrofuran by volume, and unleaded
gasoline base.
-Test One-
ACCUMULATIVE HC NOx
TEST TIME fhrs) ~mC ~m
0.7 2770 147
12.5 3030 158
22.0 3720 236
24.0 4160 210
24.8 4350 200
36.8 4290 204
-Test Two-
(not conducted)
G. HIGH MN FUEL 0.5284 grams Manganese of MMT per
liter, 5% Methanol by volume, and unleaded gasoline base.
-Test One-
ACCUMULATIVE HC NOx
TEST TIME (hrs) EE~ ppm
1.0 3320 169
2.2 3740 167
6.0 4630 206
7.5 5480 182
12.5 5970 268
14.5 5860 281
19.5 6680 198
21.8 6520 195
23.0 6300 167
24.0 6880 174
25.0 7670 167
-Test Two-
(not conducted)
094/04636 2 I g 2 9 9 1 PC~r/US93/07962
-17-
H. MANGANESE FUEL O. 033025 grams Manganese of MMT per
liter, and unleaded gasoline base.
-Test One-
ACCU~ULATIVE HC NOx
TEST TIME fhrs) EE~ ppm
0.0 2750 87
2.0 2780 92
4.0 2820 86
10.0 2800 87
20.0 2530 85
24.0 2740 88
26.0 3060 91
28.0 2830 109
34.0 3750 64
44.0 3590 64
--Test Two--
ENGINE GAS HC NOx FUEL ECON
TEMP F/C qPm ppm (mpq) COMMENTS
707 (375C) 2.35 5.54 n/a 50 mph (80. 5 kph)
@ 15 ihp
768(409C) 2.41 5.67 n/a 50 mph(80.5 kph)
@ 17 ihp
I. BASE FUEL Unleaded gasoline base (Clear fuel).
-Test One-
ACCUMULATIVE HC NOx
TEST TIME (hrs) EE~ E~_
0.0 2090 70
1.0 2200 90
3.0 2170 91
5.0 2130 96
10.0 2420 90
21.0 2200 84
24.0 2200 88
27.0 2220 89
29.0 3180 68
34.0 3020 76
44.0 3030 60
-Test Two-
ENGINE GAS HC NOx FUEL ECON
TEMP F/C qPm E ~ (mPq/kpl)COMMENTS
707 (375C) 1.91 5.86 18.3/7.79 50 mph (80.5 kph)
@ 15 ihp
828(442C) 2.07 5.73 13.2/5.62 50 mph(80.5 kph)
@ 20 ihp
2~4299~
W 0 94/04636 :- ` '' ' PC~r/US93/079
-18-
T~RT.~ 1
~YDROCARBON EMISSIONS OVER TIMB
~UMMaRY OF RESULTS (TE8T ONE)
A B C D
HC CHANGE HC CHANGE AVE LATER STAGE
~UEL T~ST 20 HOURS(1) TOTAL (2) HC (~pmc) (3)
BASE 38% 45% 3076 (15 hrs)
MANGANESE 41% 31% 3390 (16 hrs3
THF 41% 55% 4130 (14 hrs)
HIGH MN 31% 1310% 6554 (12.5 hrs)
ISO/HEX 15% 10% 2024 (16 hrs~
MEOH 15% 24% 2953 (16 hrs)
MTBE 4% 17% 2400 (16 hrs~
METHYLAL <9%> <15%> 2680 (16 hrs)
DMC <25%> <16%> 2417 (16 hrs)
NOTES:
(1) This column represents the % change of HC
emissions for the last half of Test One.
(2) This column represents total % change of HC
emissions over the entire test.
(3) This represents the average of the more
stabilized, later stage HC emissions. Average
calculated for the final hours of the test, as
indicated.
~ 094/04636 2 1 ~ ~ 9 Y 1 PCT~US93/07962
--19--
Analysis of TABL~ 1
TABLE 1 sets forth the HC emission results of Test
One. It shows that the Manganese, THF, and High Mn fuels
result in significant increases in HC emissions over time.
It shows that these increases are much greater than the
ISO/HEX, MEOH, MTBE, h~lnYLAL, and DMC fuels.
Applicant notes that the HC emission changes of the
last 20 hours of the test (column B) are fairly indicative
of long term HC emission degradation. Even the Base fuel
(absent Mn) experienced significant HC emission degradation
during this period. The BASE fuel shows an increase of 38%,
slightly lower than the Manganese, and THF fuels, which
each showed 41% increases in HC's (Column B).
In stark contrast, the ISO/HEX, MEOH, MTBE, ~l~YLAL,
and DMC fuels did not show the same significant HC emission
increases. Their increases were substantially lower than
the Manganese fuel, ranging from a 15% increase for MEOH,
to a 25% decrease for DMC. HC emission decreases were
noted for the METHYLAL and DMC fuels at 9% and 25%,
respectively. (Column B). Incredibly the ISO/HEX, MEOH,
MTBE, h~l~YLAL, and DMC fuels (all containing Mn)
experienced HC increases much lower than even the Base fuel
(absent Mn).
The Manganese, High Mn, and THF fuels all showed
materially higher later stage (Column D) HC emissions
(3390, 6554, and 4130 ppmc, respectively), than the Base
fuel (3067 ppmc).
W O 94/04636 2 1 ~ 2 9 9 1 PC~r/US93/079
-20-
In contrast, the ISO/HEX, MEOH, MTBE, ~l~YLAL, and
DMC fuels did not show the high HC emission levels of the
Manganese fuel (3390 ppmc) (Column D). Their HC emission
levels, instead, were materially lower ranging from 2953 to
2024 ppmc for MEOH and ISO/HEX, respectively (Column D).
Aston;ch;ngly, the ISO/HEX, MEOH, MTBE, M~l~Y~AL, and DMC
hydrocarbon emissions (Column D) are all also below the
Base (clear) fuel (3076 ppmc). These results are most
unexpected.
In summary, the Manganese, THF, High Manganese test
fuels show considerably high increases in HC emissions over
time. In contrast, the ISO/HEX, MEOH, MTBE, METHYLAL, and
DMC fuels do not. They control HC emissions, not only
better than the Manganese fuel, but even better than the
BASE fuel (absent Mn).
DESCRIPTION OF TEST TWO FIGURES 1 THROUGH 6
FIGURE 1
Combustion Temperature Differences. This Figure
compares engine exhaust gas temperatures ("EGT") of the
MANGANESE, BASE, MEOH, MTBE, ~l~YLAL, and DMC fuels as a
function of engine load. Test Two placed the engine under
load conditions at 50 mph in order to elicit differences in
fuel combustion temperatures, measured by engine exhaust
gas temperatures ("EGT").
At 15 ihp the EGT's for all tested fuels (except MTBE)
are relatively close together. The BASE and Manganese fuels
are the same at 707F (375C), while the DMC, MEOH, and
~ 0 94/04636 12 ~ ~ ~ 9 ~ 1 PC~r/US93/07962
-21-
h~l~Y~AL fuels range tightly from 717F (381C), 722F
(383C), and 724F (384C), respectively. The MTBE fuel, which
was tested at 16 ihp, measured 749F (398C).
As load was increased, EGT's increased very rapidly
for the Manganese and BASE fuels. The Manganese fuel
increase was the greatest. For example, at 20 ihp, the BASE
fuel temperature was 828F (442C) and Manganese's projected
temperature at the same ihp was 860F (460C).
In contrast, the MEOH, MTBE, M~l~Y~AL, and DMC fuels
had lower rates of EGT increase. For example, at 24 ihp,
the MEOH, h~l~YLAL, and DMC fuels were tightly grouped at
785F (418C), 795F (424C), and 798F (426C), respectively.
Similarly, MTBE showed an even lower rate of increase of
778F (414C) at 22 ihp.
The most significant aspect of Figure 1, is the strong
showing that MEOH, MTBE, METHYLAL, and DMC fuels, enjoy
markedly lower combustion temperatures than either the BASE
or Manganese fuels at the same loads. For example, Figure
1 shows that at 20 ihp the EGT for the Manganese fuel is
104F (40C) higher than the MEOH fuel. Additionally, Figure
1 shows that the fuels cont~;n;ng both Mn and an oxygenate
(i.e. the MEOH, MTBE, M~l~Y~AL, and DMC fuels) also had
significantly lower combustion temperatures than the BASE
fuel. For example, the MEOH fuel was 72F (22C) lower than
the BASE fuel.
- Figure 1 shows that the higher the load, the greater
the EGT differences between the two classes of fuels.
4~
W094/04636 ~ PCT/US93/079 ~
21~2991 -22-
FIGURE 2
Combustion Temperatures and Hydrocarbon Emissions.
This Figure shows hydrocarbon emissions of Test Two as a
function of engine gas temperatures ("EGT"). This Figure
shows that there is a direct correlation between HC
emissions and engine gas temperatures. The relationship is
most notable in the MEOH, MTBE, M~ nYhAL, and DMC fuels.
Figure 2 shows that the HC/EGT rate of change for the
various oxygenates is higher than the HC/EGT rate of change
for the non-oxygenated fuels. Figure 2 shows that the lower
the combustion temperature of a given oxygenate, the lower
the HC emissions.
FIGURE 3
Combustion TemPeratures and NOx Emissions. This
Figure shows NOx emission results as a function of EGT.
Figure 3, like Figure 2, shows a direct and significant
relationship between NOx em;ssions and EGT for MEOH, MTBE,
M~l~YLAL, and DMC fuels. This Figure clearly shows that at
lower EGT's, particularly in the case of the MEOH, MT8E,
M~l~YhAL, and DMC fuels, NOx emissions are much lower than
when compared to the BASE and Manganese fuels.
FIGURE 4
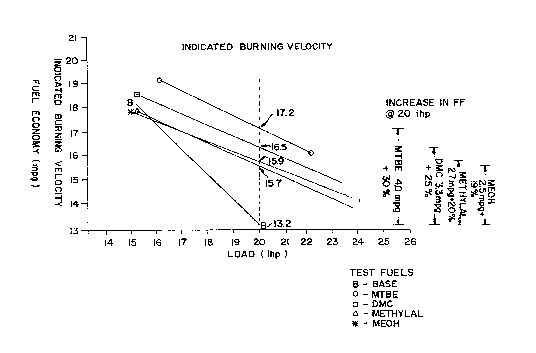
Indicated Burning Velocity. This Figure measures
burning velocity indirectly via fuel economy measurements
as a function of load. It is known in the art that
increases in fuel economy, absent a BTU boost, is an
21 1 4 2 ~ ~ 1
094/04636 PCT/US93/07962
-23-
indicator of a flame speed or burning velocity increase.
Figure 4 shows fuel economy in miles per gallon t"mpg") (or
0.42566 kilometers per liter "kpl") as a function of load
(ihp). Figure 4 shows significant fuel economy ("FE")
differences between the BASE and the oxygenated fuels,
beginning almost immediately with the application of load.
Note, that at 20 ihp the fuel economy of the Base fuel is
13.2 mpg (5.619 kpl), compared to 15.7 mpg (6.683 kpl),
15.9 mpg (6.768 kpl), 16.5 mpg (7.0234 kpl) and 17.2 mpg
(7.321 kpl) for M~lnYhAL, MEOH, DMC, and MTBE,
respectively. These material differences account for a 19%
to 30% improvement in fuel economy over the BASE fuel.
These FE improvements indicate substantial burning velocity
increases for the M~l~YLAL, MEOH, DMC, and MTBE fuels.
FIGURE 5
Burning VelocitY and HC Emissions. This Figure shows
HC emissions as a function of fuel economy, i.e. indicated
burning velocity. Figure 4 shows a strong correlation
between increased burning velocity to improvements in HC
emissions. Figure 5 clearly shows that increased burning
velocity for M~lnYLAL, MEOH, DMC, and MTBE translates into
improved HC emissions. This correlation is most apparent
for the MEOH fuel.
FIGURE 6
Burning Velocity and NOx Emissions. This Figure
shows NOx emissions as a function of fuel economy. This
W O 94/04636 : 2 1 ~ PC~r/US93/079
-24-
Figure shows a very strong correlation between increased
indicated burning velocity to improvements in NOx
emissions. This correlation exists for oxygenated fuels,
but is not noticeable for the BASE fuel.
~h~KKED PRACTICE
To the extent that oxygenated compounds are utilized
to accomplish the object of accelerating burning velocity
and/or to reduce combustion temperatures, while not
required, it is preferred that the oxygen content, as a
percent of total weight of the constituent additive
compound, be 15% or more of the total weight of the
oxygenate. While lower oxygen concentrations are
acceptable, the higher the relative oxygen content, as a
weight percentage of the total oxygenated
compound/component, the more preferred. It is believed
that the simpler the compound/component's molecular
structure the better. More complicated molecular structure
is acceptable, particularly, if the intermediate combustion
products enhance burning velocity and/or reduce combustion
temperatures.
It is preferred that the thermal efficiency (e.g. fuel
economy) of the finished fuel cont~;n;~g the
component/compound, be an improvement over the base fuel
alone.
Applicant appreciates that numerous means may be
employed to achieve the beneficial results contemplated by
Applicant's discovery of the source of the problem.
W O 94/04636 2 1 ~ 2 9 ~ 1 PC~r/US93/07962
-25-
Applicant further appreciates that with his discovery
of the source of the problem that numerous other
components, compounds, oxygenated and non-oxygenated,
including combinations thereof, and/or mechanical/chemical
means, will be identified by routine investigation to
reduce and/or eliminate heavy manganese oxide formation
during combustion.
It is contemplated that by the practice of this
invention that fuels utilizing applicant's discovery of the
source of the problem, can meet minimal environmental
standards and become eligible for 211 (f) EPA type
waivers, which have heretofore been denied organomanganese
and unleaded gasoline combinations.
It is contemplated that by the practice of this
invention that problematic, non-Mn contA;ning unleaded
fuels (absent organomanganese compounds) may benefit from
Applicant's discovery of the source of the problem. It is
contemplated that in order to control of hazardous emission
from these fuels that organomanganese compounds may be
required.
It is contemplated that through investigation that
certain ethers, phenols, esters, oxides, ketones, alcohols
and/or other chemical agents can be identified that will
solve Applicant's discovery of the source of the problem.
The production of ethers is also well known to the
art. See for example, U.S. Patents 4,262,145; 4,175,210;
4,252,541; 4,270,929; 3,482,952; 2,384,866; 1,488,605;
4,256,465; 4,267,393; 4,330,679; 4,299,999; 4,302,298;
W094/04636 ~ PCT/US93/079`~
-26-
4,310,710; 4,324,924; 4,329,516; 4,336,407; 4,320,233;
2,874,033; 3,912,463; 4,297,172; 4,334,890, et al.
Possible C2 to C6 ethers for use in the practice of
this invention may include branched and straight chain
ethers, di ethers having two oxygen and dual ether linkage,
and tri ethers having three oxygens and multiple ether
linkages. Non-limiting examples of possible C2 to C6
ethers include dimethyl ether, methyl ethyl ether, di ethyl
ether, ethyl propyl ether, methyl normal propyl ether,
ethyl isopropyl ether, methyl isopropyl ether, ethyl normal
propyl ether, propyl propyl ether, propyl isopropyl ether,
isopropyl isopropyl ether, ethyl butyl ether, ethyl
isobutyl ether, ethyl tertiary butyl ether, ethyl secondary
butyl ether, methyl normal butyl ether, methyl isobutyl
ether, methyl tertiary butyl ether, methyl secondary butyl
ether, methyl normal amyl ether, methyl secondary amyl
ether, methyl tertiary amyl ether, and methyl iso amyl
ether. Additional non-limiting examples of acceptable di
ethers (having two oxygens and dual ether linkage) include
methylene di methyl ether, methylene di ethyl ether,
methylene di propyl ether, methylene di butyl ether, and
methylene di isopropyl ether.
It is expected that ethers, where significant amounts
of free H, H2 CO, OCH3, and/or OH radicals become
intermediate combustion products, are likely to be the best
candidates.
Preferably, the ether(s) employed should be anhydrous.
Within the preferred concentration range, most C2 - C6
094/04636 PCT/US93/07962
ethers are completely miscible with petroleum hydrocarbons;
and it is preferred that such ethers be used in amounts
within their solubility limits. However, if desirable, an
amount of ether in excess of its solubility can be
incorporated in the fuel by such means, as for example, use
of mutual solvents.
Possible ketones that may be acceptable include
ketones with three to about twelve carbon atoms. Lower
alkenyl ketones are, however, likely to be slightly
preferred. Representative lower alkenyl ketones would
include diethyl ketone, methyl ethyl ketone, cyclohexanone,
cyclopentanone, methyl isobutyl ketone, ethyl butyl ketone,
butyl isobutyl ketone, ethyl propyl ketone, and the like.
Other ketones include acetone, diacetone alcohol,
diisobutyl ketone, isophorone, methyl amyl ketone, methyl
isamyl ketone, methyl propyl ketone, and the like. A
representative cyclic ketone would be ethyl phenyl
ketone. It is expected that those ketones where free H,
H2 CO, OCH3, and/or OH radicals become intermediate
combustion products are likely to be the best candidates.
Possible esters that may be acceptable include anisol
(methyl ester of benzene), isopropyl acetate, and ethyl
acrylate.
The preferred chemical means for achieving these
results is by the addition of a compound or combination of
compounds and/or components which, individually or in
combination, operate to increase flame speeds/burning
velocity and/or reduce combustion temperatures.
W094/04636 ~ 4~ ~
PCT/US93/o79
-28-
An illustrative example of a desirable fuel
composition containing an oxygenated agent, would include
the oxygenate from about 0.1 to about 20.0 weight percent
by oxygen in the composition, with or without co-solvents,
and about 0.000264 to about 0.264200 gram manganese per
liter of unleaded fuel. A more desirable composition would
include the oxygenate from about 0.5 to about 10.0 weight
percent by oxygen in the composition, with or without
cosolvents, and an organomanganese concentration from about
0.004128 to about 0.099075 grams manganese per liter of the
fuel composition. While not required, anhydrous fuel are
desirable.
Another desirable composition would include the
oxygenate from about 1.0 to about 5.0 weight percent by
oxygen in the composition, with or without cosolvents, and
an organomanganese concentration from about 0.004128 to
about 0.066050 grams manganese per liter of the fuel
composition.
Control of Hydrocarbons Emissions
Applicant has discovered that those Cyclomatic
Manganese Tricarbonyls (CMT) concentrations that heretofore
have been considered e~c~s~ive for reasons associated with
unacceptable engine out hydrocarbon (EOHC) emissions and
catalyst plugging, when constructed to solve Applicant's
discovery of the source of the problem, can prevent
unacceptable long term hydrocarbon emissions degradation
and prevent catalyst plugging. In other words, manganese
~ 094/04636 ; 2I ~ 29 9 i PCT/US93/07962
-29-
concentrations, for example, greater than 1/64, 1/32, or
even 1/16 grams of manganese per 3.785 liters are
satisfactory. In view of the prior art literature on the
subject, this result is quite unexpected. It appears that
levels equal to and exceeding 1/8 or even 3/8 gram of
manganese per 3.785 liters are quite satisfactory.
Since the heavy manganese oxides of the above
cyclomatic manganese tricarbonyls plays a leading role in
hydrocarbon deposit build-up, it is desirable to balance
the amount of cyclomatic manganese tricarbonyl compounds
employed with the efficacy of the heavy manganese oxide
alleviation means, as is necessary in order to maximize the
benefits of the invention. In the practice of Applicant's
invention, concentrations of manganese from about 0.000264
up to as high as 0.2642 gram of manganese per liter may be
used. However, concentration levels greater than 0.00825
or even 0.016505 grams but less than 0.1321 grams manganese
per liter are more preferred.
In terms of emissions benefits, concentrations of
cyclomatic manganese tricarbonyl from about 0.00825 grams
to about 0.099075 grams manganese/liter are acceptable;
concentrations in the range of about 0.033025 grams to
about 0.06605 grams manganese/ liter are preferred.
In terms of octane benefits, a desirable range is from
about 0.000264 to about 0.06605 grams manganese per liter
- of compositio~. A more desirable range is from about
0.004128 to about 0.033025 grams manganese per liter of
wo g4,04636 2 1 ~2 9 9 I PCT/US93/079 ~
-30-
composition. A preferred range is from about 0.004128 to
about 0.016512 grams manganese per liter of composition.
A preferred cyclomatic manganese tricarbonyl used in
the composition is cyclopentadienyl manganese tricarbonyl.
A more preferred cyclomatic manganese tricarbonyl is methyl
cyclopentadienyl manganese (MMT). As contemplated in this
invention, the composition can also contain homologues or
other cyclomatic manganese tricarbonyl substitutes. Non-
limiting examples of these other acceptable substitutes
include the alkenyl, aralkyl, aralkenyl, cycloalkyi,
cycloalkenyl, aryl and alkenyl groups. Illustrative and
other nonlimiting examples of acceptable cyclomatic
manganese tricarbonyl antiknock compounds include
benzyleyelopentadienyl manganese tricarbonyl; 1.2-dipropyl
3-cyclohexylcyclopentadienyl manganese tricarbonyl: 1.2-
diphenylcyclopentadienyl manganese tricarbonyl; 3-
propenylienyl manganese tricarbonyl; 2-tolyindenyl
manganese tricarbonyl; fluorenyl manganese tricarbonyl;
2.3.4.7 - propyflourentyl manganese tricarbonyl; 3-
naphthylfluorenyl manganese tricarbonyl; 4.5.6.7-
tetrahydroindenyl manganese tricarbonyl; 3-3ethenyl-4, 7-
dihydroindenyl manganese tricarbonyl; 2-ethyl 3 (a-
phenylethenyl) 4,5,6,7 tetrahydroindenyl manganese
tricarbonyl; 3 - (a-cyclohexylethenyl) -4.7
dihydroindenyl manganese tricarbonyl; l,2,3,4,5,6,7,8 -
octahydrofluorenyl manganese tricarbonyl and the like.
Mixtures of such compounds can also be used. The above
compounds can be generally prepared by methods that are
~ 0 94/04636 ~1~299~ PC~r/US93/07962
-31-
known in the art. Representative preparative methods are
described, for example, in U.S. Patents 2,819,416 and
2,818,417.
In a further effort to control hydrocarbon emissions,
Applicant also contemplates the use of other additives with
his ingredients, such as gum and corrosion inhibitors,
detergents, multipurpose additives, and scavengers, made
necessary or desirable to maintain fuel system cleanliness
and control exhaust emissions.
Applicant's invention contemplates a method for
controlling hazardous combustion emissions originating in
internal combustion engines. This method comprises the
mixing of a nonleaded gasoline base comprised of
hydrocarbons with a cyclopentadienyl manganese tricarbonyl
antiknock compound having a manganese concentration from
about 0.000264 to about 0.2642 grams of manganese per liter
of the fuel composition, together with a chemical and/or
mechAnical means for reducing the formation of heavy and/or
hazardous emission causing oxides of manganese during the
combustion of said fuel. Applicants method then
contemplates the combustion of said fuel composition in an
internal combustion engine; then emitting the resultant
engine emissions through an exhaust system, including
catalytic exhaust systems; such that the resultant
emissions and/or emission control systems meet 211 (f)
- waiver requirements.
Applicant's invention also contemplates a method for
controlling hazardous combustion emissions originating in
2l~29~
W094/04636 PCT/US93/07
-32-
internal combustion engines; comprises the mixing of a
nonleaded gasoline base comprised of hydrocarbons with a
cyclopentadienyl manganese tricarbonyl antiknock compound
having a manganese concentration from about 0.000264 to
about 0.2642 grams of manganese per liter of the fuel
composition, together with a chemical and/or mechAn;cal
means for accelerating burning velocity and/or reducing
combustion temperatures of said fuel; then combusting said
fuel composition in a spark ignited internal combustion
engine; then emitting the resultant emissions through an
exhaust system, including catalytic exhaust systems; such
that the resultant emissions meet 211 (f) waiver
requirements.
USING COSOLVENTS
It is contemplated that co-solvents may be employed
for several reasons, including, the correction of techn;cal
enleanment, vapor pressure control, and phase stability.
Cosolvent(s) may be selected from the group consisting of
C2 to Cl2 aliphatic alcohols, C3 to C12 ketones, C2 to C12
ethers, esters, oxides, phenols, and the like. It is in
the scope of this invention to employ one or more co-
solvents within a particular class of cosolvents and/or to
employ any one or more classes of cosolvents
simultaneously.
It is also within the scope of this invention to mix
different classes of cosolvents, including mixed alcohols,
ethers, esters, oxides, phenols and/or ketones. For
example, it has been found that mixed cosolvent alcohols,
0 94/04636 PC~r/US93/07962
_33_ 2 ~
particularly those in the C2 to C8 range, have a
particularly ameliorative effect on both RVP and octane
blending values.
Acceptable cosolvent concentrations will vary
depending upon the other components and their
concentrations in the composition, but will normally range
from between 0.1 to about 20.0 volume percent of the
composition. More desirable concentrations will normally
range from between 0.1 and to about 15.0% volume percent of
the composition. Preferred concentrations will range from
about 0.1 volume percent to 10 volume percent, with the
most preferred ranging from about 0.1 to about 5.0 volume
percent.
It is also within the scope of this invention to
utilize individual and/or different molecular weight
cosolvent mixtures. For example, higher molecular weight
alcohol mixtures (especially C4 - C12 in varying
combinations and concentrations) can be employed as a means
of controlling RVP, evaporative emissions, volatility,
initial and mid-range distillation depressions, to reduce
end boiling point temperatures, and to even provide for the
inclusion of hydrocarbons boiling above gasoline
temperatures in the composition.
Technical enleanment is related to depressed initial
and mid-range distillation curves. It is contemplated that
the use of cosolvents may be employed independently or in
combination with hydrocarbons boiling above midrange
boiling temperatures so as to improve distillation
W094/04636 2 1 4 2 9 9 1 PCT/US93/079~
-34-
temr~rature depression in order to mitigate techn; cal
enleanment. Such temperature depression may also be
remedied independent of co-solvents with the use of one of
more higher boiling temperature hydrocarbons, which when
added to the composition corrects the depression of
distillation temperatures.
It has also been discovered and is within the scope of
this invention to employ higher molecular weight co-
solvents, in combination with the other necessary elements
of this invention, to reduce hazardous emissions. For
example, an azeotroping co-solvent in combination with
hazardous emission causing heavier hydrocarbon, where the
resultant heavier hydrocarbon and co-solvent oxygen combust
together, under conditions where combustion is accelerated
and/or where combustion temperature is reduced, is one such
means.
UNLEADED BASE GASOLINE COMPOSITION
The gasoline to which this invention is applied is a
lead free gasoline. The gasoline bases in Applicant's fuel
composition are conventional motor fuels, boiling in the
general range of about 70 degrees to about 440 degrees F.
However, boiling ranges outside gasoline ranges are
contemplated and may be used. Substantially all grades of
unleaded gasoline employed in spark ignition internal
combustion engines are contemplated. Other non-internal
combustion engine fuel applications are also contemplated.
~ 094/04636 PCT/US93/07962
21~z~l
-35-
Generally, fuel bases may contain both straight runs
and cracked stock, with or without alkylated hydrocarbons,
~ reformed hydrocarbons, and the like. Such fuels can be
prepared from saturated hydrocarbons, e.g., straight
stocks, alkylation products, and the like, with or without
detergents, antioxidants, dispersants, metal deactivators,
lead scavengers, rust inhibitors, multi-functional
additives, emulsifiers, demulsifiers, fluidizer oils, anti-
icing, combustion catalysts, corrosion and gum inhibitors,
emulsifiers, surfactants, solvents, and/or other similar or
known additives. It is contemplated that in certain
circumstances, these additives may be included in
concentrations above normal levels, made necessary to
accommodate the ingredients of Applicant's invention.
Generally, the base gasoline will be a blend of stocks
obtained from several refinery processes. The final blend
may also contain hydrocarbons made by other procedures,
such as alkylates made by the reaction of C4 olefins;
butanes using an acid catalyst such as sulfuric acid or
hydrofluoric acid; and aromatics made from a reformer.
The olefins are generally formed by using such
procedures as thermal cracking and catalytic cracking.
Dehydrogenation of paraffins to olefins can supplement the
gaseous olefins occurring in the refinery to produce feed
material for either polymerization or alkylation processes.
- The saturated gasoline components comprise paraffins and
naphthenates. These saturates are obtained from: (1)
virgin gasoline by distillation (straight run gasoline),
21~2991
W O 94/04636 PC~r/US93/079
-36-
(2) alkylation processes (alkylates), and (3) isomerization
procedures (conversion of normal paraffins to branched
chain paraffins of greater octane quality). Saturated
gasoline components also occur in so-called natural
gasolines. In addition to the foregoing, thermally cracked
stocks, catalytically cracked stocks and catalytic
reformates contain saturated components. Preferred
gasoline bases are those having an octane rating of (R +
M)/2 ranging from 70-95. A desirable gasoline base should
have an olefinic content ranging from 1 to 30 volume
percent, and a saturate hydrocarbon content ranging from
about 40 to 80 volume percent.
The motor gasoline bases used in formulating the fuel
blends of this invention generally are within the
parameters of ASTM D-439 and have initial boiling points
ranging from about 70 degrees F to about 115 degrees F and
final boiling points ranging from about 380 degrees F to
about 437 degrees F as measured by the stAn~rd ASTM
distillation procedure (ASTM D-86). Intermediate gasoline
fractions boil away at temperatures within these ranges.
In terms of phase stability and water tolerance,
especially when employing lower molecular weight alcohols,
desirable base gasoline compositions would include as many
aromatics with C8 or lower carbon molecules as possible in
the circumstances. The ranking or aromatics in order of
their preference would be: benzene, toluene, m-xylene,
ethylbenzene, o-xylene, isoproplybenzene, N-propybenzene,
and the like. After aromatics, the next preferred gasoline
21~9~1
94/04636 PCT/US93/07962
-37-
component in terms of phase stability would be olefins.
The ranking of preferred olefins in order of their
preference would be: 2-methyl-2-butene, 2 methyl-l butene,
1 pentene, and the like. However, from the standpoint of
minimizing the high reactivity of olefins and their smog
contributing tendencies, olefinic content must be closely
watched. After olefins the least preferred gasoline
component in terms of phase stability, when using for
example alcohols, would be paraffins. The ranking of
preferred paraffins in order of their preference would be:
cyclopentane, N-pentane, 2,3 dimethylbutane, isohexane, 3-
methylpentane and the like.
In terms of phase stability, aromatics are generally
preferred over olefins; and olefins are preferred over
paraffins. Within each specific class, the lower molecular
weight components are preferred over the higher molecular
weight components.
It is also desirable t~ utilize base gasolines having
a low sulfur content, as the oxides of sulfur tend to
contribute to the irritating and choking characteristics of
smog and other forms of atmospheric pollution. To the
extent it is economically feasible, the base gasolines
should contain not more than 0.1 weight percent of sulfur
in the form of conventional sulfur-containing impurities.
Fuels in which the sulfur content is no more than about
0.02 weight percent are especially preferred for use in
this invention.
W094/04636 2 1 4 2 9 9 1 PCT/US93/079~
-38-
The gasoline bases of this invention can also contain
other high octane organic components, including phenols
(e.g., P-cresal, 2, 4 xylenal, 3-methoxyphenal), esters
(e.g., isopropyl acetate, ethyl acrylate), oxides (e.g., 2-
methylfuran), ketones (e.g., acetone, cyclopentanone),alcohols (furon, furfuryl), ethers (e.g., MTBE, TAME,
dimethyl, diisopropyl), aldehydes, and the like. See
generally "Are There Substitutions for Lead Anti-Knocks?,"
Unzelman, G. H., Forster, E. J., and Burns, A. ~., 36th
Refining Mid-Year Meeting, American Petroleum Institute,
San Francisco, California, May 14, 1971.
The gasoline may further contain antiknock quantities
of other agents, such as cyclopentadienyl nickel nitrosyl,
N-methyl aniline, and the like. Antiknock promoters such
as 2.4 pentanedione may also be included. The gasoline may
contain supplemental valve and valve seal recession
protectants. Nonlimiting examples of such additives
include boron oxides, bismuth oxides, ceramic bonded CaF2,
iron phosphate, tricresylphosphate, phosphorous and sodium
based additives, and the like. The fuel may also contain
antioxidants, such as 2,6 di-tert-butylephenol, 2,6-di-
tert-butyl-p-cresol, and phenylenediamines such as N-N-di-
sec-butyl-p-phenylenediamines, N-isopropylphenylene
diamine, and the like. The fuel may contain such additives
2S as F310, polybutene amines, aminated or polymerized
detergents, and the like.
The gasoline base may contain hydrocarbons boiling
outside normal gasoline ranges. It is contemplated in
0 94/04636 2 1 ~ 2 ~ 9 ~ PC~r/US93/07962
-39-
certain occasions these higher boiling point hydrocarbon
can be incorporated into a finished normal boiling gasoline
by utilizing the azeotroping effect of certain co-
solvents/additives. Applicant has found that higher
molecular weight C4-C12 alcohols are particularly useful in
reducing end boiling point temperatures.
Those skilled in the art will appreciate that many
variations and modifications of the invention disclosed
herein may be made without departing from the spirit and
scope thereof.
.U~ . ' 5 iF~!AP~'