Note: Descriptions are shown in the official language in which they were submitted.
Wo 94/0S670 2 1 ~ 3 6 2 5 PCr/US93/07926
1
~ITLE
ARTHROPODICIDAL TETRAHYDROPYRIMIDINES
The arthropodicidal tetrahydlo~ylilllidines of tnis invention are
S characterized by a methyl-substituted alkylene bridge. U.S. 4,831,036 discloses
tetrahydlupy,il-li(lines not sû characterized.
SUMM~R~ OF THF INVENTION
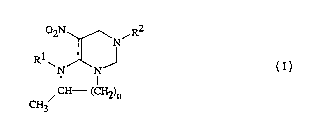
The invention pertains to compounds of Formula I, including all geometric
and stereûisûmers, ag iculturally suitable salts thereof, agricultural cûmpositions
cont~ining them and their use to control arthropods in botn agronomic and
nonagronomic environment.~. The compounds are:
02N~N,R2
R~ J
N IN
CH--(CH2)n
CH3
15 wherein:
Rl is selected from t'ne group CH3SCH2CH2-,
~N N
-CH2 ~ -CH2 ~ Cl
5 ~ Cl ~ N
R2 is selected from the group H; Cl-C20 aL~cyl optionally substituted with
1 or 2 substituents independently selected from the group CN~
C(o)R3, N02, OH, SH, Cl-C4 aLkûxy, Cl-C4 alkyltnio,
Cl-C4 haloalkoxy, N(Rs)(R6)R7-X, Cl-C4 aLkylamino,
C2-C8 diaLkylamino and C3-C8 cycloalkyl; Cl-C20 haloaLkyl:
WO 94/05670 PCI`/US93/07926
3~5
Cl-C6 alkoxy; Cl-C6 alkylthio; C2-C6 aLkenyl; C2-C6 aL~enyloxy;
C7-CIo aralkoxy; C2-C6 alkynyl; C(o)R3; N(R5)R6; C3-C8 cycloalkyl
optionally substituted with 1-3 substituents independently selected
from the group halogen, Cl-C2 alkyl and Cl-C2 haloaLkyl; phenyl and
S C7-C10 aralkyl each ring optionally substituted with a substituent
selected from the group halogen, NO2, CN, Cl-C4 alkyl, Cl-C4
alkoxy, Cl-C4 aLkylthio, Cl-C2 haloaL~yl, Cl-C2 haloaLkoxy and
Cl-C2 haloaLkylthio; Cl-C3 aLkyl substituted with a heteroaromatic
ring, the ring optionally substituted with a group selected from
halogen, N02, NH2, CN, Cl-C4 alkyl, Cl-C4 aLkoxy, Cl-C4 alkylthio,
Cl-C4 haloalkyl, Cl-C4 haloalkoxy, Cl-C4 haloalkylthio, Cl-C4
alkylamino and C2-C8 dialkylamino; a 3- to 8-membered heterocycle
attached through carbon or nitrogen COI,L~ i.,g 2-7 carbon atoms and
1-4 heteroatoms selected from the group nitrogen, oxygen and sulfur,
lS said heterocycle being fully unsaturated, partially unsaturated or fully
saturated; and (CH2CH2O)qR4;
R3 is selected from the group H, NH2, C1-C6 alkyl, OH, C1-C6 alkoxy,
Cl-C4 aLkylamino and C2-C8 dialkylamino;
R4 is selected from the group H and Cl-Cs aL~yl;
R5 and R6 are independently selected from the group H; C1-C6 alkyl; and
optionally substituted phenyl wherein the substituents are selected
from the group halogen, Cl-C6 alkyl and Cl-C6 haloaLkyl;
R7 is selected from the group C1-C6 alkyl;
X is selected from the group halogen and OH;
n is 1 or 2; and
qis 1,2,3,4OrS.
Contemplated heteroaromatic rings on the Cl to C3 alkyl R2 group include
furyl, imid:~7olyl, pyrrolyl, thienyl, pyridinyl, pyrazolyl, 1,2,3-triazolyl, 1,2,4-
triazolyl, pyrazinyl, pyrimidinyl, oxazolyl, isoxazolyl, 1,2,4-oxadiazolyl, 1,3,4-
ox~ 7Qlyl,thiazolyl,isothiazolyl, 1,2,5-thi~ 7Olyland 1,3,4-thi~ 7nlyl.
Contemplated 3- to 8-membered R2 heterocycles include morpholino,
pyrrolidinyl, imidazolidinyl, pyrazolidinyl, pyridinyl, thienyl, pyrrolinyl, and the
like.
Preferred Compounds A are compounds of Formula I wherein:
WO 94/05670 PCI /US93/07926
~ 21436~
Rl is selected from the group CH3SCH2CH2-,
--CH ~r~ Cl and ~ CH2 ~ ~
R2 is selected from the group Cl-C6 aLkyl; C3-C8 cycloalkyl;
C2-C6 alkenyl; N(R5)R6; Cl-Cs aLkyl substituted with a
group selected from C3-C6 cycloaLkyl, C2-C8 dialkylamino
and N(R5)(R6)R7-X; and (CH2CH2O)qR4; and
nis 1.
Preferred Compounds B are compounds of Preferred A wherein:
Rl is CH3SCH2CH2-; and
R2 is Cl-C6 aLkyl.
PlGrellbd Compounds C are compounds of Preferred A wherein:
Rl is
N
--CH2 ~\ ~ Cl ; and
R2 is Cl-C6 aLkyl.
Preferred Compounds D are compounds of Preferred A wherein:
Rl is
S
Cl
R2 is Cl-C6 alkyl.
Specifically preferred for biological activity are Compounds B and C,
30 respectively, which are:
W 0 94/05670 ~43~S PC~r/US93/07926
1,2,3 ,5 ,6,7-hexahydro-2,6-dimethyl- 1 -[2-(methylthio)ethyl]-8-nitro-
imidazo[l,2-c] pyrimi~in~, and
1 -[(6-chloro-3-pyridinyl)methyl]- 1 ,2,3,5,6,7-hexahydro-2,6-
dimethyl-8-nitroimid~7o [1,2-c] pyrimidine.
Compounds of this invention can exist as one or more stereoisomer. The
various stereoisomers include enantiomers, diastereomers and geometric isomers.
One skilled in the art will appreciate that one stereoisomer may be more active
and how to sepa,ate said stereoisomers. Accordingly, the present invention
comprises racemic mixtures, individual stereoisomers, and optically active
mixtures of compounds of Formula I.
In the above definitions. the term "alkyl", used either alone or in compound
words such as "alkythio" or "haloalkyl", denotes straight chain or branched alkyl
such as methyl, ethyl, n-propyl, isopropyl or the dirrcrcll~ butyl isomers.
Alkoxy denotes methoxy, ethoxy, n-propyloxy, isopropyloxy and the
various isomers of butoxy, pentoxy and hexyloxy.
Alkenyl denotes straight chain or branched alkenes such as vinyl,
l-propenyl, 2-propenyl, 3-plupellyl and the different butenyl, pentenyl and
hexenyl isomers.
Alkynyl denotes straight or branched alkynes such as ethynyl, l-propynyl,
2-propynyl and the different butynyl, pentynyl and hexynyl isomers.
Alkylamino denotes methylamino, ethylamino, n-propylamino,
isopropylamino and the different butylamino isomers. Dialkylamino denotes
nitrogen substituted with two aLkyl groups, which may be different. Examples
include N,N-dimethylamino and N-ethyl-N-methylamino
Alkylthio denotes methylthio, ethylthio, n-propylthio, isopropylthio and the
different butylthio, pentylthio and hexylthio isomers.
Aralkyl denotes a phenyl ring attached to a carbon chain, exarnples include
benzyl and phenethyl. Aralkyl is further defined to include naphthyl.
The term "halogen", either alone or in compound words as "haloalkyl",
denotes fluorine. chlorine, bromine or iodine. Further, when used in compound
words such as "haloalkyl" said alkyl can be partially or fully sllbstit~-ted with
halogen atoms. which can be the same or different. Examples of haloalkyl
include CH2CHF2, CF2CF3 and CH2CHFCl.
W O 94/OS670 2 1 ~ 3 6 2 5 PC~r/US93/07926
S
The total number of carbon atoms in a substituent group is indicated by the
"Cj-Cj" prefix where i and j are numbers from 1 to 20. For example, Cl-C3 alkyl
~lecign It~s methyl through propyl; and C2 alkoxy design~tPs OCH2CH3 and
C3 aL~oxy design:~tes OCH2CH2CH3 and OCH(CH3)2.
t 5 DFTATT ~ OF THF INVENTION
The compounds of Formula I can be prepared by the reaction of Formula II
compounds with one or more equivalents of an amine of Formula III and at least
two molar equivalents of formaldehyde in a suitable solvent as depicted in
Scheme 1. Substituents depicted in the following Schemes are as previously
defined.
Sch~.me 1
N02
N N III
~ (CH2)n CH20
Me
II
Reactions depicted in Scheme 1 are typically carried out at tempe-~lulc~s
15 ranging from 0C to the reflux temperature of the solvent, with 0C to 25C being
preferred. Suitable solvents include alcohols such as methanol and ethanol, water,
and polar aprotic solvents such as tetrahydrofuran and dimethylformamide.
Formaldehyde can be used in amounts of about 2 to 10 molar equivalents. Either
solid paraformaldehyde or aqueous solutions of formaldehyde can be used. In
20 some cases, it is desirable to use a small amount of a strong, non-oxidizing acid,
such as hydrochloric acid, as a catalyst. Alternatively, a hydrohalide or a
hydrosulfonic acid salt of amine III can be used.
Compounds of Formula II can be prepared by the reaction of imidazolidines
(n=l) or tetrahydropyrimi(lin~.s (n=2) of Formula IV with an aL~ylating agent of25 Formula V in the presence of a proton acceptor as shown in Scheme 2.
WO 94/05670 PCr/US93/07926
Sch~m~ 2
~N0
HN NH +R~Xl proton acceptor II
~ (CH2)n
Me
IV V
wherein: X1 is a leaving group such as halide or sulfonate
The reactions depicted in Scheme 2 typically involve the mixture of
equimolar amounts of IV and V in the presence of one equivalent of a base such
5 as NaH or K2CO3 in a polar, aprotic solvent such as DMF or THF at a
temperature ranging from room temperature to 150C. The product is typically
isolated by removal of the solvent followed by column chromatography on silica
gel using a suitable solvent such as chloroform, methylene chloride, methanol,
ethanol, ethylacetate, triethylamine, saturated aqueous ammonium hydroxide or
10 mixtures of these solvents.
Alternatively, compounds of Formula II can be prepared by the reaction of
t1i~min~.s of Formula VI with compounds of Formula VII as shown in Scheme 3.
Scherne 3
N02
RlNH NH2 If
~) (cH2)n + 2/~x2 Il
Me X
VI VII
wherein: x2 is a leaving group such as SCH3, OC6H5
or halogen.
Scheme 3 reactions typically involve the mixture of equimolar amounts of
r VI and VII (usually 1,1-bis(methylthio)-2-nitroethylene) in a polar solvent such
as methanol, ethanol, acetonitrile, tetrahydrofuran, water or mixtures thereof at
WO 94/05670 2 1 ~ 3 6 2 5 PCI/US93/07926
temperatures up to the reflux temperature of the solvent. A proton acceptor suchas NaOH, sodium carbonate or triethylamine can be used.
Alternatively, compounds of Formula II can be prepared by the reaction of
diamine VI with a 2,2,2-trihalonitroethane of Formula VIII as depicted in Schemev 5 4 using conditions analogous to those described for Scheme 3 reactions.
Scheme 4 reactions typically involve the use of between 1 and 10 molar
equivalents of base selected from amines such as triethylamine and pyridine,
carbonates such as Na2CO3, K2CO3 and NaHCO3, hydroxides such as LiOH,
NaOH and KOH, or like bases.
Sch~.me 4
Vl + X~ I CH2N2 base ll
X4
VIII
wherein: X3 X4 and X5 are halogen.
Formula IV compounds wherein n=l can be prepared by the reaction of
1 ,2-diaminopropane (IX, n=l ) with Formula VII compounds. Compounds of
15 Formula IV wherein n=2 can be prepared by the reaction of 1,3-diaminobutane
(IX, n=2) with Formula VII compounds in an analogous manner. Conditions for
the preparation of Formula IV compounds are analogous to those described for
Scheme 3. The preparation of Formula IV compounds is depicted in Scheme 5.
Scheme 5
H2N INH2
~ (CH2)n + VII r IV
Me
IX (n=1,2)
The preparation of ~ mine.s VI wherein n=l can be achieved using the
two-step procedure shown in Scheme 6.
W O 94/05670 PC~r/US93/07926
&~ 8
Scheme
Stepi
1 CH3CHO RlNH CN
R NH2 y
KCN Me
H+
X XI
Stepii
RlNH CN [H] RlNH NH2
Me Me
Xl VI (n=l)
In Step i of Scheme 6, amine X is treated with potassium cyanide and
acetaldehyde in the presence of one to three equivalents of acid in a solvent toform aminonitrile XI. Other cyanide salts as well as HCN can be used in the
procedure as well as hydrohalide and other acid salts of X. Suitable solvents
include methanol, ethanol, isopropanol and water, as well as combinations of
solvents. Alternative procedures for the preparation of amino nitriles such as XI
can be found in the literature (see, e.g., Synth. Commun., (1985), 15, 157;
Synthesis, (1979), 127).
In Step ii of Scheme 6, aminonitrile XI is reduced to form (1i:~mint-,
VI (n=l). This reduction can usually be achieved using lithium all-minum
hydride in amounts ranging from 0.75 to 3 molar equivalents in a solvent such asdiethyl ether or THF at a temperature ranging from -20C to the reflux
temperature of the solvent. Alternatively, the reduction of XI to VI can be
achieved using catalytic hydrogenation over a catalyst such as palladium on
carbon or Raney nickel. The addition of ammonia to the hydrogenation reaction
is sometimes useful to maximize the yield of diamine VI.
An alternative procedure for the preparation of ~ min~.s VI (n=l) is
depicted in Scheme 7.
21~362~
W O 94/05670 PC~r/US93/07926
Scheme 7
stepi R
H2N~CNH2 R8COCI R8cNH~coNH2
base
XIII XV
Step ii [H]
XV VI
(n=l)
wherein: R8 is CH SCH, 3-pyridyl, 5-thiazolyl,
2-chloro-~-pyn~yl or 2-chloro-5-thiazolyl,
In Step i of Scheme 7, alanine amide (XIII) is treated with 1 to 2 molar
equivalents of acid chloride XIV in the presence of 1 to 3 molar equivalents of a
5 base such as NaOH, KOH, K2CO3, pyridine or triethylamine. Suitable solvents
include THF, CH2Cl2, water or pyridine. The product XV can be isolated by
extraction or, more conveniently, by removal of solvent and is usually suitable for
use in Step ii of Scheme 7 in crude form. Alanine amide XIII can be used either
in neutral form as depicted or as the salt form (typically as the HCl or CF3CO2H10 salt, among others). When the salt form of XIII is used, an additional one
equivalent of base is used in Step i of Scheme 7.
In Step ii of Scheme 7, the amide XV is converted to rli:~mine VI (n=l) by
treatment with a reducing agent such as LiAlH4, BH3-THF or BH3 SMe2 in a
solvent such as THF or Et2O at temperatures ranging from 0C to the reflux
15 tempeldlulG of the solvent. Analogous procedures are well-known in the
aLult; (see e.g. Synthesis, (1981), 441).
The use of either the D- or the L- form of alanine amide XIII or its salt
provides a convenient means of obtaining enantiomerically enriched forms of
r1i~mine VI (n=l). When compounds of Formula II (n=l) are prepared as
20 described for Scheme 3 reactions usin~ enantiomerically enriched forms of VI
(n=l), the products II (n=l) are obtained in enantiomerically enriched form.
When compounds of Formula I are prepared as described for Scheme 1 reactions
W O 94/05670 PC~r/US93/07926
using enantiomerically enriched forms of II, the products I are obtained in
enantiomerically enriched form.
Alternatively, (li~min~s VI can be obtained in enantiomerically enriched
forms by resolution with enantiomeric acids, such as tartaric acid. Such
5 resolutions are well-known to one skilled in the art (see e.g. Synthesis, (1991),
789 for a related example).
The preparation of (li~min~s VI wherein n=2 can be achieved using the two-
step procedure shown in Scheme 8.
Scheme 8
Stepi 1 Me ~ CN R ~ NH
R ~nH2 ' ~ CN
X Me
XII
Stepii
Rl~ Rl~
NH ~ NIH ~nH2
~ CN
Me Me
XII Vl (n=2)
In Step i of Scheme 8, aminonitrile XII is formed when amine X and
crotononitrile are mixed either neat or in a suitable solvent. including water,
methanol, ethanol, THF or mixtures of these solvents at temp~,ldlult;s ranging
from 10C to 150C. The quantities of X used range from one to ten molar
equivalents.
The reduction of aminonitrile XII to form di:~mine VI (n=2) as depicted in
Step ii of Scheme 8 can be achieved using conditions analogous to those
previously described for Step ii of Scheme 6.
Alkylating agents of Formula V are known and include 2-
chloroethylmethylsulfide and 3-(chloromethyl)pyridine. Other representative
alkylating agents are described in EP-302,389-A2 and EP-446,913-A.
WO 94/05670 ~ 6~ ~ Pcr/US93/07926
11
Amines of Formula X are known and include 2-(methylthio)ethylamine and
3-(aminomethyl)pyridine. Other representative amines are described in
EP-302,389-A2.
F.xample I
S Step A: 2-r2-(Methylthio)ethyl~minolpropaneni~rile
A solution of 24.4 g (0.27 moles) of 2-(methylthio) ethylamine and 100 mL
of methanol was treated with 294 mL of 1 M aqueous HCl added dropwise with
ice-bath cooling over 15 min at 5-10C. The resulting solution was treated with a
solution of 17.4 g (0.27 moles) of potassium cyanide and 150 mL of water at
5-10C followed by the addition of 13 g (0.29 moles) of acetaldehyde at 5-10C.
The resulting solution was stirred at room temperature for 6 h and was then
poured into a mixture of 1 L of saturated aqueous sodium bicarbonate and
300 mL of CH2C12. The aqueous layer was extracted with two additional 200 mL
portions of CH2Cl2 and the combined organic layers were washed with 500 mL of
15 saturated aqueous NaHCO3, dried over anhydrous MgSO4, filtered and
concentrated to give 37.6 g (97%) of a pale yellow oil. lH NMR
(200 MHz, CDC13) o 3.78-3.60 (m,lH), 3.17-2.97 (m,lH), 2.94-2.74 (m,lH),
2.71-2.62 (m,2H), 2.12 (s,3H), 1.67 (br s,lH), 1.52 (d,J=7 Hz, 3H).
20 Step B: N2-r2-(Methylthio)ethyll-1.2-pro~yanediamine
A vigorously stirred (mechanical stirrer) solution of lithium aluminum hydride
(84 mL of a 1 M solution in (CH3CH2)2O, 0.084 moles) and (CH3CH2)2O
(163 mL) was treated with a solution of 6.04 g (0.042 moles) of the product fromStep A and 82 mL of (CH3CH2)20 was added dropwise at 0C. The resulting white
25 heterogeneous mixture was stirred at 0C for 1 h, at room temperature for 1 h and
then cooled to 0C and quenched by the careful, sequential addition of a solution of
3.1 mL H2O in 10 mL of tetrahydrofuran, 3.1 mL of 15% NaOH and 9.3 mL of
H2O at 0C. The resulting mixture was diluted with 155 mL of (CH3CH2)2O and
stirred at room temperature overnight. The resulting mixture was filtered and the
30 filtrate was concentrated to give 6.4 g of a dark yellow oil. lH NMR
(200 MHz, CDC13) o 2.97-2.45 (m,7H), 2.11 (s,3H), 1.75 (br s,3H), 1.05
(d,J=6 Hz, 3H).
W O 94/05670 PC~r/US93/07926
12
Step C: 5-Methyl-l-r2-(methylthio)ethyl]-2-(nitro-methylene)-;midazolidine
A solution of 2.6 g (0.018 moles) of the product from Step B, 2.9 g
(0.018 moles) of 2,2-bis-(methylthio)-1-nitroethylene and 18 mL of absolute
ethanol was heated at reflux for 5 h, cooled to room temperature and concentrated
to give 4.8 g of a brown oil. Flash chromatography of the oil on silica gel using
40:1:0.1 CH2C12:CH3CH2OH:48% NH40H gave 1.8 g of a yellow oil that
solidified on standing, m.p. 60-62C. Trituration of the solid with l-chlorobutane
gave an off-white solid that melted at 66-68C. IH NMR (200 MHz, CDC13)
8.63 (br s,lH), 6.53 (s,lH), 4.21-4.03 (m,lH), 3.90 (t,lH), 3.40-3.28 (m,3H),
2.78-2.58 (m,2H), 2.16 (s,3H), 1.36 (d,3H).
Step D: 1.23.5.6.7 - Hexahydro-2.6-dimethyl-1-r2-(methylthio)ethvll-8-nitro-
im~l~70~1.2-cl~yrimidine (Com~ound 1)
A solution of 0.50 g (0.0023 moles) of the product of Step C, 0.22 mL
(0.0025 moles) of 40% aqueous methylamine, 0.37 mL (0.005 moles) of 37%
aqueous formaldehyde and 2.3 mL of ethanol was stirred at room temperature for
20 h. Silica gel (2 g) was added to the resulting solution and the mixture was
concentrated. Flash chromatography of the residue on silica gel using 10:1:0.1
methylene chloride:ethanol:48% aqueous ammonium hydroxide gave 0.60 g of a
yellow oil. lH NMR (400 MHz, CDC13) ~ 4.40-4.32 (m,lH), 4.08-3.98 (m,2H),
3.96-3.85 (m,2H), 3.79 (apparent s,2H), 3.55-3.45 (m,lH), 3.27 (dd,lH),
2.80-2.65 (m,2H), 2.47 (s,3H), 2.11 (s,3H), 1.39 (d,lH).
Fxample 2
6-r(6-chloro-3-pyridinyl)methyl]- 1.2.3.5.6.7-hexahydro-2-rnethyl- 1 -r2-
(Jnethylthio)ethyll-8-nitro-imidazo ~ 1.2-cl-pyrimidine (Compound 2)
A solution of 1.0 g (0.0046 moles) of the product of Step C. Example 1,
0.7 g (0.0051 moles) of 3-(aminomethyl) 6-chloro-pyridine, 0.7 mL
(0.010 moles) of 37% aqueous formaldehyde and 5 mL of ethanol was stirred at
room temperature for 3 days. Silica gel (3 g) was added to the resulting solution
and the mixture was concentrated. Flash chromatography of the residue on silica
gel using 20:1:0.1 methylene chloride:ethanol:48% aqueous ammonium
hydroxide gave 0.50 g of a yellow oil. IH NMR (400 MHz, CDC13) o 8.34
(s.lH). 7.72 (d,lH), 7.35 (d,lH), 4.50-4.42 (m,lH), 4.10-4.02 (m.2H), 3.98-3.82
(m,3H), 3.82-3.70 (m.3H), 3.55-3.45 (m,lH), 3.21-3.15 (m,lH). 2.83-2.67
(m,2H), 2.13 (s,3H), 1.40 (d,3H).
W O 94/05670 2 I ~ 3 6 2 5 PC~r/US93/07926
O
13
F.xample 3
Step A: 3-rr5-Methyl-2-(nitromethylene)-1-imidazolidinyllmethyllpyridine
Aqueous hydrochloric acid (1 M, 326 mL, 326 mmoles) was added over
10 min to a solution of 3-(aminomethyl) pyridine (30.1 mL, 296 mmoles) and
methanol (110 mL). The resulting solution was treated with a solution of KCN
(19.3 g, 296 mmoles) and water (163 mL) at a temperature below 10C.
Acetaldehyde (18.1 mL, 326 mmoles) was then added at 10C and the resulting
mixture was stirred at 25C for 3.5 h. The mixture was partitioned between
saturated NaHCO3 and CH2Cl2. The aqueous layer was extracted twice with
CH2C12 and the combined organic layers were washed with saturated NaHCO3,
dried (MgSO4) and concentrated to give 38.1 g of a yellow oil.
A solution of the above product (38.1 g 236 mmoles) and diethylether
(380 mL) was added dropwise at 0C to a vigorously stirred solution of lithium
al-lminllm hydride (236 mL of a 1.0 M solution in ether, 236 mmoles) and ether
(800 mL). The resulting heterogeneous mixture was stirred at 0C for 1 h, at
25C for 1 h and then cooled to 0C and quenched by the careful, sequential
addition of 8.8 mL H2O, 8.8 mL of 15% aqueous NaOH and 26.4 mL H2O. The
mixture was diluted with ether (1000 mL) and stirred overnight at 25C. The
reslllting mixture was filtered and concentrated to give 29.2 g of a yellow oil.A solution of the above product (29.2 g, 177 mmoles) and ethanol (177 mL)
was treated with 1,1-bis (methylthio)-2-nitroethylene (29.2 g, 177 mmoles) and
heated at reflux for 2 h and then stirred overnight at 25C. The resulting solution
was concentrated to give 52.3 g of a brown oil. A 18.3 g aliquot of the crude
product was chromatographed on 1 kg of silica gel using 10: 1 :0.1
CH2Cl2-EtOH-concentrated. NH40H to give 13.3 g of the title compound as a
yellow solid that melted at 116- 118C. IH NMR (400 MHz, CDC13) o 8.75
(s,lH), 8.57 (d,lH), 8.52 (apparents,lH), 7.60 (d,lH), 7.31 (d,lH), 6.59 (s,lH),4.39 (ABq,2H), 4.02-3.93 (m,2H), 3.41 (m,lH), 1.33 (d,3H).
Step B: 1.2.3.5.6.7-Hexahydro-2.6-dimethyl-8-nitro-1-(3-pyridinylmethyl)-
imi~701 1.2-c]pyiirnidine (Compnund 22)
A solution of 1.0 g (4.3 mmoles) of the product from Step A, 0.4 mL
(4.7 mmoles) of 40% aqueous methylamine, 0.7 mL (9.4 mmoles) of 37~
aqueous formaldehyde and 5 mL of ethyl alcohol was stirred at room temperature
for 20 h. The resulting solution was concentrated to give 1.2 g of the title
compound as a yellow solid that melted at 143-146C. 1H NMR
WO 94/05670 PCr/US93/07926
~ ,.43~ 14
(400 MHz, CDC13) o 8.55 (overlapping d and s,2H total), 7.75 (d,lH), 7.29
(m,lH), 5.22 (112ABq,lH), 4.66 (1/2ABq,lH), 3.99-3.83 (m,3H), 3.82-3.74
(m,3H), 3.22 (dd,lH), 2.39 (s,3H), 1.29 (d,3H).
Fx:~rnple 4
5 Step A: Hexahydro-6-methyl-1-r2-(methylthio)ethyll-2-(nitromethylene)-
.
pyrlmltllne
A solution of 13.5 g (0.15 moles) of 2-(methylthio)ethyl amine and 12.1 mL
(0.15 moles) of crotononitrile was heated at reflux for 2 days. The resulting
mixture was cooled to room temperature, dissolved in 200 mL EtOAc, dried
(MgS04) and concentrated to give 19.5 g of an orange oil.
A solution of 19.5 g (0.12 moles) of the above product and 200 mL of
diethyl ether was added dropwise with vigorous mechanical stirring to a solutionof lithium alu.llhlulll hydride (123 mL of a 1.0 ~ solution in ether) and ether
(415 mL) at 0C. The resulting heterogeneous mixture was stirred at 0C for 1 h,at 25C for 1 h and was then cooled to 0C and quenched by the careful, dropwisesequential addition of 4.6 mL of H2O, 4.6 mL of 15% aqueous NaOH and
13.8 mL of H20. The resulting mixture was allowed to warm to 25C, diluted
with 500 mL of ether and stirred overnight at 25C. The resulting mixture was
filtered and the filtrate was dried over MgS04 and concentrated to give 16.7 g of
an orange oil.
A solution of 16.7 g (104 mmoles) of the above product, 17.1 g
(104 mmoles) of 1,1-bis (methylthio)-2-nitroethylene and 104 mL of ethyl
alcohol was heated at reflux for 2 h (effluent gases from the reaction were passed
through a bleach scrubber to trap methanethiol). The resulting solution was
concentrated to give 26.5 g of a brown oil. A 12 g sample of this oil was
chromatographed on 600 g of silica gel using 20: 1 :0.1
CH2Cl2-EtOH-concentrated NH40H to give 8.0 of the title compound as a brown
oil that later solidified. 1H NMR (400 MHz, CDC13) o 10.89 ~br s,lH), 6.62
(s,lH), 3.76-3.65 (m,lH), 3.58-3.42 (m,3H), 3.25 (quintet,lH), 2.80-2.65 (m,3H),2.17 (s,3H), 2.15-2.05 (m,2H), 1.30 (d,3H).
Step B: 1.3.4.6.7.8-Hexahydro-2.7-dimethyl-l-r2-(methylthio)ethyll-9-nitro-2H-
pyrimidino[l.6-alpyrimidine (Compound 33).
A solution of 0.7 g (3.0 mmoles) of the product from Step A, 0.3 mL
(3.3 mmoles) of 40% aqueous methylamine, 0.5 mL (6.6 mmoles) of 37%
aqueous formaldehyde and 3 mL of ethyl alcohol was stirred at 25C for 20 h and
WO 94/05670 2 1 ~ 3 6 2 S Pcr/US93,07926
then concentrated. The residue was chromatographed on 50 g silica gel using
15:1:0.1 CH2CI2-EtOH-concentrated NH40H to give 0.37 g of the title product as
a solid: m.p. 145C (dec.). lH NMR (400 MHz, CDC13) o 4.18 (br d,lH), 4.02
(d,lH), 3.78-3.57 (m,6H), 3.43-3.39 (m,lH), 2.76 (t,2H), 2.47 (s,3H), 2.30-2.22
(m,lH), 2.07 (s,3H), 1.96-1.86 (m,lH), 1.36 (d,3H).
By the general procedure described herein, or obvious modifications
thereof, the compounds of Tables 1 through 10 and Index Tables A through F can
be prt~,d. In Tables 1 through 10 and Index Tables A through F the following
notations have been used:
W O 94/05670 PC~r/US93/07926
43~
16
Me = CH3 CH2(4-Cl-Ph) = CH2 `~ Cl
Et = CH2CH3
n-Pr = (CH2)2CH3 Cl
i-Pr = CH(cH3)2~~
n-Bu = (CH2)3CH3 NH(2-CI-Ph) = NH ~0
i-Bu = CH2CH(CH3)2
s-Bu = CH(CH3)CH2CH3 Cl
t-Bu = C(CH3)3 /~
n-pentyl = (CH2)4CH3 NH(3-CI-Ph) = NH~
n-hexyl = (CH2)sCH3
c-Pr = ~
c-Bu = ~ NH(4-CI-Ph) = NH ~ Cl
c-pentyl = ~ 2-CH2-PYr = CH2 ~3
c-hexyl= ~ N
3-CH2-pyr = ~ ~I
CH2
c-heptyl = V ~
4-CH2-PYr = CH2 ~=~N
c-octyl=
Cl CH2\~
2-CH2-6-CI-pyr =
CH2(2-cl-ph) = CH2 ~
Cl +
(CH2)2 NMe3I= CH2CH2NMe3I
CH2(3-cl-ph) = CH2 ~
W O 94/05670 2 1 ~ ~ 6 2 S PC~r/US93/079Z6
3-CH2-6-CI-PYr = Cl
CH2 (CH2)2-2-Cl-Ph = CH2CH2
4-CH2-6-CI-pyr= ~/CI (CH2)2-3-cl-ph=cH2cH2~
CH2 (CH2)2~-CI-Ph = CH2CH2 ~ Cl
3-CH2-2-CI-pyr = ~3
5-CH2-2-Cl-thiazolyl = CI~CH2
5-CH2-thiazolyl = <~CH2
2-CH2-fUrYl = CH2 ~3
2-CH2-thienyl = CH
W O 94/05670 PC~r/US93/07926
.
4 3 ~3 ~ 3 18
~'l NJ
Me
R2 R2 R2
(CH2)8CH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)locH3 (CH2)20Me
n-Pr (CH2)1 1CH3 (CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)l3cH3 NEt2
i-Bu (CH2)l4cH3 N(CH2)2
s-Bu (CH2)l5cH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2CH(CH3)2(CH2) 1 8CH3 N(CH2)6
C(CH3)2CH2CH3(CH2)19CH3 N(CH2)7
CH(CH3)CH2CH2CH3 c-Pr (CH2)20H
CH2C(CH3)3 CH2(c-Pr) (cH2)3oH
n-hexyl c-Bu CH2C(CH3)CH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2CHCH2
(CH2)3c(cH3)3 c-pentyl CH2CCH
(CH2)6CH3 CH2(c-pentyl) CH2CCCH3
CH(CH3)(CH2)4cH3c-hexyl (CH2)2NMe2
CH2cH(cH3)(cH2)3cH3 CH2(c-hexyl) (cH2)
(CH2)7CH3 c-heptyl (cH2)
CH(CH3)(CH2)3CH(CH3)2 CH2(c-heptyl) (CH2)2F
CH2CH(CH2CH3)(CH2)3cH3 c-octyl (CH2)2Br
C(CH3)2CH2C(CH3)3CH2(c-octyl) (CH2)3Br
W094/OS670 21~352S PCI`/US93/07926
19
R2 R2 R2
(CH2)2N(CH2CH3)2CH2(4-CI-Ph)(CH2)2CONMe2
(cH2)2sH CH2(2-F-Ph) (CH2)2CONEt2
(CH2)3sH CH2(4-F-Ph) (cH2)2-4-cl-ph
(cH2)2sMe NH(2-CI-Ph) (cH2)2-4-F-ph
(cH2)3sMe NH(4-CI-Ph) (CH2)24-Br-Ph
(CH2)30(CH2)3CH3NH(2-F-Ph) CH2)2-2-CI-Ph
CH2C02H NH(4-F-Ph) (CH2)2-3-CI-ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F-Ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3-F Ph
CH(CH3)C02H NH(3-CI-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C02Et 2-CH2-pyr (CH2)2NMe3l
CH(i-Pr)C02H 3-CH2-PYr (CH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-PYr (CH2)2NMe3CI
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-CI-pyr (CH2)2NEt3l
CH2CH2c02H 4-CH2-6-CI-pyr (cH2)2N(Et)2
CH2CH2C2Me 3-CH2-2-CI-pyr (CH2)
CH2CH2C02Et5-CH2-2-CI-thiazolyl(CH2)3NMe2
CH2CN 5-CH2-thiazolyl(CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (CH2)3NMe3CI
(cH2)3cN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (CH2)3NMe,OH
(CH2)3No2 CH2CONH2 (cH2)2NEt3oH
CH2Ph CH2CONHMe (cH2)3NEt3I
CH2CH2Ph CH2CONMe2 (cH2)3N(Et)2
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
PC~r/~S93/07926
w o 94/05670
2 ~ 4 3 ~ 2 S 20
Table 2
02N ~\ , R2
MeS N N
Me l J
R2 R2 R2
H (cH2)8cH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)locH3 (cH2)2oMe
n-Pr (CH2)1 1CH3(cH2)3oMe
i-Pr (CH2)12CH3 NMe
n-Bu (CH2)13CH3 NEt2
i-Bu (CH2)l4cH3 N(CH2)2
s-Bu (CH2)15CH3 N(CH2)3
t-Bu (CH2)l6cH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2CH(CH3)2 (CH2)l8cH3 N(CH2)fi
C(CH3)2CH2CH3 (CH2)l9cH3 N(CH2)7
CH(cH3)cH2cH2cH3 c-Pr (CH2)~0H
CH2C(CH3)3 CH2(c-Pr) (cH2)3oH
n-hexyl c-Bu CH2C(CH3)cH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2cHcH2
(CH2)3c(cH3)3 c-pentyl CH2CCH
(CH2)6CH3 CH2(c-pentyl) CH2CCCH3
CH(CH3)(CH2)4cH3c-hexyl (CH2)2NMe2
CH2cH(cH3)(cH2)3cH3 CH2(c-hexyl) (cH2)~cl
(CH2)7cH3 c-heptyl (CH2)3Cl
CH(CH3)(CH2)3CH(cH3)2 CH2(c-heptyl) (CH2~,F
CH2cH(cH2cH3)(cH2)3cH3 c-octyl (CH2)~Br
C(CH3)2CH2C(CH3)3CH2(c-octyl)(CH2)~Br
WO 94/05670 21 q 3 6~ S PCI'/US93/07926
R2 R2 R2
(CH2)2N(CH2CH3)2 CH2(4-CI-Ph) (CH2)2CONMe2
(cH2)2sH CH2(2-F-Ph) (cH2)2coNEt2
(cH2)3sH CH2(4-F-Ph) (CH2)2-4-CI-Ph
(cH2)2sMe NH(2-CI-Ph) (CH2)2-4-F-Ph
(cH2)3sMe NH(4-CI-Ph) (CH2)2-4-Br-Ph
(CH2)3O(cH2)3cH3 NH(2-F-Ph) CH2)2-2-cl-ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-Cl-Ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F-ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3-F Ph
CH(CH3)CO2H NH(3-CI-Ph) (CH2)2-2-Br-Ph
CH(CH3)C2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C02Et 2-CH2-pyr (cH2)2NMe3l
CH(i-Pr)C02H 3-CH2-pyr (CH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-pyr (CH2)2NMe3CI
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-CI-pyr (CH2)2NEt3
CH2CH2c02H 4-CH2-6-CI-pyr (cH2)2N(Et)2Me
CH2CH2C02Me 3-CH2-2-CI-pyr (CH2)3NMe3
CH2CH2C02Et S-CH2-2-CI-thiazolyl (CH2)3NMe2
CH2CN 5-CH2-thiazolyl (CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (cH2)3NMe3cl
(cH2)3cN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (cH2)3NMe3oH
(CH2)3N02 CH2CoNH2 (CH2)2NEt30H
CH2Ph CH2CONHMe (CH2)3NEt3l
CH2CH2Ph CH2CONMe2 (CH2)3N(Et)2Mel
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
W O 94/05670 Pc~r/Us93/07926
.
~3~ Table 3
02N ~ ~ R2
N J
N Me
R2 R2 R2
(CH2)8CH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)10CH3 (cH2)2oMe
n-Pr (CH2)1 1CH3 (CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)13CH3 NEt2
i-Bu (CH2)l4cH3 N(CH2)2
s-Bu (CH2)15CH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2CH(CH3)2(cH2)l8cH3 N(CH2)6
C(CH3)2CH2CH3(CH2)19CH3 N(CH2)7
CH(CH3)CH2cH2cH3 c-Pr (cH2)2oH
CH2C(CH3)3 CH2(c-Pr) (CH2)30H
n-hexyl c-Bu CH2C(CH3)cH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2CHCH2
(CH2)3C(CH3)3 c-pentyl CH2CCH
(CH2)6CH3 CH2(c-pentyl) CH2CCCH3
CH(CH3)(CH2)4cH3c-hexyl (CH2)2NMe2
CH2cH(cH3)(cH2)3cH3 CH2(c-hexyl) (cH2)
(CH2)7CH3 c-heptyl (cH2)
CH(CH3)(CH2)3CH(CH3)2 CH2(c-heptyl) (CH2)2F
CH2CH(CH2CH3)(cH2)3cH3 c-octyl (cH2)2Br
C(CH3)2CH2C(CH3)3CH2(c-octyl)(CH2)3Br
WO 94/05670 2~ ~ 3 6~ ~ PCI'/US93/07926
23
R2 R2 R2
(CH2)2N(CH2CH3)2 CH2(4-CI-Ph) (CH2)2CONMe2
(CH2)2sH CH2(2-F-Ph) (cH2)2coNEt2
(CH2)3SH CH2(4-F-Ph) (CH2)2-4-CI ph
(cH2)2sMe NH(2-CI-Ph) (cH2)2-4-F-ph
(cH2)3sMe NH(4-CI-Ph) (CH2)2-4-Br-Ph
(CH2)3O(CH2)3CH3 NH(2-F-Ph) CH2)2-2-CI-Ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-CI-Ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3 F ph
CH(CH3)CO2H NH(3-Cl-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br Ph
CH(CH3)C02Et 2-CH2-pyr (cH2)2NMe3l
CH(i-Pr)C02H 3-CH2-PYr (cH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-PYr (CH2)2NMe3CI
CH(i-Pr)C02Et 2-CH2-6-Cl-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-Cl-pyr (CH2)2NEt3I
CH2CH2C02H 4-CH2-6-CI-pyr (CH2)2N(Et)2MeI
CH2CH2C02Me 3-CH2-2-CI-pyr (cH2)3NMe3I
CH2CH2C02Et 5-CH2-2-Cl-thia~;olyl (CH2)3NMe2
CH2CN 5-CH2-thiazolyl (cH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (CH2)3NMe3CI
(CH2)3CN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (CH2)3NMe30H
(CH2)3N02 CH2CONH2 (cH2)2NEt3oH
CH2Ph CH2CONHMe (CH2)3NEt3l
CH2CH2Ph CH2CONMe2 (cH2)3N(Et)2MeI
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
WO 94/05670 PCr/US93/07926
?,~ 43~ 3 24
Table 4
02N-- ~ R2
/~/ ~N NJ
R2 R2 R2
H (cH2)8cH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)10CH3 (CH2)20Me
n-Pr (CH2)11CH3 (CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)l3cH3 NEt2
i-Bu (CH2)l4cH3 N(CH2)2
s-Bu (CH2)15CH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2CH(CH3)2 (CH2)18CH3 N(CH2)6
C(CH3)2CH2CH3(CH2)19CH3N(CH2)7
CH(CH3)CH2CH2CH3 c-Pr (cH2)2oH
CH2C(CH3)3CH2(c-Pr) (CH2)30H
n-hexyl c-Bu CH2C(CH3)CH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2cHcH2
(CH2)3c(cH3)3c-pentyl CH2CCH
(CH2)6CH3CH2(c-pentyl)CH2CCCH3
CH(CH3)(CH2)4CH3 c-hexyl (CH2)2NMe2
CH2cH(cH3)(cH2)3cH3CH2(c-hexyl) (cH2)
(CH2)7CH3 c-heptyl (cH2)
CH(CH3)(CH2)3CH(CH3)2CH2(c-heptyl) (cH2)2F
CH2cH(cH2cH3)(cH2)3cH3c-octyl (cH2)2Br
C(CH3)2CH2C(CH3)3 CH2(c-octyl) (CH2)3Br
WO94/05670 ~ 62~ PCI'/US93/07926
.
R2 R2 R2
(CH2)2N(CH2CH3)2CH2(4-CI-Ph)(CH2)2CONMe2
(CH2)2SH CH2(2-F-Ph) (CH2)2CONEt2
(CH2)3SH CH2(4-F-Ph) (CH2)2-4-Cl Ph
(cH2)2sMe NH(2-CI-Ph) (cH2)2-4-F-ph
(CH2)3SMe NH(4-CI-Ph) (CH2)24-Br-Ph
(CH2)3O(CH2)3CH3NH(2-F-Ph) CH2)2-2-CI-Ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-CI-Ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F Ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3-F-Ph
CH(CH3)CO2H NH(3-CI-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C2Et 2-CH2-PYr (CH2)2NMe3I
CH(i-Pr)C02H 3-CH2-pyr (CH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-pyr (cH2)2NMe
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-CI-pyr (CH2)2NEt3l
CH2CH2C02H 4-CH2-6-CI-pyr (CH2)2N(Et)2Mel
CH2CH2C02Me 3-CH2-2-CI-pyr (CH2)
CH2CH2co2Et5-CH2-2-CI-thiazolyl(CH2)3NMe2
CH2CN 5-CH2-thiazolyl(CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (cH2)3NMe3cl
(CH2)3CN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (CH2)3NMe30H
(CH2)3N02 CH2CONH2 (cH2)2NEt3oH
CH2Ph CH2CONHMe (CH2)3NEt31
CH2CH2Ph CH2CONMe2 (CH2)3N(Et)2Mel
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
W o 94/05670 PC~r/US93/07926
~,~43~
26
Table 5
02N ~ , R2
~\N~N
Cl N Me
R2 R2 R2
H (CH2)8CH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)10CH3 (CH2)20Me
n-Pr (CH2)1 1CH3 (CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)13CH3 NEt2
i-Bu (CH2)14CH3 N(CH2)2
s-Bu (CH2) 1 5CH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2CH(CH3)2 (CH2)l8cH3 N(cH2)6
C(CH3)2CH2CH3 (CH2)19CH3 N(CH2)7
CH(CH3)CH2CH2CH3 c-Pr (cH2)2oH
CH2C(CH3)3 CH2(c-Pr) (cH2)3oH
n-hexyl c-Bu CH2C(CH3)cH2
CH(CH3)CH2CH(CH3)2CH2(c-Bu) CH2CHCH2
(CH2)3c(cH3)3 c-pentyl CH2CCH
(CH2)6cH3 CH2(c-pentyl) CH2CCCH3
CH(CH3)(CH2)4cH3 c-hexyl (CH2)2NMe2
CH2CH(CH3)(CH2)3CH3CH2(c-hexyl)(CH2)2CI
(CH2)7cH3 c-heptyl (CH2)3CI
CH(CH3)(CH2)3CH(CH3)2 CH2(c-heptyl) (CH2)2F
CH2cH(cH2cH3)(cH2)3cH3 c-octyl (cH2)2Br
C(CH3)2CH2C(CH3)3CH2(c-octyl) (CH2)3
W O 94/05670 21 ~ 3 62 5 PC~r/US93/07926
.
27
R2 R2 R2
(CH2)2N(CH2CH3)2CH2(4-CI-Ph)(CH2)2CONMe2
(CH2)2SH CH2(2-F-Ph) (CH2)2CONEt2
(CH2)3SH CH2(4-F-Ph) (CH2)2-4-CI-Ph
(CH2)2SMe NH(2-CI-Ph) (CH2)2-4-F-Ph
(cH2)3sMe NH(4-CI-Ph) (CH2)2~-Br-Ph
(CH2)3O(CH2)3CH3NH(2-F-Ph) CH2)2-2-CI-Ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-CI-ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F-Ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3-F-Ph
CH(CH3)CO2H NH(3-CI-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C02Et 2-CH2-pyr (CH2) ,NMe31
CH(i-Pr)C02H 3-CH2-pyr (CH2)"NMe3Br
CH(i-Pr)C02Me 4-CH2-pyr (CH2)2NMe3CI
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (cH2)2NEt2
(CH2CH20)2H 3-CH2-6-CI-pyr (CH2)2NEt3l
CH2CH2C02H 4-CH2-6-CI-pyr (CH2)2N(Et)2Mel
CH2CH2C02Me 3-CH2-2-CI-pyr (CH2)3NMe3
CH2CH2C02EtS-CH2-2-CI-thiazolyl(CH2)3NMe2
CH2CN S-CH2-thiazolyl(CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (CH2)3NMe3CI
(cH2)3cN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (cH2)3NMe3oH
(CH2)3No2 CH2CONH2 (cH2)2NEt3oH
CH2Ph CH2CONHMe (cH2)3NEt3I
CH2CH2Ph CH2cONMe2 (CH2)3N(Et)2Mel
NHPh CH2CoNEt2
CH2(2-CI-Ph) (CH2)2CONHMe
w o 94/05670 ~ Pc~r/us93/07926
2~3~
28
Table 6
02N ~ ~ R2
Cl ~t~N~ Me J J
R2 R2 R2
H (CH2)8CH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)locH3 (CH2)20Me
n-Pr (CH2)11CH3 (CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)l3cH3 NEt2
i-Bu (CH2)14CH3 N(CH2)2
s-Bu (CH2)15CH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)~7CH3 N(cH2)s
(CH2)2CH(CH3)2 (CH2)18cH3 N(CH2)6
C(CH3)2CH2CH3(CH2) I gCH3 N(CH2)7
CH(CH3)CH2CH2CH3 c-Pr (cH2)2oH
CH2C(CH3)3CH2(c-Pr) (cH2)3oH
n-hexyl c-Bu CH2C(CH3)cH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2CHCH2
(CH2)3C(CH3)3c-pentyl CH2CCH
(CH2)6CH3CH2(c-pentyl)CH2CCCH3
CH(cH3)(cH2)4cH3 c-hexyl (CH2)2NMe2
CH2CH(CH3)(CH2)3cH3CH2(c-hexyl) (cH2)
(CH2)7cH3 c-heptyl (cH2)
CH(CH3)(CH2)3CH(CH3)2CH2(c-heptyl) (CH2)2F
CH2CH(CH2CH3)(CH2)3cH3c-octyl (CH2)2Br
C(CH3)2CH2C(cH3)3 CH2(c-octyl) (CH2)3Br
WO 94/05670 ~ PCI`/US93/07926
29
R2 R2 R2
(CH2)2N(CH2CH3)2 CH2(4-CI-Ph)(CH2)2CONMe2
(CH2)2SH CH2(2-F-Ph)(CH2)2CONEt2
(cH2)3sH CH2(4-F-Ph)(CH2)2-4-CI-Ph
(cH2)2sMe NH(2-CI-Ph)(CH2)2-4-F-ph
(CH2)3SMe NH(4-Cl-Ph)(CH2)2-4-Br-Ph
(CH2)3O(CH2)3CH3 NH(2-F-Ph) CH2)2-2-cl-ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-CI-Ph
CH2C02Me CH2(3-CI-Ph)(cH2)2-2-F-ph
CH2C02Et CH2(3-F-Ph)(CH2)2-3-F-Ph
CH(CH3)CO2H NH(3-CI-Ph)(CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C02Et 2-CH2-pyr (CH2)2NMe3l
CH(i-Pr)C02H 3-CH2-PYr (cH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-pyr (cH2)2NMe
CH(i-Pr)C02Et 2-CH2-6-Cl-pyr(CH2)2NEt2
(CH2CH20)2H 3-CH2-6-Cl-pyr(CH2)2NEt3
CH2CH2C02H 4-CH2-6-Cl-pyr(cH2)2N(Et)2Me
CH2CH2C02Me 3-CH2-2-Cl-pyr (CH2)
CH2CH2C02Et 5-CH2-2-Cl-thiazolyl (CH2)3NMe2
CH2CN 5-CH2-thiazolyl(cH2)3NMe3Br
CH2CH2CN 2-CH2-furyl(CH2)3NMe3CI
(cH2)3cN 2-CH2-thienyl(cH2)2NMe3oH
(CH2)2N02 (CH2)2CONH2(cH2)3NMe?~oH
(CH2)3N02 CH2CONH2(cH2)2NEt3oH
CH2Ph CH2CONHMe(CH2)3NEt3l
CH2CH2Ph CH2CONMe2(CH2)3N(Et)2Mel
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
PC~r/US93/07926
W O 94/05670
~3~S 30
Table 7
02N ~ ~ R2
<~/\ N~_~N
Me
R2 R2 R2
H (cH2)8cH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)10CH3 (cH2)2oMe
n-Pr (CH2)l 1CH3(CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)13CH3 NE12
i-Bu (CH2)l4cH3 N(CH2)2
s-Bu (CH2)l5cH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2CH(CH3)2 (CH2)l8cH3 N(CH2)6
C(CH3)2CH2CH3(CH2)19CH3N(CH2)7
CH(cH3)cH2cH2cH3 c-Pr (CH2)20H
CH2C(CH3)3CH2(c-Pr) (CH2)30H
n-hexyl c-Bu CH2C(CH3)cH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2CHCH2
(CH2)3c(cH3)3c-pentyl CH2CCH
(CH2)6CH3CH2(c-pentyl)CH2CCCH3
CH(CH3)(CH2)4cH3 c-hexyl (CH2)2NMe2
CH2cH(cH3)(cH2)3cH3CH2(c-hexyl) (cH2)
(CH2)7CH3 c-heptyl (cH2)
CH(CH3)(CH2)3CH(CH3)2CH2(c-heptyl) (CH2)2F
CH2CH(CH2CH3)(cH2)3cH3c-octyl (cH2)2Br
C(CH3)2CH2C(CH3)3 CH2(c-octyl) (cH2)3Br
WO 94/05670 2I ~ 3 ~ 5 PCI/US93/07926
31
R2 R2 R2
(CH2)2N(CH2CH3)2CH2(4-CI-Ph) (CH2)2CONMe2
(CH2)2SH CH2(2-F-Ph) (CH2)2CONEt2
(cH2)3sH CH2(4-F-Ph) (CH2)2-4-cl-ph
(CH2)2SMe NH(2-CI-Ph) (CH2)2-4-F-ph
(cH2)3sMe NH(4-Cl-Ph) (CH2)2-4-Br-Ph
(CH2)3O(CH2)3CH3NH(2-F-Ph) CH2)2-2-CI-Ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-CI Ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F-Ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3-F Ph
CH(CH3)CO2H NH(3-CI-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C2Et 2-CH2-PYr (CH2) ,NMe3l
CH(i-Pr)C02H 3-CH2-pyr (CH2),,NMe3Br
CH(i-Pr)C02Me 4-CH2-pyr (CH2)2NMe3CI
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-CI-pyr (CH2)2NEt31
CH2CH2c02H 4-CH2-6-CI-pyr (CH2)2N(Et)2Mel
CH2CH2C02Me 3-CH2-2-CI-pyr (CH2)
CH2CH2C02Et5-CH2-2-CI-thiazolyl(CH2)3NMe2
CH2CN 5-CH2-thiazolyl(CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (CH2)3NMe3CI
(cH2)3cN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (CH2)3NMe30H
(CH2)3N02 CH2CONH2 (cH2)2NEt3oH
CH2Ph CH2CONHMe (CH2)3NE~t3l
CH2CH2Ph CH2CONMe2 (CH2)3N(Et)2Mel
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)zCONHMe
W O 94/05670 Pc~r/Us93/07926
~ 36?~ 32
Table 8
02N ~`~ , R2
--N N
Nl/ J`J
Me
R2 R2 R2
H (CH2)8CH3 OMe
Me (CH2)gcH3 CH20Me
Et (CH2)10CH3 (CH2)20Me
n-Pr (CH2)11CH3 (CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)13CH3 NEt2
i-Bu (CH2)14CH3 N(CH2)2
s-Bu (CH2)15CH3 N(CH2)3
t-Bu (CH2) 1 6CH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2CH(CH3)2 (CH2)18CH3 N(CH2)6
C(CH3)2CH2CH3 (CH2)l9cH3 N(CH2)7
CH(CH3)CH2cH2cH3 c-Pr (CH2)20H
CH2C(CH3)3 CH2(c-Pr) (CH2)30H
n-hexyl c-Bu CH2C(CH3)cH2
CH(CH3)CH2CH(cH3)2 CH2(c-Bu) CH2CHCH2
(CH2)3c(cH3)3 c-pentyl CH2CCH
(CH2)6CH3 CH2(c-pentyl) CH2CCCH3
CH(CH3)(CH2)4cH3 c-hexyl (CH2)2NMe2
CH2CH(CH3)(CH2)3CH3CH2(c-hexyl) (cH2)
(CH2)7cH3 c-heptyl (cH2)
CH(CH3)(CH2)3CH(CH3)2CH2(c-heptyl) (CH2)2F
CH2cH(cH2cH3)(cH2)3cH3c-octyl (CH2)2Br
C(CH3)2CH2C(CH3)3 CH2(c-octyl) (cH2)3Br
WO 94/05670 2 1 ~ ~ ~ 2 ~ PCI'/US93/07926
.
33 ;,
R2 R2 R2
(CH2)2N(CH2CH3)2 CH2(4-CI-Ph) (CH2)2CONMe2
(cH2)2sH CH2(2-F-Ph) (CH2)2CONEt2
(CH2)3SH CH2(4-F-Ph) (CH2)24-Cl-ph
(CH2)2sMe NH(2-CI-Ph) (CH2)24-F-Ph
(cH2)3sMe NH(4-CI-Ph) (CH2)24-Br-Ph
(CH2)3O(CH2)3cH3 NH(2-F-Ph) CH2)2-2-cl-ph
CH2CO2H NH(4-F-Ph) (cH2)2-3-cl-ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F-Ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3-F-Ph
CH(CH3)cO2H NH(3-CI-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C02Et 2-CH2-PYr (CH2)2NMe3l
CH(i-Pr)C02H 3-CH2-pyr (CH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-pyr '(CH2)2NMe3CI
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-CI-pyr (CH2)2NEt3l
CH2CH2C02H 4-CH2-6-CI-pyr (CH2)2N(Et)2Mel
CH2CH2C02Me 3-CH2-2-CI-pyr (CH2)
CH2CH2C02Et5-CH2-2-CI-thiazolyl (cH2)3NMe2
CH2CN S-CH2-thiazolyl (CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (CH2)3NMe3Cl
(cH2)3cN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N2 (cH2)2coNH2 (cH2)3NMe3oH
(CH2)3N02 CH2CONH2 (CH2)2NEt30H
CH2Ph CH2CONHMe (cH2)3NEt3l
CH2CH2Ph CH2CONMe2 (CH2)3N(Et)2Mel
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
W O 94/05670 . PC~r/US93/07926
.
~ ~ ~ 34
02N 1~ R2
>J
Me
R2 R2 R2
H (cH2)8cH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)10CH3 (CH2)20Me
n-Pr (CH2)1 1CH3 (CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)13CH3 NE~2
i-Bu (CH2)14CH3 N(CH2)2
s-Bu (CH2)15CH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)17CH3 N(CH2)s
(CH2)2cH(cH3)2(CH2)18CH3 N(CH2)6
C(CH3)2CH2CH3(CH2)l9cH3 N(CH2)7
CH(CH3)CH2CH2CH3 c-Pr (CH2)20H
CH2C(CH3)3 CH2(c-Pr) (cH2)3oH
n-hexyl c-Bu CH2C(CH3)CH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2CHCH2
(CH2)3c(cH3)3c-pentyl CH2CCH
(CH2)6CH3CH2(c-pentyl) CH2CCCH3
CH(CH3)(CH2)4cH3 c-hexyl (CH2)2NMe2
CH2CH(CH3)(CH2)3CH3 CH2(c-hexyl) (CH2)2CI
(CH2)7cH3 c-heptyl (CH2)3CI
CH(CH3)(CH2)3CH(CH3)2 CH2(c-heptyl~ (CH2)2F
CH2CH(CH2CH3)(cH2)3cH3 c-octyl (cH2)2Br
C(CH3)2CH2C(CH3)3 CH2(c-octyl) (cH2)3Br
WO 94/05670 21 ~ 3 6~ PCI'/US93/07926
R2 R2
(CH2)2N(CH2CH3)2 CH2(4-CI-Ph) (CH2)2CONMe2
(CH2)2SH CH2(2-F-Ph) (CH2)2CONEt2
(CH2)3SH CH2(4-F-Ph) (CH2)2-4-CI-Ph
(cH2)2sMe NH(2-Cl-Ph) (CH2)2-4-F-ph
(cH2)3sMe NH(4-Cl-Ph) (cH2)2-4-Br-ph
(CH2)3O(CH2)3cH3 NH(2-F-Ph) CH2)2-2-Cl-Ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-CI-Ph
CH2C02Me CH2(3-Cl-Ph) (CH2)2-2-F-Ph
CH2C02Et CH2(3-F-Ph) (CH2)2-3-F-ph
CH(CH3)CO2H NH(3-Cl-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (cH2)2-3-Br-ph
CH(CH3)C02Et 2-CH2-PYr (CH2)2NMe3I
CH(i-Pr)C02H 3-CH2-pyr (CH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-pyr (CH2)2NMe3CI
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-Cl-pyr (CH2)2NEt3I
CH2CH2C02H 4-CH2-6-Cl-pyr (CH2)2N(Et)2MeI
CH2CH2C02Me 3-CH2-2-Cl-pyr (CH2)
CH2CH2C02Et 5-CH2-2-Cl-thiazolyl (CH2)3NMe2
CH2CN 5-CH2-thiazolyl (CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (CH2)3NMe3CI
(cH2)3cN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (CH2)3NMe30H
(CH2)3N02 CH2CONH2 (cH2)2NEt3oH
CH2Ph CH2CONHMe (cH2)3NEt3I
CH2CH2Ph CH2CONMe2 (CH2)3N(Et)2MeI
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
W O 94/05670 Pc~r/us93/07926
.
36
21~ ~2 Table 10
02N ~ ~ R2
Cl S N N
~N~--lJ
R2 R2 R2
H (cH2)8cH3 OMe
Me (cH2)9cH3 CH20Me
Et (CH2)10CH3 (cH2)2oMe
n-Pr (CH2)l 1CH3(CH2)30Me
i-Pr (CH2)12CH3 NMe2
n-Bu (CH2)13CH3 NEt2
i-Bu (CH2)14CH3 N(cH2)2
s-Bu (CH2)lscH3 N(CH2)3
t-Bu (CH2)16CH3 N(CH2)4
n-pentyl (CH2)l7cH3 N(cH2)s
(CH2)2CH(CH3)2 (CH2) 1 8CH3 N(CH2)6
C(CH3)2CH2CH3(CH2)19CH3N(CH2)7
CH(CH3)CH2cH2cH3 c-Pr (CH2)2oH
CH2C(CH3)3CH2(c-Pr) (cH2)3oH
n-hexyl c-Bu CH2C(CH3)CH2
CH(CH3)CH2CH(CH3)2 CH2(c-Bu) CH2cHcH2
(CH2)3c(cH3)3c-pentyl CH2CCH
(CH2)6CH3CH2(c-pentyl)CH2CCCH3
CH(CH3)(CH2)4CH3 c-hexyl (CH2)2NMe2
CH2CH(CH3)(CH2)3CH3CH2(c-hexyl) (CH2)2CI
(CH2)7cH3 c-heptyl (cH2)
CH(CH3)(CH2)3CH(CH3)2CH2(c-heptyl) (CH2)2F
CH2CH(CH2CH3)(cH2)3cH3c-octyl (cH2)2Br
C(CH3)2CH2C(CH3)3 CH2(c-octyl) (cH2)3Br
WO 94/05670 PCr/US93/07926
;3~
R2 R2 R2
(CH2)2N(CH2CH3)2 CH2(4-Cl-Ph) (CH2)2CONMe2
(cH2)2sH CH2(2 F-Ph) (cH2)2coNEt2
(cH2)3sH CH2(4-F-Ph) (CH2)2-4-Cl Ph
(CH2)2SMe NH(2-Cl-Ph) (CH2)2-4-F-Ph
(cH2)3sMe NH(4-CI-Ph) (CH2)2-4-Br-Ph
(CH2)3O(CH2)3CH3 NH(2-F-Ph) CH2)2-2-cl-ph
CH2CO2H NH(4-F-Ph) (CH2)2-3-Cl Ph
CH2C02Me CH2(3-CI-Ph) (CH2)2-2-F-ph
CH2C02Et CH2(3-F-Ph) (cH2)2-3-F-ph
CH(CH3)CO2H NH(3-CI-Ph) (CH2)2-2-Br-Ph
cH(cH3)co2Me NH(3-F-Ph) (CH2)2-3-Br-Ph
CH(CH3)C02Et 2-CH2-pyr (cH2)2NMe3l
CH(i-Pr)C02H 3-CH2-~yr (CH2)2NMe3Br
CH(i-Pr)C02Me 4-CH2-PYr (cH2)2NMe
CH(i-Pr)C02Et 2-CH2-6-CI-pyr (CH2)2NEt2
(CH2CH20)2H 3-CH2-6-CI-pyr (CH2)2NEt3I
CH2CH2C02H 4-CH2-6-CI-pyr (CH2)2N(Et)2Mel
CH2CH2C02Me 3-CH2-2-CI-pyr (cH2)3NMe3I
CH2CH2C02Et 5-CH2-2-CI-thiazolyl (CH2)3NMe2
CH2CN 5-CH2-thiazolyl (CH2)3NMe3Br
CH2CH2CN 2-CH2-furyl (CH2)3NMe3CI
(CH2)3CN 2-CH2-thienyl (CH2)2NMe30H
(CH2)2N02 (CH2)2CONH2 (cH2)3NMe3oH
(CH2)3N02 CH2CONH2 (CH2)2NEt30H
CH2Ph CH2CONHMe (CH2)3NEt31
CH2CH2Ph CH2CONMe2 (CH2)3N(Et)2Mel
NHPh CH2CONEt2
CH2(2-CI-Ph) (CH2)2CONHMe
Forrnulatiorl/Utility
Compounds of this invention will generally be used in formulation with an
agriculturally suitable carrier comprising a liquid or solid diluent. Useful
5 formulations include dusts, granules, baits, pellets, solutions, suspensions,
emulsions, wettable powders~ emulsifiable concentrates, dry flowables and the
W O 94/05670 PC~r/US93/07926
~,~ 43~ 38
like, consistent with the physical properties of the active ingredient, mode of
application and environment~l factors such as soil type, moisture and temperature.
Sprayable formulations can be extended in suitable media and used at spray
volumes from about one to several hundred liters per hectare. High strength
5 compositions are primarily used as intermediates for further formulation. The
formulations will typically contain effective amounts of active ingredient, diluent
and surfactant within the following approximate ranges which add up 100 weight
percent.
Weight Percent
Active
~ redient Dil~lent Smf:lr~s~nt
Wettable Powders 5-90 0-74 1-10
Oil S~ )r.. ~i~ .. c, Fmnlcionc,5 50 40-95 0-15
Soluhons, (;..rl~.~l;",~ F.ml~l.Cifi~hle
C-m-~çntr~t~)
Dust~. 1-25 70-99 0-5
~n~nl-l.os, Baits and Pellets 0.01-99 5-99 99 0-15
High Strength Compositions 90-99 0-10 0-2
Typical solid diluents are described in Watkins, et al.. Handbook of
Insecticide Dust Diluents and Carriers, 2nd Ed., Dorland Books, Caldwell, New
Jersey. Typical liquid diluents and solvents are described in Marsden, Solvents
Guide, 2nd Ed., Interscience, New York, 1950. McCutcheon's Detergents and
Emulsifiers Annual, Allured Publ. Corp., Ridgewood, New Jersey, as well as
15 Sisely and Wood, Encyclopedia of Surface Active Agents, Chemical Publ.
Co., Inc., New York, 1964, list surfactants and recommended uses. All
formulations can contain minor amounts of additives to reduce foam. caking,
corrosion, microbiological growth, and the like.
Solutions are prepared by simply mixing the ingredients. Fine solid
20 compositions are made by blending and, usually, grinding as in a hammer mill or
fluid energy mill. Water-dispersible granules can be produced by agglomerating
a fine powder composition; see for example. Cross et al., Pesticide Fonnulations,
Washington, D.C., 1988, pp 251-259. Suspensions are prepared by wet-milling;
see, for example, U.S. 3,060,084. Granules and pellets can be made by spraying
25 the active material upon preformed granular carriers or by agglomeration
techniques. See Browning, "Agglomeration", Chemical Engineerin~,
W O 94/05670 2 1 ~ 3 ~ ~ ~ PC~r/~'S93/07926
.
39
December 4, 1967, pp 147-148, Perry's Chemical Engineer's Handbook. 4th Ed.,
McGraw-Hill, New York, 1963, pages 8-57 and following, and WO 91/13546.
For further information regarding the art of formulation, see
U.S. 3,235,361, Col. 6, line 16 through Col. 7. line 19 and Examples 10-41;
U.S. 3,309,192, Col. 5, line 43 through Col. 7, line 62 and Examples 8, 12. 15,
39, 41, 52, 53, 58, 132, 138 -140, 162-164, 166, 167 and 169-182;
U.S. 2,891,855, Col. 3, line 66 through Col. 5, line 17 and Examples 1-4:
Klingm~n, Weed Control as a Science, John Wiley and Sons, Inc., New York,
1961, pp 81-96; and Hance et al., Weed Control Handbook, 8th Ed., Blackwell
Scientific Publications, Oxford, 1989.
In the following Examples, all percentages are by weight and all
formulations are prepared in conventional ways. Compound numbers refer to
compounds in Index Table A.
F.xample A
Wettable Powder
Compound 1 65.0Ci'c
dodecylphenol polyethylene glycol ether 2.0cjtc
sodium ligninsulfonate 4.0Cic
sodium silicoaluminate 6.0cjtc
montmorillonite (calcined) 23.0Cic.
Fx~m~ple B
~r:~nule
Compound 1 10.0C~c
attapulgite granules (low volatile
matter, 0.71/0.30 mm; U.S.S. No.
25-50 sieves) 90.0Cic.
Fx:~mple C
E~xtruded Pellet
Compound 1 25.0C/c
anhydrous sodium sulfate 10.0C~c
crude calcium ligninsulfonate 5.0'~c
sodium aIkylnaphth~lenesulfonate 1.0C/c
calcium/m~gn~sillm bentonite 59 oc/c
WO 94/05670 PCI/US93/07926
2~36?,s 40
F.xample D
Fmulsifiable Concentrate
Compound 1 ~ 20.0~7c
blend of oil soluble sulfonates
and polyoxyethylene ethers 10.0~c
isophorone 70.0~c.
The compounds of this invention exhibit activity against a wide spectrum of
foliar-feeding, fruit-feeding, stem or root feeding, seed-feeding, aquatic and
soil-inhabiting arthropods (term "arthropods" includes insects, mites and
10 nematodes) which are pests of growing and stored agronomic crops, forestry,
greenhouse crops, ornamentals, nursery crops, stored food and fiber products,
livestock, household, and public and animal health. Those skilled in the art will
appreciate that not all compounds are equally effective against all growth stages
of all pests. Nevertheless, all of the compounds of this invention display activity
15 against pests that include: eggs, larvae and adults of the Order Lepidoptera; eggs,
foliar-feeding, fruit-feeding, root-feeding, seed-feeding larvae and adults of the
Order Coleoptera; eggs, imm,.tllres and adults of the Orders Hemiptera and
Homoptera; eggs, larvae, nymphs and adults of the Order Acari; eggs, imm~tl-res
and adults of the Orders Thysanoptera, Orthoptera and Dermaptera; eggs,
20 imm~hlres and adults of the Order Diptera; and eggs, junveniles and adults of the
Phylum Nematoda. The compounds of this invention are also active against pests
of the Orders Hymenoptera, Isoptera, Siphonaptera, Blattaria, Thysanura and
Psocoptera; pests belonging to the Class Arachnida and Phylum Platyhelminthes.
Specifically, the compounds are active against southern corn rootworrn
25 (Diabrotica undecimpunctata howardi), aster leafhopper (Mascrosteles
fascifrons), boll weevil (Anthonomus grandis), two-spotted spider mite
(Tetranychus urticae), fall armyworm (Spodoptera frugiperda), black bean aphid
(Aphis fabae), tobacco budworm (Heliothis virescens), rice water weevil
(Lissorhoptrus oryzophilus). rice leaf beetle (Oulema orYzae)~ whitebacked
30 planthopper (Sogatella furcifera), green leafhopper (Nephotettix cincticeps),brown planthopper (Nilaparvata lugens). small brown planthopper (Laodelphax
striatellus), rice stem borer (Chilo suppressalis), rice leafroller (Cnaphalocroci.s
medinalis), black ;ice stink bug (Scotinophara lurida), rice stink bug (Oebalus
pugnax), rice bug (Leptocorisa chinensis), slender rice bug (Cletus puntiger), and
35 southern green stink bug (Nezara viridula). The compounds are active on mites,
demonstrating ovicidal, larvicidal and chemosterilant activity against such
WO 94/05670 2 1 4 3 6 ~ PCr/US93/07926
41
families as Tetranychidae including Tetranychus urticae, Tetranychus
cinnabarinus, Tetranychus mcdanieli, Tetranychus pacificus, Tetranychus
turkestani, Byrobia rubrioculus, Panonychus ulmi, Panonychus citri,
Eotetranychus carpini borealis, Eotetranychus, hicoriae, Eotetranychus
S sexmacu/(7tl~, Eotetranychus yumensis, Eotetranychus banksi and Oligonychus
pratensis; Tenuipalpidae including Brevipalpus lewisi, Brevipalpus phoenicis,
Brevipalpus californicus and Brevipalpus obovatus; Eriophyidae including
Phyllocoptruta oleivora, Eriophyes sheldoni, Aculus cornutus, Epitrimerus pyri
and Eriophyes mangiferae. See WO 90/10623 and WO 92/00673 for more
10 detailed pest descriptions.
Compounds of this invention can also be mixed with one or more other
insecticides, fungicides, nematocides, bactericides, acaricides, growth regulators,
chemosterilants, semiochemicals, repellants, attractants, pheromones, feeding
stimulants or other biologically active compounds to form a multi-component
15 pesticide giving an even broader spectrum of agricultural protection. Examples of
other agricultural protectants with which compounds of this invention can be
formulated are: insecticides such as avermectin B, monocrotophos, carbofuran,
tetrachlorvinphos, malathion, parathion-methyl, methomyl, chlordimeform,
diazinon, deltamethrin, oxamyl. fenvalerate, esfenvalerate, permethrin,
20 profenofos, sulprofos, triflumuron, diflubenzuron, methoprene, buprofezin,
thiodicarb, acephate, azinphosmethyl, chlorpyrifos, dimethoate, fipronil,
flufenprox, fonophos, isofenphos, methidathion, metha-midophos, phosmet,
phosphamidon, phosalone, pirimicarb, phorate, terbufos, trichlorfon.
methoxychlor, bifenthrin, biphenate, cyfluthrin, tefluthrin, fenpropathrin,
25 fluvalinate, flucythrinate, tralomethrin, imidacloprid. metaldehyde and rotenone;
fungicides such as carbend:~7im, thiuram, dodine, maneb, chloroneb, benomyl,
cymoxanil, fenpropidine, fenpropimorph, triadimefon, captan, thiophanate-
methyl, thiabendazole, phosethyl-Al, chlorothalonil, dichloran, metalaxyl,
captafol, iprodione. oxadixyl, vinclozolin, kasugamycin, myclobutanil,
30 tebuconazole, difenoconazole. diniconazole, fluquinconazole, ipconazole,
metconazole, penconazole, propiconazole, uniconzole, flutriafol, prochloraz,
pyrifenox, fenarimol, triadimenol, diclobutrazol, copper oxychloride, furalaxyl,folpet, flusilazol. blasticidin S. diclnm~.7ine, edifenphos. isoprothiolane,
iprobenfos, mepronil, neo-asozin. pencycuron, probenazole, pyroquilon,
35 tricyclazole, validamycin, and flutolanil; nematocides such as aldoxycarb,
fenamiphos and fosthietan; bactericides such as oxytetracyline. streptomycin and
WO g4/05670 PCr/US93~07926
42
tribasic copper sulfate; acaricides such as binapacryl, oxythioquinox,
chlorobenzilate, dicofol, dienochlor, cyhexatin, hexythiazox, amitraz, propargite,
tebufenpyrad and fenbutatin oxide; and biological agents such as
entomopathogenic bacteria, virus and fungi.
In certain instances, combinations with other arthropodicides having a
similiar spectrum of control but a different mode of action will be particularlyadvantageous for resistance management.
Arthropod pests are controlled and protection of agronomic, horticultural
and specialty crops, animal and human health is achieved by applying one or
l() more of the compounds of this invention, in an effective amount, to the
environment of the pests including the agronomic and/or nonagronomic locus of
infestation, to the area to be protected, or directly on the pests to be controlled.
Thus, the present invention further comprises a method for the control of foliarand soil inhabiting arthropods and nematode pests and protection of agronomic
and/or nonagronomic crops, comprising applying one or more of the compounds
of Formula I, or compositions cont~ining at least one such compound, in an
effective amount, to the environment of the pests including the agronomic and/ornonagronomic locus of infestation, to the area to be protected, or directly on the
pests to be controlled. A preferred method of application is by spraying.
Alternatively, granular formulations of these compounds can be applied to the
plant foliage or the soil. Other methods of application include direct and residual
sprays, aerial sprays, seed coats, microenr~ps~ tions, systemic uptake. baits,
eartags, boluses, foggers, f~lmig~nt~, aerosols, dusts and many others. The
compounds can be incorporated into baits that are consumed by the arthropods or
in devices such as traps and the like.
The compounds of this invention can be applied in their pure state, but most
often application will be of a formulation comprising one or more compounds
with suitable carriers, diluents, and surfactants and possibly in combination with a
food depending on the contemplated end use. A preferred method of application
involves spraying a water dispersion or refined oil solution of the compounds.
Combinations with spray oils, spray oil concentrations, spreader stickers.
adjuvants, and synergists and other solvents such as piperonyl butoxide often
enhance compound efficacy.
I'he rate of application required for effective control will depend on such
factors as the species of arthropod to be controlled, the pest's life cycle, life stage,
its size, location, time of year, host crop or animal, feeding behavior~ mating
W O 94/05670 PC~r/US93/07926
21~3~2~
43
behavior, ambient moisture, temperature, and the like. Under normal
circumstances, application rates of about 0.01 to 2 kg of active ingredient per
hectare are sufficient to control pests in agronomic ecosystems, but as little as
0.001 kg/hectare may be sufficient or as much as 8 kg hectare may be required.
S For nonagronomic applications, effective use rates will range from about 1.0 to
50 mg/square meter but as little as 0.1 mg/square meter may be sufficient or as
much as 150 mg/square meter may be required.
The following Tests demonstrate the control efficacy of compounds of this
invention on specific pests. The pest control protection afforded by the
10 compounds is not limited, however, to these species. See Index Tables A-F for compound descriptions.
W O 94/05670 PC~r/US93/07926
.
36~ 44
Tndex Table A
02N~,~ ,R2
MeS ~/\ N )~N
~J
Me
Compound R2 m.p. C
Me oil
\~ N--~ Cl oil
3 CH2 ~ oil
4 Et oil
c-pentyl oil
6 s-butyl oil
7 n-hexyl oil
8 t-butyl oil
9 CH2CO2Me oil
CH2Ph oil
11 CH2CO2Na oil
12 CH2CH2Cl oil
13 (CH2)8CH3 oil
14 (cH2)3cl oil
(cH2)2F oil
16 N(cH2)s oil
17 i-Pr tacky solid
18 CH2CH2NMe2 oil
19 CH2CH2NEt2 84-86
(cH2)3Br oil
21 (CH2)2NMe31 151-155
W O 94/05670 2 1 ~ 3 ~ ~ ~ PC~r/US93/07926
Index Table B
~) Me
Compound R2 m.p. C
22 Me 143-146
23 Et 121-123
24 c-Pentyl oil
s-Bu oil
26 n-hexyl 97-99
27 CH2Ph tacky solid
28 t-Bu tacky solid
29 (C H2)gC H3 tacky solid
i-Pr oil
31 CH2C02CH3 1 18-121
32 N(C H2)s tacky solid
Tndex Table C
02N `~ N ' R2
MeS ~N
Me
Compound R2 m.p. C
33 Me 145 (dec)
34 Et oil
s-Bu oil
36 t-Bu oil
37 (CH2)8CH3 tackysolid
W O 94/05670 PC~r/US93/07926
%~43~ 46
38 c-Pentyl oil
(C H2)sCH3 oil
CH2Ph oil
41 C H2CO2Me lOS-108
42 CH2C O2Na tacky solid
43 N(C H2)s oil
Tndex Table D
02N~N~R2
\ ~ N
N Me
Compound R2 m.p. C
44 Me 149-151
(C H2)8C H3 oil
46 (C H2)scH3 oil
47 Et oil
48 s-Bu oil
49 i-Pr oil
Index Table E
02N N, R2
1~ J
\ N / N
Me
Compound R2 m.p. C
¦ Me ¦ 165-169
W O 94/05670 PC~r/US93/07926
21~3~2~
47
Tndex Table F
02N~N~R2
S N)~N
Cl~ ,)J
N Me
Compound R2 m.p. C
Sl ¦ Me ¦ 148-149
Cmpd No. IH ~MR Datal
s
3 8.55 (s,lH), 8.54 (s,lH), 7.74 (dd,lH), 7.30 (dd,lH), 4.44 (dt.lH).
4.10-4.00 (m,2H), 3.95-3.83 (m,3H), 3.80 (s,2H), 3.78 (d,lH),
3.51 (dt,lH), 3.17 (dd,lH), 2.85-2.67 (m,2H), 2.14 (s,3H). 1.39
(d,3H).
4 4.38-4.28 (m. lH), 4.00 (overlapping
s,2H and t,lH), 3.93 (d,lH), 3.83-3.78 (m,2H), 3.47 (apparent
quintet,lH), 3.21 (dd,lH), 2.81-2.58 (m,4H), 2.12 (s.3H), 1.39
(d,3H), 1.15 (t,3H)-
4.35-4.25 (m,lH), 4.09-3.95 (m,4H), 3.85-3.80 (m~2H), 3.51
(apparent quintet,lH), 3.21 (dd,lH), 2.93 (quintet,lH), 2.82-2.64
(m, 2H), 2.12 (s,3H), 1.92-1.82 (m,2H), 1.80-1.65 (m,2H),
1.63-1.53 (m,2H), 1.50-1.40 (m,2H), 1.39 (d,3H).
6 4.33-4.24 (m,lH), 4.04-3.97 (m,3H). 3.93 (d,lH), 3.89-3.80
(m,2H), 3.51 (quintet,lH), 3.19 (dd,lH), 2.81-2.64 (m,3H). 2.12
(s,3H), 1.67-1.53 (m,2H), 1.39 (d,3H), 1.07 (dd,3H), 0.90 (t,3H).
7 4.42-4.34 (m,lH), 4.04-3.93 (m,3H), 3.91 (d,lH), 3.84-3.78
(m,2H), 3.53-3.45 (m,lH), 3.22 (dd,lH), 2.81-2.65 (m,2H). 2.55
WO 94/05670 PCI/US93/07926
~43~ ~ 48
(t,2H), 2.11 (s,3H), 1.57-1.47 (m,2H), 1.39 (d,3H), 1.29 (m,6H),
0.89 (t,3H).
8 4.20-4.11 (m,lH), 4.08 (d,lH), 4.01 (m,lH), 3.97 (d,lH), 3.84
(t,lH), 3.81 (d,lH), 3.59-3.51 (m,lH), 3.20 (dd,lH), 2.84-2.66
(m,2H), 2.14 (s,3H), 1.40 (d,3H), 1.17 (s,9H).
9 4.43-4.33 (m,lH), 4.21-4.13 (m,2H), 4.08-3.97 (m,2H), 3.86
(t,lH), 3.76 (s,3H), 3.75-3.70 (m,lH), 3.55-3.42 (m,3H), 3.22
(t,lH), 2.80-2.65 (m,2H), 2.11 (s,3H), 1.39 (d,3H).
7.38-7.27 (m,SH), 4.43 (dt,lH), 4.07-3.98 (m,2H), 3.96 (s,2H),
3.85 (d,lH), 3.81-3.72 (m,3H), 3.52 (dt.lH), 3.13 (dd,lH),
2.83-2.68 (m,2H), 2.15 (s.3H). 1.38 (d,3H).
11 (Partial, 200 MHz, D2O, HDO=4.68) 1.97 (s,3H), 0.94 (d,3H).
12 4.40 (dt,lH), 4.08-3.97 (m,3H), 3.93 (d,lH), 3.85 (t,lH), 3.63
(t,2H), 3.48 (dt,2H), 3.22 (dd,lH), 2.95 (t,2H), 2.80-2.63 (m,2H),
2.11 (s,3H), 1.39 (d,3H).
13 4.41-4.33 (m,lH), 4.04-3.85 (m,3H), 3.83-3.62 (m,3H), 3.53-3.44
(m,lH), 3.22 (dd,lH), 2.81-2.60 (m,2H), 2.57-2.50 (m,2H), 2.11
(s,3H), l.S (m,2H), 1.39 (d,3H), 1.33-1.20 (m,12H), 0.88 (t,3H).
14 4.43-4.35 (m,lH), 4.08 (d,lH), 4.04-3.97 (m,2H), 3.92-3.82
(m,3H), 3.68-3.60 (m,2H), 3.47 (dt,lH),3.21 (dd,lH), 2.79-2.64
(m,4H), 2.11 (s,3H), 2.02-1.94 (m,2H), 1.39 (d,3H).
lS 4.67 (m,lH), 4.57 (m,lH), 4.43-4.35 (m,lH), 4.12 (s,2H),
4.04-3.84 (m,4H), 3.50 (dt,lH), 3.22 (dd,lH), 3.08-2.64 (m,4H),
2.11 (s,3H), 1.39 (d,3H).
16 4.33-4.23 (m,lH), 4.22-4.12 (m,2H), 4.08-3.82 (m,4H), 3.47
(dt,lH), 3.18 (dd,lH), 2.77-2.58 (m,6H), 2.11 (s,3H), 1.6 (m,4H),
1.42-1.25 (overlapping d and s,SH total).
W O 94/05670 2 1 4 3 6 2 ~ PC~r/US93/07926
49
17 4.30-4.21 (m,lH), 4.08-3.95 (m,4H), 3.87-3.78 (m,2H), 3.51
(quintet,lH), 3.18 (t,lH), 2.92 (quintet,lH), 2.80-2.63 (m,2H),
2.11 (s,3H), 1.38 (d,3H), 1.13 (m,6H).
18 4.40-4.33 (m,lH), 4.09 (s,2H), 4.03-3.98 (m,lH), 3.90 (s,2H), 3.84
(t,lH), 3.49 (dt,lH), 3.21 (dd,lH), 2.81-2.64 (m,4H), 2.52-2.40
(m,2H), 2.25 (s,6H), 2.11 (s,3H), 1.39 (d,3H).
19 4.40-4.33 (m,lH), 4.08 (s,2H), 4.00 (m,lH), 3.92-3.82 (m,3H),
3.51 (dt,lH), 3.21 (dd,lH), 2.81-2.53 (m,lOH), 2.11 (s,3H), 1.39
(d,3H), 1.02 (t,6H).
4.37 (m,lH), 4.10-3.97 (m,3H), 3.90-3.80 (m,3H), 3.55-3.46
(m,3H), 3.23 (dd,lH), 2.73 (m,4H), 2.11 (s,3H), 2.04 (m,2H), 1.39
(d,3H).
21 (400 MHz, d6 DMSO) 4.27-4.00 (4H,m), 3.85-3.65 (m,3H)
3.55-3.42 (m,3H), 3.11 (s,9H), 3.03-2.85 (m,2H), 2.73-2.62
(m,2H), 2.03 (s,3H), 1.26 (d,3H).
23 8.55 (overlapping s and d,2H), 7.78 (d,lH), 7.29 (m,lH), 5.09
(1/2 ABq,lH), 4.68 (1/2 ABq,lH), 3.98 (s,2H), 3.90 (overlapping
doublets,2H), 3.77 (overlapping doublets,2H), 3.22 (dd,lH), 2.51
(q,2H), 1.28 (d,3H), 1.13 (t,3H).
24 8.55 (br s,2H), 7.78 (d,lH), 7.29 (m,lH), 5.14 (1/2 ABq,lH), 4.70
(1/2 ABq,lH), 4.07-3.90 (m,4H), 3.78 (overlapping doublets,2H),
3.22 (dd,lH), 2.66 (quintet,lH), 1.87-1.63 (m,4H), 1.62-1.48
(m,2H), 1.47-1.38 (m,2H), 1.27 (d,3H).
8.54 (overlapping s and d,2H total), 7.65 (d,lH) ca. 7.27 (m,lH),
5.14 (d,lH). 4.67 (d,lH), 3.99 (s,2H), 3.91-3.75 (m,4H), 3.19
(dd,lH), 2.66-2.58 (m,lH), 1.62-1.45 (m,lH), 1.40-1.28 (m,lH),
1.27 (d,3H), 1.01 (apparent t,3H), 0.89 (t,3H).
W O 94/05670 PC~r/US93/07926
2 ~ ~ 3 6 2 S 50
26 8.55 (overlaping s and d,2H), 7.75 (d,lH), 7.29 (m,lH), 5.20
(1/2 ABq,lH), 4.66 (1/2 ABq,lH), 4.02-3.84 (m,4H), 3.80-3.68
(m,2H), 3.21 (dd,lH), 2.39 (t,2H), 1.47 (m,2H), 1.35-1.22 (m,9H),
0.88 (t,3H).
27 8.59 (overlapping s and d,2H total), 7.77 (d,lH), 7.37-7.22 (m,6H),
5.22 (1/2 ABq,lH), 4.66 (1/2 ABq,lH), 4.01-3.82 (m,SH),
3.74-3.55 (m,3H), 3.12 (dd,lH), 1.30 (d,3H).
28 ca. 8.58 (overlapping singlets,2H), 7.82 (d,lH), 7.33 (dd,lH), 4.99
(1/2 ABq,lH), 4.79 (1/2 ABq,lH), 4.09 (d,lH), 3.99 (d,lH),
3.95-3.70 (m,4H), 3.19 (apparent t,lH), 1.22 (d,3H), 1.14 (s,9H).
29 ca. 8.54 (overlapping singlets,2H),7.75 (d,lH), ca. 7.3 (m,lH),
lS 5.19 (1/2 ABq,lH), 4.65 (1/2 ABq,lH), 4.02-3.82 (m,4H),
3.80-3.68 (m,2H), 3.22 (br t,lH), 2.41 (t,2H), 1.45 (m,2H),
1.35-1.18 (m,14H), 0.88 (distorted t,3H).
8.54 (br s,2H), 7.78 (d,lH), 7.30 (m,lH), S.10 (1/2 ABq,lH), 4.71
(1/2 ABq,lH), 4.03 (d,lH), 3.99-3.83 (m,3H), 3.82-3.75 (m,2H),
3.20 (dd,lH), 2.82 (quintet,lH), 1.26 (d,3H), 1.09 (d,6H).
31 8.55 (br s,2H), 7.56 (d,lH), ca. 7.30 (m,lH), 5.12 (1/2 ABq,lH),
4.68 (1/2 ABq,lH), 4.13 (s,2H), 4.01-3.82 (m,3H), 3.80-3.67
(m,4H), 3.36 (ABq,2H), 3.20 (t,lH), 2.17 (s,3H), 1.28 (d,3H).
32 (partial) ca. 7.68 (m,lH), ca. 7.30 (d,lH), 1.34 (d,3H).
34 4.18 (d,lH), 3.97 (d,lH), 3.84 (d,lH), 3.75 (d,lH), 3.70-3.54
(m,4H), 3.44-3.36 (m,lH), 2.76 (t,2H), 2.66-2.59 (m,2H),
2.29-2.21 (m,lH), 2.07 (s,3H), 1.94-1.85 (m,lH), 1.35 (d,3H),
1.15 (t,3H).
4.08 (m,lH), 3.93 (d,lH), 3.83-3.50 (m,6H), 3.42 (m,lH),
2.82-2.65 (m,3H), 2.25-2.18 (m,lH), 2.08 (s,3H), 1.92-1.83
WO 94/05670 PCr/US93/07926
51 21 ~
(m,lH), 1.68-1.48 (m,lH), 1.42-1.30 (overlapping d and m,4H),
1.05 (d,3H), 0.91 (distorted t,3H).
-
36 (partial) 2.10 (s,3H), 1.37 (d,3H), 1.15 (s,9H).
37 4.22 (d,lH), 3.98 (d,lH), 3.82 (d,lH), 3.78-3.53 (m,5H), 3.43-3.35
(m,lH), 2.74 (t,2H), 2.60-2.47 (m,2H), 2.28-2.20 (m,lH), 2.06
(s,3H), 1.92-1.84 (m,lH), 1.55-1.45 (m,2H), 1.35 (d,3H), ca. 1.28
(m,12H), 0.88 (t,3H).
38 4.15 (d,lH), 4.00-3.83 (m,3H), 3.70-3.53 (m,4H), 3.42-3.35
(m,lH), 3.00-2.89 (m,lH), 2.82-2.70 (m,2H), 2.27-2.20 (m,lH),
2.07 (apparent s,3H), 1.92-1.82 (m,3H), 1.80- 1.63 (m,2H),
1.62-1.40 (m,4H), 1.35 (m,3H).
39 4.21 (d,lH), 3.98 (d,lH), 3.84 (d,lH), 3.78-3.52 (m,6H), 3.41
(m,lH), 2.75 (dist t,2H), 2.55 (distorted q,2H), 2.24 (m, lH), 2.05
(m,3H), 1.88 (m,lH), 1.50 (m,2H), 1.38-1.22 (m,8H), 0.89
(dist t,3H).
7.35-7.27 (m,5H), 4.23 (d,lH), 4.15 (d,lH), 3.82-3.59 (m,7H),
3.48 (m,lH), 3.24 (m,lH), 2.78 (m,2H), 2.25 (m,lH), 2.09 (s,3H),
1.88 (m,lH), 1.36 (m,3H).
41 4.30 (d,lH), 4.18 (d,lH), 4.02 (d,lH), 3.78-3.38 (m,l lH), 2.77
(m,2H), 2.25 (m,lH), 2.07 (s,3H), 1.90 (m,lH), 1.35 (m,3H).
42 (400 MHz, D20, pa;tial spectrum, HDO-4.66) 1.87 (s,3H). 1.18
(d,3H).
43 (partial spectrum) 2.08 (s,3H), 1.33 (d,3H).
44 8.54 (overlapping s and d,2H), 7.62 (apparent d,lH), 7.29 (m,lH),
4.67 (d,lH), 4.59 (m,lH), 4.14 (d,lH), 3.85 (m,lH), 3.74 (d,lH),
3.63 (d,lH), 3.52-3.38 (m,3H), 2.37 (s,3H), 2.04 (m,lH), 1.93
(m,lH), 1.35 (m,3H).
WO 94/05670 PCr/US93/07926
2~ 43~ 52
8.53 (m,2H), 7.62 (m,lH), ca. 7.27 (m,2H). 4.67 (d,lH), 4.58
(m,lH), 4.17 (d,lH), 3.83 (m,2H), 3.69 (d,lH), 3.52-3.37 (m,3H),
2.38 (m,2H), 2.05 (m,lH), 1.94 (m,lH), 1.46 (m,2H), 1.36 (m,3H), .
ca. 1.25 (m,12H), 0.88 (t,3H).
46 ca. 8.53 (overlapping d and s,2H), 7.62 (d,lH), 7.29 (m,lH), 4.67
(d,lH), 4.59 (m,lH), 4.18 (d,lH), 3.84 (apparent d,2H), 3.69
~d,lH), 3.43 (m,3H), 2.39 (m,2H), 2.06 (m,lH), 1.83 (m,lH), 1.48
(m,2H), 1.37 (d,3H), c~. 1.29 (m,6H), 0.88 (distorted t,3H).
47 (partial spectrum) 8.55 (s,lH), 8.52 (s,lH), 1.34 (distorted d,3H),
1.12 (distorted t,3H).
48 8.55 (d,lH), 8.51 (s,lH), 7.64 (d,lH), 7.29 (m,lH), 4.69-4.52
(m,2H), 4.13 (m,lH), 4.00-3.80 (m,2H), 3.72 (m,lH), 3.47
(m,3H), 2.64 (m,lH), 2.00 (m,lH), 1.86 (m,lH), 1.63-1.45
(m,lH), 1.43-1.28 (m,4H), 1.03 (m,3H), 0.88 (m,3H).
49 (partial spectrum) 8.56 (d,lH), 8.52 (s,lH), 2.82 (m,l~), 1.33
(d,3H), 1.10 (apparent s,6H).
8.32 (s,lH),7.82 (d,lH), 7.34 (d,lH), 5.05 (1/2 ABq,lH), 4.75
(1/2 ABq,lH), 3.94-3.72 (m,6H), 3.22 (dd,lH), 2.42 (s,3H). 1.26
(d,3H).
51 7.44 (s,lH), 5.18 (1/2 ABq,lH), 4.79 (1/2 ABq,lH), 3.98-3.74
(m,6H), 3.22 (dd,lH), 2.43 (s,3H), 1.30 (d,3H).
Unless in(lic~t~d otherwise, spectra were obtained in CDC13 at 400 MHz.
30 s = singlet, d = doublet. t = triplet, m = multiplet, coupling constants (J) are in
~Iertz.
W O 94/05670 PC~r/US93/07926
~1~36~
53
TFST A
Southern Corn Rootworm
Test units consisting of an 8-ounce (230 mL) plastic cup cont~ining 1 one-
inch square of a soybean-wheatgerm diet were prepared. Solutions of each of the
5 test compounds (acetone/distilled water 75/25 solvent) were sprayed into the cup.
Spraying was accomplished by passing the cup, on a conveyor belt, directly
beneath a flat fan hydraulic nozzle which discharged the spray at a rate of
0.5 pounds of active ingredient per acre (about 0.55 kg/ha) at 30 psi (207 kPa).After the spray on the cups had dried, five second-instar larvae of the southern10 corn rootworm (Diabrotica undecimpunctata howardi) were placed into each cup.The cups were then covered and held at 27C and 50% relative humidity for 48 h,
after which time mortality readings were taken. Of the compounds tested, the
following gave mortality levels of 80% or higher after 48 h: 1*,2,3,4,5,6,7,8,10,
11,12,13,14,15,16,18,19,20,21,22,23,24,25,26,27,28,29,30,33,36,37,42,47,50,51.
* test concentration was 250 ppm
T~ST B
Boll Weevil
Five adult boll weevils (Anthonomus grandis grandis) were placed into each
of a series of 9-ounce (260 mL) cups. The test units were sprayed as described in
Test A with individual solutions of the below-listed compounds. Each cup was
then covered with a vented lid and held at 27C. and 50% relative humidity for
48 h, after which time mortality readings were taken. Of the compounds tested,
the following gave mortality levels of 80% or higher: 1*,3,5,6,11,12,14,15,16,
28,50,51.
* test concentration was 250 ppm
TFSTC
Black Bean A~hid
Individual na~LulLiulll leaves were infested with 10 to 15 aphids (all stages
of Aphis fabae) and sprayed with their undersides facing up as described in
Test A. The leaves were then set in three 8-inch diameter vials cont:~ining 4 mLof sugar water solution and covered with a clear plastic l-ounce portion cup to
prevent escape of aphids that drop from the leaves. The test units were held at
27C and 50% relative humidity for 48 h, after which time mortality readings
were taken. Of the compounds tested, the following gave mortality levels of 80%
W O 94/05670 PC~r/US93/07926
2~,~3~S
54
orhigher: 1*,2,3,5,6,7,8,9,10,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,
27 ,3 1 ,36,38,39,40,42,44,45,46,47 ,48,49,5 1 .
* test concentration was 250 ppm
TEST D
5 Acter T ~fhop~per
Test units were prepared from a series of 12-ounce (350 mL) cups, each
containing oat (Avena sativa) seedlings in a l-inch (2.5 cm) layer of sterilized soil
and a 1/2 inch layer of sand. The test units were sprayed as described in Test Awith individual solutions of the compounds. After the oats had dried from the
10 spraying, between 10 and 15 adult aster leafhoppers (Mascrosteles fascifrons)were aspirated into each of the cups covered with vented lids. The cups were held
at 27C and 50% relative humidity for 48 hours, after which time mortality
readings were taken. Of the compounds tested, the following gave mortality
levels of 80% or higher:
15 2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,36,38,40,47.
TFST F
Solution Systemic ~ctivity ~inst the Rice Water Weevil ~dult~
The test compound is added directly to 50 mL of distilled water and
dissolved completely to yield a concentration of 100 ppm. This chemical solution20 is poured into a scintillation vial. Three or four rice (Oryza sativa) seedlings, 1.5
to 2.0 leaf stage and about 7 to 9 cm tall, are then p~sitiont~d in the unit by a
nonabsorbent cotton plug at the vial collar. This allows complete immersion of
the seedling root systems in the chemical solution, while the aerial portion of the
plant is isolated above the solution. The cotton also prevents the test insects from
25 accidentally contacting the ch~mic~l solution beneath the cotton. Care is taken to
avoid accidental chemic~l cont~ tion of the cotton. A clear, l-inch diameter,
plastic tube is positioned over the vial. The rice seedlings are allowed to absorb
the chemical from the solution for 24 h in the laboratory at 22C under
continuous light. Five feral adult rice water weevils (Lissorhoptrus oryzophilus30 kuschel) that have been starved for about 24 h are then transferred into the test
units. The top of the tube is sealed with a plastic cap to prevent the test insects
from escaping. The infested units are held at 27C and 65% relative humidity.
Counts of the number of live and dead adults are taken at 48 and 72 h
po~ r~,clion. Insects which do not respond to being probed or pinched with
W O 94/05670 2 1 ~ 3 6 2 5 PC~r/US93/07926
forceps are classified as dead. Of the compounds tested, the following gave
mortality levels of 80% or higher at 72 h: 1,2,5,6,7.
TF..~T F
Solution Systemic Activity A~ainst Green Leafhopper Nymphs
S The test ch~.mic~l is added directly to 10 mL of distilled water and dissolved
completely. This chto.mic~l solution is poured into a conical shaped test unit.
Three rice seelllingc are then positioned in the unit by a notched sponge disk. The
sponge disk allows a complete immersion of the seedling root systems in the
chemic~l solution, while the aerial portion of the plant is isolated above the
solution. The sponge also p.cvGnts the test nymphs from accidentally contacting
the test solution. A 7 to 10 mm space, between the surface of the chemical
solution and the bottom of the sponge disk, PIG~eI1~ accidental chemical
c~ tion of the sponge. The concentration of the test chemical in the
chemical solution is lO0 ppm. The rice seedlings are allowed to absorb the
chPmis~l from the solution for 24 h ill a growth chamber held at 27C and 65%
relative hllmi(lity. Eight to ten 3rd-instar nymphs of the green leafhopper
(Nephotettix cincticeps) are transferred into the test units using an aspirator. The
infested units are held under the same le.npelatu~G and humidity conditions
described above. Counts of the number of live and dead nymphs are taken at 24
and 48 h post-infestation. Insects which cannot waL~ are classified as dead. Of
the compounds tested, the following gave mortality levels of 80% or higher at
48 h post-infestation. 1,2,3,4,5,6,7,8,9,10,12,13,14,15,16,18,19,33,36,38,39,40,41,50,51.
I~ST G
Solution Systernic Activity ~inct Brown Pl~nthopper Nymphs
Same methods as used for Solution Systemic Activity against green
leafhopper nymphs, except that the brown planthopper (Nilaparvata lugens) is thetest species. Of the compounds tested, the following gave mortality levels of 80%
or higher at 48 h post-infestation: 1,2,3,4,5,6,7,8,9,10,13,14,15,16,18,19,22,23,
24,25,26,27,29,30,31,41,45,46,47,48,49,50,51.