Note: Descriptions are shown in the official language in which they were submitted.
- 2145473
HOECHST AKTIENGESELLSCHAFT HOE 94/F 077 Dr.FI/PP
Description
Substituted heterocyclic carboxamidoesters, their prep-
aration and their use as pharmaceuticals
The invention relates to substituted heterocyclic carbox-
amidoesters, their preparation and their use as
inhibitors of collagen biosynthesis and their use as
pharmaceuticals for the treatment of fibrotic disorders.
Compounds which inhibit the enzymes proline hydroxylase
and lysine hydroxylase cause a very selective inhibition
of collagen biosynthesis by affecting the collagen-spe-
cific hydroxylation reactions. In the course thereof,
protein-bound proline or lysine is hydroxylated by the
enzymes proline hydroxylase or lysine hydroxylase. If
this reaction i8 suppressed by inhibitors, a
nonfunctional, underhydroxylated collagen molecule is
formed which can be released into the extracellular space
by the cells only to a small extent. The under-
hydroxylated collagen can additionally not be incorpor-
ated into the collagen matrix and is very easily degradedproteolytically. As a result of these effects, altogether
the amount of extracellularly deposited collagen is
reduced.
Inhibitorn of proline hydroxylase are therefore suitable
substances in the therapy of disorders in which the
deposition of collagens contributes significantly to the
clinical picture. These include, inter alia, fibroses of
the lungs, liver and skin (scleroderma) and
~therosclerosis.
It is known that the enzyme proline hydroxylase is
inhibited by pyridine-2,4- and -2,5-dicarboxylic acid
(K. Majamaa et al., Eur. J. Biochem. 138 (1984) 239-245).
However, these compounds are active in cell culture as
21~5173
-- 2
inhibitors only at very high concentrations (Tschank, G.
et al., Biochem. J. 238 (1987) 625-633).
Prodrugs of pyridine-2,4(5)-dicarboxylates are also
known. These are described in the earlier German
Applications P 42 33 124.2, P 42 38 506.7 and
P 42 09 424Ø
N-Oxalylglycines as inhibitors of proline-4-hydroxylaæe
are known from J. Med. Chem. 1992, 35, 2652-2658
(Cunliffe et al.), and EP-A-O 457 163 (RAA~er et al.).
Hydroxyisoquinoline- and hydroxycinnolinecarboxylic acid
glycyl amides are known from Biochem. Soc. Trans. 1991,
19, 812-815 (Franklin et al.).
Surprisingly, it has now been found that heterocyclic
carboxamidoesters having an ether, a thioether or an
amino 6ubstituent in the ortho-position and an acidic
group in the para-position to the amide function are
highly effective inhibitors in vivo of collagen
biosynthesis.
The compounds are ester prodrugs of the correspo~;ng
carboxylic acids of the formula 1, in which B i8 a
carboxyl group, as are described in the application
HOE 94/F 074 filed at the same time.
The compounds of the formula 1 are cleaved in the living
organism (in vivo) and in cell cultures (in vitro) to
give compounds of the formula 1 in which B is a carboxyl
group or their salts.
The substances of the formula 1 according to the inven-
tion lead in vivo to an inhibition of collagen
biosynthesis.
After administration of the compounds of the formula 1,
they cause the inhibition of collagen biosynthesis to be
observed in vivo and in vitro in that they release
2145473
-- 3
compounds of the formula 1 in which B is a carboxyl group
or their salts. Thèse compounds inhibit proline-4-
hydroxylase and therefore lead to an inhibition of
collagen biosynthesis.
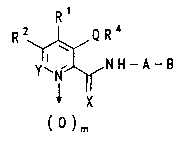
The compounds according to the in~ention correspond to
the formula 1
R 2~Q R ~
Y' N"b,N H-A-~ ( 1 )
X
( o )m
in which
Q is O, S or NRY,
X is O or S,
10 Y is C-R3 or N,
m iB O or 1,
A iæ (C1-C4)-alkylene which iB optionally substituted
by one or two substituents from the series halogen,
in particular fluorine, chlorine or bromine, cyano,
nitro, trifluoromethyl, (Cl-C6)-alkyl,
(C1-C6)-hydroxyalkyl, (C1-C6)-alkoxy,
-O-~CH2]x-CfH(2f~l g)Halg, preferably (C1-C8)-fluoro-
alkoxy,(Cl-C8)-fluoroalkenyloxy,(C1-C8)-fluoroalky-
nyloxy, -OCF2Cl or -O-CF2-CHFCl, (Cl-C6)-alkyl-
mercapto, (C1-C6)-alkylsulfinyl, (Cl-C6)-alkylsul-
fonyl,(Cl-C6)-alkylcarbonyl,(Cl-C6)-alkoxycarbonyl,
carbamoyl, N-(C1- C4 ) - alkylcarbamoyl, N,N-di-(Cl- C4 ) -
alkylcarbamoyl, (C1-C6)-alkylcarbonyloxy, (C3-C8)-
cycloalkyl, phenyl, benzyl, phenoxy, benzyloxy,
anilino, N-methylanilino, phenylmercapto, phenyl-
æulfonyl, phenylsulfinyl, sulfamoyl, N-(Cl-C4)-
alkylsulfamoyl, N,N-di-(C1-C4)-alkylsulfamoyl or
2145~7~
-- 4
by a substituted (C6-Cl2)-aryloxy, ( C7 -C1l)-aralkyl-
oxy, (C6-Cl2)-aryl or (C7-Cll)-aralkyl radical which,
in the aryl moiety, carries 1, 2, 3, 4 or 5 ident-
ical or different substituents from the series
halogen, cyano, nitro, trifluoromethyl, (Cl-C6)-
alkyl, (Cl-C6)-alkoxy, ~O~~CH2]x~CfH(2f+l g)Halg~
-OCF2Cl, -O-CF2-CHFCl, (Cl-C6)-alkylmercapto,
(Cl-C6)-alkylsulfinyl, (Cl-C6)-alkylsulfonyl,
(Cl-C6)-alkylcarbonyl, (Cl-C6)-alkoxycarbonyl,
carbamoyl, N-(Cl-C4)-alkylcarbamoyl, N,N-di-(Cl-C4)-
alkylcarbamoyl, (Cl-C6)-alkylcarbonyloxy, (C3-C8)-
cycloalkyl, sulfamoyl, N-(Cl-C4)-alkylsulfamoyl or
N,N-di-(Cl-C4)-alkylsulfamoyl, or
by a substituent Rx of the ~-C atom of an ~-amino
acid which includes the natural L-amino acids and
their D-isomers;
B is -CO2V, where
V is the radical of an alcohol VOH, in which V is
(Cl-ClO)-acyloxy-(Cl-C6)-alkyl, preferably (Cl-C10)-
alkanoyloxy-(C1-C6)-alkyl, benzyloxy-(Cl-C6)-alkyl,
benzyloxycarbonyloxy-(C1-C6)-alkyl or alkoxycarbon-
yloxy (Cl-C6)-alkyl, or
a branched, unbr~nche~ or cyclic aliphatic (Cl-C20)-
alkyl radical, or
a branched, unbr~nc~e~ or cyclic (C2-C20)-alkenyl
radical, a retinyl radical, a (C2-Cl6)-alkynyl
radical or a correspo~;ng (C4-Cl6)-alkenynyl rad-
ical, where the radicals can in each case contain
one or more multiple bonds, or
a (C6-Cl6)-aryl radical, a (C7-C16)-aralkyl radical
or a 5- or 6-membered, preferably nitrogen-contain-
ing heteroaryl radical or a 5- or 6-membered prefer-
ably nitrogen-containing heteroalkyl radical, where
the above radicals can in particular carry one or
more substituents from the series
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
2145473
carboxyl, (Cl-Cl2)-alkyl, (C3-C8)-cycloalkyl,
( C3 - C8 ) - CyC l o a l ky l - ( Cl - C12 ) - a l ky l ~
( C3 - C8 ) - cycloalkoxy, (C3 - C8 ) - cycloalkyl - (Cl - C12 ) -
alkoxy, ( C3 - C8 ) - cycloalkyloxy-(C1-C12) -alkyl,
( C3 - C8 ) - cycloalkyloxy-(C1-C12)-alkoxy, ( C3 - C8 ) - CyC lo-
alkyl- (C1-C8) -alkyl- (C1-C6) -alkoxY~ (C3-C8) -
cycloalkyl-(cl-c8)-alkoxy-(cl-c6)-alkyl~ (C3-C8)-
cycloalkyloxy-(C1-C8)-alkoxy-(C1-C6)-alkyl, (C3-C8)-
cycloalkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy, (C6-C12)-
aryl~ (C7-Cl6)-aralkYl~ (c2-cl2)-alkenyl~ (C2-C12)-
alkynyl~ (Cl-Cl2)-alkoxy, (cl-cl2)-alkoxy-(cl-cl2)-
alkyl, (cl-cl2)-alkoxy-(cl-cl2)-alkoxy~ (Cl-Cl2)-
alkoxy-(cl-c8)-alkoxy-(cl-c8)-alkyl~ (C6-Cl2)-
aryloxy, (C7-Cl6)-aralkyloxy, (C6-Cl2)-aryloxy-
(Cl-C6)-alkoxy, (C7-Cl6)-aralkoxy-(Cl-C6)-alkoxy,
(Cl-C8)-hydroxyalkyl, (C6-Cl6)-aryloxy-(Cl-C8)-alkyl,
( C7 -Cl6)-aralkoxy-(Cl-C8)-alkyl (C6-C12)-aryloxy-
(cl-c8)-alkoxy-(cl-c6)-alkyl~ ( C7 - Cl2 ) - aralky
(Cl-C8)-alkoxy-(Cl-C6)-alkyl, ~O~[CH2]x~CfH(2f+l g)Fg,
-OCF2Cl, -OCF2-CHFCl,
(Cl-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
(C6-C12)-arylcarbonyl, (C7-C16)-aralkylcarbonyl,
c;nnamoyl, (C2-C12)-alkenylcarbonyl, (C2-Cl2)-
alkynylcarbonyl,
(C1-C12)-alkoxycarbonyl, (cl-cl2)-alkoxy-(cl-cl2)-
alkoxycarbonyl, (C6-Cl2)-aryloxycarbonyl, (C7-Cl6)-
aralkoxycarbonyl, (C3-C10)-cycloalkoxycarbonyl,
(C2-C12)-alkenyloxycarbonyl, (C2-Cl2)-alkynyloxy-
carbonyl,
(Cl-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkyl-
carbonyloxy, (C6-Cl2)-arylcarbonyloxy, (C7-C16)-
aralkylcarbonyloxy, cinnamoyloxy, (C2-C12)-alkenyl-
carbonyloxy, (C2-C12)-alkynylcarbonyloxy,
(C1-C12)-alkoxycarbonyloxy, (C1-C12)-alkoxy (C1 C12)
alkoxycarbonyloxy, (C6-Cl2)-aryloxycarbonyloxy,
21~5473
(C7-C16)-aralkyloxycarbonyloxy, (c3-c8)-cyc10_
alkoxycarbonyloxy, (C2-Cl2)-alkenyloxycarbonyloxy,
(C2-C12)-alkynyloxycarbonyloxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di
(Cl-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl, N-
(C1-C10)-alkyl-N-( C3 - C8 ) - CyC loalkylcarbamoyl, N-
( ( C3 - C8 ) - cycloalkyl-(C1-C6)-alkyl)carbamoyl, N-
(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl, N-
(Cl-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl, N-
(C6-C12)-arylcarbamoyl, N-( C7 - Cl6 ) - aralkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C6-Cl6)-arylcarbamoyl, N-(C1-C1o)~
alkyl-N-(C7-Cl6)-aralkylcarbamoyl, N-~(Cl-clO)-
alkoxy-(C1-C10)alkyl)carbamoyl, N-((C6-C16)-aryloxy-
(C1-C10)alkyl)carbamoyl, N-((C7-C16)-aralkyloxy-
(C1-C10)-alkyl)-carbamoyl, N-(C1-C10)-alkyl-N-
~(C1-C10)-alkoxy-(Cl-ClO)-alkyl)carbamoyl, N-(C1-Clo)~
alkyl-N-((c6-cl2)-aryloxy-(cl-clo)-alkyl)carbamoyl,
N-(cl-clo)-alkyl-N-((c7-cl6)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl, CON(CH2)h, in which a CH2 group can
be replaced by O, S, N-(C1-C8)-alkylimino, N-(C3-C8)-
cycloalkylimino, N-(C3-C8)-cycloalkyl-(C1-C4)-
alkylimino, N-(C6-C12)-arylimino, N-(C7-Cl6)
aralkylimino or N-(C1-C4)-alkoxy-(C1-C6)-alkylimino
and h i 8 3 to 6, or by
a carbamoyl radical of the formula II
RX ~
--C O N R ~-- ~ ( I I ) .
S
in which
Rx i~ the ~ubstituent of an ~-amino acid which
includes the L- and D-amino acids,
2195973
-- 7
S i8 1, 2, 3, 4 or 5 and
T i~ OH, OR or NR R ,
R being hydrogen or (C1-C4)-alkyl,
R and R being identical or different and being
hydrogen, (C6-C12)-aryl, (C7-cll)-aralkyl~
(C1-C8)-alkyl, (C3-C8)-cycloalkyl, (+)-dehydro-
abietyl~ (cl-c8)-alkoxy (C1-C8)-alkYl, (C7-C12)-
aralkoxy_(C1-C8)-alkyl, (c6-cl2)-aryloxy_(cl-c8)-
alkyl, (C1-C10)-alkanoyl, optionally ~ubstituted
(C7-C16)-arAlk~noyl, optionally ~ubRtituted
( C 6 - Cl2 ) - aroyl, or
R and R together are -[CH2]h, in which a CH2 group
can be replaced by O, S, SO, SO2, N-acylimino, N-
(C1-C10)-alkoxycarbonylimino, N-(C1-C8)-alkyl-
imino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)cyclo-
alkyl-(C1-C4)-alkylimino,N-(C6-C12)-arylimino,N-
(C7-C16)-aralkyliminoorN-(C1-C4)-alkoxy-(C1-C6)-
alkylimino and h is 3 to 7, or are Rub~tituted by
carbamoyloxy, N-(C1-C12)-alkylcarbamoyloxy, N,N-di-
(C1-C12)-alkylcarbamoyloxy, N-(C3-C8)-cycloalkylcar-
bamoyloxy, N-(C6-C12)-arylcarbamoyloxy, N- (C7 - C16 ) -
aralkylcarbamoyloxy, N-(C1-C1o)-alkYl-N-(C6-cl2)-
arylcarbamoyloxy, N-(cl-clo)-alkyl-N-(c7-cl6)
aralkylcarbamoyloxy, N-(C1-C10)-alkylcarbamoyloxy,
N-((C6-C12)-aryloxy-(C1-C10)-alkyl)carbamoyloxy, N-
((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyloxy, N-
(C1-Cl0)-alkyl-N-((Cl-Cl0)-alkoxy-(Cl-Cl0)-
alkyl)carbamoyloxy, N-(C1-C1o)~alkyl-N-((C6_C12)_
aryloxy-(C1-C10)-alkyl)carbamoyloxy, N-(C1-Clo)~
alkyl-N- ((C7-C16)-aralkyloxy-(C1-Cl0)-
alkyl)carbamoyloxy,
amino, (C1-C12)-alkylamino, di-(C1-C12)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-Cl2)-alkenylamino,
( C3 - Cl2 ) - alkynylamino, N-(C6-C12)-arylamino,
2145973
N-(C7-C11)-aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (C1-C12)-alkoxyamino, (C1-C12)-
alkoxy-N-(C1-C10)-alkylamino,
(Cl-C12)-alkanoylamino, (C3-C8)-cycloalkanoylamino,
(C6-C12)-aroylamino, (C7-C16)-aralkanoylamino,
(C1-C12)-~lkAnoyl-N-(C1-C1O)-alkylamino, (C3 -C8) -
cycloalkanoyl-N-(C1-C10)-alkylamino, (C6-C12)-aroyl-
N-(C1-C10)-alkylamino, (C7-C11)-arAl~Anoyl-N-(C1-C10)-
alkylamino,
(C1-C12)-AlkAnoylamino-(C1-C8)-alkyl, (C3-C8)-cyclo-
alkanoylamino-(C1-C8)-alkyl, (C6-C12)-aroylamino-
(C1-C8)-alkyl, (C7 -C16)-aralkanoylamino-(C1-C8)-
alkyl, amino-(Cl-C10)-alkyl, N-(C1-C10)-alkylamino-
(C1-C10)-alkyl, N,N-di-(C1-C10)-alkylamino-(Cl-ClO)-
alkyl, (C3-C8)-cycloalkylamino-(C1-C10)-alkyl,
(C1-C12)-alkylmercapto, (C1-C12)-alkylsulfinyl,
(Cl-C12)-alkylsulfonyl, (C6-C12)-arylmercapto,
(C6-C12)-arylsulfinyl,
(C6-C12)-arylsulfonyl, (C7-C16)-aralkylmercapto,
(C7-C16)-aralkylsulfinyl, (C7-C16)-aralkylsulfonyl,
sulfamoyl, N-(C1-C10)-alkylsulfamoyl, N,N-di-
(Cl-C10)-alkylsulfamoyl, (C3-C8)-cycloalkylsulfamoyl,
N-(C6-C12)-arylsulfamoyl, N-( C7 - C16 ) - aralkyl-
sulfamoyl, N-(C1-C10)-alkyl-N-(C6-Cl2)-arylsulfamoyl,
N-(C1-C10)-alkyl-N-(C7-Cl6)-aralkylsulfamoyl,
(C1-C10)-alkylsulfonamido, N-(C1-C10)-alkyl-(Cl-ClO)-
alkylsulfonamido, (C7-C16)-aralkylsulfonamido or N-
(C1-C10)-alkyl-(C7-Cl6)-aralkylsulfonamido,wherethe
radicals which contain an aryl radical can for their
part be substituted on the aryl by 1 to 5 identical
or different radicals from the series:
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (C1-C12)-alkyl, (C3-C8)-cycloalkyl,
(C6-C12)-aryl, (c7-cl6)-aralkyl~ (Cl-C12)-alkoxy,
( C 1 - C 12) - a l k o x y - ( C 1 - C 12) - a l k y
2145q73
(cl-cl2)-alkoxy-(cl-cl2)-alkoxy~ (C6-Cl2)-aryloxy,
(C7-C16)-aralkyloxy, (Cl-C8)-hydroxyalkyl,
(Cl-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
(C6-Cl2)-arylcarbonyl, (C7-C16)-aralkylcarbonyl,
(Cl-Cl2)-alkoxycarbonyl, (cl-cl2)-alkoxy-(cl-cl2)-
alkoxycarbonyl, (C6-Cl2)-aryloxycarbonyl, (C7-Cl6)-
aralkoxycarbonyl, (C3-C8)-cycloalkoxycarbonyl,
(C2-Cl2)-alkenyloxycarbonyl, (C2-Cl2)alkynyloxy-
carbonyl,
(Cl-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkyl-
carbonyloxy, (C6-Cl2)-arylcarbonyloxy, (C7-Cl6)-
aralkylcarbonyloxy, cinnamoyloxy, (C2-Cl2)-alkenyl-
carbonyloxy, (C2-Cl2)-alkynylcarbonyloxy,
(cl-cl2)-alkoxycarbonyloxy~ (Cl-Cl2)-alkXY (Cl C12)
alkoxycarbonyloxy, (C6-Cl2)-aryloxycarbonyloxy~
(C7-C16)-aralkyloxycarbonyloxy, (C3-C8)-cycloalkoxy-
carbonyloxy, (C2-Cl2)-alkenyloxycarbonyloxy,
(C2-Cl2)-alkynyloxycarbonyloxy,
carbamoyl, N-(Cl-Cl2)-alkylcarbamoyl, N,N-di-
(Cl-Cl2)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl, N-
(Cl-C10)-alkyl-N- (C3 - C8 ) - cycloalkylcarbamoyl, N-
((C3-C8)-cycloalkyl-(Cl-C6)-alkyl)carbamoyl, N-
(Cl-C6)-alkyl-N-((C3-C8)-cycloalkyl-(Cl-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl, N-
(Cl-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl, N-
(C6-Cl2)-arylcarbamoyl, N-( C7 - C16 ) - aralkylcarbamoyl,
N-(Cl-Cl0)-alkyl-N-(C6-Cl6)-arylcarbamoyl, N-(Cl-Clo)~
alkyl-N-(C7-Cl6)-aralkylcarbamoyl, N-((Cl-clO)-
alkoxy-(Cl-C10)-alkyl)carbamoyl, N-((C6-Cl6)-aryloxy-
(Cl-C10)-alkyl)carbamoylJ N-((C7-Cl6)-aralkyloxy-
(Cl-C10)-alkyl)carbamoyl, N-(Cl-C10)-alkyl-N_
((Cl-C10)-alkoxy-(Cl-ClO)-alkyl)carbamoyl, N-(Cl-Clo)~
alkyl-N-((c6-cl2)-aryloxy_(cl-clo)-alkyl)carbamoyl~
21~5473
- 10 -
N-(cl-clo)-alkyl-N-((c7-cl6)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl, CON(CH2) h in which a CH2 group can
be replaced by O, S, N-(C1-C8)-alkylimino, N-(C3-C8)-
cycloalkylimino, N-(C3-C8)-cycloalkyl-(Cl-C4)-alkyl-
imino, N-(C6-C12)-arylimino, N-(C7-Cl6)-aralkylimino
or N-(Cl-C4)-alkoxy-(C1-C6)-alkylimino, and h i~ 3
to 7,
carbamoyloxy, N-(C1-C12)-alkylcarbamoyloxy, N,N-di-
(Cl-Cl2)-alkylcarbamoyloxy, N- (C3 -C8 ) - cycloalkylcar-
bamoyloxy, N-(C6-C16)-arylcarbamoyloxy, N-(C7-C16)-
aralkylcarbamoyloxy, N-(C1-Clo)-alkYl-N-(C6-cl2)-
arylcarbamoyloxy, N~(C1~Clo)-alkyl-N-(C7-Cl6)_
aralkylcarbamoyloxy, N-(Cl-C10)-alkylcarbamoyloxy,
N-((C6-Cl2)-aryloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
((C7-Cl6)-aralkyloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
(Cl-C10)-alkyl-N-((Cl-ClO)-alkoxy-(Cl-ClO)-alkyl)-
carbamOyloxy~ N-(cl-clo)-alkyl-N-((c6-cl2) aryloxy
(Cl-C10)-alkyl)carbamoyloxy, N-(C1-C10)-alkyl-N-
((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyloxy,
amino, (Cl-Cl2)-alkylamino, di-(Cl-Cl2)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-Cl2)-alkenylamino,
(C3-Cl2)-alkynylamino, N-(C6-Cl2)-arylamino, N-
( C7 - Cll ) - aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (Cl-Cl2)-alkoxyamino, (Cl-C12)-
alkoxy-N-(C1-C10)-alkylamino,
(Cl-C12) -Al kAnoylamino, (C3-C8) -cycloAl kAnoylamino,
( C 6 - Cl2 ) - aroylamino, ( C7 - Cl 6 ) - aralkanoylamino,
(Cl-Cl2)-AlkAnoyl-N-(C1-C10)-alkylamino, (C3-C8)-
cycloalkanoyl-N-(Cl-C10)-alkylamino, (C6-C12)-aroyl-
N-(C1-C10)-alkyl A~; no, (C7-cll) -arAl kAnOyl-N- (Cl-clo) -
alkylamino,
(C1-C12)-alkanoylamino-(Cl-C8)-alkyl, ( C3 - C8 ) - cyclo-
alkanoylamino-(Cl-C8)-alkyl, (C6-Cl2)-aroylamino-
(Cl-C8)-alkyl, ( C7 - Cl 6 ) - aralkanoylamino-(C1-C8)-
a l k y l , a m i n o - ( C 1 - C lo ) a l y
21g5473
11 -
N-(C1-C10)-alkylAm;no-(Cl-ClO)-alkyl, N,N-di-(C1-C1o)-
alkylamino-(C1-C10)-alkyl, (C3-C8)-cycloalkylamino-
(C1-C10)-alkyl,
(Cl-C12)-alkymercapto,(Cl-C12)-alkylsulfinyl,
(C1-C12)-alkylsulfonyl,(C6-C16)-arylmercapto,
(C6-C16)-arylsulfinyl,(C6-C16)-arylsulfonyl,
(C7-C16)-aralkylmercapto, (C7-C16)-aralkylsulfinyl,
(C7 - Cl6 ) -aralkyl8ulfonyl,
R1 and R3 are identical or different and are hydrogen,
(C1-C8)-alkyl, (C1-C8)-alkoxy, halogen, in particular
fluorine, chlorine or bromine, nitrile, hydroxyl,
amino, optionally mono- or disubstituted by (C1-C6)-
alkyl or hydroxy-(C1-C6)-alkyl, (C1-C6)-alkyl-
carbonyloxy,
R2 is a carboxylic acid-isosteric group, preferably 2-
imidazolyl, 5-tetrazolyl, 3-hydroxypyrazolyl, 3-
hydroxyisothiazolyl, 3-hydroxyisoxazolyl or a rad-
ical -[CH2]p-Co-NR5R6, where
p = 0, 1, 2, 3 or 4,
R5 is hydrogen, (C1-C6)-alkyl or an N-protective
group such as (C1-C8)-alkanoyl, (C1-C6)-alkyl-
carbamoyl, (C1-C6)-alkoxycarbonyl, benzyloxy-
carbonyl, (C1~clo)~acYloxy-(cl-c6)-alkyl~
preferably (C1-C10)-alkanoyloxy-(Cl-C6)-alkyl,
benzoyloxy-(C1-C6)-alkyl, benzyloxycArho~yloxy-
(C1-C6)-alkyl or (C1-C6)-alkoxycarbonyloxy-
(C1-C6)-alkyl, a 1-, 2-, 3- or 4-valent
physiologically utilizable cation, in
particular Na~, K~, Mg2~, Ca2~, Al3~ or an
ammonium ion, optionally substituted 1-3 times
by (C1-C8)-hydroxyalkyl, (C1-C4)-alkoxy-(C1-C8)-
alkyl, phenyl, benzyl or (C1-C8)-alkyl, which
for its part can be substituted 1-3 times by
hydroxyl or (C1-C4)-alkoxy or a cation of a
basic amino acid derivative,
R6 is a radical of the formula I, excluding -S02H,
2145473
-G-K-~C-U]r-D-W (I)
in which
G i 8 - S -, - S2 - or -CO-,
K is a bond or -NR7-,
C is a bond or
a branched or unbrAnche~ aliphatic (C1-Cl6)-
alkanediyl or cycloaliphatic (C3-C10)-
alkanediyl radical,
a branched or unbranched (C2-C16)-alkenediyl or
cyclo~l~en ediyl radical, a (C2-Cl6)-alkynediyl
radical or a (C2-C16)-alkenynediyl radical
which in each case can contain one or more C-C
multiple bonds,
U is a bond, hydrogen or
a radical from the series of following
heteroatom groups
-CO-, -O(CO)-, -(CO)-O-, -(CO)NR-, -NR(CO)-,
-O-, -SO-, -SO2, -NR,
in which R is (C1-C3)-alkyl or hydrogen,
r is 1, 2, 3 or 4,
D is a bond, hydrogen or
a branched or unbranched aliphatic (C1-C10)-
alkanediyl radical,
a branched or unbranched (C1-C10)-alkenediyl
radical,
a (C2-C10)-alkynediyl radical or a (C2-C10)-
alkenynediyl radical, which in each case can
contain one or more C-C multiple bonds,
W is a bond, hydrogen or
a (C3-C10)-cycloaliphatic alkyl, alkenyl,
alkynyl or alkenynyl radical,
a (C6-C16)-aryl radical or a 5- or 6-membered
heteroaryl radical, where at least one of the
variables C, D or W is not a bond and U is only
a heteroatom group if C is not a bond or if D
and/or W are not a bond and
C, D and/or W, if these are not a bond or hydrogen,
preferably are for their part substituted by a
2145g73
- 13 -
combination of up to 5 identical or different
substituents from the series
hydroxyl, halogen, cyano, trifluoromethyl,
nitro, carboxyl, (C1-C12)-alkyl, (C3-C8)-cyclo-
alkyl, (C3-C8)-cycloalkyl-(C1-C12)-alkyl,
(C3-C8)-cycloalkoxy, (C3-C8)-cycloalkyl-
(C1-C12)-alkoxy,( C3 - C8 ) - cycloalkyloxy-(C1-C12)-
alkyl, ( C3 - C8 ) - cycloalkyloxy-(C1-C12)-alkoxy,
(C3-C8)-cycloalkyl-(C1-C8)-alkyl-(C1-C6)-alkoxy,
(C3-C8)-cycloalkyl-(C1-C8)-alkoxy-(C1-C6)-alkyl,
(C3-C8)-cycloalkyloxy-(C1-C8)-alkoxy-(C1-C6)-
alkyl, (C3-C8) -cycloalkoxy-(C1-C8)-alkoxy-
(Cl-C8)-alkoxy, (C6-C12)-aryl, (C7-Cl6)-aralkyl,
(C2-C12)-alkenyl, (C2-C12)-alkynyl, (C1-C12)-
alkoxy, (cl-cl2)-alkoxy-(cl-cl2)-alkyl,
(cl-cl2)-alkoxy-(cl-cl2)-alkoxy~ (C1-C12)-
alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkyl, (C6-C12)-
aryloxy, (C7-C16)-aralkyloxy, (C6-C12)-aryloxy-
(C1-C6)-alkoxy, (c7-cl6)-aralkoxy-(cl-c6)
alkoxy, (C1-C8)-hydroxyalkyl, (C6-C16)-aryloxy-
(C1-C8)-alkyl, (C7-C16)-aralkoxy-(C1-C8)-alkyl,
(C6-C12)-aryloxy-(C1-C8)-alkoxy-(C1-C6)-alkyl,
(C7-C12)-aralkyloxy-(C1-C8)-alkoxy-(C1-C6)-
alkyl, -O-[CH2]x-cfH(2fll-g)Fg~ -OCF2Cl,
-OCF2-CHFCl,
(Cl-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcar-
bonyl, (C6-C12)-arylcarbonyl, ( C7 -C16)-aralkyl-
carbonyl, c; nn~moyl~ (C2-Cl2)- alkenylcarbonyl,
(C2-C12)-alkynylcarbonyl,
(C1-C12)-alkoxycarbonyl, (C1-C12)-alkoxy-
(C1-C12)-alkoxycarbonyl, (C6-C12)-
aryloxycarbonyl, ( C7-Cl6)- aralkoxycarbonyl,
(C3-C10)-cycloalkoxycarbonyl, (C2-C12)-
alkenyloxycarbonyl, (C2-C12)-alkynyloxycar-
bonyl,
( C 1 ~ C 1 2 ) ~ a l k y l c a r b o n y l o x y ,
2145473
- 14 -
(C3-C8)-cycloalkyl-carbonyloxy, (C6-C12)-
arylcarbonyloxy, (C7-C16)-aralkylcarbonyloxy,
cinnamoyloxy, (C2-C12)-alkenylcarbonyloxy,
(C2-C12)-alkynylcarbonyloxy,
(C1-C12)-alkoxycarbonyloxy, (C1-Cl2)-alkoxy-
(C1-Cl2)-alkoxycarbonyloxy, (C6-C12)-aryloxy-
carbonyloxy, (C7-C16)-aralkyloxycarbonyloxy,
(C3-C8)-cycloalkoxycarbonyloxy, (C2-C12)-
alkenyloxycarbonyloxy, (C2-Cl2)-alkynyloxy-
carbonyloxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl,
N-(C1-Cl0)-alkyl-N-(C3-C8)-cycloalkylcarbamoyl,
N-((C3-C8)-cycloalkyl-(C1-C6)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N-(C7-C16)-aralkyl-
carbamoyl, N-(cl-clo)-alkyl-N-(c6-cl6)-aryl
carbamoyl, N-(C1-C10)-alkyl-N-(C7-C16)-aralkyl-
carbamoyl, N-((cl-clo)-alkoxy-(cl-clo)alkyl)-
carbamoyl, N-((C6-C16)-aryloxy-(C1-Cl0)alkyl)-
carbamoyl, N-((c7-cl6)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl, N-(Cl-Cl0)-alkyl-N-((Cl-Cl0)-
alkoxy-(Cl-C1O)-alkyl)carbamoyl, N-(Cl-clo)
alkyl -N- ( ( C6 - Cl2) - aryloxy - ( Cl - C10) -
- alkyl)carbamoyl, N-(cl-clo)-alkyl-N-((c7 -C16) -
aralkyloxy-(Cl-Cl0)-alkyl)carbamoyl, CON(CH2) h~
in which a CH2 group can be replaced by O, S,
N-(Cl-C8)-alkylimino, N-(C3-C8)-cycloalkyl-
imino, N-(C3-C8)-cycloalkyl-(C1-C4)-alkylimino,
N-(C6-Cl2)-arylimino, N-(C7-C16)-aralkylimino or
N-(Cl-C4)-alkoxy-(Cl-C6)-alkylimino and h i~3 3
to 6, or by
a carbamoyl radical of the formula II
- 2145473
R" H
--CO--NR ~-- --T ( I I ) '
in which
Rx is the substituent of an ~-amino acid which
includes the L- and D-amino acids,
8 is 1, 2, 3, 4 or 5 and
T is OH, OR or NR R ,
R being hydrogen or (Cl-C4)-alkyl,
R and R being identical or different and being
hydrogen, (C6-Cl2)-aryl, (C7-Cll)-aralkyl,
(Cl-C8)-alkyl, (C3-C8)-cycloalkyl, (+)-dehydro-
abietyl, (Cl-C8)-alkoxy-(Cl-C8)-alkyl, (C7-Cl2)-
aralkoxy-(Cl-C8)-alkyl, (C6-Cl2)-aryloxy-
(Cl-C8)-alkyl, (Cl-Cl0)- Al kAnoyl, optionally
substituted (C7-Cl6)-arAlkAnoyl, optionally
substituted (C6-Cl2)-aroyl, or
R and R together are -[CH2] h~ in which a CH2 group
can be replaced by O, S, SO, SO2, N-acylimino,
N-(Cl-Cl0)-alkoxycarbonylimino, N-(Cl-C8)-alkyl-
imino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-
cycloalkyl-(Cl-C4)-alkylimino, N-(C6-Cl2)-aryl-
imino, N-(C7-Cl6)-aralkylimino or N-(Cl-C4)-
alkoxy-(C1-C6)-alkylimino and h is 3 to 7, or
are substituted by
carbamoyloxy, N-(C1-Cl2)-alkylcarbamoyloxy, N,N-di-
(C1-C12)-alkylcarbamoyloxy, N- (C3 - C8 ) - cycloalkylcar-
bamoyloxy, N-(C6-C12)-arylcarbamoyloxy, N-(C7-Cl6)-
aralkylcarbamoyloxy, N-(C1-Clo)-alkYl-N-(C6-cl2)-
arylcarbamoyloxy, N-(C1-Cl0)-alkyl-N-(C7-C16)-aral-
kylcarbamoyloxy, N-(C1-Cl0)-alkylcarbamoyloxy,
2145473
N-((c6-cl2)-aryloxy-(cl-clo)-alkyl)carbamoyloxy~ N-
((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyloxy, N-
(C1-C1O)-alkyl-N-((Cl-ClO)-alkoxy-(Cl-ClO)-
alkyl)carbamoyloxy, N-(cl-clo)-alkyl-N-((c6-cl2)-
aryloxy-(Cl-C1O)-alkyl)carbamoyloxy, N-(Cl-clO)-
alkyl-N- ((C7-C16)-aralkyloxy- (C1-C10) -
alkyl)carbamoyloxy,
amino, (C1-C12)-alkylamino, di-(C1-C12)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-C12)-alkenylamino,
(C3-C12)-alkynylamino, N-(C6-C12)-arylamino, N-
( C7 - Cll ) - aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (C1-C12)-alkoxyamino, (C1-C12)-
alkoxy-N-(C1-C1O)-alkylamino,
(C1-C12)-alkanoylamino, (C3-C8)-cycloAl~Anoylamino,
(C6-Cl2)-aroylamino~ (C7-C16)-aralkanoylamino,
(C1-C12)-alkanoyl-N-(C1-C1O)-alkylamino, ( C3 - C8 ) -
cycloAlkAnoyl-N-(C1-C10)-alkylamino, (C6-C12)-aroyl-
N-(C1-C1O)-alkylamino, (C7 -cll) -arAl kAnOyl -N-(C1-C1o)-
alkylamino,
(C1-C12)-alkanoylamino-(C1-C8)-alkyl, ( C3-C8) -CyC lo-
alkanoylamino-(C1-C8)-alkyl, (C6-C12)-aroylamino-
(C1-C8)-alkyl, (C7-C16)-aralkanoylamino-(C1-C8)-
alkyl, amino-(C1-C1O)-alkyl, N-(C1-C1O)-alkylamino-
(C1-C1O)-alkyl, N,N-di-(C1-C1O)-alkylamino-(Cl-ClO)-
alkyl, ( C3-C8) -CyC loalkylamino-(C1-C1O)-alkyl,
(Cl-C12)-alkylmercapto, (Cl-C12)-alkyl~ulfinyl,
(Cl-Cl2)-alkyl~3ulfonyl, (C6-C12)-arylmercapto,
(C6-C12)-aryl 8ul finyl,
(C6-Cl2)-aryl~ulfonyl, (C7-C16)-aralkylmercapto,
(C7-C16)-aralkyl~ulfinyl, (C7-C16)-aralkylsulfonyl,
sulfamoyl, N-(C1-C10)-alkylsulfamoyl, N,N-di-
(C1-C1O)-alkylsulfamoyl, (C3-C8)-cycloalkyl~ulfamoyl,
N-(C6-C12)-aryl~ulfamoyl, N-(C7-C16)-aralkyl-
sulfamoyl, N-(C1-C1O)-alkyl-N-(C6-Cl2)-aryl~ulfamoyl,
- 2145473
- 17 -
N-(C1-C10)-alkyl-N-(C7-Cl6)-aralkylsulfamoyl,
(C1-C10)-alkylsulfonamido,N-(Cl-ClO)-alkyl-(Cl-ClO)-
alkylsulfonamido, (C7-C16)-aralkylsulfonamido or N-
(C1-C10)-alkyl-(C7-Cl6)-aralkylsulfonamido,wherethe
radicals which contain an aryl radical can for their
part be substituted on the aryl by 1 to 5 identical
or different radicals from the series:
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (C1-C12)-alkyl, ( C3 - C8 ) - CyC loalkyl,
(C6-C12)-aryl, (c7-cl6)-aralkyl~ (Cl-Cl2)-alkoxy,
(cl-cl2)-alkoxy-(cl-cl2)-alkyl~ (C1-Cl2)-alkoxy-
(Cl-C12)-alkoxy, (C6-C12)-aryloxy, (C7-C16)-aralkyl-
oxy, (C1-C8)-hydroxyalkyl,
(Cl-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
( C6 - Cl2 ) - arylcarbonyl, ( C7 - Cl6 ) - aralkylcarbonyl,
(C1-C12)-alkoxycarbonyl, (cl-cl2)-alkoxy-(cl-cl2)
alkoxycarbonyl, (C6-Cl2)-aryloxycarbonyl, (C7-C16)-
aralkoxycarbonyl, (C3-C8)-cycloalkoxycarbonyl,
(C2-C12)-alkenyloxycarbonyl, (C2-C12)alkynyloxy-
carbonyl,
(Cl-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkylcarbony-
1 OXy, ( C 6 - Cl2 ) - arylcarbonyloxy, ( C7 - Cl 6 ) - aralkylcar-
bonyloxy, cinnamoyloxy, (C2-Cl2)-alkenylcarbonyloxy,
(C2-C12)-alkynylcarbonyloxy,
(cl-cl2)-alkoxycarbonyloxy~ (Cl-cl2)-alkoxy-(cl C12)
alkoxycarbonyloxy, (C6-C12)-aryloxycarbonyloxy,
(C7-C16)-aralkyloxycarbonyloxy, (C3-C8)-cyclo~lkoYy-
carbonyloxy, (C2-C12)-alkenyloxycarbonyloxy,
(C2-Cl2)-alkynyloxycarbonyloxy,
carbamoyl, N-(Cl-Cl2)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-( C3 - C8 ) - cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl, N-
(C1-C1O)-alkyl-N- (C3 -C8 ) - cycloalkylcarbamoyl, N-
( (C3 -C8) -cycloalkyl-(C1-C6)-alkyl)carbamoyl,
2145~73
- 18 -
N-(C1-C6)-alkyl-N-((C3-CB)-cycloalkyl-(Cl-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl, N-
(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl, N-
(C6-C12)-arylcarbamoyl, N-( C7 - Cl6 ) - aralkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C6-Cl6)-arylcarbamoyl, N-(C1-C1o).
alkyl-N-(C7-C16)-aralkylcarbamoyl, N-((Cl-clo)
alkoxy-(C1-C10)-alkyl)carbamoyl, N-((C6-C16)-aryloxy-
(C1-C10)-alkyl)carbamoyl, N-((C7 -C16)-aralkyloxy-
(C1-C10)-alkyl)carbamoyl, N-(cl-clo)-alkyl-N-
((C1-C10)-alkoxy-(Cl-ClO)-alkyl)carbamoyl, N-(C1-C1o)~
alkyl N-((c6-cl2)-aryloxy_(cl-clo)-alkyl)ca
N-(cl-clo)-alkyl-N-((c7-cl6)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl, CON(CH2)h in which a CH2 group can
be replaced by O, S, N-(C1-C8)-alkylimino, N-(C3-C8)-
cycloalkylimino, N-(C3-C8)-cycloalkyl-(C1-C4)-alkyli-
mino, N-(C6-C12)-arylimino, N-(C7-C16)-aralkylimin
or N-(C1-C4)-alkoxy-(C1-C6)-alkylimino, and h i8 3 to
7,
carbamoyloxy, N-(C1-C12)-alkylcarbamoyloxy, N,N-di-
(C1-C12)-alkylcarbamoyloxy, N-(C3-C8)-cycloalkylcar-
bamoyloxy, N-(C6-C16)-arylcarbamoyloxy, N- (C7 -C16) -
aralkylcarbamoyloxy, N-(C1-C10)-alkyl-N-(C6-Cl2)-
arylcarbamoyloxy, N-(cl-clo)-alkyl-N-(c7-cl6)
aralkylcarbamoyloxy, N-(C1-C10)-alkylcarbamoyloxy,
N-((C6-C12)-aryloxy-(C1-C10)-alkyl)carbamoyloxy, N-
( (C7 -C16) -aralkyloxy-(C1-C10)-alkyl)carbamoyloxy, N-
(C1-C10)-alkyl-N-((Cl-ClO)-alkoxy-(Cl-ClO)-alkyl)-
carbamoyloxy, N-(C1-C10)-alkyl-N-((C6-Cl2)-aryloxy-
(C1-C10)-alkyl)carbamoyloxy, N-(C1-C10)-alkyl-N_
((C7 -C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyloxy,
amino, (C1-C12)-alkylamino, di-(C1-C12)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-C12)-alkenylamino,
(C3-C12)-alkynylamino, N-(C6-C12)-arylamino, N-
( C7 - Cll ) - aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (C1-C12)-alkoxyamino, (C1-C12)-
alkoxy-N-(C1-C10)-alkylamino,
2145473
- 19 -
(Cl-C12) -Al kAnoylamino, (C3-C8) -cycloalkanoylamino,
(C6-C12)-aroylamino, (C7-C16)-aralkanoylamino,
(C1-C12)-alkanoyl-N-(C1-C1O)-alkylamino, (C3-C8)-
cycloAlkAnoyl-N-(C1-C10)-alkylamino, (C6-C12)-aroyl-
N-(C1-C1O)-alkylamino, (C7-C11)-arAlkAnoyl-N-(C1-C10)-
alkylamino,
(C1-C12)-alkanoylamino-(C1-C8)-alkyl, (C3-C8)-cyclo-
alkanoylamino-(C1-C8)-alkyl, (C6-C12)-aroylamino-
(C1-C8)-alkyl, (C7-C16)-aralkanoylamino-(Cl-C8)-
alkyl, amino-(C1-C1O)-alkyl, N-(C1-C1O)-alkylamino-
(C1-C1O)-alkyl, N,N-di-(C1-C1O)-alkylamino-(Cl-ClO)-
alkyl, (C3-C8)-cycloalkylamino-(C1-C1O)-alkyl,
(Cl-Cl2)-alkymercapto, (Cl-C12)-alkylsulfinyl,
(Cl-C12)-alkylsulfonyl, (C6-C16)-arylmercapto,
(C6-C16)-arylsulfinyl, (C6-C16)-aryl~ulfonyl,
(C7-C16)-aralkylmercapto, (C7-C16)-aralkylsulfinyl,
(C7-C16)-aralkylsulfonyl,
R7is hydrogen, (C1-C8)-alkYl~ (C6-C12)-arYl~ (C7 C11)
aralkyl, heteroaryl,
20 where the above radicals can carry 1, 2 or 3 identical or
different ~ubstituents from the series
fluorine, chlorine, trifluoromethyl, nitrile,
(Cl-C6)-alkyl, (Cl-C6)-alkoxy, (Cl~c6)-alkyl-
carbamoyl, di-(C1-C6)-alkylcarbamoyl, (C3-C6)-cyclo-
alkylcarbamoyl, (C1-C6)-alkoxycarbonyl,
R4 iB a branched or unbranched (C7-C20)-alkyl radical,
a (C6-C16)-aryl radical, a (C7-C16)-aralkyl radical,
a heteroaryl radical or a heteroaralkyl radical,
where theRe radicals are sub~tituted by one or more
30 radicals from the series
(Cl-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
(C6-C12)-arylcarbonyl, (C7-C16)-aralkylcarbonyl,
c innamoyl, ( C2 - C12 ) - alkenylcarbonyl,
2145473
- 20 -
(C2-C12)-alkynylcarbonyl,
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (Cl-C12)-alkyl, (C3-C8)-cycloalkyl, (C3-C8)-
cycloalkyl-(C1-Cl2)-alkyl, (C3-C8)-cycloalkoxy, (C3-C8)-
cycloalkyl-(C1-C12)-alkoxy, (C3 -C8) -cycloalkyloxy-(C1-C12)-
alkyl, (C3-C8)-cycloalkyloxy-(C1-C12)-alkoxy, (C3-C8)-
cycloalkyl-(C1-C8)-alkyl-(C1-C6)-alkoxy, (C3-C8)-cyclo-
alkyl-(C1-C8)-alkoxy-(C1-C6)-alkyl,(C3-C8)-cycloalkyloxy-
( 1 C8) alkoxy_(C1-C8)-alkyl,(C3-C8)-cycloalkoxy-(C1-C8)-
alkoxy-(C1-C8)-alkoxy, (C6-C12)-aryl, (C7-C16)-aralkyl,
(C2-Cl2)-alkenyl, (C2-C12)-alkynyl, (Cl-Cl2)-alkoxy,
(cl-cl2)-alkoxy-(cl-cl2)-alkyl~ (Cl-cl2)-alkoxy-(cl-cl2)
alkoxy, (C1-C12)-alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkyl,
(C6-C12)-aryloxy, (C7-C16)-aralkyloxy, (C6-C12)-aryloxy-
(C1-C6)-alkoxy, (C7-C16)-aralkoxy-(C1-C6)-alkoxy, (C1-C8)-
hydroxyalkyl, (c6-cl6)-aryloxy-(cl-c8)-alkyl~ (C7~cl6)~
aralkoxy-(C1-C8)-alkyl, (C6-C12)-aryloxy-(C1-C8)-alkoxy-
(cl-c6)-alkyl, (c7-cl2)-aralkyloxy-(cl-c8)-alkoxy-(cl-c6)
alkyl~ -~[CH2]xcfH(2f~l g)Fg~ -OCF2Cl, -OCF2-CHFCl,
(C1-C12)-alkylcarbonyl, ( C3 - C8 ) - CyC loalkylcarbonyl,
(C6-C12)-arylcarbonyl, (C7 - Cl6 ) - aralkylcarbonyl,
cinnamoyl, (C2-C12)-alkenylcarbonyl, (C2-C12)-alkynyl-
carbonyl,
(C1-C12)-alkoxycarbonyl, (C1-C12)-alkoxy-5C1-C12)-alkoxy-
carbonyl, (C6-C12)-aryloxycarbonyl, ( C7 -C16)-aralkoxycar-
bonyl, (C3-C10)-cycloalkoxycarbonyl, (C2-C12)-alkenyloxy-
carbonyl, (C2-C12)-alkynyloxycarbonyl,
(Cl-Cl2)-alkylcarbonyloxy, (C3-C8)-cycloalkylcarbonyloxy,
(C6-Cl2)-arylcarbonyloxy, (C7-Cl6)-aralkylcarbonyloxy,
c;nn~moyloxy, (C2-C12)-alkenylcarbonyloxy, (C2-C12)-
alkynylcarbonyloxy,
(C1-C12)-alkoxycarbonyloxy, (cl-cl2)-alkoxy-(cl-cl2)
alkoxycarbonyloxy, (C6-C12)-aryloxycarbonyloxy, (C7-C16)-
aralkyloxycarbonyloxy, (C3-C8)-cycloalkoxycarbonyloxy,
2145 ~73
- 21 -
(C2-C12)-alkenyloxycarbonyloxy, (C2-Cl2)-alkynyloxy-
carbonyloxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-(C1-C12)-
alkylcarbamoyl, N-( C3-C8) -CyC loalkylcarbamoyl, N,N-
dicyclo-( C3-C8) - alkylcarbamoyl, N-(cl-clo)-alkyl-N-
(C3-C8)-cycloalkylcarbamoyl, N-((C3-C8)-cycloalkyl-
(C1-C6)-alkyl)carbamoyl, N-(C1-C6)-alkYl~N~((C3~cs)
cycloalkyl-(C1-C6)-alkyl)carbamoyl, N-(+)-dehydroabietyl-
carbamoyl, N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N-(C7-C16)-aralkylcarbamoyl, N-
(C1-C10)-alkyl-N-(C6-C16)-arylcarbamoyl, N-(C1-C10)-alkyl-
N-(C7-C16)-aralkylcarbamoyl, N-((C1-C10)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-((c6-cl6)-aryloxy-(cl-clo)
alkyl)carbamoyl, N-((c7-cl6)-aralkyloxy-(cl-clo)
alkyl)carbamoyl, N~(C1~C1o)~alkYl-N-((C1-C10)-alkoxy_
(C1-C10)-alkyl)carbamoyl, N-(cl-clo)-alkyl-N-((c6 c12)
aryloxy-(C1-C10)-alkyl)carbamoyl, N-(C1-C10)-alkyl_N_
((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyl, CON(CH2)h,
in which a CH2 group can be replaced by O, S, N-(C1-C8)-
alkylimino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-cyclo-
alkyl-(C1-C4)-alkylimino, N-(C6-C12)-arylimino, N-(C7-C16)-
aralkylimino or N-(C1-C4)-alkoxy-(C1-C6)-alkylimino and h
i~ 3 to 6, or by
a carbamoyl radical of the formula II
RX H
--CO NR ~ ~ ( I I ) .
O
5 in which
Rx is the ~ubstituent of an ~-amino acid which
includes the L- and D-amino acids,
s i~ 1, 2, 3, 4 or 5 and
T is OH, OR or NR R ,
- 2145473
- 22 -
R being hydrogen or (Cl-C4)-alkyl,
R and R being identical or different and being
hydrogen, (C6-Cl2)-aryl, ( C7 - Cll ) - aralkyl,
(Cl-C8)-alkyl, (C3-C8)-cycloalkyl, (+)-dehydro-
abietyl, (Cl-C8)-alkoxy-(Cl-C8)-alkyl, (C7-Cl2)-
aralkoxy-(Cl-C8)-alkyl, (C6-Cl2)-aryloxy-
(Cl-C8)-alkyl, (Cl-C10)-alkanoyl, optionally
substituted (C7-Cl6)-arAlkAnoyl, optionally
~ubstituted (C6-Cl2)-aroyl, or
R and R together are -[CH2] h~ in which a CH2 group
can be replaced by O, S, SO, SO2, N-acylimino,
N-(Cl-C10)-alkoxycarbonylimino, N-(Cl-C8)-alkyl-
imino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-
cycloalkyl-(Cl-C4)-alkylimino, N-(C6-Cl2)-aryl-
imino, N-(C7-Cl6)-aralkylimino or N-(Cl-C4)-
alkoxy-(Cl-C6)-alkylimino and h is 3 to 7, or
are substituted by
carbamoyloxy, N-(Cl-Ci2)-alkylcarbamoyloxy, N,N-di-
(Cl-Cl2)-alkylcarbamoyloxy, N-( C3 - C8 ) - CyC loalkylcar-
bamoyloxy, N-(C6-Cl2)-arylcarbamoyloxy, N-(C7-Cl6)-
aralkylcarbamoyloxy, N-(Cl-Clo)-alkYl-N-(C6-cl2)-
arylcarbamoyloxy, N-(Cl-C10)-a-lkyl-N-( C7 - C16 ) -
aralkylcarbamoyloxy, N-(Cl-C10)-alkylcarbamoyloxy,
N-((C6-Cl2)-aryloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
((C7-Cl6)-aralkyloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
(Cl-C10)-alkyl-N-((Cl-ClO)-alkoxy-(Cl-ClO)-alkyl)-
carbamoyloxy, N-(Cl-C10)-alkyl-N-((C6-Cl2)-aryloxy-
(Cl-C10)-alkyl)carbamoyloxy, N-(cl-clo)
((C7-Cl6)-aralkyloxy-(Cl-C10)-alkyl)carbamoyloxy,
amino, (Cl-Cl2)-alkylamino, di-(Cl-Cl2)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-Cl2)-alkenylamino,
(C3-Cl2)-alkynylamino, N-(C6-Cl2)-arylamino, N-
(C7-Cll)-aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (Cl-Cl2)-alkoxyamino, (Cl-Cl2)-
alkoxy-N-(Cl-C10)-alkylamino,
2145~73
-
- 23 -
(Cl-C12) -Al kAnoylamino, (C3-c8) -cycloalkanoylamino,
(C6-Cl2)-aroylamino, (C7-C16)-aralkanoylamino,
(C1-C12)-AlkAnoyl-N-(C1-C10)-alkylamino, (C3-C8)-
cycloalkanoyl-N-(C1-C10)-alkylamino, (C6-C12)-aroyl-
N-(C1-C10)-alkylamino, (C7-Cl1)-arAlkAnoyl-N-(Cl-C10)-
alkylamino,
(Cl-C12) -Al kAnoylamino- (Cl-c8) -alkyl, (C3 -C8) -cyclo-
alkanoylamino-(C1-C8)-alkyl, (C6-C12)-aroylamino-
(C1-C8)-alkyl, ( C7 - Cl6 ) - aralkanoylamino-(Cl-C8)-
lkyl~ amino-(Cl-C10)-alkyl, N-(cl-clo)-alkylamino-
(C1-C10)-alkyl, N,N-di-(C1-C10)-alkylamino-(Cl-ClO)-
alkyl, ( C3 - C8 ) - cycloalkylamino-(C1-C10)-alkyl,
(Cl-C12)-alkylmercapto, (Cl-C12)-alkylsulfinyl,
(Cl-C12)-alkylsulfonyl, (C6-C12)-arylmercapto,
(C6-C12)-arylsulfinyl,
(C6-Cl2)-arylsulfonyl, (C7-Cl6)-aralkylmercapto,
(C7-C16)-aralkylsulfinyl, (C7-C16)-aralkylsulfonyl,
sulfamoyl, N-(C1-C10)-alkylsulfamoyl, N,N-di-
(C1-C10)-alkylsulfamoyl, (C3-C8)-cycloalkylsulfamoyl,
N-(c6-cl2)-arylsulfamoyl~ N-(C7-C16)-aralkyl-
sulfamoyl, N-(C1-C10)-alkyl-N-(C6-Cl2)-arylsulfamoyl,
N-(C1-C10)-alkyl-N-(C7-Cl6)-aralkylsulfamoyl,
(C1-C10)-alkyl-sulfonamido, N-(C1-C10)-alkyl-(Cl-ClO)-
alkylsulfonamido, (C7-C16)-aralkylsulfonamido or N-
(C1-C10)-alkyl-(C7-Cl6)-aralkylsulfonamido, wherethe
radicals which contain an aryl radical can for their
part be substituted on the aryl by 1 to 5 identical
or different radicals from the series:
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (C1-C12)-alkyl, ( C3 - C8 ) - CyC loalkyl,
(C6-C12)-aryl, (C7-Cl6)-aralkyl, (Cl-C12)-alkoxy,
(cl-cl2)-alkoxy-(cl-cl2)-alkyl~ (C1-C12)-alkoxy-
(Cl-Cl2)-alkoxy, (C6-Cl2)-aryloxy, (C7-C16)-
aralkyloxy, (C1-C8)-hydroxyalkyl,
(C1-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
21~S473
- 24 -
(C6-C12)-arylcarbonyl, (C7-C16)-aralkylcarbonyl,
(C1-C12)-alkoxycarbonyl, (cl-cl2)-alkoxy-(cl-cl2)-
alkoxycarbonyl, (C6-C12)-aryloxycarbonyl, (C7-C16)-
aralkoxycarbonyl, ( C3-C8)-CyC loalkoxycarbonyl;
(C2-C12)-alkenyloxycarbonyl, (C2-C12)alkynyloxy-
carbonyl,
(Cl-Cl2)-alkylcarbonyloxy, (C3-C8)-cycloalkylcarbony-
loxy, (C6-C12)-arylcarbonyloxy, (C7-C16)-aralkylcar-
bonyloxy, cinnamoyloxy, (C2-Cl2)-alkenylcarbonyloxy,
1 ( C2 - Cl2 ) - alkynylcarbonyloxy,
(C1-C12)-alkoxycarbonyloxy, (Cl-C12)-alkoxy-(C1-C12)-
alkoxycarbonyloxy, (C6-C12)-aryloxycarbonyloxy,
(C7-C16)-aralkyloxycarbonyloxy, (C3-C8)-cycloalkoxy-
carbonyloxy, (C2-C12)-alkenyloxycarbonyloxy,
(C2-C12)-alkynyloxycarbonyloxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl, N-
(C1-C10)-alkyl-N- (C3 - C8 ) - cycloalkylcarbamoyl, N-
((C3 -C8) -cycloalkyl-(C1-C6)-alkyl)carbamoyl, N-
(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl, N-
(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl, N-
(C6-C12)-arylcarbamoyl, N-( C7 -C16)-aralkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C6-Cl6)-arylcarbamoyl, N-(C1-C1o)~
alkyl-N-(C7-C16)-aralkylcarbamoyl, N-((Cl-clo)-
alkoxy-(C1-C10)-alkyl)carbamoyl, N-((C6-C16)-aryloxy-
(C1-C10)-alkyl)carbamoyl, N-((C7 -C16)-aralkyloxy-
(C1-C10)-alkyl)carbamoyl, N-(cl-clo)-alkyl-N-
((C1-C10)-alkoxy-(Cl-ClO)-alkyl)carbamoyl, N-(C1-C1o)~
alkyl-N-((c6-cl2)-aryloxy-(cl-clo)-alkyl)carbam
N-(cl-clo)-alkyl-N-((c7-cl6)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl, CON(CH2)h in which a group CH2 can
be replaced by O, S, N-(C1-C8)-alkylimino, N-(C3-C8)-
c y c l o a l k y l i m i n o
2145473
-
- 25 -
N-(C3-C8)-cycloalkyl-(Cl-C4)-alkylimino, N-(C6-Cl2)-
arylimino, N-(C7-Cl6)-aralkylimino or N-(Cl-C4)-
alkoxy-(Cl-C6)-alkylimino and h i~ 3 to 7,
carbamoyloxy, N-(C1-C12)-alkylcarbamoyloxy, N,N-di-
(Cl-Cl2)-alkylcarbamoyloxy, N-(C3-C8)-cycloalkylcar-
bamoyloxy, N-(C6-C16)-arylcarbamoyloxy, N-(C7-Cl6)-
aralkylcarbamoyloxy, N-(Cl-Clo)-alkYl-N-(C6-cl2)-
arylcarbamoyloxy, N-(Cl-Clo)~alkYl N ( 7 16
aralkylcarbamoyloxy, N-(Cl-C10)-alkyl)carbamoyloxy,
N-((C6-Cl2)-aryloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
( (C7 -Cl6) -aralkyloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
(Cl-C10)-alkyl-N-((Cl-ClO)-alkoxy-(Cl-ClO)-alkyl)-
carbamoyloxy, N-(Cl-C10)-alkyl-N-((C6-Cl2)-aryloxy-
(Cl-C10)-alkyl)carbamoyloxy, N-(Cl-Cl0)-alkyl-N-
((C7-Cl6)-aralkyloxy-(Cl-C10)-alkyl)carbamoyloxy,
amino, (Cl-Cl2)-alkylamino, di-(Cl-Cl2)-alkylamino,
(C3-C8)-cyc-loalkylamino, (C3-C12)-alkenylamino,
(C3-Cl2)-alkynylamino, N-(C6-Cl2)-arylamino, N-
(C7-Cll)-aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (Cl-Cl2)-alkoxyamino, (Cl-Cl2)-
alkoxy-N-(Cl-C10)-alkylamino,
(Cl-Cl2)-alkanoylamino, (C3-C8)-cycloAl~Anoylamino,
(C6-Cl2)-aroylamino, (C7-C16)-aralkanoylamino,
(Cl - C12 ) - A 1 kAnoyl -N-(Cl-C10)-alkylamino, (C3-C8)-
cycloalkanoyl-N-(Cl-C10)-alkylamino, (C6-C12)-aroyl-
N-(Cl-C10)-alkylamino, (C7-Cll)-arAlkAnoyl-N-(Cl-C10)-
alkylamino,
(Cl-Cl2)-alkanoylamino-(Cl-C8)-alkyl, (C3-C8)-cyclo-
alkanoylamino-(Cl-C8)-alkyl, (C6-Cl2)-aroylamino-
(Cl-C8)-alkyl, (C7-Cl6)-aralkanoylamino-(Cl-C8)-
alkyl, amino-(Cl-C10)-alkyl, N-(Cl-C10)-alkylamino-
(Cl-C10)-alkyl, N,N-di-(Cl-C10)-alkylamino-(Cl-ClO)-
alkyl, (C3-C8)-cycloalkylamino-(Cl-C10)-alkyl,
(Cl-C12)-alkymercapto, (Cl-C12)-alkyl~ulfinyl,
21~5473
- 26 -
(Cl-C12)-alkylRulfonyl,(C6-C16)-arylmercapto,
(C6-C16)-arylsulfinyl,(C6-Cl6)-aryl~ulfonyl,
(C7-C16)-aralkylmercapto,(C7-Cl6)-aralkyl~3ulfinyl,
(C7-Cl6)-aralkylsulfonyl,
Rl and R4 can form a chain [CH2]o~ in which one or two CH2
groups of the chain which is saturated or unsatu-
rated by a C=C double bond are optionally replaced
by O, S, SO, SO2 or NR', o = 3, 4 or 5 and
RY iS (C6-C12)-aryl, (C5-C8)-alkyl, (Cl-C8)-alkoxy-
10(C1-C8)-alkyl, (C7-C12)-aralkoxy-(C1-C8)-alkyl,
(C6~Cl2)~arYloxy-(cl-c8)-alkyl~ (C1-C10)-alkanoyl,
optionally substituted (C7-Cl6)-arAlkAnoyl, or
optionally substituted (C6-Cl2)-aroyl.
If Q is NRY, R4 is RZ, RY and RZ being identical or
15different and being (C6-Cl2)-aryl, (Cl-C8)-alkyl, (C1-C8)-
alkoxy-(Cl-C8)-alkyl, (C7-Cl2)-aralkoxy-(Cl-C8)-alkyl,
(C -Cl2)-aryloxy-(Cl-C8)-alkYl, (Cl-C10) alkanoY ~ P
nally substituted (C7-Cl6)-arAlkAnoyl~ or optionally
substituted (C6-C12)-aroyl, or
RY and RZ together represent -~CH2] h~ in which a CH2 group
can be replaced by O, S, N-acylimino or N-(C1-C10)-alkoxy-
carbonylimino, and
f = 1 to 8,
g = 0, 1 to (2f + 1),
x = 0 to 3,
h = 3 to 6.
Aryl, aryloxy, heteroaryl and heteroaryloxy compounds are
understood in particular as meAn;ng phenyl, biphenyl or
naphthyl or un~ubstituted 5- and 6-membered heteroarom-
atic rings having 1, 2 or 3 nitrogen and/or oxygen and/orsulfur atoms, ~uch as pyridyl, pyridazyl, pyrimidyl,
pyrazolyl, imidazolyl, triazolyl, thienyl, oxazolyl and
thiazolyl derivatives, and their benzo-fused derivatives,
2145~73
- 27 -
and halogen is understood as me~n;ng fluorine, chlorine,
bromine and iodine, in particular fluorine, chlorine and
bromine.
The invention furthermore comprises salts of the com-
pounds of the formula 1.
Mono-, di- or trisalts with basic reagents can be formed
in the acidic groups of the compounds of the formula 1,
in particular in the radicals B, R2 and R4.
Reagents used are, for example, alkoxides, hydroxides,
carbonates, hydrogen carbonates, hydrogen phosphates,
organometallic compounds of the alkali metal and alkaline
earth metal elements, the element~ of the 3th and 4th
main group of the Periodic Table and the elements of the
transition metals.
Amines, optionally substituted 1 to 3 times by (Cl-C8)-
hydroxyalkyl, (C1-C4)-alkoxy-(Cl-C8)-alkyl, phenyl,benzyl
or (Cl-C8)-alkyl, which for its part can be substituted 1
to 3 times by hydroxyl or (Cl-C4)-alkoxy,
for example tromethane (tris buffer), 2-aminoethanol, 3-
aminopropanol, hydroxylamine, dimethylhydroxylamine, 2-
methoxyethylamine, 3-ethoxypropylamine, and
basic amino acids and amino acid derivatives, such as
amino acid esters, histidine, arginine and lysine and
their derivatives, and also
pharmaceuticals which contain a basic group, ~uch as, for
example, ~Amilorid, ~Verapamil and beta blockers.
The invention furthermore relates to the compounds of
formula 1, for use as pharmaceuticals.
Preferred compounds of the formula 1 are those in which
Q = 0,
X = O,
Y = N or CR3,
m = 0 or 1,
214547~
- 28 -
A is (C1-C3)-alkylene which can optionally be ~ubsti-
tuted once or twice by . halogen, cyano,
tri f luoromethyl, ( Cl - C6 ) - alkyl, ( C1 - C6 ) -hydroxyalkyl,
(C1-C6) -alkoxy, -O- ~CH2] x-CfH(2f 11-g) Fg or
5 A is -CHRX-, where Rx i8 one of the substituents of
the ~-C atom of an cY-amino acid, in particular of a
natural L-amino acid and of its D-isomer,
B is -CO2V, where
V iB the radical of an alcohol VOH, in which V is
(C1-C10) -acyloxy- (C1-C6) -alkyl, preferably (C1-C10) -
alkanoyloxy- (C1-C6) -alkyl, benzyloxy- (C1-C6) -alkyl,
benzyloxycarbonyloxy - ( C1 - C6 ) - alkyl or
( C1 - C6 ) - alkoxycarbonyloxy- ( C1 - C6 ) - alkyl, or
a branched, unbranched or cyclic aliphatic (C1-C20)-
alkyl radical, or
a branched or unbranched (C2-C20)-alkenyl radical, a
retinyl radical, or
a (C2-C12)-alkynyl radical which in each case can
contain one or more C-C multiple bonds, or
a (C6-C12)-aryl radical, a (C7-C1l)-aralkyl radical
or a heteroaryl or heteroaralkyl radical,
where the above radicals can carry one or two sub-
stituents from the series (C1-C8)-alkyl, (C3-C8)-
cycloalkyl, fluorine, chlorine, hy(lroxyl, (Cl-C6)-
alkoxy, (C1-C6) -alkoxy- (C1-C6) alkoxy, (C6-C12) -
aryloxy, (C7-C12)-aralkyloxy,
(C1-C8)-alkylcarbonyl, (C3 -C8)-cycloalkylcarbonyl,
(C6-Cl2)-arylcarbonyl, (C7-C12)-aralkylcarbonyl,
(Cl-C8) - alkoxycarbonyl, (C1-C6) -alkoxy- (C1-C 6) -
alkoxycarbonyl, (C6-C12)-aryloxycarbonyl, (C7-C12)-
aralkoxycarbonyl, ( C3 - C8 ) - CyC 10A 1 koxycarbonyl,
(Cl-C8)-alkylcarbonyloxy, (C3-C8)-cycloalkylcarbonyl-
oxy, ( C6 - C12 ) - arylcarbonyloxy,
2145473
- 29 -
(C7-C12)-aralkylcarbonyloxy,
(C1-CB)-alkoxycarbonyloxy, (cl-c6)-alkoxy-(cl-c6)
alkoxycarbonyloxy, (C6-C12)aryloxycarbonyloxy,
(C7-C12)aralkyloxycarbonyloxy, (C3-C8)-cycloalkoxy-
carbonyloxy,
carbamoyl, N-(C1-C8)-alkylcarbamoyl, N,N-di-(C1-C8)-
alkylcarbamoyl, N-(C3-C8)-cycloalkylcarbamoyl,
N-((C1-C6)-alkoxy-(C1-C6)-alkyl)carbamoyl,
amino, (C1-C6)-alkylamino, di-(C1-C6)-alkylamino,
(C3-C8)-cycloalkylamino, N-(C6-C12)-arylamino, N-
(C7-C11)-aralkylamino, N-(cl-c5)-alkyl-(c6-cl2)
arylamino,
(Cl-C8)-alkanoylamino, (C3-C8)-cycloalkanoylamino,
(C6-C12)-aroylamino, (C7-C12)-aralkanoylamino,
(C1-C8)-alkanoyl-N-(C1-C6)-alkylamino, (C3-C8)-cyclo-
k~noyl-N-(C1-C6)-alkylamino, (C6-C12)-aroyl-N-
(C1-C6)-alkylamino, (C7-C11)-aralkanoyl-N-(C1-C6)-
alkylamino,
where the radicals which contain an aryl radical are
in particular substituted by up to 3 substituents
from the series
hydroxyl, fluorine, chlorine, cyano, trifluoro-
methyl, (C1-C6)-alkyl, (C3-C8)-cycloalkyl, (C1-C8)-
alkoxy,
(C1-C6)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
(C1-C6)-alkoxycarbonyl, (C3-C8)-cycloalkoxycarbonyl,
(Cl-C6)-alkylcarbonyloxy, (C3-C8)-cycloalkyl-
carbonyloxy,
(Cl-C6)-alkoxycarbonyloxy, (C3-C8)-cycloalkoxy-
- 2145 473
- 30 -
carbonyloxy,
carbamoyl, N-(Cl-C6)-alkylcarbamoyl,
N,N-di-(C1-C6)alkylcarbamoyl, N-(C3-C8)
cycloalkylcarbamoyl,
N-((Cl-C6)-alkoxy-(Cl-C6)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((Cl-C6)-alkoxy-(Cl-C6)-
alkyl)carbamoyl,
carbamoyloxy, N-(Cl-C6)-alkylcarbamoyloxy, N,N-di-
(Cl-C6)-alkylcarbamoyloxy, N- (C3 -C8 ) -cycloalkyl-
carbamoyloxy,
(Cl-C6)-alkanoylamino, (C3-C8)-cycls~lkAnoylamino,
(Cl-C6)alkylmercapto, (Cl-C6)-alkylsulfinyl, (Cl-C6)-
alkylsulfonyl and
Rl and R3 are hydrogen,
R2 is a radical -Co-NR5R6, where
R5 is hydrogen, (Cl-C6)-alkyl or an N-protective group
such as (Cl-C8)-~lk~noyl, (Cl-C6)-alkylcarbamoyl,
(Cl-C6)-alkoxycarbonyl, benzyloxycarbonyl, (Cl-C10)-
acyloxy-(Cl-C6)-alkyl, preferably (Cl-C1O)-alkanoyl-
oxy-(Cl-C6)-alkyl, benzoyloxy-(Cl-C6)-alkyl, benzyl-
oxycarbonyloxy-(Cl-C6)-alkyl or (Cl-C6)-alkoxycar-
bonyloxy-(Cl-C6)-alkyl, a 1-, 2-, 3- or 4-valent
physiologically utilizable cation, in particular
Na~, R~, Mg2~, Ca2~, Al3~ or an ammonium ion, option-
ally ~ubstituted 1-3 times by (Cl-C8)-hydroxyalkyl,
(Cl-C4)-alkoxy-(Cl-C8)-alkyl, phenyl, benzyl or
(Cl-C8)-alkyl, which for its part can be substituted
1-3 times by hydroxyl or (Cl-C4)-alkoxy or a cation
of a basic amino acid derivative,
R6 iB a radical of the formula I, excluding -SO2H,
-G-R-~C-U] r -D -W ( I )
in which
G is -SO2- or -CO-,
2195473
- 31 -
K is a bond,
C i8 a bond or
a branched or unbranched aliphatic (C1-Cl2)-
alkanediyl radical, or
a branched or l~nhrAnche~ (C2-C12)-alkenediyl
radical,
a (C2-C12)-alkynediyl radical or a (C2-C12)-
alkenynediyl radical which can contain one or
more C-C multiple bonds,
U is a bond, hydrogen or a radical from the
series of following heteroatom groups
-(CO)NR-, -NR(CO)-, -O-, -SO-, -SO2, in which
R is (C2-C3)- alkyl or hydrogen,
r is 1 or 2,
D is a bond, hydrogen or
a branched or unbranched aliphatic (C1-C8)-
alkanediyl radical,
a branched or unbranched (C2-C8)-alkenediyl
radical or
(C2-C8)-alkynediyl radical and
W is a bond, hydrogen or
a (C3-C10)-cycloaliphatic alkyl, alkenyl,
alkynyl or alkenynyl radical,
a (C6-C16)-aryl radical or a 5- or 6-membered
heteroaryl radical, where at least one of the
variables C, D or W is not a bond and U is only
a heteroatom group if C i8 not a bond or if D
and/or W are not a bond and
C, D and/or W, if these are not a bond or hydrogen,
preferably are for their part substituted by a
combination of up to 5 identical or different
substituents from the series
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (C1-C12)-alkyl, (C3-C8)-cycloalkyl,
(C 3 - C 8 ) - C y C 1 0 a l k y l - (C1- C 12 ) - a l k y l ~
( C3 - C8 ) - cycloalkoxy, (C3 - C8 ) - CyClOalkYl - (Cl - C12 ) -
alkoxy, ( C3 - C8 ) - cycloalkyloxy-(C1-C12)-alkyl,
(C3-C8)-cycloalkyloxy-(C1-C12)-alkoxy,(C3-C8)-cyclo-
alkyl-(C1-C8)-alkyl-(C1-C6)-alkoxy, (C3-C8)-cyclo-
2145~73
- 32 -
alkyl-(cl-c8)-alkoxy-(cl-c6)-alkyl~ (C3-C8)-cyclo-
alkyloxy-(C1-C8)-alkoxy-(C1-C6)-alkyl, (C3 -C8) -cyclo-
alkoxy-(cl-c8)-alkoxy-(cl-c8)-alkoxy, (C6-C12)-
(C7-C16)-aralkyl, (C2-C12)-alkenyl, (C2-C12)-alkynyl,
(Cl-Cl2)-alkoxy, (cl-cl2)-alkoxy-(cl-cl2)-alkyl~
(cl-cl2)-alkoxy-(cl-cl2)-alkoxy~ (Cl-Cl2)-alkoxy-
(cl-c8)-alkoxy-(cl-c8)-alkyl~ (C6-Cl2)-aryloxy,
(C7 - Cl6 ) -aralkyloxy,(C6-Cl2)-aryloxy-(Cl-C6)-alkoxy,
(C7-C16) -aralkoxy- (Cl-C6) -alkoxy (Cl-C8) -
hydroxyalkyl, (C6-C16)-aryloxy (cl-c8)-alkyl~
(C7 -C16) -aralkoxy-(Cl-C8)-alkyl, (C6-Cl2)-aryloxy-
(cl-c8)-alkoxy-(cl-c6)-alkyl~ ( C7 -C12)-aralkyloxy-
(C1-C8)-alkoxy-(C1-C6)-alkyl, ~O~[CH2]xCfH(2f+l g1Fg,
-OCF2Cl, -OCF2-CHFCl,
(C1-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
( C 6 - Cl2 ) - arylcarbonyl, ( C7 -C16)-aralkylcarbonyl,
C;nnAm~yl, (C2-Cl2)-alkenylcarbonyl, (C2-Cl2)-
alkynylcarbonyl,
(C1-C12)-alkoxycarbonyl, (cl-cl2)-alkoxy-(cl-cl2)-
alkoxycarbonyl, (C6-C12)-aryloxycarbonyl, ( C7 - Cl6 ) -
aralkoxycarbonyl, (C3-C10)-cycloalkoxycarbonyl,
(C2-C12)-alkenyloxycarbonyl, (C2-Cl2)-alkynyloxy-
carbonyl,
(Cl-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkylcarbony-
loxy, (C6-Cl2)-arylcarbonyloxy, (C7-Cl6)-aralkyl-
carbonyloxy, c;nnA~oyloxy, (C2-Cl2)-alkenyl-
carbonyloxy, (C2-Cl2)-alkynylcarbonyloxy,
carbamoyl, N-(Cl-Cl2)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-( C3 - C8 ) - cyc loalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl, N-
(C1-C1O)-alkyl-N-( C3 - C8 ) - CyC loalkylcarbamoyl, N-
( ( C3 - C8 ) - CyC loalkyl-(Cl-C6)-alkyl)carbamoyl, N-
(Cl-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl, N-
(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
214547~
- 33 -
N-(C6-C12)-arylcarbamoyl, N-(C7-C16)-aralkylcar-
bamoyl~ N-(cl-clo)-alkyl-N-(c6-cl6)-arylcarbam
N-(C1-C10)-alkyl-N-(C7-C16)-aralkylcarbamoyl,
N-((C1-C10)-alkoxy-(Cl-C10)alkyl)carbamoyl, N-
((c6-cl6)-aryloxy-(cl-clo)alkyl)carbamoyl~ N-
((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyl, N-
(C1-C10)-alkyl-N-((C1-C10)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-(cl-clo)-alkyl-N-((c6-cl2)
aryloxy-(C1-C10)-alkyl)carbamoyl,N-(C1-C10)-alkyl-N-
((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyl,
CON(CH2) h~ in which a CH2 group can be replaced by
O, S, N-(C1-C8)-alkylimino, N-(C3-c8)
cycloalkylimino, N-(C3-C8)-cycloalkyl-(C1-C4)-alkyl-
imino, N-(C6-C12)-arylimino, N-(C7-C16)-aralkylimino
or N-(C1-C4)-alkoxy-(C1-C6)-alkylimino and h is 3
to 6, or by
a carbamoyl radical of the formula II
RX H
\/
--C O--N R ~
S
in which
Rx is the substituent of an ~-amino acid which
includes the L- and D-amino acids,
8 is 1, 2, 3, 4 or 5 and
T is OH, OR or NR R ,
R being hydrogen or (C1-C4)-alkyl,
R and R being identical or different and being
hydrogen, (C6-C12)-aryl, (C7-C11)-aralkyl,
(C1-C8)-alkyl, (C3-C8)-cycloalkyl, (+)-dehydro-
abietyl~ (C1-C8)-alkXY (C1-C8)-alkYl~ (C7-C12)-
aralkoxy-(C1-C8)-alkyl, (C6-C12)-aryloxy-
(C1-C8)-alkyl, (C1-C10)-alkanoyl, optionally
substituted (C7-C16)-arAlkanoyl, optionally
- 21gS473
- 34 -
substituted (C6-Cl2)-aroyl, or
R and R together are -[CH2] h~ in which a CH2 group
can be replaced by O, S, SO, SO2, N-acylimino,
N-(C1-C10)-alkoxycArhQnyl;~;no, N-(Cl-C8)-alkyl-
imino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-
cycloalkyl-(Cl-C4)-alkylimino, N-(C6-Cl2)-aryl-
imino, N-(C7-C16)-aralkylimino or N-(Cl-C4)-
alkoxy-(Cl-C6)-alkylimino and h i~ 3 to 7, or
are substituted by
carbamoyloxy, N-(Cl-Cl2)-alkylcarbamoyloxy, N,N-di-
(Cl-Cl2)-alkylcarbamoyloxy, N- (C3 -C8)-cycloalkylcar-
bamoyloxy, N-(C6-Cl2)-arylcarbamoyloxy, N-(C7-Cl6)-
aralkylcarbamoyloxy, N-(C1-Clo)-alkYl-N-(C6-cl2)-
arylcarbamoyloxy, N (Cl-clo)-alkyl-N-(c7-cl6)
aralkylcarbamoyloxy, N-(Cl-C10)-alkylcarbamoyloxy,
N-~(c6-cl2)-aryloxy-(cl-clo)-alkyl)carbamoyloxy~ N-
((C7-Cl6)-aralkyloxy-(Cl-C10)-alkyl)carbamoyloxy, N-
(Cl-ClO)-alkyl-N-((Cl-ClO)-alkoxy-(Cl-ClO)-
alkyl)carbamoyloxy~ N-(Cl-C10)-alkyl N ( (C6 12)
aryloxy-(Cl-C10)-alkyl)carbamoyloxy, N-(cl-clo)
alkyl-N- ((C7-Cl6)-aralkyloxy- (Cl-C10)-
alkyl)carbamoyloxy,
amino, (Cl-Cl2)-alkylamino, di-(Cl-Cl2)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-C12)-alkenylamino,
(C3-Cl2)-alkynylamino, N-(C6-Cl2)-arylamino, N-
( C7 -Cll)-aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (Cl-Cl2)-alkoxyamino, (Cl-Cl2)-
alkoxy-N-(Cl-C10)-alkylamino,
(Cl-C12) _Al kAnoylamino, (C3-C8) -cycloAl kAnoylamino,
(C6-Cl2)-aroylamino, (C7-Cl6)-aralkanoylamino,
(Cl-Cl2)-AlkAnoyl-N-(Cl-C10)-alkylamino, (C3-C8)-
cycloalkanoyl-N-(Cl-C10)-alkylamino, (C6-Cl2)-aroyl-
N-(Cl-C10)-alkylA~;no, (C7-Cll)-arAlkAnQyl-N-(Cl-C10)-
alkylamino,
- 21~5473
- 35 -
(C1-C12)-alkanoylamino-(C1-C8)-alkyl, (C3 - C8 ) - cyclo-
alkanoylamino-(Cl-C8)-alkyl, (C6-Cl2)-aroylaminO-
(Cl-C8)-alkyl, ( C7 -Cl6)-aralkanoylamino-(Cl-C8)-
alkyl, amino-(Cl-C10)-alkyl, N-(Cl-C10)-alkylamino-
(C1-C10)-alkyl, N,N-di-(C1-C10)-alkylamino-(Cl-ClO)-
alkyl, ( C3 - C8 ) - cycloalkylamino-(Cl-C10)-alkyl,
(Cl-C12)-alkylmercapto,(Cl-C12)-alkylsulfinyl,
(Cl-C12)-alkyl~ulfonyl,(C6-C12)-arylmercapto,
( C 6 - C12 ) - aryl~ulfinyl, (C6-Cl2) -aryl~ulfonyl,
(C7-C16)-aralkylmercapto, (C7-C16)-aralkylsulfinyl,
(C7 -C16) -aralkyl8UlfOIlyl,
where the radical~ which contain an aryl radical can
for their part be ~ub~tituted on the aryl by 1 to 5
identical or different radicals from the ~erie~:
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (Cl-Cl2)-alkyl, ( C3-C8)-CyC loalkyl,
(C6-C12)-aryl, (c7-cl6)-aralkyl~ (Cl-Cl2)-alkoxy,
(cl-cl2)-alkoxy-(cl-cl2)-alkyl~ (cl-cl2)-alkoxy-
(Cl-Cl2)-alkoxy, (C6-C12)-aryloxy, (C7-C16)-
a r a 1 k y 1 o x y, ( C 1 - C 8 ) - h y d r o x y a 1 k y 1,
-O-[CH2]xCfH(2f+l g)Fg~ OCF2Cl, OCF2-CHFCl,
(Cl-C12)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
( C 6 - Cl2 ) - arylcarbonyl, ( C7 - Cl 6 ) - aralkylcarbonyl,
(C1-C12)-alkoxycarbonyl, (cl-cl2)-alkoxy-(cl-cl2)-
alkoxycarbonyl, (C6-Cl2)-aryloxycarbonyl, (C7-Cl6)-
aralkoxycarbonyl, (C3-C8)-cycloalkoxycarbonyl,
(C2-C12)-alkenyloxycarbonyl, (C2-C12)alkynyloxy-
carbonyl,
(Cl-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkylcarbony-
loxy, (C6-Cl2)-arylcarbonyloxy, (C7-Cl6)-aralkylcar-
bonyloxy, cinnamoyloxy, (C2-Cl2)-alkenylcarbonyloxy,
(C2-Cl2)-alkynylcarbonyloxy,
carbamoyl, N-(Cl-Cl2)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl,
- 21~5~73
- 36 -
N-(Cl-C10)-alkyl-N-(C3-C8)-cycloalkylcarbamoyl,
N-((C3 - C8 ) - cycloalkyl-(C1-C6)-alkyl)carbamoyl,
N-(Cl-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl;
N-(C6-Cl2)-arylcarbamoyl, N-( C7 - C16 ) - aralkyl-
carbamoyl,N-(C1-C10)-alkyl-N-(C6-Cl6)-arylcarbamoyl,
N-(cl-clo)-alkyl-N-(c7-cl6)-aralkylcarbamoyl~ N-
((cl-clo)-alkoxy-(cl-clo)-alkyl)carbamoyl~ N-
((c6-cl6)-aryloxy-(cl-clo)-alkyl)carbamoyl~ N-
( (C7 -cl6) -aralkyloxy-(C1-C10)-alkyl)carbamoyl, N-
(cl-clo)-alkyl-N-((cl-clo)-alkoxy-(cl-clo)-
alkyl)carbamoyl, N-(Cl-C1o)~alkyl-N-((C6_Cl2)_
aryloxy-(Cl-C10)-alkyl)carbamoyl,N-(Cl-ClO)-alkyl-N-
((C7-C16)-aralkyloxy-(Cl-C10)-alkyl)carbamoyl,
CON(CH2)h in which a CH2 group can be replaced by O,
S, N-(C1-C8)-alkylimino, N- (C3 -C8)-cycloalkylimino,
N-(C3-C8)-cycloalkyl-(Cl-C4)-alkylimino, N-(C6-Cl2)-
arylimino, N-(C7-Cl6)-aralkylimino or N-(C1-C4)-
alkoxy-(C1-C6)-alkylimino and h i~ 3 to 6,
amino, (C1-C12)-alkylamino, di-(C1-C12)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-Cl2)-alkenylamino,
(C3-C12)-alkynylamino, N-(C6-C12)-arylamino, N-
(C7-Cll)-aralkylamino, N-alkyl-aralkylamino, N-
alkyl-arylamino, (Cl-C12)-alkoxyamino, (Cl-Cl2)-
alkoxy-N-(C1-C10)-alkylamino,
(Cl-C12) -Al kAnoylamino, (C3-C8) -cycloalkanoylamino,
(C6-C12)-aroylamino, (C7-C16)-aralkanoylamino,
(Cl-C12)-alkanoyl-N-(Cl-C10)-alkylamino, ( C3 - C8 )
cycloAlkAnoyl-N-(C1-C1O)-alkylamino, (C6-C12)-aroyl-
N-(C1-C10)-alkyl A~; no~ (C7 -cll) -arAl kAnOyl -N-(C1-C1o)~
alkylamino,
(Cl-C12) -Al kAnoylamino- (cl-c8) -alkyl, (C3-C8)-cyclo-
alkanoylamino-(Cl-C8)-alkyl, (C6-C12)-aroylamino-
(C1-C8)-alkyl, (C7-C16)-aralkanoylamino-(Cl-C8)-
a l k y l , a m i n o - ( C 1 - C 1o ) - a l k y l ,
2145473
N-(Cl-C1O)-alkylamino-(Cl-ClO)-alkyl, N,N-di-(Cl-Clo)-
alkylamino-(Cl-C1O)-alkyl, ( C3 - C8 ) - cycloalkylamino-
(cl-clo)-alkyl~
(Cl-Cl2)-alkymercapto,(Cl-Cl2)-alkyl 8ul finyl,
(Cl-Cl2)-alkylsulfonyl,(C6-Cl6)-arylmercapto,
(C6-Cl6)-arylsulfinyl,(C6-C16)-arylsulfonyl,
(C7-C16)-aralkylmercapto,(C7-C16)-aralkylsulfinyl,
(C7 -Cl6) -aralkyl~ulfonyl,
R4 is a branched or unbranched (C7-C20)-alkyl radical
which can contain up to 3 C-C multiple bonds, or
(cl-cl2)-alkoxy-(cl-c8)-alkyl~ (cl-cl2)-alk
(C1-C8)-alkoxy-(Cl-C8)-alkyl, [CH2]XCfH(2f+l g) g,
( C 6 - C12 ) - aryl, ( C7 - Cll ) - aralkyl, heteroaryl,
heteroaralkyl, (C3-C8)-cycloalkyl, (C3-C8)-cyclo-
alkyl-(C1-C6)-alkyl, (C6-C14)-aryloxy-(C1-C8)-alkyl,
heteroaryloxy-(Cl-C8)-alkyl, (C3 - C8 ) - cycloalkyloxy-
(Cl-C8)-alkyl, ( C7 - Cl6 ) - aralkyloxy-(C1-C8)-alkyl,
heteroaralkyloxy-(C1-C8)-alkyl,
(C6-Cl4)-aryloxy-(Cl-C8)-alkoxy-(Cl-C8)-alkyl,
heteroalkyl-(Cl-C8)-alkoxy-(Cl-C8)-alkyl, (C3 - C8 ) -
cycloalkoxy-(Cl-C8)-alkoxy-(Cl-C6)-alkyl,
(C7-Cl6)-aralkyloxy-(Cl-C8)-alkoxy-(Cl-C8)-alkyl,
heteroaralkyloxy-(Cl-C8)-alkoxy-(Cl-C8)-alkyl,
(C3-C8)-cycloalkyl-(Cl-C6)-alkoxy-(Cl-C6)-alkyl, or
a radical of the formula Z
[CH21y~[0]w~[CH2]t~E (Z)~
in which E is a substituted phenyl radical of the
formula F
- 21~5473
- 38 -
R~ R~
~R~ (F),
Rl R9
or a substituted heteroaryl radical, or a sub~tituted
( C3 - C8 ) - cycloalkyl radical and
where
v = 0, 1, 2, 3, 4, 5 or 6, w = 0 or 1 and t = 0, 1,
2 or 3, with the restriction that v is not equal to
0 if w = 1, and
R6, R7, R8, R9 and R10 are identical or different and
are hydrogen, halogen, cyano, nitro, trifluoro-
methyl, (C1-C6)-alkyl, (C3-C8)-cycloalkyl, (C1-C6)-
alkoxy, -O-tCH2]xCfH(2ffl g)Fg, -OCF2Cl, -O-CF2-CHFCl,
(Cl-C6)-alkylmercapto, (Cl-C6)-hydroxyalkyl, (Cl-C6)-
alkoxy-(C1-C6)-alkoxy, (C1-C6)-alkoxy-(C1-C6)-alkyl,
(C1-C6)-alkylsulfinyl, (C1-C6)-alkylsulfonyl,
(Cl-C6)-alkylcarbonyl, (Cl-C8)-alkoxycarbonyl,
carbamoyl, N- (Cl-C8) - alkylcarbamoyl, N,N-di- (Cl-C8) -
alkylcarbamoyl, (C7-C11)-aralkylcarbamoyl optionally
substituted by fluorine, chlorine, bromine,
trifluoromethyl and (C1-C6)-alkoxy, N- (C3-C8) -
cycloalkylcarbamoyl, N-(C3-C8)-cycloalkyl-(C1-C4)-
alkylcarbamoyl, (C1-C6)-alkylcarbonyloxy, phenyl,
benzyl, phenoxy, benzyloxy, NR'Rn, such as amino,
~ anilino, N-methylanilino, phenylmercapto,
phenylsulfonyl, phenylsulfinyl, sulfamoyl, N-
(C1-C8)-alkylsulfamoyl or N,N-di-(C1-C8)-
alkylsulfamoyl or two adjacent substituents together
are a chain -tCH2 ]n or -CH=CH-CH=CH-, where one CH2
group of the chain is optionally replaced by O, S,
SO, SO2 or NR' where
R' and R" together are -tCH2~ h-~ in which one CH2 group
can be replaced by O, S, N- (Cl-C4) -alkanoylimino or
- 21~S473
- 39 -
N-(Cl-C4)-alkoxycarbonylimino, and
f = 1 to 8,
g = O, 1 to (2f + 1),
h = 3 to 6,
x = O to 3, and
n = 3 or 4.
Particularly preferred compounds of the formula 1 are
those in which
Q = O,
X = O,
Y = CR3,
m = O,
A is -CHRX-, where Rx i~ the substituent of the ~-C
atom of an ~-amino acid, in particular of a natural
L-amino acid or its D-isomer,
B = CO2V, where
V is the radical of an alcohol VOH and is a branched,
unbranched or cyclic aliphatic (Cl-Cg)-alkyl rad-
ical, a (C3-CB)-cycloalkyl-(Cl-C4)-alkyl radical,
a branched or unbranched (C2-C8)-alkenyl radical or
(C2-C8)-alkynyl radical, or a phenyl, benzyl, phen-
ethyl, phenylpropyl or phenylbutyl radical,
where the above radicals contain a substituent from
the series fluorine, chlorine, hydroxyl, (Cl- C4)-
alkoxy,(Cl-C6)-alkylcarbonyloxy,(C3-C8)-cycloalkyl-
carbonyloxy, benzyloxy, phenylalkylcarbonyloxy,
Rl and R3 are hydrogen,
R2 i~ a radical -CoNR5R6, where
R5 i~ hydrogen, (Cl-C3)-alkyl, (Cl-C4) -Al k~noyl or
a 1-, 2- or 3-valent phy~iologically utilizable
cation, in particular Na~, K~, Mg2~, Ca2~ or an
ammonium ion, optionally substituted 1-3 times
by (Cl-C8)-hydroxyalkyl, (Cl-C4)-alkoxy-(Cl-C8)-
2145~73
- 40 -
alkyl, phenyl, benzyl or (C1-C8)-alkyl, which
for its part can be substituted 1-3 times by
hydroxyl and/or (C1-C4)-alkoxy,
R6 i8 a radical of the formula I, excluding -SO2H,
- G-K- ~C -U] r -D -W ( I )
in which
G is -SO2-,
K is a bond,
C is a bond or
a (C1- C6 ) - alkanediyl radical,
U is a bond, hydrogen or -O-,
r is 1,
D is a bond, hydrogen or
an unbranched aliphatic (C1-C8)-alkanediyl rad-
ical, and
W is a bond, hydrogen, a (C6-C12)-aryl radical or
a 5- or 6-membered heteroaryl radical, where at
least one of the variables C or D or W is not
a bond and U is only a heteroatom group if C is
not a bond or if D and/or W are not a bond and
C, D and/or W, if the~e are not a bond or hydrogen,
are for their part preferably substituted by up
to 3 identical or different ~ubstituents from
the series
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (C1-C12)-alkyl, ( C3 - C8 ) - cycloalkyl,
( C 3 - C 8 ) - c y c 1 o a 1 k y l - (Cl- C1 2 ) ~ a l k y l ,
(C3-C8)-cycloalkoxy, (c3-c8)-cycloalkyl-(cl-cl2)
alkoxy, (C3-C8)-cycloalkyloxy-(C1-C12)-alkyl,
(C3 - C8 ) - cycloalkyloxy-(C1-C12)-alkoxy, (C3 -C8 ) - cyclo-
alkyl-(cl-c8)-alkyl-(cl-c6)-alkoxy~ (C3-C8)-cyclo-
alkyl-(cl-c8)-alkoxy-(cl-c6)-alkyl~ (C3-C8)-cyclo-
alkyloxy-(C1-C8)-alkoxy-(C1-C6)-alkyl, (C3-C8)-cyclo-
alkoxy-(C1-C8)-alkoxy-(C1-C8)-alkoxy, (C6-C12)-aryl,
( C7 - Cl 6 ) - aralkyl, (C2-Cl2)-alkenyl, (C2-Cl2)-alkynyl,
(Cl-C12)-alkoxy, (cl-cl2)-alkoxy-(cl-cl2)-alk
- 2145473
- 41 -
(cl-cl2)-alkoxy-(cl-cl2)-alkoxy~ (Cl-Cl2)-alkoxy-
(C1-C8)-alkoxy-(C1-C8)-alkyl, (C6-C12)-aryloxy,
( C7 -C16)-aralkyloxy,(C6-C12)-aryloxy-(C1-C6)-alkoxy,
(C7-C16)-aralkoxy-(C1-C6)-alkoxy, (C1-C8)-
hydroxyalkyl, (C6~Cl6)~arYloxy-(cl-c8)-alkyl~
( C7 - Cl6 ) - aralkoxy-(C1-C8)-alkyl, (C6-C12)-aryloxy-
(cl-c8)-alkoxy-(cl-c6)-alkyl~ ( C7 - Cl2 ) - aralkyloxy-
(C1-C8)-alkoxy-(C1-C6)-alkyl, ~O~~CH2~XCfH(2f+l g)Fg,
(Cl-Cl2)-alkoxycarbonyl, (cl-cl2)-alkoxy-(cl-cl2)-
alkoxycarbonyl, (C6-Cl2)-aryloxycarbonyl, (C7-Cl6)-
aralkoxycarbonyl, (C3-C8)-cyclo~lkoYycarbonyl,
(Cl-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkylcarbony-
loxy, (C6-Cl2)-arylcarbonyloxy, (C7-C16)-aralkylcar-
bonyloxy, cinnamoyloxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl, N-
(C1-C10)-alkyl-N-(C3-C8)-cycloalkylcarbamoyl, N-
((C3-C8)-cycloalkyl-(Cl-C6)-alkyl)carbamoyl, N-
(Cl-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl, N-
(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl, N-
(C6-C12)-arylcarbamoyl, N-(C7-C16)-aralkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C6-Cl6)-arylcarbamoyl, N-(C1-C1o)~
alkyl-N-(C7-C16)-aralkylcarbamoyl, N-((Cl-clO)-
alkoxy-(C1-C10)alkyl)carbamoyl, N-((C6-C16)-aryloxy-
(Cl-C10)alkyl)carbamoyl, N-((C7-C16)-aralkyloxy-
(C1-C10)-alkyl)carbamoyl, N-(cl-clo)-alkyl-N-
((C1-C10)-alkoxy-(Cl-ClO)-alkyl)carbamoyl, N-(Cl-Clo)~
alkyl-N-((c6-cl2)-aryloxy-(cl-clo)-alkyl)car
N-(cl-clo)-alkyl-N-((c7-cl6)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl, CON(CH2)h, in which a CH2 group can
be replaced by O, S, N-(Cl-C8)-alkylimino, N-(C3-C8)-
cycloalkylimino, N-(C3-C8)-cycloalkyl-(Cl-C4)-alkyl-
imino~ N-(c6-cl2)-arylimino~ N-(C7-Cl6)-aralkYli
or N-(Cl-C4)-alkoxy-(Cl-C6)-alkylimino and h i~ 3
to 7, or by
21q5473
-
- 42 -
a carbamoyl radical of the formula II
_
RX H
--CO NR ~-- --T (I 1),
s
in which
Rx is the substituent of an ~-amino acid which
includes the L- and D-amino acids,
8 is 1, 2, 3, 4 or 5 and
T is OH, OR or NR R ,
R being hydrogen or (Cl-C4)-alkyl,
R and R being identical or different and being
hydrogen, phenyl, benzyl, phenethyl, (C1-C8)-
alkyl, (C3-C6)-cycloalkyl, (+)-dehydroabietyl,
(C1-C8)-alkoxy-(Cl-C8)-alkyl, phenyl-(Cl-C6)-
alkoxy-(Cl-C8)-alkyl, (C6-Cl2) -ph~no~y_ (Cl-C8) -
alkyl, or
R and R together are -[CH2] h~ in which a CH2 group
can be replaced by O, S, SO, SO2, N-acylimino,
N-(Cl-C10)-alkoxycArho~yl;m;no, N-(C1-C8)-alkyl-
imino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-
cycloalkyl-(C1-C4)-alkylimino, N-phenylimino,
N-benzylimino or N-(cl-c4)-alkoxy-(cl-c6)-
alkylimino and h is 3 to 7, or are sub~tituted
by
amino, (C1-C12)-alkylamino, di-(C1-C12)-alkylamino,
(C3-C8)-cycloalkylamino, N-(C6-C12)-arylamino, N-
(C7 -Cll) -aralkylamino,
(Cl-Cl2)_Al ~Anoylamino, (C3-C8) -cycloAl kAnoylamino,
(C6-C12)-aroylamino, (C7-C16)-aralkanoylamino,
(Cl-cl2) -alkanoyl-N- (Cl-ClO) -alkYlam
2145~73
- 43 -
(C3-C8) cycloalkanoyl-N- (Cl-ClO) -alkYlamin
(C6~Cl2)~arYl-N-(cl-clo)-alkylamino~ (C7-C11)-
aralkanoyl-N-(C1-C10)-alkylamino,
(C1-C12)-AlkAnoylamino-(C1-C6)-alkyl, (C3-C8)-cyclo-
alkanoylamino, (C1-C8)-alkyl, (C6-C12)-aroylamino-
(C1-C6)-alkyl, ( C7 - Cl6 ) - aralkanoylamino-(C1-C6)-
alkyl~ amino-(C1-C10)-alkyl, N-(cl-clo)-alkylamino-
(C1-C10)-alkyl, N,N-di-(C1-C10)-alkylamino-(Cl-ClO)-
alkyl, ( C3 - C8 ) - cycloalkylamino-(C1-C10)-alkyl,
(C1-C12)-alkylmercapto,(C1-C12)-alkylsulfinyl,
(Cl-C12)-alkylsulfonyl,(C6-C12)-arylmercapto,
(C6-C12)-arylsulfinyl,(C6-C12)-arylsulfonyl,
(C7-C12)-aralkylmercapto,(C7-C12)-aralkylsulfinyl,
( C7 - Cl2 ) - aralkylsulfonyl,
where the radicals which contain an aryl radical can
for their part be substituted on the aryl by 1 to 5
identical or different radicals from the series:
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (Cl-C8)-alkyl, (C3-C8)-cycloalkyl,
(c6_cl2)-aryl, (C7-cl6)-aralkyl~ (c2-cl2)-alkenYl~
(C2-Cl2)-alkYnYl, (Cl-C8)-alkoxy~ (Cl-C6)-alkoxy-
-c6)-alkyl, (c6-cl2)-aryloxy~ (C7-Cl6)-aralkYlOXY'
O-[CH2]xcfH(2f+l-g)Fs~
(C1-C12)-alkoxycarbonyl, ( C3 - C8 ) - cycloA 1 koYycarbonyl,
(C1-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkyl-
carbonyloxy,
carbamoyl, N-(Cl-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl, N-
(C1-C10)-alkyl-N-( C3-C8) -cyc loalkylcarbamoyl, N-
((C3-C8)-cycloalkyl-(Cl-C6)-alkyl)carbamoyl, N-
(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
2145473
- 44 -
N-(Cl-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl, N-
(C6-Cl2)-arylcarbamoyl, N-(C7-C16)-aralkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C6-Cl6)-arylcarbamoyl, N-(Cl-Clo)~
alkyl-N-(C7-Cl6)-aralkylcarbamoyl, N-((Cl-clo)
alkoxy-(Cl-C10)alkyl)carbamoyl, N-((C6-C16)-aryloxy-
(Cl-C10)alkyl)carbamoyl, N-((C7-Cl6)-aralkyloxy-
(Cl-C10)-alkyl)carbamoyl, N-(cl-clo)-alkyl-N-
((Cl-C10)-alkoxy-(Cl-ClO)-alkyl)carbamoyl, N-(Cl-Clo)~
alkyl-N-((c6-cl2)-aryloxy-(cl-clo)-alkyl)carbam
N-(cl-clo)-alkyl-N-((c7-cl6)-aralkyloxy-(cl-clo)-
alkyl)carbamoyl, CON(CH2)h, in which a CH2 group can
be replaced by O, S, N-(Cl-C8)-alkylimino, N-(C3-C8)-
cycloalkylimino,N-(C3-C8)-cycloalkyl-(Cl-C4)-alkyl-
imino, N-(c6-cl2)-arylimino~ N-(C7-Cl6)-aralkYlim
or N-(Cl-C4)-alkoxy-(Cl-C6)-alkylimino and h is 3
to 7,
amino, (Cl-C6)-alkylamino, di-(Cl-C6)-alkylamino,
(C3-C8)-cycloalkylamino, N-(C6-Cl2)-arylamino, N-
(C7-Cll)-aralkylamino, N-(cl-c3)-alkyl-(c7-cll)
aralkylamino, N~(Cl~C3)~alkYl-(C6-Cl2)-arylaminO,
(Cl-C8)-alkoxyamino,
(Cl-C8)-alkanoylamino, (C3-C8)-cycloalkanoylamino,
(C6-l2)-aroylamino, (C7-C12)-aralkanoylamino,
(Cl-C8)-~lkanoyl-N-(Cl-C6)-alkylamino,(C3-C8)-cyclo-
alkanoyl-N-(Cl-C6)-alkylamino, (C6-Cl2)-aroyl-N-
(Cl-C10)-alkylamino, (C7-Cll)-aralkAnoyl-N-(Cl-C6)-
alkylamino,
(Cl-C8) -Al kAnoylamino- (Cl-C4) -alkyl, (C3-C8)-cyclo-
alkanoylamino-(Cl-C4)-alkyl, (C6-Cl2)-aroylamino-
(Cl-C4)-alkyl and (C7-Cl2)-aralkAnoylamino-(Cl-C4)-
alkyl,
R4 is a branched or unbranched (C7-C20)-alkyl radical
which can contain one or two C-C multiple bonds, or
(cl-c8)-alkoxy-(cl-c6)-alkyl~(cl-c6)-alkoxy-(cl-c4)
alkoxy-(Cl-C4)-alkyl or a radical of the formula Z
- 2145~73
-
- 45 -
- [CH2] V- []W- [CH2] t-E ( ) ~
where E is a sub~tituted phenyl radical of the
formula F
R6 R7
~RJ (F),
R10 R9
or a (C3-C8)-cycloalkyl radical, where
v = 0, 1, 2 or 3, w = 0 or 1 and t can be 0 or 1,
with the restriction that v is not equal to 0 if w
= 1, and
i hich R6 R7 R8 R9 and R10 are identical or
different and are
hydrogen, fluorine, chlorine, cyano, trifluoro-
methyl, (C1-C6)-alkyl, (C1-C6)-alkoxy,
O-[CH2]xcfH(2f+l-g)Fs~ N-(C1-C8)-alkylcarbamoyl,
N,N-di-(C1-C8)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, or (C7-C11)-phenylalkylcarbamoyl
optionally substituted by fluorine, chlorine, tri-
fluoromethyl and (C1-C6)-alkoxy.
Very particularly preferred compounds of the formula 1
are those in which
Q = O,
X = O,
Y = CR ,
m = 0,
A is -CHRX-, where Rx is the substituent of the ~-C
atom of an ~-amino acid, in particular hydrogen,
B = CO2V, where
V is the radical of an alcohol VOH and is a branched
or unbranched or cyclic aliphatic (C1-Cg)-alkyl
radical, a (C5-C6)-cycloalkyl-(C1-C4)-alkyl radical,
a branched or llnhranched (C2-C8)-alkenyl radical or
2145~73
- 46 -
a phenyl, benzyl, phenethyl, phenylpropyl or phenyl-
butyl radical,
where the abo~e radicals contain a substituent from
the series hydroxyl, (C1-C4)-alkoxy, (Cl-C6)-alkyl-
carbonyloxy, (C5-C6)-cycloalkylcarbonyloxy, benzoyl-
oxy, phenylalkylcarbonyloxy,
R1 and R3 are hydrogen,
R2 i6 a radical -CoNR5R6, where
R5 is hydrogen or a 1-, 2- or 3-valent physiologi-
cally utilizable cation, in particular Na~, K~,
Mg2~, Ca2~ or H3N~C(CH20H)3 (tris salt),
R6 is a radical of the formula I, excluding -SO2H,
-G-K-[C-U] -D-W (I)
in which
lS G is -SO2-,
K is a bond,
C is a bond,
U is a bond,
r is 1,
D is a bond or
( Cl - C4 ) - alkanediyl,
W is hydrogen or
a phenyl radical,
where this is substituted by 1, 2, 3, 4 or 5
substituents from the series fluorine,
chlorine, nitrile, trifluoromethyl, (C1-C6)-
alkyl, phenyl, (C1-C6)-alkoxy, phenoYy, -O-
[CH2]xcfH(2f+l-g)Fs~
carbamoyl, N-(C1-C8)-alkylcarbamoyl, N,N-di-
(C1-C8)-alkylcarbamoyl, N-(C3-C6)-
cycloalkylcarbamoyl,N-(C1-C4)-alkyl-N-(C3-C6)-
cycloalkylcarbamoyl, N-((C3-C6)-cycloalkyl-
(C1-C4)-alkyl)carbamoyl, N-(C1-C6)-alkyl-N-
((C3-C6)-cycloalkyl-(C1-C4)-alkyl)carbamoyl,
2145473
- 47 -
N-(+)-dehydroabietylcarbamoyl, N-(C6-Cl2)-
phenylcarbamoyl, N-phenyl-(Cl-C4)-
alkylcarbamoyl, N-(C1-C6)-alkyl-N-
phenylcarbamoyl, N-(C1-C6)-alkyl-N-phenyl-
(Cl-C4~-alkylcarbamoyl, N-((C1-C6)-alkoxy-
(Cl-C6)-alkyl)carbamoyl, N-phenoxy-(cl-c6)-
alkyl)carbamoyl, CON(CH2)h, in which a CH2
group can be replaced by O or N-(C1-C6)-
alkylimino and h iB 3 to 5, or
a carbamoyl radical of the formula II
RX H
--C O N R ~
S
in which
Rx is the substituent of an ~-amino acid which
includes the L- and D-amino acids,
s is 1, 2, 3, 4 or 5 and
T is OH, OR or NR R,
R being hydrogen or (C1-C4)-alkyl,
R and R being identical or different and being
hydrogen, phenyl, benzyl, phenethyl, (C1-C8)-
alkyl, ( C3-C6)- cycloalkyl, (+)-dehydroabietyl,
(cl-c8)-alkoxy-(cl-c8)-alkyl~ (C7-C12)-
phenylalkoxy-(C1-C8)-alkyl, (C6-C12)-phenoxy-
(C1-C8)-alkyl, or
R and R together are -[CH2~ h~ in which a CH2 group
can be replaced by O, S, SO, SO2, N-acylimino,
2 5 N- ( Cl - Clo ) - alkoxycarbonylimino, N-(Cl-C8)-alkyl-imino, N- (C3 - C6 ) - cycloalkylimino, N- (C3 - C6 ) -
cycloalkyl-(Cl-C4)-alkylimino, N-phenylimino,
N - b e n z y l i m i n o o r
21~5473
- 48 -
N-(Cl-C4)-alkoxy-(C1-C6)-alkylimino and h i8 3
to 5,
(Cl-C8)-alkanoylamino, (C3-C8)-cyclo~lk~noylamino,
(C6-Cl2)-phenylamino, (C7-Cll)-phenylAlk~noylamino,
(Cl-C8)-alkanoyl-N-(Cl-C10)-alkylamino, (C3-C6)-
cycloalkanoyl-N-(Cl-C6)-alkylamino, benzoyl-N-
(Cl-C10)-alkylamino, (C7-Cll)-phenylalkanoyl-N-
(C1-C6)-alkylamino,
(C1-C10)-~lk~noylamino-(Cl-C2)-alkyl, (C3-C8)-cyclo-
alkanoylamino- (Cl -C2 ) -alkyl, benzoylamino- (Cl -C2 ) -
alkyl, (C7-C14)-phenyl~lk~noylamino-(Cl-C2)-alkyland
where the radical~ which contain an aryl radical can
for their part be substituted by a substituent from
the serie~ hydrogen, hydroxyl, fluorine, chlorine,
trifluoromethyl, (Cl-C6)-alkyl, (Cl-C8)-alkoxy and
R4 is a branched or unbr~nche~ (C7-Cl2)-alkyl radical
which can contain one or
two C-C multiple bonds, or
(Cl-C4)-alkoxy-(Cl-C6)-alkyl,(Cl-C4)-alkoxy-(Cl-C4)-
alkoxy-(Cl-C4)-alkyl
or a radical of the formula Z
-[CH2]V-[O]W-[cH2]t-E (Z)'
where E is a substituted phenyl radical of the
formula F
R6 R~
>~<
~RJ (F),
Rl R
or a (C3-C8)-cycloalkyl radical, where
v = 0, 1, 2 or 3, w = 0 and t = 0, and
2145~73
- 49 -
i hich R6 R7 R8 R9 and R10 are identical or
different and are hydrogen, fluorine, chlorine,
cyano, trifluoromethyl, (C1-C6)-alkyl, (C1-C6)-
alkoxy, -o-[cH2]xcfH(2f+l-g)Fg~ N-(C1-C8)-alkyl-
carbamoyl, N,N-di-(C1-C8)-alkylcarbamoyl, N- (C3-C8) -
cycloalkylcarbamoyl, or (C7-C11)-phenylalkyl-
carbamoyl optionally substituted by fluorine,
chlorine, trifluoromethyl and (C1-C6)-alkoxy and
n is 0,
10 f is 1 to 5,
g i8 O, 1 to (2f+1) and
x is 0 or 1.
The invention relates to the use of compound~ of the
formula 1 and to the physiologically tolerable salts for
the inhibition of collagen biosynthesis.
The invention furthermore relates to the u~e of compounds
of the formula 1 and to the physiologically tolerable
~alts for the production of a pharmaceutical against
fibrotic disorders, in particular in the liver, in the
lungs and on the skin (scleroderma).
The invention in particular relates to the compounds of
the formula 1 for use as inhibitors of proline-4-
hydroxylase.
The invention finally relates to the compounds of the
formula 1 for use as pharmaceuticals.
The invention furthermore comprises prodrugs for the
compounds of the formula 1 which cause an inhibition of
collagen biosynthesis in vivo by release of compounds of
the formula 1 or their salts.
The invention finally also comprises prodrugs which cause
an inhibitory action on proline-4-hydroxylase due to
release of compounds of the formula 1 or their salts.
~ 21~5~73
- 50 -
Prodrug groups are chemical groups which in vivo
- are converted to the carboxylate group of the com-
pounds of the formula 1 and/or
- can be removed from the amide N atom and/or
- can be converted to a pyridine ring.
Suitable prodrug groups are known to the person skilled
in the art.
The following prodrug groups are mentioned in particular:
for the carboxylate group ester, amide or hydroxymethyl
groups and their derivatives and aldehyde groups and
their derivatives, for the pyridine N atom N-oxides and
N-alkyl derivatives and for the pyridine ring 1,4-di-
hydropyridine derivatives.
The invention furthermore relates to a process for the
preparation of compounds of the formula 1.
Compounds of the formula 1 in which A is a substituted
alkylene moiety, B = CO2H, Y = CR3 and m = O or 1 are
prepared by carrying out the reactions described in
Schemes 1, 2 and 3.
2145473
-
- 51 -
Scheme 1
RO2C~ ACoH/H2o2 RO2C~
CO2R t~`CO2R
2 3 ~lln~Pn ~ n
( R ~ H, Lower alkyl)
MQR~or
RO2C~QR4 ~)QR~ RO2C~L
CO2R CO2R
Sa: R ~ H
L ~ tl, ~r
S b : R ~ Loweralky
HO2C~QR~RO2C~QR4
CO2RCO2H
6 R ~ Lower alkyl 7 R ~ Lower alkyl
Re Scheme 1
The 3-Qubstituted 5-carboxypyridine-2-carboxylic acid
esterQ of the formula 6 are prepared from the pyridine-
2,5-dicarboxylic acid diesters of the formula 2.
The oxidation of the pyridine-2,5-dicarboxylates of the
formula 2 i~ described in J. Chem. Soc. Perkin Trans. 2,
1978, 34-38 and in J. Org. Chem. 25 (1960) 565-568
(M.L. Peterson).
The halogenation (chlorination) of the pyridine-N-oxides
of the formula 3 iQ carried out u~ing thionyl chloride,
preferably in a nonpolar ~olvent and optionally using a
catalyst, and the reaction of the 3-chloropyridine-2,5-
2145473
- 52 -
dicarboxylic acid diester (formula 4) with A1kox;des MQR4
(M = metal, Q = O) in the alcohol R40H or in a dipolar
aprotic solvent.
The pyridine-2-carboxylic acid ester-5-carboxylates of
5 the formula 6 can be prepared under esterification
conditions from substituted pyridine-2,5-dicarboxylic
acids (see CA: Vol. 68, 1968, 68840 h). Suitable condi-
tions are e.g. esterification with methanol in the
presence of sulfuric acid, the reaction time being
10 selected such that complete esterification to the diester
product only takes place to a minor extent, or it i8
posRible to separate the diester products as by-products.
Scheme 2
H N R 5 R 6 R ~ N R 5
HO2C~¢~,QR~ QR~
N ~` C 2 Lower alkyl N~` C 2 Lower alkyl
6 9
The compounds of the formula 9 are prepared from the
15 compounds of the formula 6 and the amide derivatives of
the formula 8, where it may be expedient to activate both
reactants with auxiliary reagents (Houben-Weyl: Methoden
der Organischen Chemie ~Methods of Organic Chemistry],
Volume IX, Chapter 19, pages 636 to 637); cf. Scheme 2.
20 Reagents used for carboxylic acid activation can be
substances known to the person skilled in the art, such
as thionyl chloride, oxalyl chloride, pivaloyl chloride
or chloroformic acid ester derivatives. It i8 not always
necessary to isolate these activated derivatives of the
25 compounds of the formula 7. It is usually expedient to
react them with the sulfonamide derivatives of the
formula 8 after preparation in situ or as crude products.
Expediently, the compounds of the formula 8 are first
2145473
- 53 -
reacted with an inorganic or organic base, such as e.g.
sodium or potassium hydroxide, carbonate, ~lkoY;de,
hydride or amide, ammonia, triethylamine, tributylamine
or pyridine at -20 to +150C, preferably at 0 to -80C,
and this reaction mixture is reacted with a compound of
the formula 6 or its activated form. The reaction is
carried out in an inert solvent, such as e.g. methylene
chloride, methanol, ethanol, acetone, ethyl acetate,
toluene, tetrahydrofuran, acetonitrile, N,N-dimethylform-
amide, N,N-dimethylacetamide, nitromethane, dimethyl
sulfoxide or mixtures of these solvents.
Scheme 3
R 1 H 2 N - ~ - C 2 R 1 2 Q I
R~C02R1~ i2 ) ~ '~NH-A-C02R~2
9 1 0
/ --
lli) / ~I)
R2 I QH
~ ~ NH-A-C02R 1 2
R 2 ~Q R ~'
R~N~NH-~-C02R I 2
o
R11 = H, (C1-C3)-alkyl or PG
R12 = H, (C1-C8)-alkyl or benzyl
X = a leaving group, in particular halogen, OS02Me or
OS02phenyl
- _ 2145~73
- 54 -
Re Scheme 3
il.) Pyridine-2-carboxylic acids of the formula 9
(Rl1 = H) are reacted with the amino esters of the
formula 10 to give the amido esters of the
formula 1, or
i2.) pyridine-2-carboxylic acid esters of formula 9 (R
= lower alkyl) are reacted under aminolysis condi-
tions with the compounds of the formula 10 to give
the compounds of the formula 1;
ii) the compounds of the formula 1 are prepared by
alkylation of compounds of the formula 11 with R4X
and if desired
iii) the compounds of the formula 1 if Q = 0 or NRY are
converted to their pyridine-N-oxides.
Suitable processes for amide formation (Reaction il) are
the methods of carbonyl activation and the condensation
reactions known from peptide chemistry.
The reagents u~ed for carboxylic acid activation can be
the substanceæ known to the person skilled in the art,
such as thionyl chloride, oxalyl chloride, pivaloyl
chloride, chloroformic acid ester derivatives or N,N'-
carbonyldiimidazole. The activated derivatives of the
compounds of the formula 9 are reacted, after preparation
in situ, with the amide derivatives of the formula 10.
A æuitable con~enRing agent is, for example, the
combination of N,N'-dicyclohexylcarbodiimide/N-hydroxy-
lH-benzotriazole and N-ethylmorpholine.
Suitable ~olvents are dichloromethane, tetrachlorometh-
ane, butyl acetate, ethyl acetate, toluene, tetrahydro-
furan, dimethoxyethane, 1,4-dioxane, acetonitrile, N,N-
dimethylformamide, N,N-dimethylacetamide, dimethyl
21~5~73 -
- 55 -
~ulfoxide, nitromethane and/or pyridine.
The compounds of the formula I are inhibitors of proline-
4-hydroxylase. The inhibition of this enzyme was deter-
mined as described by Raule and Gunzler in Anal. Biochem.
184, 291-297 (1990).
The compounds of the formula 1 according to the invention
have useful ph~rm~cological properties and in particular
show antifibrotic activity, in particular in the liver,
in the lungs and on the skin (scleroderma).
The antifibrotic action can be determined in the carbon
tetrachloride-induced liver fibrosis model. To do thi6,
rats are treated twice weekly with CCl4 (1 ml/kg)
dissolved in olive oil. The test substance i8 adminis-
tered daily, if desired even twice daily, orally or
intraperitoneally - dissolved in a suitable tolerable
solvent. The extent of the liver fibrosis is determined
hiætologically and the amount of collagen in the liver is
analyzed by hydroxyproline determination - as described
in Kivirikko et al. (Anal. Biochem. 19, 249 f. (1967)).
The fibrinogenesis activity can be determined by radio-
immunological determination of collagen fragments and
procollagen peptides in the serum. The compounds accord-
ing to the invention are active in this model at concen-
trationæ of 1 to 100 mg/kg.
The fibrinogenesis activity can be determined by radioim-
munological determination of the N-terminal propeptide of
collagen type III or of the N- or C-terminal croggl;nk;ng
~- ~; n of the collagen type IV (7s-collagen or type IV
collagen NCl) in the serum.
For this purpose, the hydroxyproline, procollagen III
peptide, 7s-collagen and type IV collagen NC concentra-
tions are measured in the liver of
a) untreated rats (control)
b) rats to whom carbon tetrachloride has been
- 2145473
- 56 -
administered (CCl4 control)
c) rats to whom first CCl4 and then a compound accord-
ing to the invention has been administered
(this test method is described by Rouiller, C., Experi-
mental Toxic Injury of the Liver; in The Liver,
C. Rouiller, Vol. 2, p. 335 to 476, New York, Academic
Press, 1964).
The compounds of the formula 1 can be used as medicaments
in the form of pharmaceutical preparations which contain
them, optionally with tolerable pharmaceutical excipi-
ents. The compounds can be used as medicines, e.g. in the
form of pharmaceutical preparations which contain these
compounds in a mixture with a pharmaceutical, organic or
inorganic excipient, such as e.g. water, gum arabic,
gelatin, lactose, starch, magnesium stearate, talc,
vegetable oils, polyalkylene glycols, petroleum jelly
etc., suitable for enteral, percutaneous or perenteral
administration.
They can be administered orally for this purpose in doses
of 0.1 to 25 mg/kg/day, preferably 1 to 5 mg/kg/day or
parenterally in doses of 0.01 to 5 mg/kg/day, preferably
0.01 to 2.5 mg/kg/day, in particular 0.5 to
1.0 mg/kg/day. In severe cases the dose can also be
increased. In many cases, however, lower doses also
suffice. These details relate to an adult of weight about
75 kg.
~ Example 1
3-Benzyloxy-5-[((4-((((+)-dehydroabietylamino)-L-
valinyl)carbonyl)phenylsulfonyl)amino)carbonyl~pyridine-
2-carboxylic acid (glycylethyl ester)amide
Example 2
3-Benzyloxy-5-[((4-(((2-(4-fluorophenyl)ethyl)amino)-
carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-2-
carboxylic acid (glycylethyl ester)amide
21g5473
- 57 -
Example 3
3-Benzyloxy-5-[((4-((cyclohexylamino)carbonyl)phenylsul-
fonyl)amino)carbonyl~pyridine-2-carboxylic acid
(glycylethyl ester)amide
Example 4
3-Benzyloxy-5-t((4-(((diethylamino)-L-~alinyl)carbonyl)-
phenylsulfonyl)amino)carbonyl]pyridine-2-carboxylic acid
(glycylethyl ester)amide
Example 5
3-Benzyloxy-5-~((4-(N,N-diethylaminocarbonyl)phenyl-
sulfonyl)amino)carbonyl]pyridine-2-carboxylic acid
(glycylethyl ester)amide
Example 6
3-Benzyloxy-5-~((4-((2-chloro-5-methoxybenzoyl)amino)-
ethyl)phenylsulfonyl)amino)carbonyl]pyridine-2-carboxylic
acid (glycylethyl ester)amide
Example 7
3-Benzyloxy-5-[((4-(2-propyloxy)phenyl)sulfonyl)amino)-
carbonyl]pyridine-2-carboxylic acid (glycylethyl
ester)amide
Example 8
3-(4-(Fluorobenzyloxy)-5-[((4-(2-propoxy)phenyl)sul-
fonyl)amino)carbonyl]pyridine-2-carboxylic acid (glycyl-
ethyl ester)amide
Example 9
3-Benzyloxy-5-~((n-butylsulfonyl)amino)carbonyl]pyridine-
2-carboxylic acid (glycylethyl ester)amide
Example 10
5-t((n-Butylsulfonyl)amino)carbonyl]-3-(4-fluorobenzyl-
oxy)pyridine-2-carboxylic acid (glycylethyl ester)amide
2145473
Example 11
5-[((n-Butylsulfonyl)amino)carbonyl]-3-(4-chlorobenzyl-
oxy)pyridine-2-carboxylic acid (glycylethyl ester)amide
Example 12
5-[((n-Butylsulfonyl)amino)carbonyl]-3-(3-methoxybenzyl-
oxy)pyridine-2-carboxylic acid (glycylethyl ester)amide
Example 13
3-Benzyloxy-5-[((4-((((+)-dehydroabietylamino)-L-
valinyl)carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-
2-carboxylic acid(glycyl-(2-propyl) ester)amide
Example 14
3-Benzyloxy-5-[((4-(((2-(4-fluorophenyl)ethyl)amino)-
carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-2-
carboxylic acid(glycyl-(2-propyl) ester)amide
Example 15
3-Benzyloxy-5-[((4-((cylcohexylamino)carbonyl)phenyl-
sulfonyl)amino)carbonyl]pyridine-2-carboxylic
acid(glycyl-2-propyl) ester)amide
Example 16
3-Benzyloxy-5-[((4-(((diethylamino)-L-valinyl)carbonyl)-
phenylsulfonyl)amino)carbonyl]pyridine-2-carboxylic
acid(glycyl-(2-propyl) ester)amide
Example 17
3-Benzyloxy-5-[((4-(N,N-diethylaminocarbonyl)phenyl-
sulfonyl)amino)carbonyl]pyridine-2-carboxylic acid
(glycyl-(2-propyl) e~ter)amide
Example 18
3-Benzyloxy-5-~((4-(2-((2-chloro-5-methoxybenzoyl)amino)-
ethyl)phenylRulfonyl) a~; no)carbonyl]pyridine-2-carboxylic
acid (glycyl-(2-propyl) ester)amide
2145~73
- 59 -
Example 19
3-Benzyloxy-5-~((4-((((+)-dehydroabietylamino)-L-
valinyl)carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-
2-carboxylic acid (glycyl-(3-pentyl) ester)amide
Example 20
3-Benzyloxy-5-[((4-(((2-(4-fluorophenyl)ethyl)amino)-
carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-2-
carboxylic acid (glycyl-(3-pentyl) ester)amide
Example 21
3-Benzyloxy-5-~((4-((cyclohexylamino)carbonyl)phenyl-
sulfonyl)amino)carbonyl]pyridine-2-carboxylic acid
(glycyl-(3-pentyl) ester)amide
Example 22
3-Benzyloxy-5-~((4-(((diethylamino)-L-valinyl)carbonyl)-
phenylsulfonyl)amino)carbonyl]pyridine-2-carboxylic acid
(glycyl-(3-pentyl) ester)~amide
Example 23
3-Benzyloxy-5-~((4-(N,N-diethylaminocarbonyl)phenyl-
sulfonyl)amino)carbonyl]pyridine-2-carboxylic acid
(glycyl-(3-pentyl) ester)amide
Example 24
3-Benzyloxy-5-t((4-(2((2-chloro-5-methoxybenzoyl)amino)-
ethyl)phenylsulfonyl)amino)carbonyl]pyridin-2-carboxylic
acid (glycyl-(3-pentyl) ester)amide
Example 25
3-Benzoyloxy-5-[((n-butylsulfonyl)amino)carbonyl]-
pyrimidine-2-carboxylic acid (glycyl-(3-pentyl)
ester)amide
Example 26
5-[((n-Butylsulfonyl)amino)carbonyl]-3-(4-fluoro-
benzyloxy)pyridine-2-carboxylic acid (glycyl-(3-pentyl)
ester)amide
2145473
- 60 -
Example 27
5-[((n-Butyl 8ul fonyl)amino)carbonyl]-3-(4-chloro-
benzyloxy)pyridine-2-carboxylic acid (glycyl-(3-pentyl)
ester)amide
Example 28
5-[((n-Butylsulfonyl)amino)carbonyl]-3-(3-methoxy-
benzyloxy)pyridine-2-carboxylic acid (glycyl (3-pentyl)
ester)amide
Example 29
3-Benzyloxy-5-[((4-((((+)-dehydroabietylamino)-L-
~alinyl)carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-
2-carboxylic acid (glycyl-(1-butyl) ester)amide
Example 30
3-Benzyloxy-5-[((4-(((2-(4-fluorophenyl)ethyl)amino)-
carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-2-
carboxylic acid (glycyl-(1-butyl) ester)amide
Example 31
3-Benzyloxy-5-[((4-((cyclohexylamino)carbonyl)phenyl-
sulfonyl)amino)carbonyl]pyridin-2-carboxylic acid
(glycyl-(l-butyl) ester)amide
Example 32
3-Benzyloxy-5-[((4-(((2-(4-fluorophenyl)ethyl)amino)-
carbonyl)phenylsulfonyl)amino)carbonyl]pyridine-2-
carboxyic acid (glycyl-(4-heptyl) ester)amide
Example 33
3-Benzyloxy-5-~((4-((cyclohexylamino)carbonyl)phenyl-
sulfonyl)amino)carbonyl]pyridine-2-carboxylic acid
(glycylretinyl ester)amide