Note: Descriptions are shown in the official language in which they were submitted.
CA 02146130 2001-06-12
4'V0 94/08904
PCT/US93/09762
Antifouling Coating Composition and Method
BACKGROUND OF THE 11~1VENZ'ION
This invention relates generally to protection of underwater surfaces from
fouling by aquatic organisms. This invention was made with government support
awarded by the C)ffice of Naval Research under contract No. N00014-86-K-0261.
The government has certain rights in the invention.
DESCRIPITON OF THE PRIOR ART
In marine, brackish, and freshwater environments, organisms collect, settle,
attach, and grow on submerged structures. Organisms which do so can include
algae, and aquatic: animals, such as tunicates, hydroids, bivalves, bryozoans,
polychaete worms, sponges, and barnacles. Submerged structures can include the
underwater surfaces of ships, docks and piers, pilings, fish nets, heat
exchangers,
dams, piping structures, such as intake screens, and cooling towers. The
presence
of these organisms, known as the "fouling" of a structure, can be harmful in
many
respects. They can add to the weight of the structure, hamper its
hydrodynamics,
reduce its operating efficiency, increase susceptibility to corrosion, and
degrade or
even fracture the structure.
The common method of controlling the attachment of fouling organisms is
by protecting the structure to be protected with a paint or coating which
contains
an antifouling agent. Exemplary antifouling coatings and paints are described
in
U.S. Patent No. 4596,724 to Lane, U.S. Patent No. 4,410,642 to Layton, and
U.S.
Patent No. 4,788,302 to Costlow. Application of a coating of this type
inhibits the
attachment, or "settling", of the organism, by either disabling the organism
or
providing it with an unattractive environment upon which to settle.
Of the fouling organisms noted above, barnacles have proven to be among
the most difficult to control. Typically, commercsal antifouling coatings and
paints
include a toxic metal-containing compound such as tri-n-butyl tin (TBT), or
cuprous oxide, which leaches from the coating. Although these compounds
exhibit moderate success in inhibiting barnacle settlement, they degrade
slowly in
marine environments, and therefore are ecologically harmful In fact, TBT is
sufficiently toxic that its release rate is limited by legislat;on in some
countries.
Some experimental non-toxic compounds have been tested with limited
success in barnacle settlement inhibition. See, e.g., Gerhart et al., J. Chem.
Ecol.
14:1905-1917 (1988), which discloses the use of pukalide, epoxypukalide, and
an
extract produced by the octocoral Leptogorgia airgulata, to inhibit barnacle
settlement, and Sears et al., J. Chem. Ecol. 16:791-799 (1990), which
discloses the
CA 02146130 2001-06-12
WO 94/08904 PCT/US93/09762
2
use of ethyl acetate extracts of the sponge Lissodendoryx isodictylais to
inhibit
settlement.
Japanese Patent Disclosure No. 54-44018A of April 7,1979 (Patent
Application No. '~2-109110 of September' 10, 1977), discloses gamma-
methylenebutenolide lactone and alkyl gamma-methylenebutenolide lactone
derivatives having the general structure
R
C O O
Rz
wherein Rl and R2 are hydrogen or saturated or unsaturated alkyl groups
of 1-8 carbon atoms. The compounds are natural products from terrestrial
plants.
SLTMMARY OF THE nVVENTION
In view of the foregoing, it is an object of the present invention to provide
an antifouling composition which is effective in inhibiting the settlement of
fouling organisms on an underwater surface.
Another object of the present invention is to provide an antifouling paint or
coating composition which is effective in protecting underwater structures
Erom
fouling by barnacles, and other aquatic organisms.
A further object is to provide structures which are effectively protected
against fouling by aquatic organisms.
These and other objects are accomplished by the present invention which in
one aspect comprises a composition for use as a marine or freshwater
antifoulant
comprising a protective carrier component functioning to release antifouling
agent and, as an antifouling agent, at least one compound selected from the
group
consisting of
~3
R2 R3 ~C O O
w /
R~ ~O R4 I
O OCH3
(1) (2)
~~4~~30
WO 94/08904 PCT/US93/09762
3
O O O
RI CH2
RII O
R10 2
(3) ~ (4) (5)
CH3 ~ H
O
O
O
CH3 H CH2
w (7)
O
R23 R24
R ~~2 O
1! 1
RI 9 120 ~3
(8) (g)
wherein Rl~ R2, R3, and R4 are independently selected from -C(O)R5;
C(O)OR6, (C1-Cg)allcyl, phenyl, phenyl substituted with (C1-C4)alkyl, (C1-
C4)alkoxy, (C2_Cg)alkenyl, (C2-Cg)alkynyl, halogen, and hydrogen, provided
that
at least one of R1, R2~ R3, and R4 is not hydrogen;
wherein R5 is R6 or NR7Rg;
wherein R6 is (C1-Cg)alkyl, (C2-Cg)alkenyl, (C2_Cg)alkynyl, phenyl,
phenyl substituted with (C1-C4)alkyl, (Cl_C4)alkoxy, or halogen;
wherein R7 and Rg are independently selected from hydrogen or R6;
wherein R9, R10. R11. R12. R13. R14. R15. R16. R17. R18. R19. R20. and R21
are independently selected from the group consisting of hydrogen and (C1-C10)
alkyl; and
wherein R22, R23 and R24 are each independently selected from hydrogen,
(C1-C3) alkyl groups, (C1-C3) alkoxy groups, halogens, and hydroxyl groups.
A second aspect of the present invention comprises a method of protecting
a marine or freshwater structure against fouling by marine or freshwater
fouling
2~.~~~.3~
WO 94/08904 . PG'T/US93/09762
4
organisms comprising applying at least one of the aforementioned compounds on
and/or into said structure.
Another invention is a marine or freshwater structure protected against
fouling organisms wherein said protection is afforded by at least one of the
aforementioned compounds having been applied on and/or into said structure.
BRIEF DESCRIPTION OF THE DRAWINGS
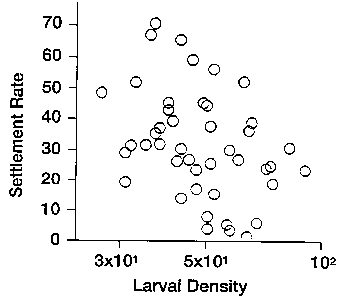
Figure 1 is a graph plotting settlement rate of untreated controls as a
function of larval density. The least squares regression equation for the data
is Y
= 47.4 (log X) - 41.3, where Y represents settlement rate and X represents
larval
density.
Figure 2 is a graph plotting settlement rate of treated samples as a function
of larval density. The least squares regression equation for the data is Y = -
51.7
(log X) + 118.6, where Y represents settlement rate and X represents larval
density).
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to controlling the attachment of
unwanted organisms to submerged surfaces by contacting the organisms with one
or more compounds having antifouling activity selected from the group
consisting of those of formulae (1) through (9).
It has been discovered that such compounds inhibit the settlement of
fouling organisms, particularly barnacles. As used herein, "settlement" refers
to
the attachment of aquatic organisms to an underwater structure. Contacting an
organism with a compound of the invention in the area adjacent a submerged
surface prevents the settling of the organism on that submerged surface.
In the practice of the method of the present invention, the antifouling
compound may be contacted to the organism by coating the object to be
protected
with a coating containing the antifouling compound, which then releases the
compound into the aquatic environment immediately adjacent the external
surfaces of the article, by including the antifouling compound within material
formed into an aquatic article which then releases the compound, by releasing
the
compound directly into the aquatic environment surrounding the protected
object, or by any other method wherein the compound contacts the organism
prior to its attachment to the surface. As used herein, the term "contacting"
means
that an amount of antifouling compound sufficient to inhibit settlement of the
organism on the surface of interest physically contacts the organism, whether
by
direct external contact, inhalation, respiration, digestion, inhibition, or
any other
process.
Preferred compounds are selected from the group consisting of 2
ethylfuran; 2-methylfuran; methyl-2-furanoate; ethyl-3-furoate; 2-furyl-n-
pentyl
WO 94/08904 ~ ~ ~ PGT/US93/09762
ketone; 2-acetylfuran; khellin; y decalactone; a-angelica lactone; a-santorun;
a-
methylene-~butyrolactone; coumaranone; alantolactone; and 3-methyl-2-
cyclohexene-1-one.
The amount of compound to be used in the method will vary depending on
5 a number of factors, including the identity of the antifouling compound, the
identity of the organism to be inhibited, and the mode of contact. In
addition, the
rate at which the compound is released into the surrounding aquatic
environment
can be a major factor in determining both the effectiveness of the method and
the
duration of protection. If the compound is released too rapidly, it will be
exhausted quickly, and the coating must be re-applied for the surface to be
protected. If, on the other hand, the release rate of the antifouling compound
is
too slow, the concentration of the compound in the aquatic environment
immediately surrounding the surface to be protected may be insufficient to
inhibit
settlement. Preferably, the antifouling compound is released into the
environment
adjacent the protected surface at the rate of between about 0.0001 and 1000
g/cm2-hr, and more preferably is released at a rate of between about 0 01 and
100
g/cm2-hr. Compositions of the invention preferably comprise one or more
compounds of the invention in a concentration of about 0.01 weight percent to
about 50 weight percent based on said composition, more preferably in a
concentration of about 0.1 to 20 weight percent based on said composition.
The organisms against which a surface can be protected by the present
method can be any organism which can attach to a submerged surface. Exemplary
organisms include algae, including members of the phyla Chlorophyll and,
fungi,
microbes, tunicates, including members of the class Ascidiancea, such as Ciona
intestinalis, Diplosoma listerianium, and BotryiIus sclosseri, members of the
class
Hydrozoa, including Clava squamata, Hydractinia echinata, Obelia geniculata,
and
Tubularia larnyx, bivalves, including Mytilus edulis, Crassostrea virginica,
Ostrea
edulis, Ostrea chilensia, and Lasaea rubra, bryozoans, including Ectra pilosa,
Bugula
neritinia, and Bozverbankia gracilis, polychaete worms, including Hydroides
norvegica, sponges, and members of the class Cirripedia (barnacles), such as
Balanus amphitrite, Lepas anatifera, Balanus balanus, Balanus balanoides,
Balanus
hameri, Balanus crenatus, Balanus improvisus, Balanus galeatus, and Balanus
eburneus.
Organisms of the genus Balanus are particularly frequent foulers of aquatic
structures. Specific fouling organisms to which this invention is especially
directed include barnacles, zebra mussels, algae, bacteria, diatoms, hydroids,
bryzoa, ascidians, tube worms, and asiatic clams.
In addition to the compounds of the invention, the composition can
comprise additional antifouling agents which may act in combination or
synergistically; said additional antifouling agent can be, for example:
manganesE
WO 94/08904 PGT/US93/09762
6
ethylene bisdithiocarbamate; a coordination product of zinc ion and manganese
ethylene bisdithiocarbamate; zinc ethylene bisdithiocarbamate; zinc dimethyl
dithiocarbamate; 2, 4, 5, 6-tetrachloroisophthalonitrile; 2-methylthio-4-t-
butylamino-6-cyclopropylamino-s-triazine; 3-(3,4-dichlorophenyl)-1,1-dimethyl
urea; N-(fluorodichloromethylthio)-phthalimide; N,N-dimethyl-N'-phenyl-(N-
fluorodichloromethylthio)-sulfamide; tetramethylthiuram disulfide; 2, 4, 6-
trichlorophenyl maleimide; zinc 2-pyridinthiol-1-oxide; copper thiocyanate; Cu-
10% Ni alloy solid solution; and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one.
The protective carrier component functioning to release antifouling agent
can be a film-forming component, an elastomeric component, vulcanized rubber,
or a cementitious component. The protective carrier component can be any
component or combination of components which is applied easily to the surface
to
be protected, adheres to the submerged surface to be protected, and permits
the
release of the antifouling compound into the water immediately surrounding the
coated surface. Different components will be preferred depending on the
material
comprising the underwater surface, the operation requirements of the surface,
the
configuration of the surface, and the antifouling compound. Exemplary film-
forming components include polymer resin solutions. Exemplary polymer resins
include unsaturated polyester resins formed from (a) unsaturated acids and
anhydrides, such as malefic anhydride, fumaric and, and itaconic acid; (b)
saturated acids and anhydrides, such as phthalic anhydride, isophthalic
anhydride, terephthalic anhydride, tetrahydrophthalic anhydride,
tetrahalophthalic anhydrides, chlorendic acid, adipic acid, and sebacic acid;
(c)
glycols, such as ethylene glycol,1,2 propylene glycol, dibromoneopentyl
glycol,
Dianol 33~, and Dianol 22~; and (d) vinyl monomers, such as styrene, vinyl
toluene, chlorostyrene, bromostyrene, methylinethacrylate, and ethylene glycol
dimethacrylate. Other suitable resins include vinyl ester-, vinyl acetate-,
and vinyl
chloride-based resins, elastomeric components, vulcanized rubbers, and
urethane-
based resins. The cementitious compounds are used to protect certain types of
underwater structures, as are the elastomeric materials and vulcanized rubber.
The percentage of the antifouling compound of the invention in the coating
required for proper release of the compound into the aquatic environment
surrounding the surface to be protected will vary depending on the identify of
the
antifouling compound, the identity of the film-forming component of the
coating
and other additives present in the coating which may affect release rate. As
described above, the release rate of the antifouling compomd can be a major
factor in determining both the effectiveness of the method and the duration of
protection. It is preferred that the coating be released into the surrounding
water
at a rate of between about 0.0001 and 1,000 ~.g/cm2-hr; more preferably, the
CA 02146130 2001-06-12
WO 94/08904 PCT/US93/09762
7
compound comprises between about 0.01 and 100 ~.g/cm2--hr. Preferably, the
antifouling compound comprises between about 0.001 and 80 percent of the
coating by weight, and more preferably comprises between 0.01 and 20 percent
of
the coating.
Those skilled in this art will appreciate that a coating of the present
invention can comprise any number of forms, including a paint, a gelcoat, or
varnish, and the :like. The coating can include components in addition to the
antifouling coatisig and film-forming component which confer a desirable
property, such as hardness, strength, rigidity, reduced drag, impermeability,
or
water resistance.
The present invention encompasses any article which contains a surface
coated with a coating containing at least one of the aforementioned compounds.
Those articles which are particularly suitable for protection with the coating
are
those. which, either intentionally or inadvertently, are submerged for a least
the
I5 duration required for an organism to settle on a submerged object. Coated
articles
can comprise any material to which aquatic organisms are known to attach, such
as
metal, wood, con~ete, polymer, and stone. Exemplary articles which may require
antifouling protection include boats and boat hulls, fish nets, recreational
equipment, such as surfboards, jet skis, and water skis, piers and pilings,
buoys,
offshore oil rigging equipment, and decorative or functional stone formations.
The composition of the invention c:an be a cementitious composition which
includes at least one of said antifouling compounds and a cementitious matrix.
Such a composition is suitable for use in submerged structures, such as piers,
pilings, and offshore oil rigging equipment and scaffolding, upon which
fouling
organisms tend to settle. Exemplary cementitious matrix compositions include
portland cement and calcium aluminate based compositions. As those skilled in
this art will appreciate, the cementitious matrix should be able to release
the
antifouling compound, and the antifouling compound must be present in
sufficient concentration that the release rate of the compound into the
surrounding aquatic environment inhibits settling of organisms on the
submerged
surface of an article formed from the composition.
The invention is now desczibed in more detail in the following examples
which are provided to more completely disclose the information to those
skilled
in this art, but should not be considered as limiting the invention.
WO 94/08904
PGT/LJS93/09762
8
EXAMPLES
Collection and Culture of Experimental Specimens
Adult individuals of the acorn barnacle Balanus amphitrite Darwin were
collected from the Duke University Marine Laboratory seawall in Beaufort,
North
Carolina. Collected specimens were crushed, and the nauplius stage larvae
released therefrom were cultured to cyprid stage for cyprid-stage assays
according to the methods of Rittschof et al., 1. Exp. Mar. Biol. Ecol. 82:131-
146
(1984).
Settlement As_ sT for Cvpr~ id-Stake Larvae
Settlement assays were performed as previously described by Rittschof et
al. J. Chem. Ecol.11: 551-563 (1985). Three-day old cyprid larvae were used.
All compounds were tested for their ability to inhibit settlement by cyprid
larvae of the barnacle Balanus amphitrite. Larvae were added to 50 x 9 mm
polystyrene Petri dishes containing 5 ml of aged seawater that had been passed
through a 100 kDa cut-off filter and varying levels of test compound. Controls
consisted of barnacle larvae and filtered seawater added to the dishes without
test
compound. Dishes were then incubated for 20-24 hrs at 28° C with light
for
approximately 15 hours and in darkness for approximately 9 hours. The dishes
were then removed from the incubator, examined under a dissecting microscope
to determine whether larvae were living or dead. Larvae were then killed by
addition of several drops of 10% formalin solution. Settlement rate was
quantified
as number of larvae that had attached to the dish surface, expressed as a
percentage of total larvae in the dish. Experiments were performed in
duplicate.
The lower the percent settlement, the more efficacious the test compound.
EXAMPLE 1
Ethyl-3-furoate (9.63 N.l) was diluted to 20 ml with seawater. Aliquots of
this stock solution were added to separate dishes containing seawater to
provide
the concentrations shown in Table 1. The larvae were added and the test
conducted as described above.
TABLE 1
Control of Barnacle Settlement with Ethyl-3-furoate
Concentration % Settlement
0 (Control) 53
500 ~.g/ml 0
50 ~.g/ml 11
5 ~.g/ml 18
500 ng/ml 52
WO 94/08904 ~ ~ ~ ~ ~ ~ ~ PCT/US93/09762
9
EXAMPLE 2
Methyl-2-furoate (8.48 ~.l) was diluted to 20 ml with seawater. Aliquots of
this stock solution were added to separate dishes containing seawater to
provide
the concentrations shown in Table 2. The larvae were added and the test
condu~aed as described above.
TABLE 2
Control of Barnacle Settlement with Methyl-2-furoate
Concentration % Settlement
0 (Control) 53
500 ~.g/ml 2
50 ~.g/ml 46
5 ~.g/ml 53
EXAMPLE 3
2-Ethylfuran (6.94 ~.1) was diluted to 20 ml with seawater. Aliquots of this
stock solution were added to separate dishes containing seawater to provide
the
concentrations shown in Table 3. The larvae were added and the test conducted
as
described above.
TABLE 3
Control of Barnacle Settlement with 2-Eth, lfuran
Concentration % Settlement
0 (Control) 53
500 ~.g/ml 41
50 ~tg/ml 54
EXAMPLE 4
2-Methylfuran (10.98 ~.1) was diluted to 20 ml with seawater. Aliquots of
tlus stock solution were added to separate dishes containing seawater to
provide
the concentrations shown in Table 4. The larvae were added and the test
conducted as described above.
WO 94/08904 PGT/US93/09762
TABLE 4
Control of Barnacle Settlement with 2-Methvlfuran
Concentration % Settlement
0 (Control) 53
5 50 ~.g/ml 39
5 ~.g/mI 61
EXAMPLE 5
2-Acetylfuran (9.11N.1) was diluted to 20 ml with seawater. Aliquots of this
10 stock solution were added to separate dishes containing seawater to provide
the
concentrations shown in Table 5. The larvae were added and the test conducted
as
described above.
TABLE 5
Control of Barnacle Settlement with 2-Acetvlfuran
Concentration % Settlement
0 (Control) 53
500 ~.g/ml 54
EXAMPLE 6
2-Furyl-n-pentyl ketone (0.909 ~t.l) was diluted to 20 ml with seawater.
Aliquots of tlus stock solution were added to separate dishes containing
seawater
to provide the concentrations shown in Table 6. The larvae were added and the
test conducted as described above.
TABLE 6
Control of Barnacle Settlement with 2-Fur,~pe~ ketone
Concentration % Settlement
0 (Control) 42
500 ~,g/ml 0
50 ~.g/ml 1
5 ~,g/ml 2
500 ng/ml 14 '
50 ng/ml 26
5 ng/ml 20 '
500 ng/ml 22
WO 94/08904 PCT/US93/09762
11
EXAMPLE 7
2-Furyl-n-pentyl ketone (0.909 ~,1) 2-ethylfuran (6.94 ~.1) and 2-acetylfuran
(9.11 ~,1) were each diluted to 20 ml with seawater. Aliquots of these stock
solutions were added to separate dishes containing seawater to provide the
respective test substances in concentrations of 500 ~.g/ml. The larvae were
added
and the test conducted as described above. These data are presented in Table
7.
TABLE 7
Control of Barnacle Settlement with Furan Compounds at 500 ~, ml
Compound % Settlement
Control 61
2-Furyl-n-pentyl ketone 0
2-Ethylfuran 34
2-Acetylfuran 41
EXAMPLE 8
A number of lactones were tested for control of barnade settlement. All
lactones were tested at a concentration of 3 x 10-6 M in dishes containing
seawater. The larvae were added and the test conducted as described above.
These data are presented in Table 8.
TABLE 8
Control of Barnacle Settlement with Lactones
Compound % Settlement
Control 35
'y undecalactone 0
8-undecalactone 40
E-caprolactone 41
8-dodecanolactone 38
'y octanolactone 39
'y decalactone 3
y valerolactone 26
, EXAMPLE 9
Solutions of a-methylene-'y butyrolactone were prepared in seawater at the
concentrations shown in the table below. Five ml of each solution were added
to
duplicate dishes. The larvae were then added to the dishes and the test
conducted
as described above. These data are presented in Table 9.
WO 94/08904 PCT/US93/09762
12
TABLE 9
Control of Barnacle Settlement with ~-Methylene-y butvrolactone
Concentration _% Settlement
0 (Control) 62
500 ~.g/ml 0
50 ~.g/ml 0
5 ~tg/ml 36
500 ng/ml 58
50 ng/ml 62
5 ng/ml 61
EXAMPLE 10
A second test was performed with a-methylene-'y butyrolactone. A series of
solutions of a-methyleneJy butyrolactone in seawater at concentrations ranging
from 500 ~.g/ml to 50 ng/mI were prepared. Aliquots of these solutions were
taken and added to duplicate dishes. The actual concentrations tested appear
in
the table below. The larvae were then added to the dishes and the test
conducted
as described above. These data are presented in Table 10.
TABLE 10
Control of Barnacle Settlement with a-Meth lay Butvrolactone
Concentration ~% Settlement
0 (Control) 66
500 ~,g/ml 5
50 ~g/ml 29
5 ~g/ml 47
500 ng/ml 53
50 ng/ml 49
EXAMPLE 11
Solutions of a-angelica lactone were prepared in seawater at the
concentrations shown in the table below. Five ml of each solution were added
to
duplicate dishes. The larvae were then added to the dishes and the test
conducted
as described above. These data are presented in Table 11.
WO 94/08904 PCT/US93/09762
13
TABLE 11
Control of Barnacle Settlement with a-An elica lactone
Concentration _% Settlement
0 (Control) 62
500 ~tg/ml 0
50 ~.g/ml 39
P 5 ~.g/ml 54
500 ng/ml 60
50 ng/ml 65
5 ng/ml 62
EXAMPLE 12
A second test was performed with a-angelica lactone. A series of solutions
of a-angelica lactone in seawater at concentrations ranging from 500 ~g/ml to
5
ng/ml were prepared. Aliquots of these solutions were taken and added to
duplicate dishes. The actual concentrations tested appear in the table below.
The
larvae were then added to the dishes and the test conducted as described
above.
These data are presented in Table 12.
TABLE 12
Control of Barnacle Settlement with ,~-An eg lica lactone
Concentration _% Settlement
0 (Control) 66
500 ~,g/ml 0
50 ~.g/ml 3
5 ~.g/ml 33
500 ng/ml 33
50 ng/ml 53
5 ng/ml 41
EXAMPLE 13
A solution of 2-coumaranone (25 ~.g in 50 ml of filtered, aged seawater) was
prepared. Aliquots of this solution were taken and added to duplicate dishes
to
provide the nominal concentrations shown in the table below. The larvae were
then added to the dishes and the test conducted as described above. These data
are presented in Table 13.
~.~~~13~ ~ !
WO 94/08904 PCT/US93/09762
14
TABLE 13
Control of Barnacle Settlement with 2-Coumaranone
Concentration _% Settlement
0 (Control) 58
500 ~.g/ml 0
50 ~.g/ml
64
5 ~.g/ml 51 '
500 ng/ml 59
50 ng/ml 61
5 ng/ml 54
EXAMPLE 14
A series of solutions of 'y decalactone in seawater at concentrations ranging
from 500 ~.g/ml to 5 pg/ml were prepared. Aliquots of these solutions were
taken
and added to duplicate dishes. The actual concentrations tested appear in the
table below. The larvae were then added to the dishes and the test conducted
as
described above. These data are presented in Table 14.
TABLE 14
Control of Barnacle Settlement with
y Decalactone
Concentration _% Settlement
0 (Control) 66
500 ltg/ml 0
50 ~.g/ml 1
5 ~.g/ml 25
500 ng/ml 8
50 ng/ml 25
5 ng/ml 32
500 pg/ml 44
50 pg/ml 52
5 pg/ml 45
EXAMPLE 15
A series of solutions of y valerolactone in seawater at concentrations
ranging from 500 ~g/ml to 500 pg/ml were prepared. Aliquots of these solutions
were taken and added to duplicate dishes. The actual concentrations tested
appear in the table below. The larvae were then added to the dishes and the
test
conducted as described above. These data are presented in Table 15.
WO 94/08904 PGT/US93/09762
TABLE 15
Control of Barnade Settlement with ~Valerolactone
Concentration % Settlement
0 (Control) 66
5 500 ~.g/ml 52
50 ~g/ml 37
5 ~g/ml 42
500 ng/ml 48
50 ng/ml 42
10 5 ng/ml 43
500 pg/ml 55
EXAMPLE 16
Toxicity assays were conducted by adding nauplius stage larvae to 50 x 5
15 mm polystyrene Petri dishes or glass vials containing 5 ml of 100 kDa
filtered
seawater. Experimental dishes received doses of 'y decalactone, a-angelica
lactone,
a-methylene-~butyrolactone, a-santorun and alantolactone. Dishes or vials
receiving no test compound served as controls. The dishes or vials were
incubated
at 28° C with a 15:9 light:dark cycle. After incubation, the dishes or
vials were
exarnined under a dissecting microscope to determine whether the larvae were
alive or dead. Larvae which did not respond to an emission of visible light
were
considered dead. The number of living and dead larvae were then counted.
Probit
analysis was used to obtain concentrations corresponding to half-maximal
inhibition (EC50 values). These data are summarized in Table 16.
TABLE 16
Lactone Half maximal Inhibition Values
Comuound EC_~0
Y decalactone 4 ng/ml (plastic)
a-angelica lactone 70 ~,g/ml (plastic)
a-methylene-~-butyrolactone 6 ~.g/ml (plastic)
a-methylene-~butyrolactone 40 ~.g/ml (glass]
a-santorun 14 ~.g/ml (glass)
alantolactone 500 ng/ml (glass)
EXAMPLE 17
Settlement Assay Procedure
Laboratory experiments were performed with day 3 cyprid larvae of the
acorn barnacle Balanus amphitrite cultured as described in Rittschof et al.,
j. Exp.
Marine Biol. ~ Ecol. 82:131-146 (1984). Settlement experiments were performed
using polystyrene dishes as described in Rittschof et al., J. Chem.
Ecol.11:551-563
WO 94/08904 ~ ~ ~ ~ ~ PCT/US93/09762
16
(1985) and in Sears et al., j. Chem. Ecol. 16:791-799 (1990). Larvae were
added to
polystyrene dishes containing 5 ml of aged seawater that had been passed
through
a 100 kilo-Dalton cut-off filter and varying levels of 3-methyl-2-cyclohexene-
1-one.
Controls consisted of barnacle larvae and filtered seawater added to
polystyrene
dishes without 3-methyl-2-cyclohexene-1-one. After addition of larvae, the
dishes
were incubated for 20 to 24 hours at 28°C on a 15:9 light:dark cycle.
The dishes were then removed from the incubator, examined under a
dissecting microscope to determine if larvae were living (moving) or dead (not
moving). Larvae were then killed by addition of several drops of 10% formalin
solution.
Settlement rate was quantified as number of larvae that had attached to the
dish surface, expressed as a percentage of total larvae in the dish. Dishes
were
more than 200 larvae were excluded from subsequent analysis, since extremely
high larval densities may ixthibit settlement rates. Linear regressions were
performed using percentage settlement as the dependent (Y) variable and log of
larval density (larvae per dish) as the independent variable. Each dish was
treated as a single replicate.
Settlement Assav Results
Data were combined for all control dishes (n=65 for combined data set). In
the controls, barnacle settlement increased as a linear function of larval
density
(Figure 1). The least squares regression equation for the data is: Y=47.4 (log
X) -
41.3, where Y represents settlement rate and X represents larval density. Data
also
were combined for treatments generated by addition of 3-methyl-2-cyclohexene-1-
one at concentrations of 9, 90, and 900 picomolar (n=44 for combined data
set). At
these concentrations, barnacle settlement decreased as a linear function of
barnacle density (Figure 2). The least squares regression equation for this
data set
is: Y= -51.7 (log X) + 118.6, where Y represents settlement rate and X
represents
larval density).
Data also were combined Eor all higher concentrations of 3-methyl-2-
cyclohexene-1-one (n=107) for combined data set - data not shown). The
regression line for these replicates was not significantly different from that
of the
untreated controls (regression equation: Y = 48.5 (log X) - 49.1, where Y
represents settlement rate and X represents larval density). The decrease in
effectiveness at higher concentrations is not atypical for anti-aggregative
pheromones.
While the invention has been described with reference to specific examples
and applications, other modifications and uses for the invention will be
apparent
to those skilled in the art without departing from the spirit and scope of the
invention as defined in the following claims.