Note: Descriptions are shown in the official language in which they were submitted.
~. 214635
WO 94/08896 PCT/US93/09906
I
SLOW RELEASE FERTILIZER AND
ACTIVE SYNTHETIC SOIL
Origin of the Invention
The invention described herein may be manufactured and used by
or for the Government of the United States of America for governmental
purposes without the payment of any royalties thereon or therefor.
Field of the Invention
The present invention relates to an active synthetic soil for
horticulture. More particularly the present invention relates to an active
synthetic soil made from synthetic apatite and natural zeolite having a
complete spectrum of agronutrients necessary for plant growth.
Background of the Invention
Synthetic soils for horticulture (i. e., solid substrates for plant
support) include two general categories-inert and active. Inert
substrates are commonly used in nutriculture (e. g., hydroponics) and
are designed to provide mechanical support, proper root aeration and
drainage. Quartz sand is a good example of an inert soil. Plant
nutrients are added separately as, for example, liquid fertilizers such as
Hoagland's solution. Soils which are defined as "active" have the ability
to provide nutrient retention and release (i. e., incorporate fertilizing
capability) in addition to the other primary soil functions of the above
mentioned inert soils.
It is known that nutrient retaining activity in natural soils is due to
the presence of organic matter and clay components. Such components
have charge sites suitable for ion exchange. Prior to release, the
nutrient elements are held at the charge sites as "exchange ions."
Recent introduction of ion exchange media (that are not normally found
in natural soils) having a high exchange ion holding capacity have made
feasible the development of active synthetic soil-fertilizers which can
supply plant nutrients over a long period of time.
Mineral zeolites have been found to be a class of very useful ion
exchange media. Many natural species are prevalent and numerous
synthetic species have been made in the laboratory. Zeolites are
hydrated aluminosilicates of alkali and alkaline-earth rations that
possess infinite, three-dimensional crystal structures (i.e.,
2~463~9
'~O 94/0889b ~ f ~ PCT/US93/09906
2
tektosilicates). The primary building units of the zeolite crystal structure
are (AI,Si)04 tetrahedra. When A13+ and sometimes Fe3+ substitute for
Si4+ in the central ration position of the tetrahedron, a net-negative
charge is generated. This negative charge is counterbalanced primarily
by monovalent and divalent "exchange rations." Zeolites have shown
the ability to exchange most of their constituent exchange rations as
well as hydrateldehydrate without major changes in the structural
framework. Most zeolites have large channels andlor cages that allow
exchange rations easy access to charge sites and provide unique ration
selectivity.
The use of zeolites as a major soil component has a relatively
recent past. U. S. Patent 4,337,078 to Petrov et al. describes the use of
a natural zeolite clinoptilolite with vermiculite and peat in a synthetic
soil. The term zeoponics has been coined to describe synthetic soils
containing zeolites in horticulture.
Agronomists and botanists have long recognized the vital function
of sixteen nutrients needed by growing plants including the trace
elements or micronutrients - zinc, chlorine, iron, manganese, copper,
molybdenum and boron. It is also known that the optimal spectrum and
concentration of micronutrients in a particular soil can vary depending on
the plants being grown, soil properties, climate, and the stage of the
plant growth cycle.
While most natural soils contain micronutrients at least to some
extent and the overall need is small, depletion can occur with intensive
agricultural activity. Even when the soil concentration is putatively
adequate, other factors can prevent micronutrient uptake by the plant.
Since micronutrients must be available as soluble ions, such ions can be
immobilized in low solubility alkaline soils andlor can be trapped on
clays or organic materials as insoluble complexes.
It has been common practice to supplement phosphorus-
impoverished soil by using a mineral fertilizers such as rock phosphate
or natural apatite Such minerals, however, do not supply the required
micronutrients and can contain toxic elements such as fluorine and '
cadmium.
Rock phosphate as mined is relatively insoluble in water.
TherEfore, the raw product is generally pretreated to enhance phosphate
solubility prior to use. Such processes, however, are considered too
2146359
3
expensive for farmers in underdeveloped nations. Yet, fertilizer use is
necessary to promote economic development.
Summary of the Invention
The present invention provides a synthetic soil and fertilizer composition
for horticulture which contains an entire spectrum of nutrients essential for
plant
growth. The soil combines a ration exchange medium charged with ammonium
and potassium exchange rations and an apatite composition ~ comprising
magnesium, sulfur and plant micronutrients. The apatite is preferably
synthetic
which, unlike natural varieties is essentially free of toxic elements. The
presence of moisture mobilizes the plant nutrients at a slow, steady rate. In
addition, the nutrient release rate can be closely tailored to the
horticultural
requirements. These features and others offer potential for use in lunar and
other agriculture applications.
In one embodiment, the present invention provides a slow release
fertilizer. The fertilizer is made from apatite comprising a matrix of calcium
phosphate having a dispersion of one or more agronutrients and a cationic
exchange medium having a charge of one or more agronutrients. The apatite
and cationic exchange medium are preferably essentially free of agrotoxins,
such as, for example, fluorine, cadmium and sodium, in amounts detrimental to
the growth of most plants. Agronutrients include, for example, potassium,
ammonium-nitrogen, magnesium, sulfur, zinc, chlorine, iron, manganese,
copper, molybdenum and/or boron. The fertilizer can further include a pH
buffer
to maintain a pH balance of from about 5.5 to about 7. The cationic exchange
medium can comprise natural or synthetic zeolite, phyllosilicate or a
combination thereof including clinoptilolite, chabazite, mordenite,
phillipsite,
Linde type A, Linde type X, vermiculite, smectite or a combination thereof.
The
ration exchange medium has a ration exchange capacity (CEC) of at least 50
cmolclkg, preferably at least 100 cmolclkg, and more preferably ~at least 150
cmolclkg. The ration exchange medium preferably has a charge of ammonium
and potassium ions at a weight ratio of from about 1 to about 5:1 of
ammonium:potassium. The fertilizer preferably comprises from about 5 to
about 100 parts by weight of the synthetic apatite per 100 parts by weight of
the
cationic exchange medium.
In a preferred embodiment, the apatite of the fertilizer has a generally
uniform composition and corresponds to the formula:
(~a5-xm/2 Mx)((P~4)3-yq/3 Qy)((~H)1-zXz)
AMENDED SHEET
CA 02146359 2001-02-13
3A
wherein M is a ration containing an element selected from potassium, zinc,
iron,
manganese, magnesium, or copper or a combination thereof; wherein m is the
molar average valence of M according to the equation m = (Emixi)/(Exi) where
each mi is the valence of an itn ration comprising M and xi is the relative
molar
proportion of the ~ ration; wherein Q is an anion of carbonate, silicate or
containing an element selected from boron, molybdenum, or sulfur, or a
combination thereof; wherein q is the molar average valence of Q according to
the equation q = (Eqiyi)l(Eyi) where each qi is the valence of an ltn anion
comprising Q and yi is the relative molar proportion of the ~tnanion; wherein
X is
chloride, fluoride or a combination thereof; and wherein x has a value of 0 -
0.82, y has a value of 0 - 0.76, and z has a value of 0 - 0.15, provided that
at
least one of x and y are greater than zero and the amount of fluoride does not
exceed 3000 ppm by weight, and also provided that when x is zero Q includes
an anion of boron, rr~alybdenum andlor sulfur. Preferably Mx has the formula:
~ KxKMgxMgFexFeZnxZnMnxMnCuxCu
wherein xK S 0.205; xMg <_ 0.412; xFe S 0.144; xZn S 0.0123; xMn S 0.044; xCu
<_ 0.0038; x = xK + xMg + xFe + xZn + xMn + xCu~ and wherein x > 0. More
preferably, 0.051 S xK S 0.?_~~5; 0.165 S xMg S 0.412; 0.0359 S xFe S 0.144;
0.006 _< xzn <_ 0.0123; 0.018 S xMn S 0.044; and 0.0016 S xCu S 0.0038.
Especially, 0.102 S xK <_ 0. ~i 54; 0.247 S xMg S 0.33; 0.072 S xFe S 0.108;
0.0061 S xzn S 0.0092; 0.018 S xMn S 0.036; and 0.0025 S xCu S 0.0032. Qy
preferably has the formu!~::
(C03)yC{Si04)ySi(Mo04)yMo (B03)yB (S04)yS
wherein yC has a value up to about 0.5, y ,i has a value up to about 0.218,
YMo
has a value up to about 0.000052, yB has a value up to about 0.0093, and yS
has a value up to about 0.25; and wherein y = yC + YSi +YMo + YB + YS, and
{YMo + YB + YS) > 0. More preferably, 0.00002 <_ yMo <_ 0.000042; 0.00185 S
yg <_ 0.00741; and 0.125 <_ yS <_ 0.25. Especially, 0.000021 s yMo S
0.0000313; 0.0037 <_ yg _< 0.0056; and 0.156 S yS S 0.219. Where the
solubility
control agent is carbonate, preferably 0.0668 <_ yC S 0.334, and especially
0.134 S yC 50.2; and where it is silicate, preferably 0.0435 5 ySi <_ 0.131,
and
especially 0.0653 <_ ySi <_ 0.109. Xz preferably has the formula:
CIzCI FZF
_ _ __
38
wherein zCl has a value up to about 0.071, zF has a value less than about
0.08,
and z = zCl + zF. More preferably, 0.0283 S zCl s 0.071; and zF S 0.008.
Especially, 0.0565 5 zCl S 0.064; and zF S 0.00008.
In a particularly preferred embodiment, the apatite of the fertilizer in the
present invention is an agronutrient-substituted hydroxylapatlte of the
formula:
(Ca5-xmI2KxKMgxMgFexFe~xZnMnxMnCuxCu) I(P04)3-yql3
(C03)yC(Si04)ySi(Mo04)yMo (B03)yB (S04)yS]I(OH)1-z CIzCIFzF)
wherein m is the molar average valence of the potassium, magnesium, iron,
zinc, manganese and copper rations according to the equation:
m = (xK + 2xMg+2xFe+2xZn+2xMn+?.xCu)~x
wherein q is the molar average valence of the anions C03, Si04, Mo04, B03
and S04 according to the equation:
q = (2YC + 4YSi +2YMo + 3YB + 2YS)~Y
wherein x = xK + xMg + xFe + xZn + xMn + xCu, Y = YC + YSi +YMo + YB + YS.
z = zCl + zF, and at least one of x, yMo, yg and yS is greater than zero; and
wherein xK S 0.21; xMg S 0.41; xFe 5 0.14; xZn s 0.012; xMn S 0.044; xCu s
0.0038; yC S 0.5; ySi 5 0.218; yMo S 0.000052; yg S 0.0093; yS S 0.25; zCl 5
0.071; and zF _< 0.08. Preferably, 0.051 s xK s 0.205; 0.165 5 xMg S 0.412;
0.0359 S xFe 5 0.144; 0.006 5 x~ 5 0.0123; 0.018 s xMn 5 0.044; 0.0016 5
xCu S 0.0038; 0.00002 5 yMo 5 0.000042; 0.00185 S yg 5 0.00741; 0.125 S yS
5 0.25; 0.0283 S zCl S 0.071; and zF 5 0.008. Especially, 0.102 5 xK S 0.154;
0.247 5 xMg s 0.33; 0.072 5 xFe < 0.108; 0.0061 S x~ S 0.0092; 0.018 S xMn s
0.036; 0.0025 s xCu S 0.0032; 0.000021 S yMo S 0.0000313; 0.0037 S yB s
0.0056; 0.157 S yS S 0.219; 0.0565 S zCl S 0.064; and zF S 0.00008.
* *
AfIA~f~ED SHEET
~214G359
WO 94/08896 ~ PCT/US93/09906
4
In another embodiment, the present invention provides a
horticultural method: In one step, a botanical species is planted in a
sufficient amount of the fertilizer composition described above. In
another step, the fertilizer is contacted with moisture to mobilize the
agronutrients.
In a further embodiment, the present invention provides a method
of making an active synthetic fertilizer. In one step, a synthetic apatite is
prepared by admixing in an aqueous medium from about 1.0 to about
1.6 moles per liter of a soluble ionic calcium compound and a solution
mixture comprising from about 0.5 to about 0.8 moles per liter of a
soluble ionic phosphate compound and an agronomic amount of, one or
more soluble agronutrients selected from magnesium, zinc, sulfur,
chlorine, iron, manganese, copper, molybdenum and boron to form a
crystalline calcium phosphate precipitate having agronutrients dispersed
therein. The precipitate is recovered, dried and suitably granulated. As
another step, individual zeolite portions are charged with ammonium
and potassium rations to displace native rations. The precipitate is
blended with the charged zeolites at a proportion of from about 5 to
about 100 part by weight of the precipitate per 100 parts by weight of the
ammonium and potassium charged zeolites. The weight ratio of
ammonium charged zeolite to potassium charged zeolite is from about 1
to about 5:1. The zeolite is preferably clinoptilotite. The fertilizer blend
preferably includes from 0 to about 10 parts by weight of a pH buffer per
100 parts by weight of the ammonium and potassium charged zeolites.
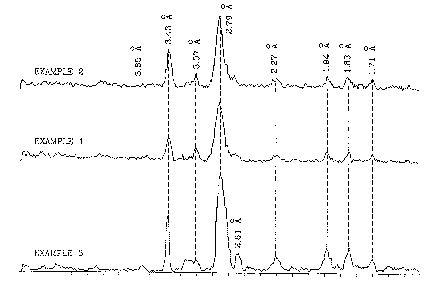
Brief Description of the Figure
The Figure shows diffractographs with peak spacing for three
different synthetic apatite compositions of the present invention. The
diffractographs indicate that the present synthetic apatite has a
crystalline structure similar to naturally occurring hydroxyapatite and
carbonate hydroxyapatite.
Detailed Description of the Invention
An entire spectn.im of essential agronomic nutrients including .
nitrogen, potassium, magnesium, sulfur and micronutrients are
incorporated into an active synthetic soil for horticulture. Upon contact
by moisture, the nutrients are slowly released, as required, for plant use.
214~3~9
WO 94/08896 PGT/US93/09906
In addition, a fertilization rate can be controlled and the soil tailored to
horticultural needs.
The major component of the synthetic soil composition is a
synthetic apatite fertilizer. The apatite has a calcium phosphate matrix
' 5 which is at least slightly soluble in water. Water solubility is necessary
to give mobility to nutrient elements contained in the apatite matrix.
Examples of suitable calcium phosphates include dicalcium
orthophosphate (CaHP04), monocalcium orthophosphate
(Ca(H2P04)2), tricalcium orthophosphate (Ca3(P04)2), hydrates
thereof and calcium pyrophosphate pentahydrate (Ca2P207~5H20).
Preferably, from about 30 to about 50 parts by weight phosphorus are
used per 100 parts calcium, and more preferably, from about 40 to
about 45 parts by weight phosphorus per 100 parts calcium.
One or more essential agronomic nutrients besides calcium and
phosphorus are dispersed within the crystal structure of the synthetic
apatite. Essential agronomic nutrients (agronutrients) in addition to
calcium and phosphorus, include potassium, nitrogen, magnesium,
sulfur, zinc, chlorine, iron, manganese, copper, molybdenum and boron.
The latter seven elements (zinc, chlorine, iron, manganese, copper,
molybdenum and boron) are generally referred to as micronutrients and
are needed by plants in lower amounts than the other essential
agronutrients.
Agronutrients are provided in the present composition as water
soluble inorganic (ionic) compounds. The inorganic compounds should
not have acute toxicity (e. g. cyanide salts), or other undesirable
properties and should be free of excessive amounts of agrotoxins
including unwanted elements and organic toxins. Undesirable elements
typically include most heavy metals such as lead, cadmium, mercury,
and the like, and other elements such as fluorine, sodium, arsenic,
antimony, selenium, tin, and the like. The synthetic apatite can,
however, contain a relatively small amount of any of these toxins below
a toxic level for plants and, where appropriate, grazing animals. For
example, natural apatite contains about 6 percent fluorine and has only
limited potential as a soil supplement because of the fluorine toxicity,
particularly to grazing animals such as sheep which can ingest the
fluorine, e.g. by licking the soil containing the supplement. Prior art
phosphatic fertilizers, in contrast, can contain about 3000 ppm fluorine,
whereas natural soils average about 300 ppm and plants typically
214f 3~9
WO 94/08846 - ~ PCT/US93/09906
6
contain about 3 ppm fluorine. Thus, the present synthetic apatite
composition should generally contain no more than 10 parts fluorine per
100 parts calcium, by weight, but preferably contains no more than 3000
ppm fluorine, more preferably no more than 300 ppm, and especially no
more than 3 ppm. Tolerance levels of specific plants and animals for
other agrotoxins can be found in the literature or determined empirically.
The amount of agrotoxins in the synthetic apatite should be less than an
amount which would result in release into the environment of the
agrotoxins in excess of a given tolerance level.
Examples of suitable water soluble compounds of agronutrients
used in the preparation of the synthetic apatite include potassium
compounds such as potassium chloride, potassium nitrate, potassium
nitrite, potassium sulfate, and potassium phosphate; magnesium
compounds such as magnesium nitrate, magnesium chloride,
magnesi~rm nitrite, magnesium chlorate, magnesium perchlorate and
hydrates thereof; sulfur compounds such as sodium sulfate, ammonium
sulfate, potassium sulfate, and hydrates thereof; zinc compounds such
as zinc chloride, zinc nitrate, zinc nitrite, zinc sulfate and hydrates
thereof; chlorine compounds such as sodium chloride, potassium
chloride, ammonium chloride; iron compounds such as ferric nitrate,
ferrous ~ nitrate, ferrous nitrite, ferric nitrite, ferric chloride, ferrous
chloride, ferric sulfate, ferrous sulfate and hydrates thereof; manganese
compounds such as manganese(II) nitrate, manganese(II) nitrite,
manganese dichloride, manganese(II) sulfate and hydrates thereof;
copper compounds such as copper(II) chloride, copper(III) chloride,
copper(II) nitrate, copper(II) nitrite and hydrates thereof; molybdenum
compounds such as ammonium paramolybdate, ammonium
permolybdate, sodium trimolybdate, sodium tetramolybdate, sodium
paramolybdate, sodium octamolybdate, potassium molybdate and
hydrates thereof; and boron compounds such as sodium tetraborate,
sodium metaborate, potassium tetraborate, potassium metaborate,
ammonium tetraborate, hydrates thereof and orthoboric acid. The
preferred, more preferred and optimum amounts of the agronomic '
nutrient elements per 100 parts calcium in the synthetic apatite, are set
forth in Table 1.
CA 02146359 2001-02-13
7
Table 1
AgronutrientPreferred More Optimum
Element Amount Preferred Amount
(parts Amount (parts
by (parts by
Weight) by weight)
wei ht
Ca 100 100 100
P 30-50 40-45 40-42
K 0-4 1-4 2-3
M 0-5 2-5 3-4
S 0-4 2-4 2.5-3.5
Zn 0-0.4 0.2-0.4 0.08-0.3
CI 0-1.25 0.5-1.25 0.1-0.13
Fe 0-4 1-4 2-3
Mn 0-1.2 0.5-1.2 0.5-1
Cu 0-0.12 0.05-0.12 0.08-0.1
Mo 0-0.0025 0.001-0.0020.001-
0.0015
B 0-0.05 0.01-0.04 0.02-0.03
The synthetic apatite composition can also comprise a silicon and/or carbonate
solubility control agent dispersed in the apatite matrix. The solubility
control agent
increases or decreases the water solubility and permits enhanced control over
the rate
at which nutrient elements are released. The effect of carbonate content on
natural
apatites is described in several publications including Caro, J., Journal of
Agricultural
Food Chemistry, 4:684-687, 1956; McClellan, G., American Mineralogist, 54:1374-
1391, 1969; and Lehr R., National Fertilizer Development Center Bulletin, Y-
43, Vol. 8
published by the Tennessee Valley Authority, Muscle Shoals, Alabama.
The solubility control agent is provided in the synthetic apatite composition
as a
water soluble inorganic or organic compound. Examples of suitable water
soluble
carbonate compounds include sodium carbonate, sodium bicarbonate, ammonium
carbonate, ammonium bicarbonate, potassium carbonate and potassium
bicarbonate.
Examples of water soluble silicon compounds include inorganic silicates such
as
sodium silicate, sodium disilicate, sodium metasilicate, sodium orthosilicate,
potassium disilicate, potassium metasilicate, potassium hydrogen disilicate,
ammonium silicate, and hydrates thereof, and organic silicates such as ethyl
orthosilicate and propyl orthosilicate.
A solubility control agent can comprise from 0 to about 15 parts by weight per
100 parts calcium. A carbonate agent is preferably used in an amount of 0 to
about
15 parts by weight per 100 parts calcium,
_~~463~9
'~'VO 94/08896 ~ PGT/US93/09906 ,
8
more preferably from about 2 to about 10 parts by weight and optimally
from about 4 to about 6 parts by weight. A silicon agent is preferably
used in an amount of 0 to about 10 parts by weight per 100 parts
calcium, more preferably from about 2 to about 6 parts by weight and
optimally from about 3 to about 5 parts by weight.
The present synthetic apatite composition can optionally include a
binder agent to assist processing of the calcium phosphate into pellet
form. Examples of such processing aid binders include calcium-
lignosulfonate, cellulose, and the like. The binder comprises from 0 to
70 about 10 percent by weight or more of the synthetic apatite.
The present synthetic apatite composition is the precipitated
product of a water soluble calcium compound and a water soluble
phosphate mixture comprising a water soluble phosphate compound and
one or more water soluble agronutrients. The resulting product has
nutrient elements incorporated into the structure of the calcium
phosphate matrix.
The second component of the present synthetic soil is a cationic
exchange medium saturated with a charge of exchange rations of one or
more agronutrients. Suitable cationic exchange media have a ration
exchange capacity (CEC) greater than about 50 cmolclkg. Cationic
exchange media preferably have a CEC of at least about 100 cmolclkg,
but more preferably at least about 150 cmolclkg. In addition, suitable
cationic exchange media are substantially chemically inert, have low
solubility in water and are essentially free of elements toxic to plant
growth.
A most preferred class of suitable cationic exchange media are
mineral zeolites. Zeolites as mentioned previously are hydrated
aluminosilicates of alkali and alkaline-earth rations that possess infinite,
three-dimensional crystal tetrahedral structures. ~ Natural zeolites are a
common mineral matter widely found in a relatively pure state.
Synthetic zeolites have also been manufactured. Zeolites generally
have a theoretical CEC of from about 200 cmolc/kg to about 600
cmolclkg or more for some synthetically produced varieties. '
Representative examples of common natural zeolites include
clinoptilolite (Na3,K3){AIgSi30072}~24H20, chabazite
(Na2,Ca)6{AI12Si24072}~40H20, mordentite Nag{AIgSi300g6}~24H20,
phillipsite (Na,K)5{AI5Si11032}~20H20, and the like.
214539
WO 94/08896 PCT/US93/09906
9
Representative examples of synthetic zeolites include Linde Type
- A Nag6{AIg6Sigg0384}~216H20, Linde Type X
Na86{AI86Si106~384}~2~H20, and the like.
Due to desirable sand-like mechanical properties, a high degree
of internal tunneling for favorable nutrient retention capacity and relative
abundance in nature, a most preferred natural zeolite is clinoptilolite
which is widely found in a relatively pure state. Clinoptilolite has been
found to have good drainage and water holding characteristics, and a
high theoretical ration exchange capacity of about 200 cmolclkg.
Clinoptilolite also has a high affinity for NH4+ and the ability to hold the
ion internally away from nitrifying bacteria. Hence nitrification rates are
slow and the amount of leached N is low. Clinoptilolite is commercially
available as sand-sized particles.
While zeolites are preferred cationic exchange media, other types
can be used. Examples of other natural mineral exchange media are
phyllosilicate clays such as vermiculite and smectite. Ion exchange
resins can also be used though more expensive. For convenience of
illustration, the cationic exchange medium will be referred to
hereinbelow as the preferred but non-limiting zeolite embodiment.
The zeolite in the present synthetic soil is wholly or partially
saturated with a charge of exchange rations of one or more agronomic
nutrients so that existing native rations such as Na~ are replaced with
the desired agronutrient rations. Applicable agronutrients which can be
charged on the zeolite generally include potassium, ammonium,
manganese(II), zinc, iron(II), copper(II), calcium and magnesium.
Selectivity (i. e. retention capacity) of exchange rations can vary
depending on the type and variety of the ration exchange medium in
question. However, as a rule of thumb, the adsorption selectivity in
clinoptilolite favors monovalent exchange rations over divalent rations
and among these, ion selectivity generally decreases with increasing ion
hydration radius. For a clinoptilolite sample mined in the Wyoming
region, selectivity for agronutrients and sodium was determined as
follows: potassium > ammonium » sodium > manganese(II) -
copper(II) = iron(II) > zinc > calcium > magnesium.
In the practice of the prESent invention, the zeolite is preferably
saturated with ammonium and potassium rations (totally replacing
native rations) at a weight ratio of from about 1 to about 5:1 of
ammonium:potassium. As used herein, agronomic nutrients saturated
- 2~.45~59 .
WO 94/08896 PCT/US93/09906
on the zeolite will be referred to by the preferred but non-limiting
potassium and ammonium embodiment.
The present soil comprises from about 5 to about 100 parts of the
synthetic apatite per 100 parts by weight of the K+, NH4+ saturated
5 zeolite.
A third optional but preferred component of the present soil
composition is a pH buffer to maintain a soil pH in the range of from
about 5.5 to about 7. Examples of suitable pH buffers include weak
acids (e. g., humic acid). The pH buffer is used at from about 0 to about
10 10 parts per 100 parts by weight of the K+, NH4+ saturated zeolite.
The synthetic apatite is conveniently made, for example, by
preparing two or more aqueous stock solutions containing the
appropriate compounds and mixing the stock solutions together. An
inorganic replacement reaction occurs in the solution mixture to produce
a precipitate. The precipitate can be recovered, e. g. by filtration, and
dried.
A first stock solution is made by dissolving a suitable quantity of
the water soluble calcium compound in a neutral or basic aqueous
medium. Examples of suitable calcium compounds include calcium
nitrate, calcium nitrite, calcium chloride, calcium chlorate, hydrates
thereof, and the like. Calcium nitrate tetrahydrate is a preferred
compound. The first solution preferably includes the calcium compound
in an amount of from about 1.0 to about 1.6 moles per liter.
A second stock solution is prepared by dissolving a suitable
quantity of the soluble phosphate compound and suitable quantities of
the soluble anionic nutrient compounds) in a neutral or basic aqueous
medium. Examples of suitable soluble phosphate compounds include
ammonium orthophosphate-mono-H, ammonium orthophosphate-di-H,
ammonium orthophosphate, ammonium hypophosphate and the like.
The second solution preferably includes the phosphate compound in an
amount of from about 0.5 to about 0.8 moles per liter. The amount of
anionic nutrient compounds) in the second solution will depend on the
desired concentration in the synthetic apatite end product which, in tum, '
will depend on the agronomic application. Generally, the second stock
solution can include one or more anionic nutrient compounds each in an
amount of from about 0.002 to about 0.4 moles per liter.
A third stock solution is prepared, where appropriate, by
dissolving a suitable quantity of the soluble cationic nutrient
z~~~3~s
WO 94/08896 PCT/US93/09906
11
compounds) in a neutral or basic aqueous medium. The quantity of the
cationic nutrient compounds) in the third solution will again depend on
the desired concentration in the synthetic apatite end product which, in
tum, will depend on the agronomic application. Generally, the third
solution includes one or more cationic nutrient compounds, each in an
amount of from about 0.05 to about 5 moles per liter.
The optional silicon andlor carbonate solubility control agent can
be added to the second (anionic) stock solution in an amount of from
about 0.002 to about 0.4 moles per liter.
When preparing the above stock solutions, it is desirable to avoid
mixing salts together which can undergo unwanted inorganic
replacement reactions in the stock solutions. Therefore, ionic
compounds having a desired component element in the anion are held
in solution separately from ionic compounds having a desired
component element in the ration. Liquid organic compounds (e. g. ethyl
orthosilicate), however, can be added to any of the stock solutions or
added separately before or after the stock solutions are mixed together.
A preferred basic aqueous medium comprises a solution of from
about 18 to about 30 percent by weight of ammonium hydroxide in
deionized water. A preferred neutral aqueous medium comprises
deionized water.
Typically, the third stock solution is mixed with the second stock
solution and the combined solution is then mixed with the first stock
solution. The resulting mixture is then maintained at ordinary
temperature and pressure for a sufficient time period for the crystalline
precipitate to form.
The precipitate is recovered by ordinary means, such as, for
example, by decanting the supernatant and filtering in a Buchner funnel.
The precipitate is preferably washed with deionized water.
The washed precipitate can be dried at room temperature.
Preferably, however the precipitate is dried at a temperature ranging
from about 200°C to about 600°C for a time period of from about
2 to
' about 20 hours in drying equipment such as an oven, wherein the
temperature is preferably boosted in steps of 200°C after 2 hour
intervals. The drying procedure can simultaneously dry the precipitate
and dehydrate or partially dehydrate the calcium phosphate endproduct.
Solubility is also partially dependent on the degree of hydration of the
calcium phosphate crystals, i. e., crystal size and degree of crystallinity.
2I4G3~ 9
WO 94/08896 ~ PGT/US93/09906
12
Since solubility is reduced by dehydration, the drying procedure
specified can be used to adjust the solubility of the final product. The
actual drying procedure used is not particularly critical so long as care is
exercised in obtaining the desired degree of dehydration. The dried
precipitate is preferably cooled in a low humidity environment.
The precipitate can be crushed, granulated or pelletized by
conventional means to produce a suitable particle size for use in soil
treatment. Binding agents can be used to assist the formation of a
relatively consistent granulation particle size and avoid the production of
fines. Preferably, non-reactive binders are used.
As indicated above, the type of nutrient elements incorporated
into the calcium phosphate crystal structure can vary from a single
nutrient element to all seven micronutrients as well as potassium, sulfur
and magnesium. The quantity of each nutrient element incorporated can
be specified based on the agronomic factors involved.
Prior to use, native rations of the zeolite exchange medium are
replaced with rations of agronutrients, preferably NH4+, K+ as
mentioned above. Various methods can be employed. Generally,
zeolite particles having a size from about 50 mm to about 1000 mm are
preferably divided into individual portions for each agronutrient used.
Each portion is then preferably individually charged with the desired
agronutrient until saturation. The agronutrient charge is conveniently
provided by a sufFiciently concentrated (e. g., 1 M) aqueous solution of
an ionic compound such as a chloride, nitrate, sulfate, and the like of the
agronutrient. Typically, the zeolite and nutrient solution are contacted at
a suitable weight ratio, such as, for example, from about 1:2 to about 1:5
zeolite:nutrient solution. To ensure that the exchange sites of the zeolite
are saturated with the agronutrient, the mixture is preferably agitated in
a suitable vessel for a period of time such as 24 hours, the solution is
decanted, and the zeolite is washed an additional two times with the
appropriate solution. Afterward, the supernatant is decanted and the
zeolite is washed with deionized water to remove excess nutrient
solution. The wash supernatant can be tested with an indicator
compound to determine the presence of excess solution in the zeolite.
Silver nitrate, for example, is a good indicator for chloride ions.
After each portion of the zeolite is saturated with the desired
agronutrient charge and excess solution is removed, the saturated
zeolites are dried in an oven, for example, at a temperature on the order
. 2146359
WO 94/08896 PCT/US93/09906
13
of 105°C for a time period on the order of 24 hours. Once dried, the
synthetic apatite and various saturated zeolite components can be dry
blended in suitable equipment at a desired ratio.
When the instant synthetic soil comes in contact with moisture,
' 5 nutrient elements become mobilized as the apatite is dissolved.
As a
first step, nutrients dispersed in the apatite matrix (magnesium,
sulfur
and micronutrients in addition to phosphorus and calcium)
are slowly
released as dissolution proceeds. Calcium ion production
is adsorbed
by the zeolite which acts as a Ca2+ sink. Removal of Ca2+
from the
solution phase shifts the equilibrium towards increased apatite
dissolution and phosphate fertilization in the soil. Adsorbed
calcium
ions compete with the K+ and NH4+ ion charge at zeolite exchange
sites causing the release of K+ and NH4+ into the soil. The
pH buffer
maintains a mildly acidic soil pH to further assist the rate
of apatite
dissolution and nutrient release.
Desired apatite solubility and nutrient release rate are
usually
determined empirically based on type of plant being grown,
growth cycle
requirements, and the like agronomic factors.
The present fertilizing soil can be used in conventional
agronomic
applications by direct addition by conventional means to
a suitably
prepared field but is preferably used in horticultural applications
such as
zeoponics and hydroponics.
The present synthetic soil has potential for lunar applications
since zeolite synthesis from minerals found on the moon is
thought to be
feasible. Furthermore, plant-essential elements occur in
trace quantities
in lunar rock and can be extracted.
To conduct a zeoponics culture, for example, a suitable
greenhouse or culture environment has the present synthetic
soil and
fertilizer appropriately blended and spread to a sufficient
depth to
support the root structure of seedlings planted therein.
The soil is kept
moist to fertilize the plants.
The present invention is further illustrated by the following
examples:
Examples 1-3
Three synthetic apatite compositions having nutrient elements
incorporated into the crystalline structure were synthesized by an
inorganic replacement reaction to simulate a naturally occurring
~~~fi3~9
JVO 94/08896 PCT/US93/09906
14
hydroxyapatite mineral. Initially, three stock solutions (A, B and C) were
prepared using laboratory reagent grade chemicals. Each reaction was
run using 500 ml of stock solutions A and B and 20 ml of stock solution
C. The composition of the solutions is shown in Table 2.
Table 2
Compound Concmtratim
(grams)
Ex le 1 Exa (e Ex le 3
2
Solution A (0.5
liters 20 rrt X
NH OH in deionized
water)
Calcium nitrate 141.52 141.52 141.52
tetrahydrate
(Ce(N ) '4H 0)
Solution B (0.5
Liters 20 xt X
NH OH in deionized
water)
Ammonium orthophosphate-43.32 43.32 43.32
mono-N
((NH ) HPO )
Mmbnium carbonate 11.93 11.93
((NH ) )
Ammonium chloride 1.011 1.011 1.011
((NH )Cl)
Orthoboric acid 0.779 0.779 0.779
(H BO )
Amaonium paramolybdate0.00098 0.00098 0.00098
((NH ) N '4H 0)
Ammonium sulfate 2.4974 2.4974 2.4974
NH ) SO )
Solution C (20 ml
deionized water)
Nagneaium nitrate 13.499 3.374 12.972
(Ng(Jl ) )
Iron(IL) nitrate 3.627 3.627 3.627
hexahydrate
(Fe(N ) '6H 0)
lianganese(11) sulfate0.5408 0.5408 0.5408
monohydrate
(llnSO ' H 0 )
Zinc nitrate 0.5652 0.5652 0.5652
(zn(N ) )
Copper(II) nitrate 0.1464 0.1464 0.1464
2.5hydrate
(Cu(N ) )'2.5H 0)
Other additive (ml)
Ethyl orthosilicate- S -
After stock solutions A, B and C were prepared, solution C was
quickly added to solution B and vigorously mixed for several seconds.
This combined solution (B and C) was then added to solution A. In
Example 2, the ethyl orthosilicate liquid was also added to solution A
concurrently with solutions B and C. In all the examples the final mixture
was vigorously stirred for 5 minutes and then allowed to stand for 18
2146359
WO 94/08896 PCT/US93/09906
hours to precipitate the calcium phosphate product. The clear
supernatant was decanted and disposed of. The precipitate was
washed 4 times with 3 liters of deionized water each washing. The
precipitate was filtered using a Biichner funnel and Whatman #41 filter
' S paper, and washed again with an additional 500 ml of deionized water.
The precipitate was removed from the filter paper and placed into a
' glass beaker for drying. The precipitate was dried in an oven at
200°C
for 17 hours, lightly crushed in an agate mortar and stored in a
desiccator.
10 The three synthetic materials were characterized by powder x-ray
diffraction and by electron microprobe analysis. The Figure shows
diffractographs of the compositions. The peaks (d-spacing) correspond
to peaks for natural hydroxyapatites. Peak width was narrow suggesting
that individual crystals have a width of from about 200-500 angstroms.
15 The chemical analysis of the composition is shown in Table 3.
Table 3
Cortponent Frectton
(X)
Ex le Exa le Ex le 3
1 2
Ua 0 0 0 0
K 0 0 0 0
S 0.439 0.139 2.584
Ca0 46.165 47.789 45.211
P 33.461 35.205 36.116
Fe 1.001 1.217 1.175
M 2.839 0.700 2.562
S1 - 0.9838
OH 3.163 3.265 3.401
C 6.T 5.7 -
Nn 2028 2468 2635
Cu 38 75 T9
Cl 350 140 100
2n 303 849 587
H 4 53 0
..-
1237 ppm 768 pp" 716 ~,
- ~
Examples 4-12
In the following examples, the apatite compositions prepared in
Examples 1-3 were contacted with deionized water to determine the
equilibrium ion concentration after dissolution. At the end of each run,
pH and the ion concentrations of the various elements were measured.
Concentrations of manganese, iron, copper and zinc ions were
determined using DTPA chelating agent (pH=7.3). The procedure
_21~~3~9 ~v .
WO 94/08896 PCT/US93/09906
16
consisted of placing a 0.5 g sample of the synthetic apatite composition
in a covered glass bottle containing 80 ml of deionized water. The
bottles and samples were placed in an environmentally controlled
reciprocal shaker at a setting of 100 rpm and shaken for 96 hours. The
temperature was held at 25°C. Results are given in Table 4.
Table 4
Ex. Sale pH Element
No. Concrntration
mg/l mg/kg ~/l
P Ca Ng S Mn* Fe* Cu' 2ntB Mo Cl
4 1 8.701.3313.629.85.6 121 244 6 31 4.4 <0.020.6
5 1 8.701.3013.638.86.0 118 249 b 29 4.4 <0.021.7
6 1 8.701.3013.539.65.8 118 250 6 29 4.3 <0.021.1
7 2 8.130.1715.23.682.4 152 250 7 5b 1.9 <0.022.3
8 2 8.160.2214.83.272.7 163 302 7 59 1.9 <0.02t.b
9 2 8.160.1T14.13.222.3 152 246 7 5b 1.9 <0.023.1
3 7.002.9510.32.6717.8 160 402 10 57 0.8 <0.020.4
11 3 6.953.8010.32.T5t7.6 158 40b 10 55 0.8 <0.020.4
12 3 7.123.4510.02.7117.6 158 408 10 55 0.8 <0.020.5
* DTPA extractable, pH=7.3.
Example 40
In the following examples, the apatite compositions
10 prepared in Examples 1-3 were contacted with an aqueous
medium wherein the pH was varied between 5 and 7 to
determine the equilibrium ion concentration after
dissolution. The procedure was similar to Examples 4-12
except that a 0.5 M sodium acetate solutions buffered
with acetic acid to the desired pH were used instead of
deionized water. Results are given in Table 5. As
expected, the synthetic apatite dissolved to a greater
extent in a more acidic medium.
214G3~9
WO 94/08896 PCT/US93/09906
17
Table 5
Ex. Sample pH Element
Ho. Concentration
Ca Ng
13 1 8.00 116.1 51.8
14 1 8.06 99.8 48.0
15 1 8.11 98.5 46.6
16 1 6.30 314.7 70.0
17 1 6.31 310.5 66.0
18 1 6.31 310.5 66.0
19 1 5.08 1216 82.8
20 1 5.08 1384 82.6
21 1 5.08 1244 84.6
22 2 7.91 74.0 9.2
23 2 7.92 73.7 9.0
24 2 7.92 73.4 9.1
25 2 6.25 287.0 15.7
2b 2 6.25 282.8 15.4
27 2 6.25 287.0 15.8
28 2 5.07 1098 20.7
29 2 5.07 1056 20.5
30 2 5.07 1098 20.6
31 3 7.56 37.2 36.3
32 3 7.56 38.5 35.6
33 3 7.55 37.9 37.9
34 3 6.15 189.5 53.8
35 3 6.15 187.9 54.0
36 3 6.15 191.1 54.2
37 3 5.05 988 77.4
38 3 5.05 975 74.0
39 3 5.05 975 74.6