Note: Descriptions are shown in the official language in which they were submitted.
WO 94/07488 2 1 4 6 4 4 ~ PCI`/US93/08063
3-SUBSTITUTED 2-OXINDOLE-1 -CARBOXAMIDE
PHARMACEUTICAL COMPOSITIONS
Background of The Invention
This invention relates to pharmaceutical compositions comprising certain 3-
substituted 2-oxindole-1-carboxamides and medium chain (C8 to C10) fatty acid
triglyceride and propylene glycol diesters. These carboxamides are useful as
analgesics for use in mammals such as man and in ameliorating or alleviating pain
encountered while recovering from surgery or other trauma or in eliminating the
symptoms of chronic diseases such as inflammation and pain associated with
rheumatoid arthritis and osteoarthritis, as shown in United States Patents 4,556,672 and
5,047,554. The carboxamides are also useful for treating collagenase mediated
disorders and diseases, such as bone resorption disorders, corneal ulceration,
periodontal disease, inflammatory diseases, and wounds of the skin and burns in a
mammal, as shown in United States Patent 5,008,283.
The carboxamides described in formula I are chemically unstable in water. It is
known that the rate of hydrolytic degradation may be reduced by protection of labile
drugs e.g. sequestration in the hydrophobic core of micelles or formulation in low water
activity, non-aqueous solvent-based vehicles i.e. essential oils. In addition to hydrolytic
instability the carboxamides are also prone to oxidative degradation in aqueous e.g.
water, and non-aqueous e.g. oils, vehicles. Oxidative instability can be reduced in
saturated oils by inclusion of antioxidants or by formulation in unsaturated oils which
protect the drug by being preferentially oxidized themselves. However, the
carboxamides described in formula I are not readily stabilized in oils commonly used
in pharmaceutical preparations e.g. sesame oil, peanut oil, safflower oil, cottonseed oil.
Summary of The Invention
The present invention relates to a pharmaceutical preparation comprising:
(A) at least one triglyceride or propylene glycol diester of fractionated coconut
oil fatty acids; and
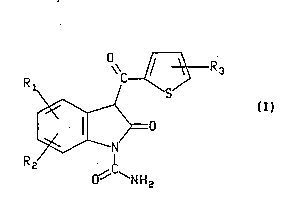
(B) at least one compound of the formula
WO 94/07488 PCr/US93/08063
44g
-2-
\\C/~i R ~3
Rl~ / S
~0
R2 C
~ ~NH2
where Rl, R2, and R3 are each independently hydrogen, fluoro, bromo, or chloro,
or a pharmaceutically acceptable salt thereof,
wherein the weight ratio of (A) to (B) ranges from 5.6 to 999.
The pharmaceutical preparation includes 85 to 99 % by weight of (A) and 0.1
to 15 % by weight of (B).
Preferably, Rl, R2, and R3 are each independently fluoro or chloro.
Preferred compounds of formula I include:
5-chloro-2,3-dihydro-2-oxo-3-(2-thienylcarbonyl)-indole-carboxamide;
6-chloro-5-fluoro-2,3-dihydro-2-oxo-3-(2-thienylcarbonyl)-in-
dole-carboxamide; and
6-chloro-5-fluoro-2 ,3-dihydro-2-oxo-3-(2-(4-chloro)-thienyl-
carbonyl)-indole-carboxamide.
The present invention also includes a method of inhibiting activation of
collagenase.
The present invention also includes a method of treating an inflammatory
disease.
The present invention also includes a method of eliciting an analgesic response.
Detailed Description of the Invention
It has been found that pharmaceutical preparations including the carboxamides
30 of formula I and C8 to C10 saturated fatty acid triglycerides and propylene glycol
diesters have superior product viability and shelf life. As the result of utilizing such a
formulation, the carboxamides are less susceptible to hydrolysis and oxidation which
can deteriorate them and ultimately render them ineffective. Stabilization of the
WO 94/07488 ~ 1 4 ~ 4 4 8 PCr/US93/08063
-3-
carboxamides in these preparations do not require the addition of an antioxidant or
other auxiliary stabilizers.
The triglycerides used in the claimed invention are neutral oils which are
composed of esters of medium chain (C8 to CI0) fatty acids, also referred to as
5 fractionated coconut oil. These fatty acids are esterified with either glycerin or
propylene glycol and are sold under the name MIGLYOL~ (i.e. MIGLYOL~ 810,
MIGLYOL~ 812, and MIGLYOL~ 840). MIGLYOLS are also described as triglycerides
of fractionated coconut oil fatty acids or caprylic acid/ capric acid triglycerides.
Fractionated coconut oil is prepared from the fixed oils obtained from the dried solid
10 part of the endosperm of Cocos nucifera L by hydrolysis, fractionation of the liberated
fatty acids and re-esterification with glycerol or propylene glycol. It consists of a
mixture of short and medium-chain saturated fatty acids, mainly octanoic and decanoic
acids. Miglyol~ is the trade name for fractionated coconut oil or caprylic acid/capric
acid triglycerides from Dynamit Nobel Ltd., Germany and the U.K. These vehicles have
15 demonstrated stability against oxidation and rancidification as well as outstanding safety
and biocompatibility. Furthermore, since only saturated fatty acids are used the oils do
not generate peroxides or otherfree radicals which could destabilize the pharmaceutical
contained therein. The low water content also minimizes the hydrolysis of the
carboxamide. In the preferred composition, a carboxamide of formula I is dispersed
20 in an oil vehicle comprising Miglyol~ 812 and other oil-soluble additives described
below under agitation to produce a homogenous suspension of the drug substance in
the oil vehicle.
Other additives which can be present in the pharmaceutical preparation can
include an anticaking agent such as, for example, propylene glycol, polyethylene glycol,
25 glycerin, sorbitol, benzyl alcohol, lecithin, or aluminum stearate. The amount of
anticaking agent can range from approximately 0.05 to 5 % by weight. The
pharmaceutical preparation can also contain preservative in an amount ranging from
0.5 to 2.0 % by weight. Such preservatives can include, for example, phenol, benzyl
alcohol, parabens, chlorbutanol, and benzyl benzoate. Gelling agents, such as
30 aluminum monostearate can also be included in the pharmaceutical preparation in an
amount ranging from o.5 to 3.0 % by volume.
The stability of these pharmaceutical preparations can be evaluated, for
example, under accelerated storage conditions after subjecting suspensions of the
W O 94/07488 PC~r/ U S93/08063
4 4 8
4-
carboxamides (6 % by weight of the drug substance) packaged in glass vials to high
temperatures of up to 70C for up to nine weeks. During the stability challenge the
level of intact drug remaining in the preparation, as well as hydrolytic and oxidative
decomposition products is quantified by high performance liquid chromatography
(HPLC). For assay, the suspension is diluted with methanol/triethylamine 100/1 volume-
by-volume to give a final drug concentration of 0.6 to 1.2 mg/mL. This solvent
dissolves the suspended drug to produce a solution which can be directly injected on
the HPLC column. For chromatography, the mobile phase is methanol/water 90/10 v/v
+ 1% triethylamine, the column is a reversed phase octadecasilane and the solvent flow
rate was 1 mL/minute. Drug detection was by UV absorbance at 246 nm. Such an
assay has shown there to be virtually no decomposition of carboxamide in suspension
after nine weeks.
When a compound of formula I or salt thereof is used in a human subject, the
daily dosage will normally be determined by the prescribing physicians. Moreover, the
dosage will vary according to the age, weight and response of the individual patient,
as well as the severity of the patient's symptoms and the potency of the particular
compound being administered. However, for acute administration to relieve pain, an
effective dose in most instances will be 0.01 to 0.25 9 as needed (e.g., one- to four-
times-a-day). For chronic administration, in most instances an effective dose will be
from 0.01 to 0.5 g per day, and preferably 0.1 to 0.25 g per day in single or divided
doses. On the other hand, it may be necessary to use dosages outside these limits in
some cases.
Preferably the pharmaceutical compositions of the present invention are
parenteral pharmaceutical compositions. The pharmaceutical compositions of this
invention may be produced by formulating a compound of formula I (as the active
ingredient) in dosage unit form. Some examples of dosage unit forms are sterile
suspensions for intramuscular, subcutaneous or intra-articular injection, sterile
ophthalmic suspensions for topical application to the eye, capsules for oral
administration, rectal suppositories, or topical lotion for application to the skin or scalp.
An example of a suitable pharmaceutical dosage for oral administration are soft
gelatin capsules. Orally administered suspensions can be delivered, e.g., upon
encapsulation of a suspension of compound I in the oil, i.e. Miglyol 812, in a soft
gelatin capsule. A rectal suppository may be formulated by dispersing the carboxamide
WO94/07488 2I~ PCr/US93/08063
-5-
in a neutral oil along with compatible suppository bases, such as cocoa butter or
Whitepsol W35, which have melting points above body temperature. A topical product
for application to the skin would contain the carboxamide as the active agent dispersed
in the neutral oil, e.g., Miglyol 812, and also containing one or more pharmaceutical
5 inactive ingredients, such as: cetyl alcohol, stearic acid, propylene glycol, aluminum
monostearate, benzyl alcohol, as diluents and preservatives. A parenteral composition
is preferably a suspension of the carboxamide in the neutral oil, and may also contain
other inactive pharmaceutical components, such as: benzyl alcohol as preservative,
aluminum monostearate as a gelling agent and propylene glycol as a dispersing agent.
The following Example illustrates how the pharmaceutical preparations can be
prepared. Commercial reagents can be utilized without further purification.
EXAMPLE 1
800 mL of Miglyol 812 was heated to 45C in a compounding vessel equipped
15 with an agitator and homogenizer. 10 g of benzyl alcohol was added to the oil under
agitation (about 60-80 R.P.M.). The oil solution was sterile filtered into a sterile
compounding vessel equipped wait an agitator and homogenizer. 120 g of micronized,
sterile carboxamide powder was dispersed into the oil phase under agitation. Thesuspension was homogenized under high shear for ten minutes and then was allowed20 to cool to room temperature under mild agitation (60-80 R.P.M.). The suspension was
brought to a total batch weight of 1000 grams with the addition of the required amount
of sterile Miglyol 812 to the suspension to give a final concentration of 12 % by weight
of carboxamide in the final formulation. The suspension was aseptically filled into 50
cc, Type 1, flint glass vials using an automated filling apparatus. The vials were capped
25 with teflon-coated rubber stoppers and crimped with aluminum shells.
E)G~MPLE 2
800 mL of Miglyol 812 was heated to 45C in a compounding vessel equipped
with an agitator and homogenizer. 10 g of benzyl alcohol was added to the oil under
agitation (-60-80 R.P.M.). The oil solution was sterile filtered into a sterile compounding
30 vessel equipped with an agitator and homogenizer. 20 g of sterile, aluminum
monostearate powder was added to the oil solution in divided portions under agitation
to gel the oil. The gelled oil was allowed to cool to room temperature and allowed to
stand for six hours without agitation. 120 g of micronized, sterile carboxamide powder
WO 94/07488 PCI'/US93/08063
2l4~448
-6 -
was then dispersed into the gelled oil under agitation. The suspension was brought to
a total batch weight of 1000 grams with the addition of the required amount of sterile,
gelled Miglyol 812 to the suspension to give a final concentration of 12% by weight of
carboxamide in the final formulation. The suspension was aseptically filled into 50 cc,
5 Type 1, flint glass vials using an automated filling apparatus. The vials were capped
with teflon-coated rubber stoppers and crimped with aluminum shells.
EXAMPLE 3
800 mL of Miglyol 812 was heated to 45C in a compounding vessel equipped
with an agitator and homogenizer. 10 g of benzyl alcohol was added to the oil under
10 agitation (about 60-80 R.P.M.). 120 g of micronized, sterile carboxamide powder was
dispersed into the oil phase under agitation. The suspension was homogenized under
high shear for ten minutes and then was allowed to cool to room temperature under
mild agitation (60-80 R.P.M.). The suspension was brought to a total batch weight of
1000 grams with the addition of the required amount of Miglyol 812 to the suspension
15 to give final concentration of 12% by weight carboxamide in the final formulation. The
suspension was filled into soft gelatin capsules using an automated filling apparatus for
oral ingestion.
EXAMPLE 4
200 g of Miglyol 812 and 800 g of Whitepsol W35 were heated to 60C in a
20 compounding vessel equipped with an agitator and homogenizer. Carboxamide
powder was dispersed into the resulting oil solution under agitation. The suspension
was allowed to filled into suppository molds and congealed by cooling to room
temperature.