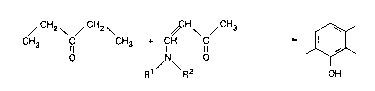Note: Descriptions are shown in the official language in which they were submitted.
2147913
Preparation Of 2,3,6-Trimethylphenol
The present invention relates to a process for the preparation of 2,3,6-
trimethylphenol, an essential starting point for the preparation of vitamin E.
2,3,6-trimethylphenol can be obtained, according to DE 2,415,930, by the
catalytical alkylation of 11-cresol in the gas phase. This process suffers from the
drawback that it produces isomer mixtures from which the desired isomer can be
obtained in a pure form only with difficulty and with loss of yield.
Other industrial processes are based on the condensation of diethyl ketone with a
,0 c4building block, such as crotonaldehyde (cf DE 1,793,037) or methyl vinyl
ketone (cf DE 1,668,874). A drawback of this otherwise advantageous process is
that it does not give direct access to 2,3,6-trimethylphenol, but first producestrimethylcyclohexenone, which must be dehydrated in an additional step to form
the trimethylphenol.
It was thus the object of the invention to provide a process for the preparation of
2,3,6-trimethylphenol starting from diethyl ketone and a readily available
C4 building block which requires only one reaction stage.
20 The invention relates to a process for the preparation of 2,3,6-trimethylphenol
wherein diethyl ketone is caused to react with a 1-amino-vinylmethylketone at
temperatures ranging from 50 to 200C, preferably from 70 to 1 50C.
The reaction thus takes place according to the following reaction scheme:
~ CH2 ~ ~ CH2 \ DCH I / CH3 ~
CH3 ICI ~h~ o J~
R1 R2 OH
The 1-amino-vinyl methyl ketones used can be theoretically all of the 1-
aminovinyl methyl ketones which have a primary, secondary, or tertiary amino
group. For economical reasons, those amino compounds are preferably used which
are cheaply available and/or are particularly simple to handle or to separate.
Examples thereof are 1-dimethylamino-, 1-diethylamino-, 1-dipropylamino-, 1-
dicyclohexylamino-, 1-diethanolamino-, 1-piperazino-, 1-N-methylpiperazino-, or
1-morpholinoamino-vinyl methyl ketones. Theoretically however, all of the 1-
21~7913
. _
amino- vinylmethylketones of the aforementioned formula can be used in which
the substituents R1 and R2 have the following meanings:
a) R1 and R2 independently denote hydrogen or
Cl-C20alkyl, preferably c,-c8alkyl, in particular c~-c4alkyl such as methyl,
ethyl"7-propyl, isopropyl, n-butyl, isobutyl and tert-butyl;
c3-c8cycloalkyl, preferably c5-c8cycloalkyl such as cyclopentyl, cyclohexyl,
cycloheptyl and cyclooctyl, in particular cyclopentyl and cyclohexyl;
c~-C2o hydroxyalkyl, preferably c2-c8 hydroxyalkyl, in particular C2-c4 hydroxy-alkyl such as 2-hydroxyethyl and 3-hydroxypropyl;
phenyl and c7-c,2alkylphenyl such as 2-methylphenyl, 3-methylphenyl, 4-
methylphenyl, 2,4-dimethylphenyl, 2,5-dimethylphenyl, 2,6-dimethylphenyl,
3,5-dimethylphenyl and 2,3,4-trimethylphenyl;
or
C7-c~2 phenylalkyl such as benzyl, phenethyl, 1 -phenylpropyl, 2-phenylpropyl,
1-phenylbutyl, 2-phenylbutyl, 3-phenylbutyl, preferably benzyl and phenethyl;
or alternatively
b) R1 and R2 together form a c2-c7alkylene chain which can be substituted by
one to four C~-C4alkyl radicals and/or interrupted by oxygen, nitrogen or
sulfur. Examples thereof are the following groups:
30 --(CH2)2-~ -(CH2)3-~ -(CH2)4-~ -(CH2)5-, -(CH2)6-, -(CH2)7-, (CHCH3)-,
-(CH2)2-O-(CH2)2-. -(CH2)2-N-(cH2)2-- and -(CH2)2-S-(CH2)2--
Since a simple process for the preparation of 1-amino-vinyl methyl ketones by the
reaction of cracked gas containing diacetylenes and formed during the generationof acetylene with secondary amines and water at temperatures ranging from 0 to
1 50C has recently been found (cf DE Patent Application P 43 08 080.4), a very
advantageous process is thus available in which the desired 2,3,6-trimethylphenol
can be directly obtained from readily available starting materials.
Furthermore, the 1-amino-vinyl methyl ketones can be prepared, for example, as
described in US 3,141,880, by the reaction of monoamines with ketone or, as
2147913
described in US 3,285,915, by the reaction of secondary amines with a 3-
alkoxycyclobutanone .
The process of the invention is successfully carried out in the presence of basic
agents.
The basic agent used can be any stronger base having a deprotonating action.
Examples thereof are hydroxides, oxides, alcoholates, amides or hydrides of alkali
metals or alkaline earth metals, metalorganic compounds, such as butyllithium orphenyllithium or quaternary ammonium hydroxides, such as benzyltrimethyl-
ammonium hydroxide.
The basic agents are generally used in stoichiometric amounts or, if desired, inslight excess.
The reaction of the invention can be carried out in the presence or absence of asolvent. When no solvent is present, it is advantageous to operate in excess diethyl
ketone acting as diluent.
Suitable solvents are, for example: aliphatic petroleum ethers, hydrocarbons such
as petroleum ether or gasoline mixtures; cycloaliphatic hydrocarbons such as
cyclohexane; aromatic hydrocarbons such as benzene, toluene, xylene, cumene
and p-diisopropylbenzene, chlorinated hydrocarbons such as chlorobenzene,
chlorotoluene and dichlorobenzene; ethers such as methyl-tert-butyl ether,
tetrahydrofuran and dioxane; sulfoxides such as dimethyl sulfoxide, acid amides
such as dimethylformamide and N-methylpyrrolidone, and higher alcohols such as
pentanols.
The reaction is particularly successful when using water-immiscible solvents.
When such water-immiscible solvents are used it is advantageous to carry out thereaction with constant removal of water.
A particular advantage of the process of the invention is that it is also possible to
use mixtures of secondary amines with water since by this means the aminovinyl
ketones can be used directly, ie without isolation, after their preparation fromdiacetylenes and the aqueous solution of a secondary amine as described in
P 43 08 080.4 for the preparation of trimethylphenol.
40 The condensation is generally effected at temperatures ranging from 50 to
200C, preferably from 70 to 1 50C.
2147913
.
The reaction times are generally from 20 to 40 hours, preferably from 25 to 30
hours - depending on the temperature of reaction and the reactants.
Usually atmospheric pressure is used, but it is possible to operate under reduced
or elevated pressure.
Diethyl ketone and the aminovinyl methyl ketone are advantageous used in a molarratio of from 1 :1 to 50:1, preferably 1 :1 zu 20:1.
To effect purification of the reaction mixture, the procedure generally adopted is to
first neutralize the resulting phenolate and then separate the reaction mixture by
fractional distillation.
The amine obtained by distillation or removed from the bottoms can be reused forthe preparation of 1-amino-vinylmethylketone.
Using the process of the invention it is possible to obtain 2,3,6-trimethylphenol as
desirable essential vitamin E precursor in a single simple reaction stage in very
good yields from readily available diethyl ketone and from aminovinyl methyl
20 ketones of the formula iii which are easy to obtain in a simple manner.
Example
In a glass flask having a capacity of 250 mL and equipped with a stirrer and water
removing means there were intermixed 16.9g (0.1 mol) of dipropylaminovinyl
methyl ketone, 172.3 g (2 mol) of diethyl ketone and 11.2 g of a 50 (Y0 strengthaqueous KoHsolution (corresponding to 0.1 mol) and the mixture was refluxed for
29 hours with the removal of water. 6.7mL of H2O were separated during this
period (expected: 5.6 mL from the caustic potash solution, 1.8 mL from the
30 reaction). The resulting very dark brown reaction solution was analyzed by gas
chromatography following neutralization with acetic acid. The content of 2,3,6-
trimethylphenol was quantified by factorization. The selectivity toward trimethyl-
phenol was 63~Yo based on converted aminovinyl methyl ketone (conversion
890~). The most important by-product was 3-ethyl-4-methylphenol at a
selectivity of 26 ~0 (by area, as measured by gas chromatography).