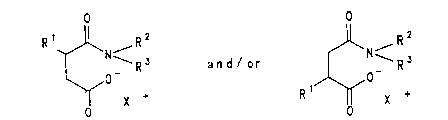Note: Descriptions are shown in the official language in which they were submitted.
214~ 8
HOECHST A~Ll:N~SELLSCHAFT HOE 94/F 122 Dr.gI/wo
Description
Salt~ of alkyl- and alkenylsuccinic acid half-amides
The invention relates to salts of alkyl- and/or alkenyl-
~uccinic acid half-amides, a process for their prepara-
tion and their use as surfactants.
Alkyl- and alkenylsuccinic acid half-amides have been
known per ~e for a long time. EP-A-0 498 178 describes
~mmo~;um salts of alkenylsuccinic acid half-amides which
are used as corrosion inhibitors and in oil and/or gas
production.
The use of alkenylsuccinic acid half-amides as corrosion-
protective agents is also described in EP-A-0 127 132 and
in EP-A-0 191 952.
EP-A-0 510 564 di~closes a process for the preparation of
surface-active agents from di- or tricarboxylic acid~.
Succinic acid, for example, is used as a dicarboxylic
acid. The products are used as surface-active agents,
preferably in co~metic formulations.
US-A-3 576 743 describes oil-soluble products which are
formed from esterification of a polycarboxylic acid
anhydride, in particular alkenylsuccinic acid anhydride,
with a polyhydric alcohol and subsequent reaction of the
ester formed with a primary amine. The amines employed
are primary amino-alcohols, such as ethanolamine, tris-
(hydroxymethyl)r ;n -thane and gl~r-;ne. The6e product~
are used as lubricant and fuel additives.
However, the abovementioned compounds have the dis-
advantage of being usable only in certain fields of use.
They often also have dermatological properties which are
not good, 80 that they are unsuitable for use as surfact-
antn in the detergents and cosmetics sector.
2148548
The object of the invention was therefore to provide
novel water-~oluble alkyl- and/or alkenyl~uccinic acid
compounds which do not have the abovementioned disadvant-
ages and also are readily biologically degradable.
The invention relates to salts of alkyl- and/or alkenyl-
succinic acid half-amides of the formulae
O O
R~ R~ '~N R
o
( I a ) ( I b )
in which
R1 i~ C6-Cloo-alkYl or C6-cloo-alkenyl~
R2 is a deoxysaccharide radical which i~ derived from
mono-, di- or oligosaccharides,
R3 i~ hydrogen, C1-C6-alkyl or R2, and
X+ is a proton, an alkali metal ion, the equivalent of
an alkaline earth metal ion, or an ammonium ion of
the formula N+HR4R5R6 (II) or of the formula
N+HR2R3R4 (III),
in which
R4, R5 and R6 independently of one another are
hydrogen, C1-C20-alkyl, a hydroxyl group, a C1-C6-
hydroxyalkyl group or a C3-C6-polyhydroxyalkyl group
and
R2, R3 and R4 have the abovementioned meAn; n~ .
According to nomenclature in organic chemi~try, a
~accharide in which a hydroxyl radical is replaced
formally by a hydrogen radical is called a deoxy-
saccharide (also desoxysaccharide) (D. Hellwinkel, "Die
~ystemati~che Nomenklatur der Organischen Chemie"
[Sy~tematic Nomenclature of Organic Chemistry]", Springer
21485~
-- 3
Verlag, Berlin, Heidelberg, New York 1974, page 70). A
more precise description is deoxy-n-saccharyl radical, in
which n identifies the position within the saccharyl
radical which links the saccharide part with, for
example, an amide nitrogen.
Preferred salts of succinic acid half-amides of the
formulae (Ia) and/or (Ib) are those in which
Rl is C6-C22-alkenyl,
R2 is a deoxysaccharide radical which is derived from
glucose, galactose, mannose, fructose, maltose,
isomaltose, isomaltulose, lactose, sorbitol, maltol,
lactol, isomaltol or starch hydrolysate,
R3 is hydrogen or C1-C6-alkyl, or equals R2, and
X+ is a lithium ion, potassium ion or sodium ion or the
equivalent of a calcium or magnesium ion.
Particularly preferred salts of succinic acid half-amides
of the formulae (Ia) and/or (Ib), however, are those in
which
Rl is C6-C22-alkenyl,0 R2 is a deoxysaccharide radical which is derived from
glucose, maltose, isomaltulose, lactose, sorbitol,
maltol or lactol,
R3 is Cl-C6-alkyl and
X+ is a sodium ion.
The invention also relates to a process for the prepara-
tion of the abovementioned salts of alkyl- and/or
alkenylsuccinic acid half-amides according to the inven-
tion, which comprises reacting an alkyl- and/or alkenyl-
succinic acid anhydride with carbohydrates cont~
amino groups in the presence of basic compounds. Alkali
metal hydroxides, alkaline earth metal hydroxides,
alkylamines and/or hydroxyalkylamines are preferably used
as the basic compounds. Sodium hydroxide is particularly
suitable. Other suitable basic compounds are amines, in
particular alkylamines, such as primary, secondary and
tertiary Cl-C6-alkylamines, for example triethylamine,
;~148548
-- 4
and hydroxyalkylamines, such as primary, secondary and
tertiary Cl-C6-hydroxyalkylamines, for example tri-
ethanolamine, and amino-sugars.
The alkenylsuccinic acid anhydrides used as starting
substances can be obtained by reaction of maleic
anhydride and olefins in the sense of an ene reaction.
The correspo~;ng alkylsuccinic acid anhydrides are
obtained by hydrogenation.
Carbohydrates contA;n;ng amino groups which are suitable
for the reaction with a succinic acid anhydride to give
the desired compounds of the formulae (Ia) or (Ib)
according to the invention and which may be mentioned are
the amino-sugars and aminopolyols, the sugar radical and
the polyol radical being designated the deoxysaccharide
radical in the context of the invention. Amino-sugars,
such as glucosamine, galactosamine and others, are known
from the prior art and are obtA;nAhle in various ways
(S. Peat, Adv. Carbohydr. Chem. 2, 37 (1946); Foster,
Stacy, Adv. Carbohydr. Chem. 7, 247 (1952); Org.
Synthesis 26, 36 (1946)). Amino polyols have likewise
been known for a long time and can be obtained by
reductive amination of the correspo~;ng mono-, di- or
oligosaccharides (US-A-2 830 983, EP-A-O 255 033 and
US-A-2 016 962), thus, for example, 1-methylamino-
1-deoxysorbitol (N-methylglucamine) or 1-amino-1-deoxy-
galactol.
Suitable solvents for the abovementioned reaction are
water, lower alcohols, such as methanol, ethanol, propa-
nols and glycols, other water-miscible polar organic
solvents, such as ketones and glycol ethers, and, as
aprotic solvents, dimethylformamide and dimethyl sulf-
oxide. The reaction is preferably carried out in an
aqueous solution or an aqueous-alcoholic solution. The
reaction of the alkyl- and/or alkenylsuccinic acid
anhydrides with the carbohydrate contA;n;ng amino groups
is carried out at a temperature of O to 100C, preferably
~148548
- - 5 -
30 to 70C.
The concentration of the carbohydrate cont~;n;ng amino
groups in the solution is 5 to 70% by weight, preferably
10 to 50% by weight.
The carbohydrates contA;n;ng amino groups are initially
introduced into the reaction vessel in an appropriate
concentration in one of the abovementioned solvents and
the correspo~;ng alkyl- and/or alkenylsuccinic acid
anhydride is then added dropwise. The duration of the
dropwise addition is between 10 minutes and 6 hours,
preferably between 30 minutes and 2 hours. The pH of the
reaction is controlled via the addition of the basic
compounds .
The molar ratio of alkyl- and/or alkenylsuccinic acid
anhydride to carbohydrate cont~;n;ng amino groups is
0.8-1.2:0.8-1.2, preferably 1:1.
The salts of alkyl- and/or alkenylsuccinic acid half-
amides according to the invention are used as surfact-
ants. The salts of alkyl- and/or alkenylsuccinic acid
half-amides are used in an amount of 1 to 25% by weight,
preferably 3 to 15% by weight, based on the total weight
of the surfactant solution.
Analysis
The total solids content of the solution is determined by
drying at 100C in a vacuum drying cabinet for 12 hours.
The infrared spectra of the dried samples show the
typical bands of the amides at about 1630 to 1650 cm~1
(secondary and tertiary amides) and additionally at about
1550 cm~l for the carboxylate anion.
The analysis of the alkyl- and/or alkenylsuccinic acid
half-amides is shown for the isolated dry substances in
2148548
_ - 6 -
Example 1. For Examples 2 to 11, the solids content i8
determined as described above. The half-amide content can
be determined therefrom by means of potentiometric
titration.
Examples
The preparation according to the invention of the
alk(en)ylsuccinic derivatives described by the formulae
Ia and Ib is described in the following examples. Product
mixtures of Ia and Ib, in which Ia and Ib in each case
form a diastereomer pair on the ba~i~ of two pos~ible
stereoisomeric arrangements of the radical Rl (R and S),
are in principle formed in the processes described. In
Example 1, the isomers resulting therefrom are named
individually, and in the subsequent examples only the
general name is given.
Example 1
Sodium salt of a mixture of
N-(l-Deoxyglucityl)-N-methyl-l-(R)-dodecenylsuccinic acid
monoamide
N-(l-Deoxyglucityl)-N-methyl-l-(S)-dodecenylsuccinic acid
monoamide
N-(l-Deoxyglucityl)-N-methyl-2-(R)-dodecenylsuccinic acid
monoamide
N-(l-Deoxyglucityl)-N-methyl-2-(S)-dodecenylsuccinic acid
monoamide
- 97.5 g of N-methylglucamine are initially introduced into
790 ml of water. 133 g of distilled dodecenylsuccinic
acid anhydride are added dropwi~e at 60C in the cour~e
of 4 hours, while stirring. The pH is kept between 9.0
and 9.5 by means of 40.3 g of 50% strength by weight
sodium hydroxide solution. Thereafter, the mixture is
subsequently stirred at 60C for a further hour. A
vigorously foaming, clear solution having a solids
content of 25.4% by weight is obt~ine~. A dried sample
shows the absorption signals at 1412, 1575 and 1630 cm~l
2148548
- 7 -
typical of formula Ia and Ib in the infrared spectrum.
10 g of the above solution (corresponds to 2.54 g of dry
substance) are chromatographed over a silica gel column
(length 50 cm, ID 5 cm). According to HPLC, the main
fraction (2.10 g) comprises the 4 components expected in
a ratio (in % areas) of 19:17:33:31. In the 13C-NMR spec-
trum (75.4 MHz, CD30D), the isomer mixture shows the
expected signal groups for the alkyl chain and C2 and C3
of the succinic acid part at 14 to 50 ppm, of the carbo-
hydrate part from 64 to 74 ppm, of the olefinic C atomsat 128 to 135 ppm and of the carbonyl C atoms of the
carboxyl and amide groups at 176 to 185 ppm.
Example 2
N-(l-Deoxyglucityl)-N-methyl-pentapropenylsuccinic acid
monoamide Na salt
274 g of pentapropenylsuccinic acid anhydride (technical
grade, M = 366) are reacted with 136 g of N-methylgluc-
amine (1-methylamino-1-deoxyglucitol) in 105 g of water
at 60C in the course of 1 hour and the mixture is
subsequently stirred for a further hour. Thereafter, 56 g
of 50% strength sodium hydroxide solution i8 added in the
course of 1 hour. 583 g of solution having a solids
content of 68% by weight are obtained. The half-amide
content of the solid is 78% by weight.
Example 3
N-(1-Deoxyglucityl)-N-methyl-tetrapropenylsuccinic acid
monoamide triethanolammonium salt
296 g of tetrapropenylsuccinic acid anhydride (technical
grade) of molar mass 296, 196 g of N-methylglucamine
(1-methylamino-1-deoxyglucitol) and 149 g of
triethylamine are reacted in 200 g of water at 60C. The
duration of the dropwise addition of the succinic acid
anhydride is 1.5 hours. A solution having a solids
content of 76% by weight is obtA;ne~. The half-amide
content of the solid is 82% by weight.
2148548
-- 8
Example 4
N-(2-Deoxyglucosamino)-dodecyl/tetradecenylsuccinic acid
monoamide Na salt
64.7 g of glucosamine hydrochloride are initially intro-
duced into 300 ml of water. 88.8 g of a mixture of
dodecenyl- and tetradecenylsuccinic acid anhydride of
average molar mass 296 are added dropwise at 10C in the
course of 1 hour. In parallel with this, a solution of
24 g of sodium hydroxide dissolved in 56 g of water is
added dropwise such that a pH of 7 to 8 exists. The
mixture is subsequently stirred at 40C for a further
hour to give 523 g of a clear, vigorously foam; ng solu-
tion having a solids content of 32% by weight. The half-
amide content of the solid is 88% by weight.
Example 5
N-(1-Deoxyglucityl)-N-methyl-dodecyl/tetradecenylsuccinic
acid monoamide 1-deoxyglucityl-dimethylammonium salt
97.5 g of N-methylglucamine and 106 g of dimethylgluc-
amine are initially introduced into 500 ml of water. The
mixture is heated to 60C and 148 g of a mixture of
dodecenyl- and tetradecenylsuccinic acid anhydride of
average molar mass 296 are added dropwise in the course
of 1.5 hours. 850 g of a clear solution having a solids
content of 43% by weight are obtained. The half-amide
content of the solid is 78% by weight.
Example 6
N-(1-Deoxyglucityl)-N-methyl-dodecyl/tetradecenylsuccinic
acid mo~o~mide triethanolammonium salt
148 g of dodecenyl/tetradecenylsuccinic acid anhydride
(technical grade) of molar mass 296, 97.5 g of N-methyl-
glucamine (1-methylamino-1-deoxyglucitol) and 74.6 g of
triethanolamine are reacted in 500 ml of water at 60C.
The duration of the dropwise addition of the succinic
acid anhydride is 2 hours. 815 g of solution having a
21 185~8
g
solids content of 40% by weight result. The half-amide
content of the solid is 77% by weight.
Example 7
N-(1-Deoxyglucityl)-N-methyl-dodecyl/tetradecenylsuccinic
acid monoamide 1-deoxyglucitylmethylammonium salt
195 g of N-methylglucamine are initially introduced into
500 ml of water. The mixture is heated to 60C and 148 g
of a mixture of dodecenyl- and tetradecenylsuccinic acid
anhydride of average molar mass 296 are added dropwise in
the course of 1 hour. The mixture is subsequently stirred
at 60C for a further 1.5 hours to give 825 g of a clear
solution having a solids content of 41% by weight. The
half-amide content of the solid is 88% by weight.
Example 8
N-(1-Deoxyglucityl)-N-methyl-dodecyl/tetradecenylsuccinic
acid monoamide Na salt
97.5 g of N-methylglucamine are initially introduced into
450 g of water. 148 g of a mixture of dodecenyl- and
tetradecenylsuccinic acid anhydride of average molar mass
296 are added dropwise at 60C in the course of 2 hours.
In parallel with this, 61 g of 30% strength sodium
hydroxide solution are added dropwise such that a pH of
9 to 10 exists. The mixture is subsequently stirred at
60C for a further hour to give 743 g of a clear, vigor-
ously foaming solution having a solids content of 34% by
weight. The half-amide content of the solid is 62% by
weight.
Example 9
N-(1-Deoxyglucityl)-dodecyl~tetradecenylsuccinic acid
monoamide Na salt
90.5 g of glucamine are initially introduced into 450 g
of water. 148 g of a mixture of dodecenyl- and tetra-
decenylsuccinic acid anhydride of average molar mass 296
2148548
- 10 -
are added dropwise at 60C in the course of 2 hours. In
parallel with this, 70 g of 30% strength sodium hydroxide
solution are added dropwise euch that a pH of 9 to 10
exists. The mixture is subsequently stirred at 60C for
a further 2 hours to give 743 g of a clear solution
having a solids content of 31% by weight. The half-amide
content of the solid is 92% by weight.
Example 10
N-(2-Deoxy-D-glycopyranosylmannityl/sorbityl)-dodecenyl-
succinic acid monoamide Na salt
34.1 g of 2-amino-2-deoxy-D-glucopyranosylmannitol/
sorbitol (2-amino-palatinit), are initially introduced
into 87 g of water. 23.1 g of a dodecenylsuccinic acid
anhydride are added dropwise at 50C in the course of
half an hour. In parallel with this, 7.5 g of 50%
strength sodium hydroxide solution are added dropwise
such that a pH of 9 to 9.5 is maintained. The mixture is
subsequently stirred at 50C for a further 2 hours to
give 140.5 g of a clear, vigorously foaming solution
having a solids content of 46% by weight. The half-amide
content of the solid is 88% by weight.
Example 11
N-(l-Deoxyglucityl)-N-methyl-dodecyl/tetradecenylsuccinic
acid monoamide trishydroxymethyl-methylammonium salt
195 g of N-methylglucamine are suspended in 800 ml of
water. 296 g of a mixture of dodecyl- and tetradecenyl-
succinic acid anhydride (average molar mass 296) are
~lowly added dropwi~e while cooling with ice (duration:
3 hours), the pH being kept at 9 to 10 during this
operation with 80 g of 50% strength sodium hydroxide
solution. The mixture is stirred at 60C for a further
hour. The now clear solution is acidified to pH 3 with
50 g of concentrated sulfuric acid, while cooling with
ice, and the sodium sulfate which has precipitated out is
filtered off. The filtrate is neutralized with 112 g of
~14~5~8
11
tri~hydroxymethylaminomethane (pH 6.6). The methanol is
distilled off and replaced successively with water. A
solution having a solids content of 39% by weight is
obtained. The half-amide content of the solid is 91% by
weight.
Examples 12 to 16
Testing of the foaming power and determination of the low
temperature turbidity point and the Zeïn value of
Examples 5 to 9.
The Zeïn values (A.R. Reng et al., Parfumerie und ~osme-
tik 68, 771 to 788, 1987) give guiding indications of the
~kin compatibility of surfactants. For mild surfactants,
it should be less than 200 mg of nitrogen/100 ml of
surfactant solution. The products of Examples 5 to 9
prepared according to the invention have Zeïn values far
below this limit and are therefore to be classified as
very mild. The results of the tests are summarized in
Table 1.
Table 1
Example Product Foama) Low tempera- Zeïn
from immediate/ ture turbidity value
Example 5 min point
12 5 -230/230 -5C 32
13 6 240/240 2C 46
14 7 260/250 -3C 26
8 250/250 -4C 49
16 9 250/250 -8C 123
a) according to Ross/Miles in mm at 1.0% of wash-active
sub~tance
Examples 17a and b
~Z11~548
- 12 -
Preparation of a hair shampoo which i8 particularly mild
to the skin using the products from Example 7 and
Example 9 (all figures in % by weight).
Example 17a
5 ~Genapol LR0 liquid (lauryl alcohol
ether-sulfate, manufacturer: Hoechst AG)40 %
Perfume oil 0.03%
Water 47.65%
Product from Example 7 (as a 41% strength by
10 weight solution) 8.5 %
~Genapol L-3 (lauryl alcohol ethoxylate,
manufacturer: Hoechst AG) 2.0 %
Dyestuff solution 0.27%
Preservative 0.05%
15 Sodium chloride 1.5 %
Example 17b
~Genapol LR0 liquid (lauryl alcohol
ether-Rulfate) 40 %
Perfume oil 0.03%
Water 44.85%
Product from Example 9 (as a 31% strength by
solution weight) 11.3 %
~Genapol L-3 (lauryl alcohol ethoxylate)2.0 %
Dyestuff solution 0.27%
25 Preservative 0.05%
Sodium chloride 1.5 %
~ Examples 18a to d
Prepa~ation of a detergent concentrate which is particu-
larly mild on the skin using the product from Example 7.
~i4~548
- 13 -
Example 18a 18b 18c 18d
Contents in % by weight
Sec-alkanesulfonate
(~Hostapur SAS, 60%45.033.3
Manufacturer: Hoechst
5 AG)
Lauryl ether sulfate
(~Genapol LRO 70% 11.4 - 38.6 14.2
Manufacturer: Hoechst
AG)
Fatty alcohol ethoxy-
late - 10.0 - 7.0
(~Genapol UD 80
Manufacturer: Hoechst
AG)
Coconut amidopropyl-
betaine - - lO.0 lO.0
(~Genagen CAB 30%
Manufacturer: Hoechst
AG)
Product from Example 7
(as a 41% ~trength by 12.2 24.4 24.4 48.8
weight solution
Ethanol 5.0 5.0 5.0 5.0
Water 26.427.3 22.0 15.0