Note: Descriptions are shown in the official language in which they were submitted.
~14~59 6
) T - 3 1l7 .~* . j
- ~ DESCRIPTION -~
PROCESS FOR POLY~ERI~ING ALP~A-OLEFIN
Technical Field. .
This invention relates ~o a process for producing ~-
olefin polymers. More particularly, the invention-relates to
a process -~at utilizes a novel high activity s~ereoregular
~olyme~ization catalyst system to produce ~-ole~in pol~Imers
having imprcved polymer properties.
ackqround Art
The use o~ a solid, transition metal based, olefin
polymerization catalyst systèm including a titanium~containing,
magnesium halide-~ased catalyst component to produce a polymer
of an ~-ole~in such as ethyl`ene, propylene, and butene-1, is
well known in the art. SUch polymerization catalyst systems
are typically obtained by the combination of a magnesium
halide-based catalyst component, an organoaluminum compound and
one or more electron donors. For..convenience of reference, the
soIid titanium-containing catal~st component is referred to
herein as "procatalyst"~.. the arganoaluminum compound, as
~ ."cocatalyst", and an electron dono~ sompoundJ wh~-ch is
typically used separately, or used ~artially or totally
complexed with the organoaluminum compound, as l'selestivi~y
control agent" ~SC~) J It is also known~to incorporate electron
donor compounds into the pro catalyst. The electron donor
which is incorporated with the titanium-containing compounds
sexves a different purpose than the electron donor referred to
as the selectiv.i~y control agent. The compounds which are used
as the electron donor are the same as or dif~erent from
compounds which are used as ~hë selectivity control agent~ The
abo~e-described stereoregular high acti~ity catalysts ~are
broadly con~en~ional and are described i~ n~merous ~atents and
other references including EP~A-02g7l63, US-A-4,728/7Q5 and
GB-A-2143834.
: Although a ~road range o~ compounds are known
generally as ~electivity control~agents, a particular catalyst
3S component may have a specific compound or groups of compounds
with whl.-~ it is s?ecially compati~le~ For anv given
~ F ~J r~ r~ F ~T
-`, 214~536
,~
procatalyst andjor cocatalyst, discovery of an appropriate type
of selectivity control agent can lead to signif~cant increases
in catalyst efficiency, hydrogen utilization efficiency as well
as an improvement in polymer product properties.
Many classes o~ selectivity control agents have been
disclosed for possible use in polymerization catalysts. One
clas~ ar such selectivity control agents is the class of
organo silanes. ~ For example, Hoppin et al, U.S. Patent
- 4,990,478, describe branched C3 C10 alkyl-t-
butoxydimethoxysilanes. Other aliphatic silanes are descri~ed
in Hoppin et al, U.Si Patent 4,829,03$. Kioka et al, U.S.
Patent 5,028,671, describe a catalyst syste~ which incorporates
various alkylalkoxysilanes, such as di-n-
octadecyldimethoxysilane and di-n-octadecyldiethoxysilane as
selectivity control agents.
Although many methods are known for producing highly
stereoregu~ar ~-olefin polymers, it is still desired to improve
the acti~ity of the catalyst and produce polymers or copolymers
that exhibit improved properties such as high ~ w and
broad molecular weight distribu~ion.-;~urther, it ls desired to
produce polymers or copolymers tha~ exhiblt a reduction in the
`. amount of ~olatiles.
Disclosure of the Invention
The invention relates to an improved process for the
production of homopolymers or copolymers of ~-oIe~ins that ha~e
improved polymer properties.
More particularly, thë present inYention is a process
for the production of polymers using a high activity olefin
polymerization catalyst system which comprises (a) a titanium
l~halide-containing procatalyst component ~tained by ,
halogenating a magnesium compound of the formula MgR'RJ',
; wherein R' is~an a~koxide group, aryloxy group or a
hydrocarbyl carbonate group and R'' is an alkoxide group, an
ar~Ioxy group, a hydrocarbyl group or a halogen, especially
an alkoxide group containing from 1 to 10 carbon a~oms with a
halogenated tetra~alent titanium compound containing 2 to 4
halogen atoms in th~: preserlce of an electron donor, and a
~: ~
AME!IDE3 ^`~EET
21~596 -
., ; 1. . .
.
- 2a -
.~ ,. ~,
:`
halohydrocarbon, (b)~.an organoaluminum cocatalyst component, :~`
and tc) an organosilane selectivity control agent having the ~"
qeneral formula:
- .:
. ~
, ~ ' : .
.. - . ::
-
.
: ::: :
:: .: : ' : : i :
A~\AEl~cD SHE~
- 21~8~9~ .
`
_.... , -,`
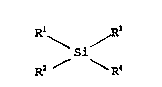
Rl / R
` \si
R2 ~ ~?.4
.," ~ ' ''.
~
- .
wherein R~ is a preferably linear, alkyl group of 13 to 30
carbon atoms, an alkaryl group of 1~ to 36 carbon atoms or
aralkyl group of 16 to 36 carbon atomsi R2 and R3 are,
independently, methyl or alkyl groups of 13 to 30 carbon
atoms, or hydrocarboxyloxy group of 1 to 6 car~on atoms; and
R~ is hydrocarbyloxy group of 1 to 6 carbon atoms.
Descri~tion of the Invention
EP-A 0455313 discloses a catalyst system derived
15- ~rom component (b) above, an organosilicon which can be one
defined for component (c) and a solid titanium catalyst which
is not obtained using a halohydrocarbon~
The magneslum compound employed in the
preparation of the solid catalyst component contains
20 alkcxide, aryloxide, hydrocar~yl carbonate or halogen. The ;
alkoxide, when present, usually ~ontains from 1 to 10 carbo~ :
atoms. Alkoxides containing from 1 to 8 carbon atoms are
preferable, with alkoxides of 2 to 4 carbon atoms being more
preferable. The aryloxide, when present, generally contains
from 6 to 10 carbon atoms, with 6 to 8 carbon atoms being
preferred. The hydro~arbyl car~onate, when present,
generally contains 1 to 10 carbon atoms. When halogèn is
present, i~ can~be present as bromine, fluorine, `iodine or r.,.
chlorine, with chlorine being preferred.
Suitable magnesium compounds are magneslum chloride,
magnesium bromid~, magnesium ~luoride, ethoxy magnesiu~
~romide, isobuto~y~ magnesium chloride, phenoxy magnesium
iodide, cumyloxy magnesium ~romide, magnesium diethoxidep
magnes um isopropoxide, magnesium ethyl carbonate, ethoxy
magnesium, magnesi ~ steara~e, magnesium laura~e, and naphthoxy
~agnesium chloride. Especially preferred as ~he magnesium
n ~ F ~T
~ 214859~ ~ `
-4-
compounds are magnesium dialkoxides. Preferred magnesium
compound is magnesium diethoxide.
Ha ~ ~ ~ ion of the magnesium compound with the
halogenated tetravalent titanium compound is generally effected
by using an excess of the titanium compound. At least 2 moles
of the titanium compoun~ are normally used per mole of the
magnesium compound. Preferably from 4 moles to 100 moles of
the titanium compound are used per mole of the magnesium
compoundj and most preferably from 4 moles to 20 moles of the
titanium compound are used per mole of the magnesium compound.
Halogenation of the magnesium compound with the
halogenated tetravalent titanium compound is usually effected
by contacting the compounds at an elevated temperature in the
range from about 60C to about 150C, preferably from about
70C to about 120C. Usually the reac~ion is allowed to
proceed over a period of 0.1 to 6 hours/ preferably from about
0.6 to about 3.5 hours. The halogenated product is solid
material which is isolated fro~ the liquid reaction medium by a
~ suitable separation meth~, such as conventional filtration.
; The halogenated tetravalent compound employed to
halogenate the magnesium compound contalns at least two halogen-
at~ms, and preferably contains ~our halogen atoms. The halogen
~atoms are chlorine atoms, bromine atoms/ iodinè ~to~s or
fluorine atoms. The halogenated tetravalent titanium compo~nd
has up to two alkoxy or aryloxy groups. Examples of suitable
halogenated tetravalent titanium compounds include
diethoxytitanium dibromide, isopropoxytitanium triiodide,
dihexoxytitaniu~dichloride, phenoxytitanium trichloride,
titanium tetrachloride and titanium-tetrabromide. The
preferred halo~enated tetravalent ti~anium compound is titanium
tetrachloride.
Halogenation~of the magneslum compound with the
~halogenated~tetravalent titanium compound, as notad, is
conducted in the presence of a~halohydrocarbon and an electron
donor. I~ desi~red, an iner~t~hydrocarbon diluent or solvent may
~ ; also be present.
:~; :
:~ ~
AMEN9E~ Sl',EET
` 214~59~
.
Suitable halohydrocarbons include aromatic or
aliphatic, including cyclic and alicyclic compounds.
Preferably the halohydrocarbon contains 1 or 2 halogen atoms,
although more may be p~esent if desired. It is prefarred that
the halogen is, independently, chlorine, bromine or ~luorine.
Exemplary of suitable aromatic halohydrocarbons are
chlorobenzene, bromobenzene, dichlorobenzene,
dichlorodihromobenzene, o-chlorotoluene, chlorotoluene,
dichlorotoluene, chloronaphthalene Chlorobenzene, o-
chlorotoluene and dichlorobenzene are the preferredhalohvdrocarbons, with chlorobenzene and o-chlorotoluene being
more preferred.
' The ~'iphatic halohydrocarbons which can be employed
suitably have 1 to 12 carbon atoms. Preferably such
halohydrocarbons have 1 to 9 carbon atoms and at least 2
halogen atoms. Most preferably the halogen is present as
chlorine. Suitable aliphatic halohydrocarbons include
dibromomethane, trichloromethane, 1,2-dichloroethane,
..,.; .,
trichloroethane, dichloro~luoroethane, hexachloroethane,
trichloropropane, chlorobutane, dichlorobutane, ehloropentane,
trichlorofluorooctane, tetrachloroisooctane, dibromodi~
fluorodecane. The pre~erred aliphatic halohydrocar~ons are
carbon tetrachloride and trichloroethane.
Aromatic halohydrocarbons arepreferred, ~articularly
those Qf 6 to 12 carbon atoms, and especial~y those of 6 to lQ
carbon atoms.
~ ypical electron d~nors that are incorpoxated within
the procatalyst include esters, particularly axomatic estexs,
; ethers, particularly aromatic ethers, ketones, phenols, amines,
am~des, imines, nitriles, phosphines, p~osphites, stibines,
arsines, phosphoramides and aleoholates. Esters of
polycarboxylic acids are ~he pre~erred electron donors~
Particularly preferred ~are alkyl as~ers of aromatic
polycar~oxyli~ acid Tllustrati~e of suitable esters of
polycarboxylic acid electron donQrs are die~hyl phthalate,
diisoamyl phthalate, ethyl p-ethoxybenzoate, methyl p-
ethoxybenzoate, diisobutyl phthalate, dimethyl
S
E~!J
8~ ~i 16 - .
`````!
-6~
napthalenedicarboxylate, diisobutyl maleate., diisopropyl
terephthalate, a~d diisoamyl phyhalate. Diisobutyl phthalate
and ethyi-p-ethoxybenzoate are the preferred alkyl ester of an
aromatic carboxylic acid.
After the solid halogenated product has been separated
from the liquid reaction medium, it can be treated one or more .,
tlmes with a~ditional halogenated tetravalen~ titanium
compound. Preferably, the halogenated product is trPated
multiple times with separate portions of the halogenated
tetravalent titanium compound. ~e~ter results are obtained i~
the halogenated product is treated twice with separate portions
of the halogenated tetravalent titanium compound. If desired,
the solid halogenated.product is treated one or more times with
a mixture of halogenated tetravalent titanium compound and a
halohydrocarbon. As In the initial halogenation,.,at least 2
moles of the titanium compound are generally.employed per mole .
of the magnesium compound, and preferably from 4 moles to 100
moles of the titanium compound are employed per'mole of the ~,
magne~ium compound. Most preferably from 4 moles to 2Q..moles
of the titanium compound 'per mole of magnesium"compound.
':'`~^. . Optionally, the solid~halogenated product is treated
at least once w~th one or more acid chlorides during~the ',
ad~itional treatments with the halogenated tetravalent titanium
compound. Suitable acid chlorides include benzoyl chloride and
phthaloyl chloride. The preferred acid chlorid,e is phtyaloyl
: chloride.
After the solid halogenated product has been treated
one ~r more times with additional~halo'genated tetravalent
titanium compound, it is desirably separated from the liquid,,l relacti`on medium,;washed 'at least once'with~an iner~ hydlroca~bon
of up to 10 carbon atoms to remove unreacted titanium
compounds, and dried. Exemplary of the iner~ hydrocarbons that
are suitable f~or the invent'ion are,isopentane, isooctane,
hexane, heptane and cyclohexane.
~ Preferred final washed~products have a titanium
content of from 0.5 percent by weight to 6,0 percent by weight.
A more preferred~final wash product has from 1.5 percent by
.
; :
21~859~
` ' ' ' '-;''
weight to 4.0 percent by weight. The a~omic ratio of titanium
to magnesium in the final product is between 0.01:1 and 0.2:1.
A praferred final product has an atomic ratio of titanium to
magnesium from about o.02:1 to o.15:1.
The cocatalyst is an organoaluminum compound which
is typically an alkyIaluminum compound. Suitable al~ylaluminum
compounds include trialkylaluminum compounds, such as triethyl-
aluminum or triisobutylaluminum; dialkylaluminum halides such
as diethylaluminum chloride and dipropylaluminum chloride; and
dialkylaluminum alkoxides such as~diethylaluminum ethoxide.
Trialkylaluminum compounds axe preferred, with triethylaluminum
being the preferred trialkylaluminum compound.
The organosilane selectivity control agents in the
cat~lyst system contain at least one silicon-oxygen-carbon
li.nkage. Suitable organosilane compounds include co,mpounds
having the following general formula~
E~ R3
4 ~ ` \ Si ' ''~
R2 / ~4 ..
wherein ~1 is a linear àlkyl-~roup of 13 to 30 carbon a~oms,
. alkary.l group of 16 to 36 carbon atoms or aralkyl g~oup of 16
to 36 carbon akoms. It is pre~erred~tha~ Rl is alkyl ~roup o~
16 to 3Q carbon at~ms, al~aryl group of 19 to 3Q carbon atoms
or aralkyl group of ~9 to 30 carbon atoms. It is further
. 2S pre~erred that Rl is alkyl ~roup.of 1.8 to 28 carbo~ atoms. R2
and R3 are, independently, methyl or alkyl group o~ 13 to 30
carbon atoms, or hydrocarbylo ~ groups of 1 to 6 carbon atoms.
:It is preferred that R2 and R3 arej independently, methyl or
alkyl group o~ 16 to 30 ca~bon atoms or alkoxy group o~ 1 to 4
ca~bon a~oms. It~:is further prefe~red that R2 is methyl or
alkyl ,group of 18 to 28:c~rbon atoms or al~oxy ~roup of 1 to 4
carbon atoms and R3.is al~oxy gro~p o~ 1 to 4 carbon a~ms~ Tt
is preferred that R~ is alkoxy group o~ 1 to 4 ~ax~on at~ms.
R4 is hydrocarbyloxy group of 1 ~o 6 carbon atoms. It is
: 35 further preferred that R2, R3 a~d ~ are ethoxy or me~hoxy
groups. Examples o~ suitable organosilane selectivity control
agents inc~ude n-o~tadecyltriethoxysilane, n-triacontyl-
AME~DEDSkEE~
214859fi
` ! - 8-
triethoxysilane, methyl-n-octadecyldimethoxysilane, methyl-n-
octadecyldiethoxysil~ne, n-octadecyltrimethoxysilane, n-
triacontyltrimethoxysilanè and mixtures thereof. The preferred
organosilan~a selectivity control.agents are n-.^.
octadecyltriethoxysilane, n-methyloctadecyldimethoxysilane and
n-octadecyltrimethoxysilane. The in~ention also contemplates
the use of mixtures of two or more selectivity control agents.
The selectivi~y co~trol agent is suitably provided in a
quantity such that the molar ratio of the selecti~ity control
agent to the titanium present in the procatalyst is fxom about
0.5 to..about 80. ~olar ratios from about 2 to about 60 are
preferred, with molar ratios from about 5 to about 40 being
more preferred.
The high acti~ity stereoregular polymerization
catalyst is utilized to effect polymerization by contacting at
least one -olefin under. polymerization conditions. In
accordance with ~he invention, the procatalyst component, .
organoaluminum cocatalyst, and selectivity control agent are
intxoduced into the polymerization reactor.~eparately or, if
desîred, two or. all of the components are partialIy or
completely mixed with each other ~efore they are introduced
into the reactor.
The partîcular type.. of polymerizatîon process
utîlized is not crîtîcal to the operation af the present
inventîon and the polymerization processes now regarded as
con~entional are suitable în the process of ~he inventîon. The ;~
polymerization is conducted under pol~merîzation condi~ions as
a liguid phase or a: gas-phase process employing a fluidized
catalyst bed.
The polymerizatîon conducted în ~he~ lîquîd~phas~
employs as reactîon diluent an added înert lî~uid diluent or
alternatively a li~uid dîluent which comprises the olefint such
as propylene or 1-butene, undergoing polymeriza~ion. If a
¢opolymer i~ prepared wherein ethyl~e îs one of th~ monomers,
e~hylene is întroduced by conventional means to a diluent.
~ îcal polymerization conditîons înclude a reactîon
temperature ~rom about 25C to about 125C, with temperatures
from about 3~C to about 90C beîng preferred, and a pressure
'!
AMENGED ~!EET
_J :,
,, , . ~llL85~
.. !
sufficient to maintain the reaction mixture in a li~uid phase.
Such pressures are from about 150 psi to about 1200 psi, with
pressures from about 250 p~i to about 900 psi are preferred~
The liquid phase reaction is operated in a batchwise manner or
.. ~ as a continuous or semi-continuous process. Subsequent to
reaction, the polymer product is recovered by cGnventional
procedures. The precise controls of the polymerization
conditions and reaction parameters of the liquid phase process :~
are within the skill of the art.
As an alternate embodiment of the invention, the -~
polymerization is conducted in a gas phase process in the
presence of a fluidized catalyst bed. One such gas phase
process polymerization process is described in U.S. Patent
4,379,7S9. The gas phase process typically involves charging
to reactor an amount of preformed polymer particles, gaseous
monomer and separately charge a lesser amount of each catalyst
component. G~seous monomer, such as propylene, ls passed
through the bed of solid particles at a high rate under
condit.Lons of temperature and pressure sufficient to initiate
2~ .and maintain polymerization. Unreacted olefin is separated and
recovered and polymerized olefin particles are separated at a
~ rate substantially equivalent to its produ~tion. The process
is conducted in a batchwise manner or a continuous or semi- ~ -
continuous process wit~ constant or intermittent addition of
2S the catalyst components and/or ~-olefin to the polymerization
~` reactor. Typical~.. polymerizati~n temperatures for a g~s phase
process are from about 30C to about 120C and typical
pressures are up to about ~70 kg/cm2 (1000 psi), with pressures
from about 7 kg/cm~ (100 psi) to about 35 kg/cm2 (500 psi)
being preferred~
In;both the liquid phase and the gas-phase poly-
merization processes, molesular hydrogen is added to the
reaction mixture as a chain transfer agent to re~ulate the
moIecular weight ~of ~hè polymeric produc~. Hy~rogen is
S typically employed for this pu~pose in a manner well known ~o
persons skilled in t~e artO The precise control of reaction
`, 2 1 4 8 ~
. .
C
conditions, the rate of addition o~ feed component and
molecular hydrogen is ~roadly within the skill of the art.
The preseIlt invention is useful in the polymerization
of ~-olefins of up to 20.carbon atoms! such as ethylene,
propylene, dodecane, including mixtures thereof. ~t is
pre~erred that ~-ole~ins of 3 carbon atoms to 8 carbon atoms,
such as propylene, buteneT1 and pentene-l and hexane-l, are
polymerized.
The polymers produced according to this invention are
predominantly isotactic. Polymer yields are high relative to
the amount of catalyst employed. The process af the invention
produce homopolymers and copolymers includ~ng both ràndom and
impact copol~mers havin~ a relativel~ high stiffness while
having a broad molecular weight distribution and maintaining an :~
15 ; o~igomers content (determined by the weight fraction of~
oligomer) of less than 300 ppm if a homopolymer and ~ess than
600 ppm if a copolymer.: The preferred homopolymers o~ the~
inventi~n ha~e an oliyomers content of less than lSO ppm is
preferred. More preferred`homopolxmers haYe:oligomers con~ent
20 of less than 80 ppm~ A reduction in oligomers conte~t is ;:
.indicati~e of a reduction in volatiles, e.g. smoke and/or oil,
~: liberated d~rin~ subsequent processing, e.g. extrus~on
Other eatures, ad~antages and embodi~ents of the
~i:nvention disclosed herein will~be readily apparent to those
; 25 exercising ordinary ~.skill after reading the foregoing ~:
disclosure. In ~his regard, while specific e~ odiments o~ ~he
invention have~ ~een described in :detail, ~ariations - and
modifica~ions of~:~these~embodiments can be effected without
departing from the spirit and scope of the invention as:
descr~bed and~dlaimed.
The inve~tion described herein is illustratedl bu~
not~ limited by ~he :~fol~owing~Illustrative~ Emb~diments and
Comparative EY~mple. :The ~ollowinq te~ms are;~sed t~roughout
the Illustrative Embodiments and Comparative Example:
35 : ODTM5~ (n-octadecyltrimethoxysilane)
ODTES (n-octa~ecyltriethoxysilane~
DNDDMS tdi-n-decyldimethoxysilane)
DDTMS (n-dodec::yltrimethoxysilane~
CTMS~(n-triacontyltrimethoxysilane~
1 0
AMEND~D SHEE~
~ 1 4 ~
MNDD~S (methyl~n-decyldimethoxysilane)
MODD~S ~methyl-n-octadecyldiethoxysilane)
PEEB tethyl-p-ethoxybenzoate)
NP~MS (n-propyl~rimethoxysilane)
S DIBDES (diisobutyldiethoxysilane) .
DIBDMS (diisobutyldimethoxysilane).
ILLUSTRATIVE EMBODXMENT I
(a) Preparation of Procatalyst Component
The procatalyst ~as prep~red by adding magnesium
diethoxide (2.17 gl 19 mmol) to 5S ml of a 50/50 (vol/vol)
mixture of TiCl4/chlorobenzene. A~ter adding diisobutyl
" .. . . .. .
phthalate (0.74 ml, 2.75 mmol), the mixture was heated in an
oil bath and stirred at 110C.for 60 minutes. The mixture was
filtered hot and the solid portion was slurried in 55 ml o~- a
50/SO (vol/vol) mixture of TiCl~/chlorobenzene~ In the
preparation of some o~ the procatalyst, phthaloyl chloride :
(0.13 ml, O.90 mmol) was added to the slurry at room ;
temperature. The resulting slurry was stirred at 110C for 60
- minutes, filtered, and~the solvent obtained was slurried again
. 20 in a fre~h ~ 50 mixtur~. of TiCl4/chlorobenzene. Af~er
: stirring at 110~ for 30 minutes, th~ mixture was filtered and
allowed to cool to.room temperature. The procatalyst slurry
-,
was washed 6 times with 125 ml portions of isooctane and ~hen
dried for 120 minu~es, at 25'C, under nitrogen.
2S ~ ) Polymerization of Propylene .` ~`
: Various catalysts were produced using several
organosilanes as the selecti~ity control agent, some of which
axe withln the scope of the invention (TCTMS, ODTES, ODTMS, and
MODDES) :and other hat are not within the scope o~ the
.3Q i~vention (NPT~S~ DNDDMS, DIBDES, DDTMS, MNDD~S and DIBDMS)..
Propylene (2iooc~) and molecular hydrogen were introduced lnto
a 1 gallon autocla~e.~ The temperature~ o~ the propylene and
. molecular hydrogen was raised~ to 67Co ~n organosilane
selectivity con~roi agent, triethylaluminum, and the :
35 ~ pro~atalyst clurry producPd above we~e:premixed for about 20
minutes and then the mixture was introduced into the autoc~aYe.
: The amount of silane utilized~ in ~he polymerization also
va~_ed. The amount of triethylaluminum (0.70 mmoles) and the
amount of the~ procatalyst slurry (sufficient quantity of
.
A~Vlr~lG~3 S`-IEET
: ~ - 214~5g6 `. ~
procatalyst to provide o~Ol mmoles of titanium) remained
constant. The autocla~e was then heated to about 67C and the
polymerization was continued at 67C for one hour. The
polypropylene product was recovered from the resulting mixture
S by conventional methods~a~d the weight of the product was used
to calculate the reaction yield in millions of grams of poIymer
product per gram (MMg/g3 of titanium in the procatal~st. The
term IIQI~ was calcu7 ated as the quotient of the weight average
molecular ~eight ~Mw) and the number a~erage molecular weight
(~), determined by gel permeation chromatography. The term
"~" as defined in "Encyclopedia of Polymer Science and
Engineering, 2nd Edition", Vol. lO, pp. 1-19 (1987~,
incorporated herein by reference, is the z-average molecula~
weight. The term "X" was calculated as the quotient of ~ and
Mw~ "Melt Flow" is determined according to ASTM D-1238-73,
condition L. "Viscosity Ratio"~was determined ~by cone and
plate rheome~ry ~dynamic viscosity me~s~rements) as a ratio of
... . . .
the viscosi~y of the pr~oduct at;a~frequency of 0.1 H~ divided
by the. viscosity o~ th~e product at a frequency of 1.0 Hz. As
q 20 the viscosity ratio of~polymer product in~reases, the molecular
weight distribution increases.
The results of a series of polymerizations are shown in T~BLE
I `' ~
:
t
:
... ... .. .. . .. . ..
~ 2 ~L 4 ~ 5 9
~ O ul
~, n ~ 3 , 3 ~ ~ ~ w w , 3
I' I~ r~ ~ ~n
o
t-t Xl ~ O O O O o ~ O O O O Q O O O O O
1~ o o ~ J o t~ o o ~ t~ ~--
Ul ~ ~ ~U~ ,p Ul ~ U~ Ul C~ ~1 ~ -CO W Ul O :.-
P.~ P) 11 0
It It ~ ''
tD tD -`~ '-
~ ~ ,............ ~
`' Y ~ ~ ~ )-' '~ 1~ 1' ~ W N ~ t`~ ~ ~ W 1
. o ~ 1 `J ~ ~ W ~i W W W ~ ~1 ~D '.
o ~ . . U~, ,' '.
~ ~ ~ . . ~
O Op~ ' O o ~,
r o t~ ) w ~ ~~ w )~ 1 3 ~
o ~ 3 't
O ~ ~ :
W o W~ ~~ W' CO ~ ~ VS l~l
~, : H
` ~D ,.
W ~ ~ Co ~' ~ ~ O ~ ~ O ~ ~ ~ W ~ W ~ g ,~
~ '
~ ,
.~..... .
t~ O C~ D ~ wG~ Ul ~ O Ul -
C:) OOOOOO~OOOOOOOOOO O , -:
o ~ ~ o ~ ~ ~ ~ ~ ~ ~ ~~ `~ ~ ~ ~ ~ 10
,p D ~ ~ O ~ I~ '~ n ~ w o ~ ~
c
~ o
O O ~ ~t
A ~ r n c~ u r ~T
-~ 214~59~- :
ComParative ExamPle
(a) Preparation of Procatalyst Component
The procatalyst was prepared by adding magnesium
diethoxide (50 mmo~) to 150 ml of a 50/50 ~vol/vol) mixture of
chlorobenzene/TiCl4. After addiIlg ethyl benzoate ~16.7 mmol),
the mixture was heated in an oil ~ath and stirred at llQC for
approximately 60 minutes... The resulting slurry was filtered i
and slurried twice with 150 ml of a fresh 50/50 (vol/vol).
Benzoyl chlor-de (0.:4 mlj was added to the ~inal slurry. After
stirring at 110C for approximately 30 minutes, the mixture was
filtered. The slurry was washed six times with 150 ml portions
of isopentane and then dried for~ 90 minutes, at 30C, under
nitrogen.
(b) Polymerization -.
Using the above-described procatalyst (section a),
propylene was polymerized as described in Illustrative
Embodiment I, section (b), except the selectivity control agent
was PEEB.
~- Illus~rative Embodiment II
20 ~ To illustrate a further advantage of ~he~catalyst
system of the invention, polymer products havi~g a melt flow of
~; a~'~ut 3.0 dg/min were produced according to the procedures
described in Illustrative Embodiment I and the Comparative
Example. In particuIar r the polymer products were~ produced
2S using 0.2 mmol of the speci~ied selectivity control agent
("SCA'I) and sufficient hydrogen necessary to produce a polymer
having a melt flow of about.3.0 dg/min. The values are shown
. in:T~BLE II. ~ . ;
-
:~ :
. . !
,
,;
'
14 `:
' :
~ ~ .
A~.i;E;11'3~E.T ~-
~:``!; 2148~6
. .
TABLE II
SCAI mmol of
H2 Required4
T~ 2
DIBDMS2 30
PEEB3 - 135
MODDES 12
ODTES 13
MNDDMS 17
TCTMS ~ ~8
ODTMS : : ~20
DDTMS 22 `
DNDDMS 29 ~`
0.2 ~mol of each selectivity control agent was used.
~ For comparison
15 . : 3 Comparative Example : . ................................... ` :
- 4 mmol of H2 required to make a polymer product having a
melt flow of about 3 dg/min
.
: As noted, the catalyst systems of the invention .
exhibit increased hydrogen utilization e~ficiency.
Illustrativ~ Embodiment III ~ :
Viscosity ratio values were~taken for the pol~mers
~ having a melt flow~o~ about 3 dg/min.~ The values are shown in
`; ~ TABLE`I I. : : :
~ : Table III
: 25 ~CA . ~_iscosit~ ~atio
MODDES ~ .85~
` . :TCrMS : `-- . 1.80`.
: :~ :ODTES ~ : ' 10 7 ~ -
: ` : ODZ~MS ~ ~ 1.60
... 30~ DNDDMS : ~ ~ 1.62
MN~ ,60
NP~MSI ~ 1.58
For comparison
It is seen f ~om: TABLE III ~hat th~ catalyst sys~ems
~of;the inventlon exhi~it a higher viscosity ratio and therefore
2 1~ 8 ~ ~ ~
a broader molecular weight distribution than conventional
cat~lyst systems using NPTMS as the selectivity control agent.
,
. ~ ,.
-;
~t :`'
.,
~ '
~ .. . , . j,,~,
.s.
`~
`
:
16
.,-
.,'
A ~ U ~ ~T