Note: Descriptions are shown in the official language in which they were submitted.
~` - 21~37
,.
94/01758 Pcr/u~s3/o~8
METHOD FC)R PREPARING CALlBRATlON CUP~VES
BACKGROUND OF TWE INVENTION
; This invention relates to a method for forming a
calibration curve for a component of interest in a fluid for each of a
plurality of optical r~ad stations in an automated, analytical
instrumen~.
Various types of chemical tests can be performed by
automated test equipment, an example of testing of considerable
interest being the assay of biological substances for human health
care. Automated test equipment allows iarge numbers of test
samples to be processed rapidly. Such equipment is employed in
health care institutions including hospitals and laboratories. Biological
; fluids, such as whole blood, plasma, serum or urine are tested to find
evidence of disease, to monitor therapeutic drug levels, etc.
In ~he automated test instrument a sample of the test
fluid is typically provided in a sample cup and all of the process steps
including pipetting of the sample onto an assay test element,
incubation and readout of the signal obtained are carried out
automatically. The test instrument typically includes a series of work
Wo 94/0175~ 1 4 ~ S ~ 7 PCr/US93/06081
- 2-
stations each of which performs one of the steps in the test
procedure. The assay element may be~transported from one work
station to the next by a conveyor such as a carousel to enable the
test steps to be accomplished sequentially. Typically, the conveyor
carries a plurality of assay modules each of which is secured to a
specific location of the surface of the conveyor. In the usual
arrangement, the assay modules are spaced apart from each other in
berths which are located along the periphery of the conveyor to
facilitate automatic insertion and extraction.
tO In certain types of instrumen~s such as those which are
designed to carry out assays based on immunornetric interactions
between analytes or metabolites and their binding partners, at least
the part of the conveyor carrying the assay modules is arranged
¦~ within the temperature controlled chamber since it is necessary that
the assay be carried out at a very precisely controlled temperature, for
example at 37 + 0.5C. The assay cartridges are maintained in the
temperature controlled chamber for a period of time sufficient to bring
them to the desired temperature prior to beginning the assay
procedure and are maintained at that temperature for the duration of
the process.
As is known in the art typically there is stored in the
control processing unit (CPU) of such automated instruments a
calibration curve for each analyte which can be analyzed by the
instrument. When a patient sample is analyzed by the instrument the
signal obtained from the sample is automatically applied to the
calibration curve and the concentration of the analyte in the sample
is calculated therefrom. New calibration curves must be prepared at
-`` 214~537 ~
~094/01758 Pcr/uss3/o6o8
-3-
vari~us intervals due to variables such as different production lots of
the assay elements which are used in the instruments, etc.
It is known in the art to provide automated analytical
instruments which include more than one optical read station. Such
5 instruments can provide a desirably higher throughput which allows
a larger number of sampies to be analyzed in a given time period. To
allow the instrument to be operated at its maximum capacity it must
have the capability to read out the signal from any assay elernent at
any of the optical read stations. Accordingly, those skilled in the art
10 will appreciate that a calibration curve must be prepared for each
analyte at each optical read station for the instrument to be operated
in this mode.
It can be seen that such instruments therefore present
considerations relating to the expense and time involved in preparing
15 the requisite calibration curves, that is, how many assay elements
and what volumes of control or calibrator compositions are required
and the time period needed to do so. One technique for preparing
such calibration curves învolves using a separate series of assay
elements for each of the optical read stations. Since each calibration
20 curve typically requires six control or calibrator compositions of
different concentrations and these are usually run in duplicate the
number of assay elements needed for that method increases
significantly as the number of optical read stations in the instrument
increases. Thus, there is a continuing need for new and
25 advantageous calibration methods for use with instruments having a
plurality of optical read stations.
214!~S37
wo 94~n1758 ji , Pcr/uss3/o6o8
SUMMARY OF TtlE INVENTION
It is therefore the object of the present invention to
provide a novel method for providing calibration curves for use with
an analytical instrument having a plurality of optical read stations.
It is another object of the invention to provide a method
for providing calibration curves whersin each assay device used tO
prepare a calibration curve is read at each of the plurality of optical
read stations.
It is a further object of the invention ~o provide such a
method wherein the assay devices are read successively at each of
the plurality of opticai read stations at different specifically defined
times.
These and other objects and advantages are
accomplished in accordance with the invention by providing a method
for preparing a calibration curve for an analyte of interest on an
automated analytical instrument which includes a plurality of optical
read stations. According to the method, each of the plurality of assay
devices to which there is applied one of the control or calibrator
- ~ cornpositions used to create the calibration curve is read successively
20 at each of the optical read stations at different times. The times set
for taking the readings at each of the optical read stations are
specifically defined and are determined, in part, by the incubation
time required for the particular analytical assay for which the
calibration curves are being provided and the number of optical read
stations in the instrument as will be described in detail below herein.
Thus, for any particular analyte, and defining the time
necessary to bring the assay device to which there has been applied
` 211~7
-~0 94/017~i8 P~US93/0608
-5-
a control or calibrator composition and any other required reagent(s~
to the desired temperature in the temperature-con$rolled chamber as
Tor the first optical reading is taken at optical read station R1 at a time
Tl which may be the same as To or a time close to To~ The second
5 optical reading is taken at optical read station R2 at time T2 which can
be defined as T1 + ~tl and a third optical reading, if required, at
- optical read station R3 at time T3 which can be defined as T2 + I~t2
(where At2 may be the same as ~tl or different).
The method of the invention can be practiced with an
10 analytical instrument which has two or more optical read stations.
Accordingiy, where the instrument has N read stations (where N is an
integer equal to or greater than 2), the method comprises the steps
of taking, on an assay element, N successive optical readings at each
of the N optical read stations, each reading being taken at a time
15 which is different for each optical read station.
As noted previously, in analytical instruments which
have multiple optical read stations it is preferred to provide, for each
optical read station, a calibration curve for each analyte which can be
analyzed by the instrument. By doing so any assa~ device which is
20 used for the analysis of a patient sample fluid for any analyte within
the instrument assay menu can be read at any of the plurality of
optical read stations thus allowing the instrument to be operated most
efficiently and maximizing the assay throughput rate as much as is
possible. As will be appreciated by those skilled in the art all the
25 optical readings taken on a patient sample fluid have to be obtained
at the same optical read station and are taken at the times established
214~37
WO g4/01758 ~ ! ~` PCI /US93/06081
-6- ~.
for such readings at the respective optical read stations by the
calibration method of ~he invention.
By using only one assay device for each of the control
or calibrator compositions used to provide the calibration curves for
a plurality of optical read stations in accordance with the method of
the invention there is provided a significant decrease in the time
- required to obtain the curves and a significant reduction in the
number of assay devices necessary to do so. In addition, lesser
volumes of calibrator and/or control compositions are needed in view
of the decreased number of assay devices. It can be appreciated
therefore that the method provides significant advantages.
BRIEF DESCRIPTION OF THE DRAWINGS
For a better understanding of the invention as well as
other objects and further features thereof, reference is made to the
following detailed description of various preferred embodiments
thereof taken in conjunction with the accompanying drawings
wherein:
Fig. 1 is a partially schematic, cross-sectional view of an
assay device which may be utilized in the method of the invention;
Fig. 2 is a partially schematic, perspective view of
another assay device which may be utilized in the method of the
invention;
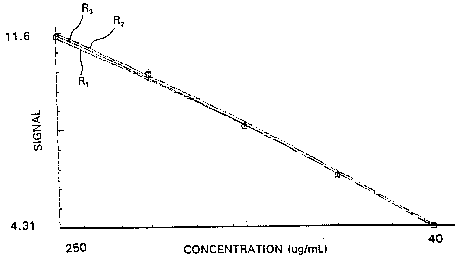
Fig. 3 is a graphical illustration showing the calibration
curves prepared for an analyte for three optical read stations utilizing
an assay device of the type shown in Fig. 1; and
~VO 94/01758 21~ 7 pcr/uss3/o6o8l t
Fig. 4 is a graphical illustration showing the calibration
curves prepared for another analyte for three optical read stations
utilizing an assay device of the type shown in Fiy. 2
DESCRIPTION OF THE PREFERRED EMBC)DIMENTS
The method of the invention may be practiced with any
assay device which includes, or to which there may be applied, one
or more reagents which will provide, in the presence of a fluid sample
including an analyte of interest, an optical signal which is a function
of the concentration of analyte in the fluid. The optical signal may be
provided by any suitable signal-generating system. Any light radiation
- emitting or absorbing species, including those which react with a
reagent which provides an optical signal can be utilized as the signal-
generating species.
The desired optical signal may be generated as a result
of chemical reactions and/or immunometric interactions. The
preferred assays with which the method is practiced are those based
on immunometric interactions between an analyte of interest and
binding partners for the analyte, particularly specific binding partners.
Particularly preferred assays are based on antigen-antibody
interactions. It is known in the art to us~ in immunornetric assays
conjugates which are made up of a light radiation- emitting or
absorbing label covalently bound to one of the members of the signal
generating system. Any of the labels known for use in immunometric
assays may be utilized including those which are directly detectabte,
- 25 for example, a fluorophore, a chemiluminophor, a radioactive material
or a light absorbing material, and those which are indirectiy detectable
2149S37 ,--
WO 94/01758 PC~/US93~06081
1': ~' . ! .,
" ~: .
-8-
such as an enzyme. Any change in fluorescence, chemilumiscence,
radioactivity or other change in visible or near visible radiation can be
detected. Where the label is an enzyme it can be one which interacts
with a substrate to cause a change in absorption where the substrate
5 is a chromogen, in fluorescence where the substrate is a fluorophore,
in chemiluminescence where the substrate is a chemiluminescent
precursor or in phosphorescence where the substrate is a phosphor.
Enzyme labels are preferred in instances where it is desirable to
amplify the signai which is obtained.
Any suitable assay device may be utilized in the practice
of the method of the invention. Suitable assay devices include
containers such as test tubes and cups to which the fluid reagent(s)
and calibrator or control composition are added, self-contained assay
elements which include the necessary reagent(s) for the assay and to
1~ which only the fluid calibrator or control composition is added and
assay elements which may inGlude a reagent and to which one or
more additional reagents as well as the calibrator or control
composition are added.
One preferred type of assay device is the "dry" assay
20 element including those which are made up of only one reagent layer
carried by a support layer and multilayer assay elements which have
at least one reagent layer and one or more other layers which may be
reagent layers, a light-blocking layer, a layer for receiving a signal-
generating species formed in, or liberated from, another layer, etc.
25 Another preferred type of assay device is that wherein the reactions
and/or interactions which are utilized to provide the desired optical
signal are carried out on a solid carrier material.
.
~ , ," ~,, " ", ,~ " ,~,, , ", ~ , ",
~` !`
`; ~0 94/01758 2 1 4 9 ~ ~ 7 PCr/VSs3/06081
g
The assay me~hod practiced with the assay devices may
be an end point assay, i.e., one where an optical reading is taken at
a defined period of time after the sample ftuid and any other required
reagent(s) are applied to the assay device and the signal applied to a
5 calibration curve to determine the concentration of analyte in the
fluid. The assay method may also be a rate assay in which a plurality
of optical readings ar~ taken at specific times after applying ~he
sampie fiuid and any other required reagent~s) to the assay device to
ob~ain a linear reaction rate relationship. The slope of the linear rate
10 relationship is taken and applied to a calibration curve to determine
the analyte concentration.
As noted pr~viously the calibration method of the
~ invention can be practiced generally with analytical instrumentsi~ having a plurality of optical read stations. The maximum number of
15 optical read stations with which the method can be practiced in any
instance is determined in part by the incubation period required for
the assay. This is so because the shape of the calibration curve
which is obtained at any optical read station may begin to change
when the time the assay device remains in the temperature-controlled
20 chamber after the last required fluid, i.e. the control or calibrator
composition or another reagent, has been applied to the assay device
exceeds some maximum period which is a function of the particular
assay method. This consideration places constraints on the amount
of time which can be allowed to pass between the end of the required
25 incubation period and the first optical reading taken at the first optical
read station and also on the time period~s) between the successive
optical readings taken at the plurality of read stations. Accordingly,
21~!3537- ~
i
WO 94/017~8 PCI`/US93/06081
`. '-10
it is preferred to obtain the first optical signal at the first optical read
staion at a time which is very close to the required incubation time,
for example, within a time which is approximately one percent (based
on the incubation time) after the expiration of the incubation period.
- 5 It is further preferred to complete the successive optical readings
taken on an assay device at the plurality of optical read stations
within a time period which is approximately ten percent (based on the
incubation time) after the expiration of the incubation period. The
calibration method is preferably practiced with an analytical
instrument having frorn two to six optical read stations.
As will be described in detail below herein the method by
which the calibration curves are formed may involve on!y the
application of the control or calibrator ~omposition to the assay
device and ~herefore only one incubation period is required. However,
the method rnay require, in addition to applying the control or
calibrator composition to the assay device, the application of one or
more reagents. In such assay methods there may be required two or
more separate incubation periods. It should be understood that the
~ incubation period to which reference has been made as being
- ~ 20 controlling with respect to setting the times for the optical readings
is that which occurs after t'ne last (which rnay be the only) fluid
rea~ent is applied to the assay element.
The method of the invention will now be described with
respect to a specific preferred embodiment wherein there is utilized
a multilayer assay element as illustrated in Fig. 1 with which there is
carried out an end point assay. Referring now to Fig. 1 there is seen
an assay element 10 which is a thin film multilayer element typically
`,~0 94/01758 PCI'/US93/06081
-1 '1 -
having a thickness of about 0.1 mm and cornprised of a transparent
- support 12 which carries in succession a reagent layer 14, a light-
blocking layer 1~ and an optical ~op coat laycr 18 which may serve
as a reagent layer, a filter layer such as for proteins, an an~iabrasion
5 layer, etc. The reagent layer 14 is very thin, typically having a
thickness of about 0.025 mm and includes a immunocomplex of a
- binding partner for the analyte of interest and a conjugate of a labeled
analyte (the same as the sample analyte, an analogue thereof or a
structurally similar material which will bind to the binding partner~.
10 The binding partner, an antibody when the sample analyte is an
antigen, is immobilized in the reagent layer 14 by being covaiently
bound to the surface of the support layer 12, which may be of any
appropriate material such as a polyester or a polystyrene, or to a
matrix material or by being physically held by the matrix material.
15 The matrix rnaterial may be hydrophilic gel material such as gelatin,
a polysaccharide, e.g. agarose, a derivatized polysaccharide, mixtures
thereof, and the like. Light-blocking layer 16 may comprise any
suitable material such as, for example, iron oxide, titanium dioxide or
the like dispersed in a binder material such as a polysaccharide. The
20 optional topcoat layer 18 may comprise an antiabrasion layer of a
material such as a polysaccharide or preferably may include buffers,
blocking and displacing agents, etc.
The assay element 10 may also include a layer or other
means tnot shown) for distributing the sample fluid uniformly across
25 the surface of the top layer of the element. Any suitable fluid
distribution technique may be used including, for example, particulate
layers, polymeric layers, fibrous layers, woven fabric layers and liquid
214~ 5 37 ~ `
WO 94/01758 PCI`/US93tO~081 --
-12- `-
transport systems which have been disclosed in the art as being
suitable for this purpose. Many such fluid distribution systerns and
materials for providing a uniform distribution of a fluid sample across
the surface of an assay element are known in the art and therefore
5 extensive discussion of such materials and systems is not required
here. A particularly preferred fluid transport system is that described
- in United States Patent 5,051,237. The distribution means, whether
a layer of fibrous material, etc. or a liquid transport system is
preferably relatively thick in comparison to reagent layer 14.
10One of the series of control or calibrator compositions is
distributed across the surface of the assay element 1 () and the fluid
diffuses throughout layers 14, 16 and 18 as well as any fluid
distribution layer or liquid transport system present and an equilibrium
is established. The analyte present in the control will compete with
15 the labeled analyte in reagent layer 14 for the available binding sites
on the antibodies immobilized in layer 14, the labeled analyte being
dissociated therefrom and replaced by the control analyte in a ratio
approximately equal to the relative amounts of control analyte and
labeled analyte. Thus, depending upon the amount of analyte in the
20 controt, some percentage of the labeled analyte initially bound to the
immobilized antibodies in layer 14 will be displaced therefrom and
distributed throughout the remainder of the assay element. The
amount of labeled analyte bound to the immobilized antibodies in
reagent layer 14 at any time in inversely proportional to the amount
25 of control analyte.
The assay element 10 is then allowed to remain in a
temperature - controlled chamber for the appropriate incubation time
214~5~7
`- ~ ~o 94/01758 Pcr/US93/o6081
-13-
and at the end of the period, or as close as possible thereto, for
example, within approximately one or two percent, a readout signal
is obtained at the first optical read station by irradiating reagent iayer
14 through support layer 12 with the appropriate electromagnetic
5 radiation. After a brief period of time a readout signal is obtained at
the seoond optical station in the same manner. `Optical readings are
then obtained at any additional optical read stations with a brief
period of time between each. The time between each of the
successive readings obtained with the assay element is preferably the
1 0 same.
The procedure is repeated for each of the series of
control or calibrator compositions required to provide the calibration
curve, each composition being applied to a different assay element
: ~ which is specific for the same analyte of interes~. The number of15 control or calibrator compositions required in any instance is
dependent in part on the assay concentration range. To illustrate, in
an assay for theophylline it is preferred to utilize six calibrator
compositions, respectively containing 0.00, 2.50, 5.00, 10.00,
20.00 and 40.00 ug/mL of theophylline to create the calibration
20 curve. In a preferred embodiment wherein calibration curves are
prepared for a theophylline assay and the analytical instrument
includes three optical read stations, each assay element to which
there is applied one of the calibrator compositions is preferably read
at the three optical read stations after residing in the temperature
25 controlled chamber for 350, 360 and 370 seconds, respectively.
The optical signal obtained from assay element 10 is
inversely proportional to the amount of analyte in the calibrator
214~53~
WO 94/01758 PCI/US93/06081
'.'''
-14-
composition, that is the signal decreases as the amount of analyte
increases. Since reagent layer 14 is relatively thin in comparison to
the combined thickness of layers 18 and 18 together with that of any
fluid distribution layer or liquid transport system present and because
5 light blocking layer 16 prevents any of the readout electromagnetic
radiation from entering layer 18 or anything above it, the second
signal obtained will be a function of the labeled analyte which is
bound to the immobilized antibodies and a small percentage of the
free labeled analyte which is distributed throughout the remainder of
10 the assay element. In a preferred embodiment the ratio of the
thickness of reagent layer 14 to the combined thickness of the light-
blocking iayer and the remainder of the assay element is from about
1:20 to about 1:100 or more.
In a particularly preferred embodiment of the method, the
15 optical signals obtained with assay elemen~ 10 are normalized by
obtaining for each assay element used, prior to any calibrator
composition being applied, an optical reading at each of the optical
read stations. This firs~, or "dry", reading is carried out by irradiating
the assay element in the same manner described above to obtain the
20 optical readings after the calibrator composition has been applied to
the assay element and the appropriate times have elapsed. The
second, or "wet", reading at each optical station is divided by the
first, or "dry" optical reading at that station to normalize the signal so
as to compensate for variations in reagent levels because of variations
25 in layer thickness frorn assay element to assay element and also for
variations in the analytical instrument position response. Copending,
commonly assigned application serial no. 382,555, filed July 19,
214~S37
- ~0 94/01758 P~r/uss3/o6o8
-15-
1989 discloses and claims this method for determining the
concentration of analyte in a sample fluid.
In another particularly preferred embodiment of the
method the first, or "dry" optical reading can be oorrected for relative
5 humidity and/or temperature variations. Copending, commonly
assigned application serial no. 533,163, filed June 4, 1990 discloses
- and claims this method for determining the concentration of analyte
in a sample fluid.
Another specific preferred embodiment of the invention
10 utilizes an assay element as illustrated in Fig. 2 with which there is
carried out a rate assay. Referrin~ now to Fig. 2 there is seen a self-
contained, capillary assay module 20 which carries all the test
reagents except for the sample fluid necessary for a particular assay.
This preferred assay element includes a plurality of chambers in a
15 housing 22 wherein a first chamber serves as a front reservoir 24 for
the storage of a labeled conjugate solution. The solution is covered
with a frangible or puncturable foil layer (not shown). A second of
the chambers serves as a back reservoir 26 for the storage of a
substrate solution which is also covered with a similar foil layer (not
20 shown). An optional third chamber serves as a mixing bowl 28 for
the mixing of reagents and a fourth charnber forms part of a
dispenser 30 which is utilized to dispense the substrate solution to
one end of the porous member 32. There is also shown a chamber
34 within the housing 22 wherein there is arranged an absorbing
25 material for absorbing fluid removed from the porous member such as
by a wash fluid as it propagates through the porous member 32.
21~S~7 - i
WO 94/01758 PCI`/US93/0~i081
-16-
., .
In this preferred embodiment the porous member 32 is
- a thin p,orous member possessing an intercommunicating ne~work of
openings throughout such that a fluid deposited on the member will
propa~ate throughout the member because the capillary action. The
thin porous member 32 may be any suitable element such as a porous
membrane, a fibrous mesh pad or the like and may be of any suitable
material such as glass, polymeric materials, paper, etc. In a
particularly preferred embodiment porous member 32 comprises a
nonwoven glass fiber mesh having very thin fibers such as on the
1 û order of about 1 micrometer.
The porous member 32 is mounted within a guide ~not
shown~ formed within the housing 22 and having top and bottom
surfaces which are spaced apart a distance sufficient to support the
member 32. By way of example, the spacing between the top and
- 15 bottom surfaces of th~ guide may be in the range of from about 0.30
mm to about 0.60 m,m; the preferred spacing is about 0.40 mm.
The porous member 32 extends from the dispenser 30
to the Ghamber 34 which holds the absorbing material. The
dispenser chamber 30 is configured as a well for hold,ing a fluid, the
;~ 20 dispenser 30 including a port at the bottom of the well and means for
allowing communication of fluid from the bottom of the well into the
porous member 32. Liquid absorbing material 36, which may be any
suitable materia!,, is located within chamber 34 and forms a part of
the chamber 34 for taking up fluid expelled from the porous member
32 and the guide area, or reaction zone. Absorbing material 36 is
located contiguous porous member 32 and in a preferred embodiment
` ~o ~4/017~8 2 1 ~ ~ 5 3 7 P~r/USg3/0~081
-17-
(as illustrated) is formed c~nveniently as an extension of the porous
material folded back and forth on itself.
The housing 22 also preferably includes a chamber 38
which is positioned immediately above the top horizontal surface of
5 porous member 32 and has a port at the bottom periphery thereof to
allow fluid to be delivered to the porus member 32. The housing 22
may include a transparent window area (now shown) positloned
immediately below the bottom horizontal surfar e of porous rnember
32 to provide access for the illumination used to measure any
10 detectable change effected in the porous member as a result of the
assay method or preferably an opening in the housing to permit
readout illumination to be directed onto the porous member without
having to pass through the material of which the housing is
comprised. A preferred assay module of the type illustrated in Fig. 2
15 is disclosed and claimed in copending, commonly assigned application
serial no. 354,026, filed May 19, 1989.
- For purposes of illustration the method will be described
with respect to an assay for human chronic gonadotropin (HCG).
Initially, the control or calibrator composition is deposited onto the
20 solid carrier 32 to which there are immobilized antibodies to HCG and
the assay element is incubated. Next the enzyme oonjugate solution
comprising an antibody to HCG covalently bound to an enzyme such
as alkaline phosphatase is aspirated by a pipette from reservoir 24
and deposited on the solid carrier through chamber 38. The assay
2~ element is then allowed to incubate for a second time. Subsequently,
a substrate solution for the particular enzyme label, for example,
methyl imbelliferyl phosphate when the enzyme is alkaline
214~5~7
WO 94/~1758 ~ Pcr/uS93/o6081
-18-
phosphatase, is aspirated by a pipette from reservoir 26 and
deposited in dispenser 30 where it is allowed to enter one end of the
porous member 32 and propagate through the member. The
substrate solution functions as a wash solution to remove free labeled
conjugate from the reaction zone and also to react with the enzyrne
label to provide a detectable species.
After a third period of incubation, the detectable species
which is liberated by the reaction between the enzyme and the
substrate material is read kinetically. It is preferred to obtain four
optical readings on each assay element. In the illustrative
embodiment wherein the analytical instrument includes three optical
read stations, each assay elemen~ to which there is applied one of the
calibrator compositions is preferably read a first time at the three
optical read stations after residing in the temperature controlled
charnber for 228, 240 and 252 seconds respectively. The second
readings are obtained at 288, 300 and 312 seconds, respectively.
: The third readings are taken at 348, 360 and 372 seconds
respectively and the fourth readings at 408, 420 and 432 seconds
respectively.
The slope of the curve for each calibrator composition at
each optical read station is calculated and these values are used to
form the calibration curve for each optical read station. In the
illustrative assay for HCG it is preferred to utilize six calibrator
compositions containing 0.00, 5.00, 15.00, 50.00, 150.00 and
500.00 mlU~mL HCG respectively.
It should be noted that although the rate assay has been
illustrated with respect to the assay for HCG where it is preferred to
2143537
~o 94/017~8 Pcr/u~93/06~81
-19-
obtain four readings on each assay element a~ each optical read
station, the kinetic reading of the liberated detectable species may
require a different number for optical readings, for examplet only two
or as many as five or more.
As noted previously, when a patient sample fluid is
analyzed with the instrurnent the optical readings taken on the assay
device can be obtained at any of the plurality of optical read stations.
Of course, it will be understood that for each assay device bearing a
patient sample fluid, whether an end point assay or a rate assay, the
optical reading(s) must be taken at the same optical read station.
Further, the readings must be taken at the times the calibrator
compositions were read at the particular optical read station. For
example, for the illustrative theophylline example described above, an
assay device bearing a patient sample fluid would be read at any one
1~ of the three optical read stations at times of 350, 36Q and 370
¦~ - seconds respectively.
¦ The invention will now be described further in detail with
respect to specific preferred embodiments by way of examples, it
being understood that these are intended to be illustrative only and
the invention is not limited to the materials, procedures, etc. recited
therein .
EXAMPLE I
Calibration curves for a theophylline assay for three
optical read- stations in an automated analytical instrument were
obtained by the following procedure. Six calibrator compositions
WO 94/01758 2 1 4 ~ 5 3 7 .~ ~ PCI`/US~3/06~81 ~
-ZO-
having, respectively, .l 2.50, 5.00, 1().00, 20.00 and 40.00
ug/mL of the theophylline were used.
An assay element of the type illustrated in Fig. 1 was
inserted in a temperature controlled charnber and an optical reading
5 obtained at each of the three optical read stations. Subsequently, a
calibrator composition was dispensed to the assay element and the
assay element was read at each of the three optical read stations
after residing in the chamber for 350, 360 and 370 seconds
respectively. The "wet" reading at each read station was then
10 divided by the "dry" reading at that station to obtain a normalized
optical signal.
- The procedure was repeated for each calibrator
composi~ion and duplicates o~ each composition were run. The
results are shown in Table 1. Each signal is the average of the two
15 signals obtained from the duplicates.
~`
, _ _ _
TABLF I
. _ ,
CALIBRATOR OPTICAL READ OPTICAL FIEAD ¦ OPTICAL READ
ug/mL) STATION #1 STATION ~2 I STATION ~3
_ _
SIGNAL (NORMALIZED)
O 00 ¦ 14.54 ¦ 14.79 114.72
I i
20 2.50 1 1 .36 1 1 .57 1 1 .50
l .1
5 00 10.02 10.21 10.09
I . i _
10.00 8.09 8.20 8.10 ~
_ l _ 3
20.00 1 6.22 1 6.33 1 6.24
40 00 4.31 4.39 4.32
I . _ .
- 21~5~7 `
--- ,iO 94/01758 Pcr/US93/o6081
The calibration curves for the three optical read stations are
shown in Fig. 3.
~1!
Calibration curves for an HCG assay for three optical
5 read stations in an automated analytical instrument were obtained
according to the method of the invention. Six calibrator composil:ions
having, respectively, 0.00, 5.00, 1 5.00, 50.00, 150.00 and 500.00
mlU/mL of HCG were used.
An assay etement of the ty,~e illustrated in Fig. 2 was
10 inserted in a temperature controlled charnber and the calibrator
composition dispensed thereto. After a ~hree minute incubation
period the enzyme conjugate solution comprising alkaline phosphatase
bound to an antibody to HCG was applied to the assay element
follovved by a nine minute incubation period. The substrate solution
15 comprising methyl um~elliferyl phosphate was then applied and four
optical readings were taken at each of the three optical read stations.
The first optical readings were taken at the three optical read stations
at 228, 240 and 252 seconds, respectively, after the substrate
solution has been applied to the solid carrier. The secorld kinetic
20 readings were obtained at 288, 300 and 312 seconds respectively.
The third readings were obtained at 348, 360 and 372 seconds
respectively and the fourth at 408, 420 and 432 seconds
respectively.
The procedure was repeated for each calibrator
25 composition and duplicates of each composition were run. The slope
for each of the calibrator compositions at each of the optical read
stations was calculated. The results are shown in Table ll. Each
214~37 ~
WO 94/017~;8 ;; - r ~ PCI /US93/06081
-22-
signal (slope) is the average of the two signals obtained from the
duplicates.
_ , -- - _ _ -- ~
Calibrator OPTICAL READ OPTICAL READ OPTICAL FiEAD
(mlU/mL) STATION #1 STATION #2 STATION #3
SIGNAL (mV/sec)
_
0.00 9.70 10.32 9.6
I . . .
5.00 35.43 38.60 37.82
I . _ _ ..
15.00 86.00 94.31 94.23
I , _
50.00 256.26 269.72 263.42
_ _ _ . .
150.00 _ 623 .00 643 .95 63 1.48
- 1 C 500.00 1262.92 1310.24 1297.37
~,,
The calibration curves for the three optical read stations
~ are shown in Fig. 4.
- Although the invention has been described with respect
to specific preferred embodiments it is not intended to be lirnited
15 thereto but rather those skilled in the art will recognize that variations
and modification may be made therein which are within the spirit of
the invention and the scope of the appended claims.
, j . .