Note: Descriptions are shown in the official language in which they were submitted.
~15032 0
The present lnventlon relates to an apparatus and
method for removlng odours, or other alr-borne contaminants,
from air.
Alr ln enclosed spaces becomes stale or develops
odours owlng to contamlnatlon by substances such as volatlle
organlc compounds, mould and inorganlc compounds. In
hospltals, anaesthetlc gases and toxlc sterlllzlng
compounds, such as ethylene oxlde, can accumulate ln the
alr. In smaller spaces, such as the lnterlors of cars,
trucks and alrcraft, accumulatlon of contamlnants, for
example from constructlon or furnlshlng material, can be
more of a problem. Tobacco combustlon also contrlbutes to
contaminatlon.
Treatment of contamlnated air has focused on
removal of solld partlcles, such as by flltratlon,
adsorptlon onto actlvated carbon or electrostatlc
preclpltatlon. Such solld removal merely transfers the
contamlnant from one place to another. In the case of
actlvated carbon, whlch is a common choice for such removal,
the contaminants are adsorbed onto the carbon surface which
eventually becomes saturated and must be replaced. Some
contamlnants, such as low molecular welght gaseous
compounds, are not satlsfactorlly adsorbed or are easlly
desorbed. Further, efflciency of contamlnant removal
decreases at elevated temperatures.
Of the many requirements of an alr remedlatlng
apparatus, a few are of fundamental lmportance and are not
achleved by commerclally available systems. These
requirements are the abllity to handle a variety of
60557-4966
2150320
contamlnants slmultaneously at room temperature and under
prolonged, long term operatlon.
Recently photocatalysls has been used to remove
contamlnants. Contamlnated alr ls passed over or through a
photocatalyst lrradlated wlth a sultable llght source.
Anatase tltanlum dloxlde (TlO2) ls a common form of
photocatalyst and ls sultably irradiated wlth UV light at
room temperature. Photocatalytlc treatment uslng tltanlum
dloxlde removes low molecular weight organlc and lnorganlc
gases compounds faster than activated carbon at continous
operation and has the advantage that the contaminants are
generally broken down lnto harmless, odourless or less toxlc
compounds.
U.S. Patent No. 5,045,288 (Raupp et al.) discloses
an apparatus whlch uses photocatalysls to remove
contamlnants from water. The water ls pumped through a bed
of photocatalyst lrradlated by UV llght through UV-
transparent walls of a reactlon vessel. Although such an
arrangement mlght be modlfled for use wlth alr lnstead of
water, the large reactlon chamber whlch ls needed for the
catalyst bed and the loose catalyst make such an apparatus
unsultable for small spaces such as rooms. Further, the
back-pressure or reslstance created by the catalyst lmpedes
the treatment of large volumes of contamlnated alr and
therefore would requlre powerful equlpment to be effectlve
for contamlnant removal.
U.S. Patent No. 4,892,712 (Robertson et al.)
dlscloses an apparatus for removal of contamlnants from
flulds whlch uses photocatalysls. The apparatus surrounds a
60557-4966
2150320
UV tube with a cyllndrical matrix containing the
photocatalyst. The UV tube and matrix are in turn enclosed
in a cylindrical fluid-tight ~acket having a small fluid
inlet tube, perpendicular to the longitudinal axis of the
cylindrical ~acket, at one end of the apparatus and a
corresponding fluid outlet tube at the other end of the
apparatus. Although the apparatus is described as being
useful for both gases and liquids, this apparatus would not
be effective for removlng contamlnants qulckly from the air
in a room or laboratory. The back-pressure generated by
movlng alr along the length of the matrix would require a
pump or fan inconveniently large.
There ls a need for arrangements whlch are
suitable to decontaminate or remove odours from small spaces
such as rooms in hospltals, houses or laboratorles or
interiors of vehicles such as cars, trucks or airplanes. To
be suitable for smaller spaces, the decontamlnatlng
equlpment should be able to treat large volumes of alr
qulckly wlthout belng too large or cumbersome. Preferably
such equipment could be placed on a bench or table and
quickly remove unpleasant or harmful contaminants.
According to one aspect of the inventlon there ls
provided an apparatus for purifying alr comprlslng a housing
having an air inlet and an air outlet;
a circulating means, mounted ln the housing, to
circulate air from outside the housing through the inlet
along an air flow path inside the housing and out of the
outlet;
a planar filter means disposed across the path of
60557-4966
2150320
circulatlng air, sald fllter means comprlslng a
photocatalyst flxed to a flbrous porous support;
and a llght source mounted ln the houslng to
dlrect llght onto the fllter means to actlvate the
photocatalyst.
In a preferred embodlment the alr flow path has an
upstream portion ln whlch alr flows ln one dlrectlon and an
adjacent downstream portlon ln whlch alr flows ln the
opposlte directlon and the planar fllter means is dlsposed
ln the houslng such that the filter means extends across the
upstream and downstream portlons.
The houslng provldes a path for the alr to
clrculate and a structure on whlch the other elements such
as the llght source and the clrculatlng means can be
mounted. The houslng may be plastlc or metal or other
sultable structural materlal. Besldes the lnlet and outlet,
the houslng should be reasonably airtight.
The inlet should be big enough to accommodate the
filter means. If the inlet ls larger than the fllter means
then alr would be allowed to pass wlthout belng exposed to
the photocatalyst. If lt ls smaller than the fllter means
thls would waste some of the avallable area of the filter.
The slze of the fllter means should be blg enough to permlt
suitable alr flow. The alr lnlet may have an addltional
fllter means, upstream of the photocatalyst fllter means,
such as ls used in furnaces, to remove dust and prevent
clogglng of the photocatalyst.
Slnce alr cannot flow as fast through the fllter
means as through an unobstructed outlet of the same
60557-4966
2150320
dimenslons, the outlet may be made smaller than the lnlet to
equallze the system. In an apparatus tested, a 33% size
dlfference was used (48 lnch2 lnlet and 36 inch2 outlet).
A protectlve grllle ls preferably placed on the
outslde of the lnlet and the outlet. The grllle should not
substantlally lnterfere wlth the alr flow.
The llght source should supply llght at sufflclent
lntenslty and wavelength band to energlze the photocatalyst
so that reactlon or breakdown of the contamlnants ls
effected. A wlde range of llght sources can be used.
Sultable llght sources include: fluorescent blackllghts,
lncandescent blackllghts, xenon lamps and low, medlum and
hlgh pressure mercury lamps. However, lf the photocatalyst
is tltanlum dloxlde, a source of UV, such as a blackllght
havlng a wavelength band of from 300 to 440 nm, ls
preferred. Whllst shorter wavelengths such as around 254 nm
have been found to be efflclent, lt ls preferred, for safety
reasons, to use llght ln the UV A reglon. A wavelength band
of around 350-380 nm is suitable. A wavelength of 366 nm is
preferred.
The intensity of light is governed by the type and
number of light sources. For titanium dioxide it has been
found that lower power 15 watt mercury bulbs, with a peak
output at wavelengths of 313 and 366 nm, are adequate. Such
bulbs provide safety and economy. Using such bulbs, it has
been found that whllst there is a significant increase in
reaction speed (measured by the reduction in contaminants as
a function of time) using 2 bulbs rather than 1 bulb, the
increase obtained by using 3, rather than 2 bulbs, is less
60557-4966
21S0320
signlflcant. Therefore for domestlc appllcatlons, 2 bulbs
may be preferred for the sake of economy.
The llght source ls arranged to one slde of the
filter means. That is, the light source is not surrounded
by the filter means. This permits the filter means to have
a larger area ln the path of the air and the llght from the
llght source radlates to reach the exposed area of the
fllter means. Preferably a reflectlng layer ls dlsposed
behlnd the light source to reflect escaplng llght back to
the filter means~
Any photocatalyst whlch effectively reduces or
removes contaminants may be used. Sultable photocatalysts
are TlO2, ZnO, CdS, WO3, SnO2, ZrO2, Sb2O4, CeO2 and Fe2O3.
A preferred photocatalyst ls the anatase form of TlO2.
Optlmlzlng odour or contamlnant removal wlll be a
balance between conflictlng needs such as lncreaslng
efflclency on the one hand and lncreaslng alr flow and
reduclng costs on the other hand. For a glven
photocatalyst, efflclency of contamlnant removal wlll be
affected by the lntenslty and wavelength of the light; the
surface area of the photocatalyst; the volumetrlc alr flow;
and the pressure dlfferentlal across the fllter means.
The alr flow determlnes the resldence tlme or the
tlme the contamlnants are in contact with the photocatalyst.
With regard to residence tlme, the longer the contamlnated
alr ls ln contact wlth the photocatalyst, the hlgher the
probablllty that the contamlnants wlll decompose. On the
other hand, lt ls deslrable to process as much alr as
qulckly as possible to reduce the level of contamlnants.
60557-4966
21S0320
The circulation means ls conveniently a fan
although other means, such as pumps, or convection might be
used. It is only required that the means ensures movement
of the air over the photocatalyst.
In a preferred embodiment the inlet is in the top
of one slde the front of the housing and the outlet is at
the end of a tunnel protruding out from the bottom of the
same side of the housing. This tunnel gives a convenient
location for the fan and, by increasing the area of the
bottom of the apparatus (the "footprint"), gives stability
to the apparatus so that it will not readily tip over. Such
a design also permits a further preferred feature in whlch
the filter can extend substantially the whole height of the
apparatus. Incoming air is then exposed to the top section
of the filter, guided around in a U-turn by the curved
configuration of the back of the housing and is then again
exposed to photocatalsyt on the bottom section of the filter
on its way through the tunnel to the outlet. A plenum is
used to separate top and bottom sections of the filter in
this arrangement.
Other arrangements are, of course, possible. For
example, the housing could be constructed so that the
circulating air travels in an essentially straight path from
an inlet on one side of the housing to an outlet at the
other side of the housing. In such an arrangement the
housing might conveniently be rectangular ln shape. The
filter means could be mounted ad~acent either the inlet or
the outlet or even two filter means could be used one
located ad~acent each of the inlet and outlet. The
60557-4966
215~320
circulating means could then be located centrally between
the inlet and outlet. Thus it will be seen that various
arrangements are possible.
The filter means includes a fllter which may be
flat or pleated. The fllter essentially comprlses two
components, the support and the photocatalyst. The support
is fibrous and should be sufficiently porous to permlt
adequate alr flow. Preferably the support has a large
surface area and ls transparent to the light used or absorbs
little of the llght. The photocatalyst should be arranged
on the support to maximlse the surface area exposed to the
circulating air. The photocatalyst could be in the form of
a coating on the support prepared conventionally by
contacting the support with a coatlng composition of the
photocatalyst in a liquid medium. An effective way to
maxlmlze the surface area ls to apply the photocatalyst in
the form of a finely divided powder to a fibrous non-woven
support which is capable of holding the photocatalyst
particles electrostatically, such as an electret materlal.
Precharged electret fibres are preferably formed from a film
of a dielectrlc polymer that is capable of belng corona
charged and then fibrillated. Suitable film forming
dlelectrlc polymers include polyolefins, such as
polypropylene, linear low denslty polyethylene, poly-1-
butene, polytetrafluoroethylene,
polytrifluorochloroethylene; or polyvinylchloride; aromatic
polyarenes, such as polystyrene; polycarbonates; polyesters;
and copolymers and blends thereof. Preferred are
polyolefins free of branched alkyl radlcals and copolymers
60557-4966
21 5032 D
thereof. Particularly preferred are polypropylene and
polypropylene copolymers.
Varlous functlonal additlves known ln the art can
be blended wlth the dlelectrlc polymers or copolymers such
as poly(4-methyl-1-pentene) as taught ln U.S. Pat. No.
4,874,399, a fatty acid metal salt, as dlsclosed ln U.S.
Pat. No. 4,789,504, or partlculates as per U.S. Pat. No.
4,456,648. Such electret flbres wlll electrostatlcally flx
partlculates. Blown flbres, partlcularly blown
polypropylene mlcroflbres are preferred as the support
materlal. It is further preferred that the flbrous support
ls polypropylene havlng a welght range of from 20 to 80
gm/ml, more preferably ln the range of from 30 to 60 gm/ml.
The preferred support materlal, blown
polypropylene mlcroflbres, ls avallable commercially in
rolls having a thlckness of 5 thousandths of an lnch ln
compressed form and 20 thousandths of an lnch ln
uncompressed form. For the present lnventlon lt is
preferred to "fluff" the material glving an uncompressed
thlckness of 60 thousandths of an inch. This material,
after having been loaded wlth the photocatalyst, ls
preferably used as a double or trlple layer.
A fllter means ln whlch the photocatalyst ls
electrostatlcally flxed to the support, ls readlly prepared
by contacting the flnely dlvlded photocatalyst powder with
the support and removlng any loosely adhered particles, or
excess partlcles, by vacuumlng.
A photocatalyst ln powder form can also be loaded
on the support by coatlng the support wlth, for example, a
60557-4966
2l ~o32D
thln water-based acrylic adhesive and then contacting the
coated support with the photocatalyst powder. Titanium
dioxide can be loaded on a polypropylene support using this
technique.
The photocatalyst powder should generally have a
particle size of less than 1 mm or the support may not be
able to hold the particles electrostatically. Further, a
smaller particle size would normally provide a larger
surface area and hence lncrease the reaction speed.
Partlcle sizes ranging from 1 nm to 1 mm (1000 microns) are
sultable, preferably from 2 nm to 1000 nm.
The filter should generally provide a surface area
of photocatalyst of at least 1 m2/g, preferably at least
5 m2/g. When the photocatalyst is in powder form and is
electrostatically fixed to the support, substantlally all
the surface area of the photocatalyst ls avallable for
reaction. Sultable tltanlum dloxlde photocatalysts ln
powder form are available from Degussa under the trade
deslgnatlon P 25, whlch has a surface area of about 50 m2/g,
and from Homblkat under the trade deslgnatlon UV100, whlch
has a surface area of about 250 m2/g. However, lt ls to be
noted that when the partlcles are extremely flne they may
clump together resultlng ln a decrease of the predlcted
surface area.
A fllter means prepared by coatlng a composltlon
of a tltanlum dloxlde photocatalyst ln a llquld medlum onto
a support would typlcally have a surface area of from about
10 to about 15 m2/g.
Uslng an electrostatlcally bound powdered
60557-4966
.. - 2150320
photocatalyst on a flbrous support, up to 50% by welght of
photocatalyst can be loaded on the support based on the
welght of the support. A preferred loadlng range ls from 10
to 25% by welght.
By use of an actlvated photocatalyst, such as
tltanlum dloxlde, both organlc and inorganlc compounds can
be decomposed. For example: hydrocarbons can be decomposed
to carbon dloxlde and water; and hydrogen sulphlde can be
decomposed to elemental sulphur. The reactlons whlch occur
do not consume the photocatalyst whlch may be contlnually
re-actlvated by the llght.
The fllter means may addltlonally comprlse a layer
of an adsorbent, such as actlvated carbon, on at least one
other flbrous porous support layers to beneflt from the
comblnatlon of both photocatalytlc and adsorptlve
contamlnant reductlon or removal. If such an addltlonal
layer ls used, lt should be on the slde of the photocatalyst
layer opposlte the llght source to avold blocklng the llght
and obstructlng actlvatlon of the photocatalyst.
Slnce the fllter means ls dlsposed across the path
of the clrculatlng air, when the apparatus ls ln operatlon,
a back pressure ls generated. The back pressure ls a
functlon of the pressure drop across the fllter. To glve an
example, ln a domestlc furnace, the pressure drop across a
fllter would be around 0.15 lnches of water at a face
veloclty of 300 ft/mln. In such a domestic furnace, lf the
pressure drop across the fllter rose to 0.5 lnches of water
at 300 ft/min, the fan mlght not have enough power to
clrculate alr.
60557-4966
2150320
For table top or bench top appllcatlons, such as
preferred for an apparatus of the present lnventlon, the
pressure-drop across the filter should preferably be in the
range of 0.3 to 0.5 lnches of water at a face veloclty of 75
ft/mln, 0.6 to 1.0 lnches at 300 ft~mln. Ideally, the
pressure drop should be as low as posslble for a glven alr
flow, conslstent wlth achlevlng effectlve contact of the alr
wlth the photocatalyst.
Preferably, the alr flow wlll range from a face
veloclty of from 10 ft/mln to 700 ft~mln, more preferably
from 25 ft/mln to 350 ft/mln.
Uslng the preferred polypropylene support materlal
a thickness of from 120 thousandths of an lnch, for a double
layer, to about 130 thousandths of an lnch for a compressed
trlple or multlple layer, ls sultable.
It ls preferred that the support ls pleated or
corrugated. Thls pleated constructlon permlts a greater
surface area wlthout slgnlflcantly lncreaslng the back
pressure or lmpedlng the alr flow. To asslst ln
constructlng such a pleated structure the support can be
mounted on a backlng materlal whlch can be conflgured to
hold the deslred shape. The backlng materlal should not
lnterfere wlth alr flow or the energlsatlon of the
photocatalyst. Expanded metal or scrlm ls sultable as a
backlng materlal. Scrlm ls a net of materlal such as
polypropylene and is avallable for example, from the Conwed
Corp. A form of scrlm commerclally avallable has a
thlckness of about 10 thousandths of an lnch. The backlng
layer may be coated wlth a layer of pressure-sensltlve
60557-4966
2150~20
adheslve to hold the support. The fllter means may have
multlple layers of the support, for example a layer of
support on each of a plurallty of backlngs, or a slngle
backlng mlght have a layer of support on each slde so the
backlng layer ls actually ln between the support layers. Of
course, havlng more layers wlll lmpede alr flow. A slngle
or double-layer, pleated, ls preferred.
The apparatus can be used to remove varlous odours
and contamlnants, for example, low molecular welght organlc
gases such as lsobutane, propane, dlethylether and
acetaldehyde and hlgher molecular welght gases such as
ethylproplonate (frulty rum smell), allylbutyrate (peach,
apricot smell) and propylbutyrate (sweaty, rancid smell).
An important contaminant which can be removed is hydrogen
sulphide. It has been sald that 80 percent of maladorous
gases contain hydrogen sulphlde.
The lnventlon wlll be further descrlbed wlth
reference to the accompanylng drawings showing, by way of
example, embodiments of the inventlon and elements thereof.
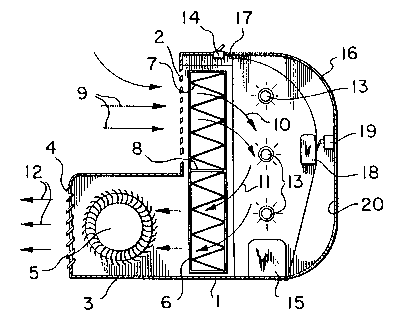
Flgure 1 ls a partlally cut-away schematlc slde
view of an apparatus according to the inventlon;
Flgure 2 ls a front vlew of the embodlment of
flgure l;
Figure 3 ls a partially cut-away schematic slde
view as in flgure 1, but showing the pivoted rear portion ln
an open posltlon;
Figure 4 ls an exploded vlew of a fllter cartrldge
containing a filter sultable for use in the apparatus of
figure l;
60557-4966
21~320
14
Figures 5 and 6 are front and slde vlews
respectlvely of the fllter cartrldge of figure 4;
Flgures 7, 8 and 9 are exploded vlews of dlfferent
embodlments of a fllter sultable for use ln the apparatus of
flgure 1.
Wlth reference to Flgure l, an apparatus for
purlfylng air accordlng to the lnventlon lncludes a houslng
1 whlch is generally L-shaped wlth a curved back. The
houslng ls made of structural plastlc materlal but could be
made of any other sultable structural materlal such as
metal. The front of the houslng has a top portlon
comprlslng an alr lnlet 2 and bottom portlon havlng an
extenslon generally lndlcated by 3 termlnatlng ln an alr
outlet 4. A fan 5 ls mounted ln the extenslon 3. The
extenslon 3 provldes a convenlent locatlon for fan 5 and
addltlonally provldes a stable deslgn. A fllter means ln
the form of a generally planar fllter cartrldge 6 ls mounted
wlthln the houslng ad~acent lnlet 2 and extends from the top
of the houslng to the bottom of the houslng generally
coplanar wlth lnlet 2. It can be seen that the fllter
cartrldge 6 extends across both the upstream and downstream
alr flow passages such that the cross-sectlonal area of the
cartrldge ls substantlally equal to the sum of the areas of
the upstream and downstream passages. Dlsposed ln cartrldge
6 ls a fllter 7 whlch comprlses one or more layers of a
support havlng a photocatalyst flxed thereon. A plenum 8
separates the fllter cartrldge 6 lnto an upper fllter
sectlon and a lower fllter sectlon so that clrculatlng alr,
shown by arrows 9, 10, 11 and 12, passes through the upper
60557-4966
2150320
section of the cartrldge 6 after enterlng lnlet 2 and then
passes through the lower section on lts way to the outlet 4.
In thls embodlment the alr ls thus exposed twlce to the
fllter. The preferred conflguratlon of fllter 7 shown ln
Flgure 1 ls a pleated arrangement ln whlch fllter 7
comprlses a plurallty of planar faces each dlsposed at an
angle to the general dlrectlon of the alr flow. The fllter
will be discussed ln more detall herelnafter.
UV lamps 13 act as the llght source and are
mounted ln the side walls of houslng 1 on one slde of fllter
cartrldge 6 and between fllter cartrldge 6 and the back of
the houslng. UV lamps are a preferred llght source for
tltanlum dloxlde as photocatalyst. The lamps shown are
conventlonal cyllndrlcal UV lamps and extend from one slde
wall of the houslng to the other slde wall. The posltlonlng
of lamps 13 ls not crltlcal, provlded they can energlze the
photocatalyst effectlvely. The lamps are actlvated by a
swltch 14 mounted at the top of houslng 1 for easy access,
and a ballast 15, mounted at the bottom of the houslng for
stablllty.
The back of houslng 1 comprlses a shroud 16 whlch
ls plvoted at plvots 17 at the top of the houslng and can be
opened upwardly for easy access to the lnslde of the
houslng. A lock 18 and catch 19 fasten the plvoted shroud
16 ln lts closed positlon. A reflectlve surface 20 of
pollshed alumlnum on the lnslde surface of shroud 16 and the
back of houslng 1, redlrects llght escaplng from the lamps
13 to the fllter 7 to maxlmlze efflclency. Pollshed
alumlnum ls the preferred materlal for reflectlon of UV
60557-4966
2150323
llght.
Flgure 2 shows a front view of the apparatus of
Flgure 1. Houslng 1 ls provlded wlth a protectlve lnlet
grllle 30, disposed across the lnlet 2 (not shown ln thls
flgure), and protectlve outlet grlll 31, dlsposed across the
outlet 4 (not shown ln this flgure).
Flgure 3 ls another slde vlew of the apparatus
shown ln Flgures 1 and 2 and is the same as Figure 1 except
that it shows the plvoted shroud 16 ln an open positlon.
In operation, fan 5 draws outside air through
lnlet 2 and through the upper portion of fllter 7. The
photocatalyst on filter 7 ls energlzed by the UV lamps 13
whlch causes breakdown and degradatlon of contamlnants in
the alr ln contact wlth lt. The alr contlnues to be drawn
through houslng 1 around the back and through the lower
sectlon of fllter 7 where further breakdown and degradatlon
are effected ln the lower sectlon of the filter. Reactlon
products and unreacted gases and alr then pass through
extension 3 and are expelled from outlet 4.
Flgures 4, 5 and 6 show the fllter cartrldge 6 of
Flgure 1 ln more detall. Cartrldge 6 has an outslde caslng
of two detachable sectlons 40 and 41 whlch contaln the
fllter 7. The casing permlts easy handllng and protectlon
of fllter 7. In the embodlment shown in the drawlngs,
filter 7 has a pleated or corrugated shape. Plenum 8
dlvldes the upper (inlet) sectlon from the lower (outlet)
section of the fllter and prevents alr from belng permltted
to bypass the fllter. Narrow llps 42 and 43 extend across
the front and back faces of the caslng sectlons to
60557-4966
2150~20
comfortably retain the filter ln the caslng.
Flgures 7, 8 and 9 are exploded vlews of three
types of fllter sultable for use ln the present lnventlon.
Flgure 7 ls a vlew of the fllter 7
shown ln Flgures 4, 5 and 6. Support 71 ls a double layer
of blown polypropylene mlcroflbres loaded wlth a
photocatalyst powder of tltanlum dloxlde electrostatlcally
flxed to the flbres. Backlng layers 72 and 73 are expanded
metal mesh whlch have been formed lnto a pleated or
corrugated shape. To achleve the fllter 7 (shown ln Flgure
1) the support layer 71 ls pressed between expanded metal
mesh layers 72 and 73 forclng the pllable flbrous support
layer 71 to adopt the same pleated shape as expanded metal
mesh layers 72 and 73.
In flgure 8, support 81 ls a slngle layer of the
same tltanlum dloxlde loaded polypropylene flbres as shown
ln flgure 7. Slnce only a slngle backlng layer 82 ls used,
support 81 ls flxed to the backlng layer 82 by applylng a
coatlng of pressure-sensltlve adheslve to elther the backlng
layer 82 or the support layer 81. ~
Figure 9 ls the same as flgure 7 except that
support 91 ls a slngle layer (as ln figure 8) between the
two backing layes 92 and 93. A further difference is that
an addltional support layer 94 is present. The additional
support layer may be an additional layer of polypropylene
flbres loaded wlth titanium dioxide photocatalyst or it may
be a porous layer contalnlng a conventional adsorbent such
as a layer of actlve carbon flxed to a porous web of
polyester flbres.
60557-4966
2150320
An apparatus according to the lnventlon was
constructed by modlfylng an LP 1500 Blonalre alr purlfier.
The UV lamps were 15 watt twelve lnch RPR-3000 lamps (such
as used ln a Rayonet Photochemical Reactor) obtained from
the Southern Ultravlolet Co. The fan had two speeds. The
hlgh speed or hlgh flow settlng provided a flow rate of 1080
L/mln. The low speed setting provlded a flow rate of 604
L/min. In the apparatus used these settlngs correspond to
face velocltles of 180 ft/mln (hlgh flow) and 100 ft/mln
(low flow).
The modlfled Blonalre LP 1500 alr purlfler whlch
was used ln the examples, and represented schematlcally by
flgures 1, 2 and 3, had a helght of 8.75 inches, a depth of
10.625 lnches and a wldth of 12.25 lnches. These dlmenslons
glve a compact devlce wlth a modest "footprlnt" suitable for
a bench or desk top.
The filter cartridge used had a width of 11.5
inches and a height of 7.75 inches. The plenum was 3.38
inches from the bottom of the cartridge. The cartridge was
1.5 lnches thick and the distance from one pleat to the
next, along the plane of the pleat was 1.58 inches. Thus a
fllter with eight pleats had 16 pleat faces making it
comparable to a flat unpleated filter spanning 16 x 1.58
inches. The lip of the cartridge was about 0.5 inches all
round.
The apparatus was tested by placing it in an air-
tlght chamber 2 feet by 2 feet by 2 feet (227 litres). A
known amount of contaminant was introduced into the chamber.
For testing purposes the initial concentration of
60557-4966
2150320
19
contaminant was sometlmes ad~usted to as much as 1500 to
2000 ppm. (In normal use, concentratlons would be about 1-
20 ppm). After a few mlnutes to allow for equlllbration,
the apparatus was activated and samples removed with a
syringe at regular intervals. The samples were analyzed
wlth a Perkln Elmer Autosystem Gas chromatogram - 9000
equipped with a methanizer. The temperature of the flame
ionlzation detector was reduced from 350C to 225C when
testlng for isobutane and propane, to improve accuracy. For
hydrogen sulphide, the concentration was measured with a
confined space monltor available from 3M under the trade
designation Dynatel CSM 500.
The apparatus was compared to a Phlllps Clean Alr
System 75 (as a reference) consldered to be an lndustry
standard. The Phlllps devlce is designed to remove odours
and lt has two actlvated carbon fllters. It has a flow rate
of 3136 L/mln. Air is drawn in from the sides of the devlce
and exhausted from the top of the device.
Preparation of Filters
Various fllters were tested. They were prepared
as follows:
Filter #l
A web of blown polypropylene non-woven fibres was
needle-packed to a web. The web was shaken in a bag with
excess titanium dioxide powder (Degussa P. 25) untll the web
was saturated with the powder. The titanium dioxide loaded
web was vacuumed in a fume hood or with a portable vacuum to
remove loose powder. The web was folded and placed on a
pleated wire mesh backing layer (0.015 inches thick and 1.25
60557-4966
215032û
lnches diamond expanded metal mesh) havlng elght pleats or
corrugatlons. A second pleated wlre mesh backlng layer was
pressed onto the other slde of the web. The fllter was
placed ln a cardboard frame (12 inches by 8 inches) for ease
of handling.
Filter #2
The procedure for Fllter #l was followed except
that only one layer of polypropylene web was used and the
web had a pleated wlre mesh backlng on only one slde. The
web was fixed to the backing by a coatlng of pressure-
sensltlve adhesive.
Filter #3
A sheet of glass fibre was used instead of the
blown polypropylene flbres. The tltanlum dloxlde was coated
on the glass flbres wlth a solgel process (such as ls used
ln US Patent No. 4,892,712). Otherwlse the procedure was as
for Fllter #1.
Fllter #4 and Fllter #5
The procedure for Fllter #l was followed except
that a scrlm materlal was used as backlng layer. The scrlm
was coated with a pressure-sensitive adheslve. Since the
scrlm ls dlfficult to pleat, this type of filter was
unpleated and flat.
Fllter #6
The procedure for Filter #l was followed except
that one layer of titanium dioxide loaded web was replaced
by a layer of a polyester flbre web coated with an actlvated
carbon adsorbent.
The apparatus was tested uslng the followlng
60557-4966
215032~
compounds as contamlnant: lsobutane, hydrogen sulphlde,
propane, dlethyl ether, acetaldehyde, ethyl proplonate,
allyl butyrate and propyl butyrate.
Unless otherwlse stated, the fllter used was
Fllter #l and the apparatus was used at the low settlng of
604 L/min.
60557-4966
215 032D
Example 1 Isobutane as Contamlnant
TA~LE 1
Time Contamlnant Concentratlon (ppm)
(Hours) Phlllps Hlgh Flow Low Flow
(reference) (1080 L/mln) (604 L/mln)
0.00 1473 1351 1406
0.50 1364 1321 1355
1.00 1355 1291 1311
1.50 1347 1259 1264
2.00 1330 1229 1221
2.50 1323 1205 1186
3.00
3.25 1299
~ 1141 1149
3.50 --
.
3.75 1291
4 00 ~ 1126 1123
. .
4.25 1276
.. ... ... .,,.. ....... , : : ---~- :
4 50 ~ 1110
4.75 1265 ::~
812
5 0O . --- 1088
5.25 1258 - :
5.75 1242
6.00 - - - 1045
6.75 1208
~ 859
6.83 -~
7.00 : ~ ; 1004 -~
7.50 :~ 982
7.75 1192
22.50 :~ - 300
22.75 ~ : 474
23.25 923
60557-4966
21~0320
Example 2 ProPane as Contamlnant
TABLE 2
Tlme ~Hours) Contamlnant Concentratlon (ppm)
Phlllps (reference) Hlgh Flow
(1080 L/mln)
0.00 1414 1703
~ 1649 .
0.50 1362 - ~-: - - : :-
~ -- 1635
0.67 . -
1.00 1348 1619
1.50 1345 1579
2.00 1338 1555
2.50 1327 1531
3.00 1316 1509
3.50 1309 -~
................................ . - - - - - - - - - - - - - - - - - - - - - - - -
3.67 : : : : - 1482
- -- .......... ....................
4.00 1300 . -
4.50 1291 - --
............... . . .. ............. ...... ......
4.75 : -- - 1408
5.00 1269 - -
6.00 ~ . 1261
. . .
6.50 ~ 1264
20.50 1008 :::-~: : :: : . -.
- - - 815
21.50 - ~
60557-4966
21S0320
24
Example 3 Dlethylether as Contamlnant
TABLE 3
Contamlnant Concentratlon (ppm)
Tlme (HUrs) Phlllps Hlgh Flow Low Flow
(reference) (1080 L/mln) (604 L/mln)
0.00 1909 1665 1524
0.50 1870 1392 1306
1.00 1880 1147 1106
1.50 1867 986 952
2.00 1858 864 810
2.50 1826 741 719
3.00 1814 654 636
3.50 1763 568 552
Example 4 Acetaldehyde as Contamlnant
TABLE 4
Contamlnant Concentratlon (ppm)
Tlme (Hours) Phlllps (reference) Hlgh Flow (1080 L/mln)
0.00 2605 2874
:
1.00 ~ 2065
2.08 2494 1655
. . .. , - .
3.00 ~ 1400
3.33 2386
- .. -.-.. , .,-... -- - .. ,
4.08 ~ 1279
4.83 2385
60557-4966
2150320
Example 5 Isobutane as Contamlnant
TABLE 5
Several dlfferent fllters were tested, as lndlcated
below, all at low flow setting of 604 L/min.
Contamlnant Concentratlon (ppm)
(Hours) Phlllps Fllter #3Fllter #1Filter #2
(reference)
0.00 1473 1380 1406 1384
0.50 1364 1352 1355 1356
1.00 1355 1314 1311 1335
1.50 1347 1301 1264 1316
2.00 1330 1262 1221 1295
2.50 1323 1265 1186 1256
3.00 - ~ 1173
3.17 ~ -1232
- - - - -
3.25 1299
.
3.50 ~ 12341149 1202
- -
3.75 1291
. .. -.-.. -.. ..... ................ . ... .~... .. .~
4.00 ~ 1224 1123
. - - -: : : . : : :: .-
4.25 1276
4.50 - ~- 1208 ~ 1150
. . -..... . .. ,.: .. .::.. .... .. . - - - .
4.75 1265
~ - -
5.25 1258
-.. -.. -. .-.. -. - - ~ - ~
5.50 ~ 1177 -~ 1113
- ~ ::
5.75 1242
60557-4966
2150320
Table 5 Cont. 26
Tlme Contaminant Concentratlon (ppm)
(Hours) Phlllps Fllter Fllter Filter
(reference) #3 #1 #2
6.50 - 1141 ~ 1083
6.75 1208
: : : . ~: - - - .
6.83 ~ 860
7.00 ~ 1126 ~ 1076
7.75 1192 :
22.50 ~ 301 -
: .- - .. - -.- : - - -
23.00 ~ 618 - ~ - ~ 572
23.25 g23
Example 6 Isobutane as Contaminant
TABLE 6
Dlfferent numbers of UV bulbs were tested.
Tlme (Hours) Contamlnant Concentratation (ppm)
3 UV Bulbs 2 UV Bulbs 1 UV Bulb
0.00 1448 1303 1262
0.50 1370 1215 1232
1.00 1371 ~ 1218
-,.. - -.--. -- . -- .. . .- .
2.00 1257 1198
3.00 ~ 1110 1221
3.50 1235
~ . - ,
4.00 1215 1070
~ .. 1144
4.08 .
5.00 1158 1033
60557-4966
2150~20
Table 6 Cont. 27
Tlme (Hours) Contamlnant Concentratlon (ppm)
3 UV Bulbs 2 UV Bulbs1 UV Bulb
5.17 ~ 1122
5.83 ~ 1093
.
6.00 1117
22.00 573 576 770
Example 7 Isobutane as Contaminant
TABLE 7
Dlfferent UV wavelengths were tested.
Tlme (Hours) Contamlnant Concentratlon (ppm)
313 nm 366 nm
0.00 1360 1292
0.50 1311 1263
1.00 1268 1229
1.50 1223 1205
2.00 1181 1169
2.50 1147 1137
3.00 1134 1092
3.50 1112 1083
4.00 1067 1070
4.50 ~ 1039
5.00 ~ 1029
5.50 ~ 990
6.00 882 982
. . - - - - - -
6.50 ~ 954
: . ~ - :
6.83 832
- - - - -
~ 936
Z2.50 291 -~
. - - - -
23.00 ~ 414
60557-4966
2ls~32a
28
Example 8 Ethyl Proplonate as Contaminant
TABLE 8
Tlme Contaminant Concentratlon (ppm)
(Mln) Phlllps (reference) Low Flow
(604 L~mln)
- 303 269
0.5 45 64
1.0 39 18
1.5 22 03
2.0 22 01
Example 9Allyl Butyrate as Contamlnant
TABLE 9
Tlme Contamlnant Concentratlon (ppm)
(Mln)
Phlllps (reference) Low Flow
(604 L/min)
0.0 84 91
11 22
07 08
05 03
03 01
60557-4g66
2150320
29
Example 10 Propyl Butyrate as Contamlnant
TABLE 10
Tlme Contaminant Concentratlon (ppm)
(Mln) Philips (reference) Low Flow
(604 L/min)
0 215 154
16 2g
11
8 5
4 2
Example 11Isobutane as Contamlnant
TABLE 11
Tlme (Hours) Contamlnant Concentrate (ppm)
Fllter #4 Fllter #5
0.00 1448 1473
- - - - -
0.25 ~ 1450
0.50 1402 1431
: :- - - - - - -
0.83 ~ 1404
1.00 1362
., ..- ... . . -.. -
1.33 ~ 1356
1.50 1327
. - . . . - - . . . - - - - -
1.83 ~ 1330
2.00 1289
- - . . . - . . ~ .
2.50 1256
2.67 ~ 1294
3 ~ 1247
- - - . . - - . . .
3.50 1186 1216
4-00 -~ 1180
4 50 ~ 1158
- . . . ., . ...... . ..... ..... - -
4.67 1124
60557-4966
21SO320
-
Table 11 cont. 30
Time (Hours) Contamlnant Concentrate (ppm)
Fllter #4 Fllter #5
~. - 1120
5.00 ; .
5.50 1077
. :: -: . . -:: .: . . . - - .
6.00 ~ 1067
.
6.50 1030 - ~ -
7 0O ~ 1009
-- ---- -- ......... . .. .. ..... .. .
7.17 1003
22.83 ~ 476
23.00 502
Example 12Hydrogen Sulphlde as Contamlnant
TABLE 12
Tlme (Hours) Contamlnant Concentratlon (ppm)
Phlllps High Flow
(reference) (1080 L/min)
0.00 40.60 40.60
0.25 39.10 33.30
0.50 37.70 22.60
0.75 36.20 14.40
1.00 34.80 8.10
1.25 33.30 4.10
1.50 31.90 1.90
1.75 30.40
. . . .
2.00 30.30
60557-4966
2150~20
31
Example 13 Hydroqen Sulphide as Contamlnant
TABLE 13
The apparatus was tested and further compared wlth a
run leavlng the UV swltched off.
Tlme Contamlnant Concentratlon (ppm)
(Hours) Low Flow (604 Hlgh Flow Hlgh Flow
L/mln) UV On (1080 L/mln) (1080 L/mln)
UV On UV Off
0.00 40 41 42
0.25 29 ~3 42
0.50 25 23 42
0.75 20 14 41
1.00 15 08 41
1.25 12 04 39
1.50 08 02 39
1.75 05 ~ 39
2 00 . ~ 38
- ~ ................. - . ...
OTE: In Examples 12 and 13 the testlng chamber was a
larger chamber of 510 lltres capaclty. Also, the
hydrogen sulphlde concentratlon was determlned
wlth a Dynatel CSM-500 (a conflned space monitor)
whlch has an electro-chemlcal sensor for hydrogen
sulphlde.
60557-4966
2lsn320
Example 14 Hydroqen Sulphide and Isobutane as
Contamlnant
Filter #6 was tested. Thls hybrld fllter has both
a photocatalyst layer and an addltlonal layer of actlvated
carbon on a polyester fibre support. A mixture of the above
two contamlnants was used.
Table 14A
(Hydrogen Sulphlde as contamlnant)
Tlme (Hours) Contamlnant Concentration
(ppm)
Hlgh Flow (1080 L/min)
0.00 39.1
0.25 21.2
0.50 9.6
0.75 3.2
1.00 1.0
1.25 0.6
1.50 0.4
Table 14B
(Isobutane as Contaminant)
Tlme (Hours) Contamlnant Concentration
(ppm)
Hlgh Flow (1080 L/min)
o.oo 1484
0.33 1090
0.67 1086
60557-4966
2Isn320
Table 14b cont. 33
Tlme (Hours) Contamlnnt Concentratlon
(ppm)
Hlgh Flow (1080 L/min)
1.00 1080
1.33 1066
1.67 1052
2.00 1039
3 0O 968
4.00 947
5.00 906
6.00 884
7.00 857
22.25 642
Example 15 Hydrogen Sulphlde and Isobutane as Contaminant
As in Example 14, the hybrid filter #6 was used.
A mixture of the above two contaminants was used.
Table 15A
(Hydrogen Sulphide as contaminant)
Time (Hours) Contaminant Concentration
(ppm)
Hlgh Flow (1080 L/min)
0.OO 40.6
0.25 33.3
0.50 26.0
0 75 20.7
1.00 16.2
1.25 12.2
1.50 9.0
60557-4966
21 S0320
Table 15a cont. 34
Tlme (Hours) Contamlnant Concentration
(ppm)
Hlgh Flow (1080 L/mln)
1.75 6.4
2.00 4.5
2.25 3.2
2.50 2.6
2.75 2.2
3.00 2.0
Table 15B
(Isobutane as Contamlnant)
Tlme (Hours) Contamlnant Concentratlon
(ppm)
Hlgh Flow (1080 L/mln)
0.00 1455
0.25 1445
0.75 1431
1.75 1366
2.75 1314
3 75 1263
4 75 1225
5.75 1189
21.25 748
60557-4966
215~320
.
The clean alr dellvery rate (CADR) ls a convenlent
parameter to compare contamlnant removal rates. If
ln (CAt/CA): ls plotted agalnst tlme, where CAt ls the
contamlnant concentratlon at a glven tlme and CA ls the
lnltlal concentratlon, then the slope of the graph wlll be
the decay constant k. The natural decay constant kn ls the
slope of the same graph for results obtalned wlthout
photocatalysls or other actlve removal. The CADR ls then
calculated as:
CADR = (k-kn)V
where V ls the volume of the test chamber.
Uslng the above examples, the followlng tables A
to E can be generated.
60557-4966
215032~
36
TABLE A
Decay constants (k) and clean alr delivery rates
(CADR) for dlfferent fllters tested wlth lsobutane (lnltlal
concentratlon approxlmately 1400 parts per mllllon).
Fllter Number Flowrate of k CADR
Fllter Devlce~l/hr) (Lthr)
Fllter #3604 0.03404 6.71
Fllter #1604 0.068g4 14.64
Fllter #11080 0.04572 9.37
Fllter #2604 0.0382 7.68
Fllter #4604 0.04565 9.35
Fllter #51080 0.04948 10.22
Fllter #61080 0.028096 5.36
Fllter #61080 0.031244 6.078
Phlllps3115 0.01812 3.10
TABLE B
Decay constants (k) and clean alr dellvery rates
(CADR) for filter Fllter #l tested wlth lnltlal
concentratlons of lsobutane at approxlmately 1400 ppm.
Number of UVWavelength of k CADR
Bulbs UV Bulbs (l/hr) (L/hr)
2 313 0.03585 7.12
1 313 0.02225 5.051
3 313 0.06894 15.65
3 366 0.04916 11.16
Note The decay constant (kn) for lsobutane was
determlned to be 0.00446 Lthr.
60557-4966
2150320
TABLE C
Decay constants ~k) and clean alr dellvery rates
(CADR) for low molecular welght gases (lnltial
concentrations of approxlmately 1500 ppm).
Gas belng Fllter Flowrate of k CADR
tested Fllter (l/hr) (L/hr)
Devlce
Propane Fllter #1 604 0.0343 7.788
Phlllps 3115 0.0155 3.515
............ ..... ..... ...... .. ... ..... .... ... . . . ... . . .. . . ...
Dlethyl- Filter #1 604 0.2895 65.72
ether
- -
Fllter #1 1080 0.3036 68.91
Phlllps 3115 0.01905 4.324
. . . . .. ..... .. .. .. . .... . . . . .. . . ... -
Acetal- Fllter #1 604 0.1974 44.8
dehyde
~ Phlllps 3115 0.01967 4.466
ote: The natural decay constant (kn) was not obtalned
for these gases and was taken to be zero. As a
result, the actual clean alr dellvery rates would
be sllghtly lower than the reported values.
60557-4966
2150320
.
38
TABLE D
Decay constants (k) and clean alr dellvery rates
(CADR) for hlgher molecular welght gases (lnitlal
concentratlons of approxlmately 100-500 ppm).
Gas belng Filter Flowrate k CADR
Tested of Fllter(l/mln) (L/mln)
Devlce
Ethyl- Fllter #1 604 0.051817 11.76
proplonate
(frulty
rum smell)
Phlllps 3115 0.019917 4.52
Allyl- Fllter #1 604 0.04633 10.52
butyrate
(apricot
smell)
~ . -
~ Phlllps 31150.032433 7.362
Propyl- Fllter #1 604 0.03715 11.08
butyrate
(sweaty
smell)
Phlllps 3115 0.0529 12.01
. -
Note: The natural decay constant (kn) was not obtalned
for these gases and was taken to be zero. As a
result, the actual clean alr dellvery rates would
be sllghtly lower than the reported values.
60557-4966
215032D
39
TABLE E
Decay constants (k) and clean air delivery rates
(CADR) for hydrogen sulphide (initial concentrations of
approximately 40 ppm).
Filter Flowrate of k CADR
Filter Device (1/min) (L/min)
Filter #1 604 0.01868 9.242
Filter #1 1080 0.03428 17.2
Filter #1 ~ . 0.000919 0.1857
(UV OFF)
- - . - . . . . .. . .. . . .. . . . . . .. . . . . . . . .
Filter #4 604 0.0202 10.02
Filter #4 1080 0.03118 15.62
- - - - - - . - - - - . . . . - . - ... . .. ...
- - - - - - ~ - - - . - . . - . - . .- . - - . . .-
Filter #6 1080 3.305522 28.10
Filter #6 1080 1.09159 9.28
Philips 3115 0.002603 1.045
ote: The natural decay constant (kn) for hydrogen
sulphide was determined to be 0.000555 L/min.
60557-4966
215032D
It should be noted that for carbon fllters the
CADR drops (less efficiency) when the flow rate is
decreased. For a photocatalytic devlce of the invention,
decreasing the flow rate generally increases the CADR.
The results demonstrate that the apparatus of the
invention outperforms the active carbon filter of the
Philips device, especlally for the removal of low molecular
weight gases such as lsobutane, propane, acetaldehyde and
hydrogen sulphide.
60557-4966