Note: Descriptions are shown in the official language in which they were submitted.
WO94/13304PCT~S93111720
2l~383
~NTACTD CO~POSITION ~ND ~FT~OD OF PRODIJCTION
FIFT~ OF TNV~NTION
This invention relates to antacid compositions,
and to a method for the preparation thereof. This
invention also relates to the use of these compositions in
methods of treating mAmm~ls.
R~KGROUND OF T~F TNVFNTION
Neutralization is the change of a solution from
acid or alkaline to neutral through the addition of an
ade~uate amount of either an alkaline or acidic substance.
Antacids belong to the class of drugs that are able to
neutralize bodily fluids. Many factors can affect
neutralization including the acidity of the solution, the
dosage form and ~arious properties, e.g., dissolution rate
of the drug. Slow rate of neutralization is a significant
problem encountered in the development of antacid
compositions, particularly those cont~;ning an active
ingredient that is poorly soluble in water. For example,
patients seeking relief for pain associated with stomach
acid by taking an antacid often must wait for at least
twenty minutes or longer after administration of the
antacid. Poorly-water soluble drugs, i.e., those having a
solubility less than about l0 mg/ml, tend to be eliminated
from the gastrointestinal tract before being able to react
with excess stomach acid.
U.S. Patent 4,533,543 (Morris et al) discloses a
chewable antacid tablet which disintegrates in the mouth
becoming a smooth creamy pleasant-tasting emulsion which
affords m~xjmllm surface contact of the particles of the
antacid. Each antacid tablet contains solid antacid
particles having a primary particle size of less than l00
WO94/133~ PCT~S93/11720
3~ --
millimicrons, which particles are thoroughly coated with a
mixture of a fatty material or oil, a surfactant and a
flavorant. Morris et al, however, do not disclose antacid t
particles consisting essentially of an aluminum-based
S neutralization agent or suggest an enhanced rate of
neutralization of their small antacid particles.
U.S. Patent 3,843,778 ~Diamond et al) describe a
process for preparing improved antacid particles. The size
of the antacid particles fall within the range of 0.05 -
10 300 microns. However, the antacid particles are coated
with an oil.
U.S. Patent No. 4,271,142 describes a portable
llquid antacid. Example 8 therein specifies an antacid
particle size of 50 millimicrons. ~owever, such particles
15 are coated with an oil.
French Patent 2,512,344 discloses an antacid
suspension prepared by mixing aluminum hydroxide and/or
magnesium hydroxide in powder form with water and then
mechanically fragmenting and dispersing the mixture until
20 the mean particle size is 5-10 microns.
It would be desirable to be able to prepare
compositions cont~i n; ng stable dispersible antacid
particles in the low micron-size range which do not
appreciably flocculate or agglomerate due to interparticle
25 attractive forces and do not require the presence of an oil
coating or a crosslinked matrix. Moreover, production of
antacid compositions displaying enhanced rates of
neutralization would be highly desirable.
~U~M~RY OF T~ INV~NTION
We have discovered stable, dispersible antacid
particles and a method for preparing such particles by wet
milling in the presence of grinding media optionally in
conjunction with a surface modifier. The particles can be
WO94/13304 2 15 0 ~ 8 3 PCT~S93/11720
.
-- 3
formulated into antacid compositions exhibiting remarkably
high rates of neutralization.
This invention provides an antacid composition
comprising particles consisting essentially of an aluminum-
based neutralizing agent having optionally a surfacemodifier adsorbed on the surface thereof, said agent having
an average particle size of less than about 3 microns.
Another embodiment of this invention provides a
method of preparing the above-described composition
comprising the steps of dispersing an aluminum-based
neutralization agent in a liquid dispersion medium and
applying mechanical means in the presence of grinding media
to reduce the particle size to less than about 3 microns.
Optionally, the particles can be reduced in size in the
presence of a surface modifier or the particles can be
contacted with a surface modifier after attrition.
Advantages of this invention include 1) the
simple and convenient method for preparing antacid
compositions having particles less than about 3 microns in
size by wet milling optionally in conjunction with a
surface modifier and 2) the unexpectedly enhanced rate of
neutralization.
RRIF.F nF.SCRIPTION OF T~F~ nRAWTNG
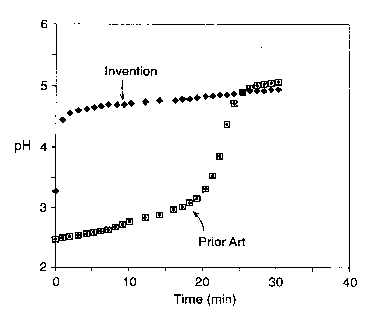
The sole Figure illustrates the increase in the
rate of neutralization of simulated gastric fluid for the
composition of this invention compared to the prior art.
nF.SCRIPTION OF PRFFFRRF.T~ IRODIMF~TS
The present invention describes improved antacid
compositions comprised of particles having a small average
particle size. These compositions are an improvement over
the prior art in that their rate of neutralization is
greatly enhanced over that of the prior art. The invention
is described herein in connection with its preferred
utility of neutralizing gastric fluid.
WO94/13304 PCT~S93/11720
The particles useful in the practice of this
invention comprise an aluminum-based neutralizing agent.
This agent exists in a discrete, crystalline phase. The
crystalline phase differs from a non-crystalline or
amorphous phase which often results from precipitation
techniques.
The agent is poorly soluble and dispersible in at
least one liquid medium. By "poorly soluble" it is meant
that the agent has a solubility in the liquid dispersion
medium of less than about lO mg/ml, and preferably of less
than about l mg/ml. A preferred liquid dispersion medium
is water. The invention can be practiced with other liquid
media in which the agent is poorly soluble and dispersible
including, for example, aqueous salt solutions, safflower
oil and solvents such as ethanol, t-butanol, hexane and
glycol. The pH of the aqueous dispersion media can be
adjusted by techniques known in the art.
The antacid composition comprises particles
consisting essentially of an "aluminum-based neutralization
agent". By "consisting essentially of" it is meant that
the particles are essentially free of, e.g., oil coatings
which are known in the art. Preferable aluminum-based
neutralization agents which may be used in the practice of
this invention include: aluminum hydroxide, aluminum
hydroxycarbonate, aluminum magnesium glycinate and
dihydroxy aluminum aminoacetate. A preferred aluminum-
based neutralization agent is aluminum hydroxide.
In preferred embodiments, the antacid
composition further comprises particles of a magnesium-
based compound. Preferable magnesium compounds includemagnesium aluminate, magnesium hydroxide, magnesium
carbonate, magnesium oxide and magnesium trisilicate. A
preferred magnesium based compound is magnesium hydroxide.
The composition of this invention contains a
discrete phase of an aluminum-based neutralizing agent as
described above optionally having a surface modifier
WO94113304 2 i S 0 3 ~ ~ PCT~S93111720
- 5 -
adsorbed on the surface thereof. Useful surface modifiers
are believed to include those which physically adhere to
the surface of the particles but do not chemically bond to
them.
Suitable surface modifiers can preferably be
selected from known organic and inorganic pharmaceutical
excipients. Such excipients include various polymers, low
molecular weight oligomers, natural products and
surfactants. Preferred surface modifiers include nonionic
and anionic surfactants. Representative examples of
excipients include simethicone, gelatin, casein, lecithin
(phosphatides), gum acacia, cholesterol, tragacanth,
stearic acid, benzalkonium chloride, calcium stearate,
glyceryl monostearate, cetostearyl alcohol, cetomacrogol
emulsifying wax, sorbitan esters, polyoxyethylene alkyl
ethers, e.g., macrogol ethers such as cetomacrogol 1000,
polyoxyethylene castor oil derivatives, polyoxyethylene
sorbitan fatty acid esters, e.g., the commercially
available Tweens, polyethylene glycols, polyoxyethylene
stearates, colloidal silicon dioxide, phosphates, sodium
dodecylsulfate, carboxymethylcellulose calcium,
carboxymethylcellulose sodium, methylcellulose,
hydroxyethylcellulose, hydroxypropylcellulose,
hydroxypropylmethylcellulose phthalate, noncrystalline
cellulose, magnesium aluminum silicate, triethanolamine,
polyvinyl alcohol and polyvinylpyrrolidone (PVP). Most of
these excipients are described in detail in the H~n~hook of
Ph~r~cel~t; ~1 F.xc;p; ents, published jointly by the
American Pharmaceutical Association and The Pharmaceutical
Society of Great Britain, The Pharmaceutical Press, 1986,
the disclosure of which is hereby incorporated by reference
in its entirety. The surface modifiers are commercially
available and/or can be prepared by techniques known in the
art.
Preferred surface modifiers include Pluronic F68 and
F108, which are block copolymers of ethylene oxide,
WO94/133~ PCT~S93/11720
3Q~ 6
Tetronic 908, which is a tetrafunctional block copolymer
derived from sequential addition of ethylene oxide and
propylene oxide to ethylène~;~m;ne, dextran, lecithin, t
Aerosol OT, which is a dioctyl ester of sodium
5 sulfosuccinic acid, available from American Cyanamid,
Duponol P, which is a sodium lauryl sulfate, available from
DuPont, Triton X-200, which is an alkyl aryl polyester
sulfonate, available from Rohm and Haas, Tween 80, which is
a polyoxyethylene sorbitan fatty acid ester, available from
ICI Specialty Chemicals, and Carbowax 33S0 and 934, which
are polyethylene glycols available from Union Carbide.
A particularly preferred surface modifier is
simethicone. Preferred surface modifiers do not chemically
réact with the agent or itself. Furthermore, the
individually adsorbed molecules of the surface modifier are
essentially free of intermolecular crosslinkages.
As used herein, particle size refers to a number
average particle size as measured by conventional particle
size measuring techniques well known to those skilled in
the art, such as sedimentation field flow fractionation,
photon correlation spectroscopy, or disk centrifugation.
By "an effective average particle size of less than 3
microns" it is meant that at least 90% of the particles
have a weight average particle size of less than about 3
microns when measured by the above-noted techniques.
The composition of this invention can be prepared in
a method comprising the steps of dispersing an aluminum-
based neutralizing agent in a li~uid dispersion medium and
applying mechanical means in the presence of grinding media
to reduce the particle size of less than about 3 microns.
In preferred embodiments, the aluminum-based neutralizing
agent has an average particle size of less than l micron.
When magnesium-based particles are present, it is preferred
that they have a particle size of less than about 3
microns, and more preferably of less than about l micron.
Optionally, the particles can be reduced in size in the
WO94/13304 Z 1~ 0 ~ 8 3 PCT~S93/11720
.
-- 7
presence of a surface modifier, or can be contacted with a
surface modifier after attrition.
A specific procedure for preparing the compositions
of this invention is set forth below. The aluminum-based
neutralizing agent selected is obtained commercially and/or
prepared by techniques known in the art in a conventional
coarse form. It is preferred, but not essential, that the
particle size of the coarse aluminum-based neutralizing
agent selected be less than about lO0 microns as determined
by sieve analysis. If the coarse particle size of the
aluminum-based neutralizing agent is greater than about lO0
microns, then it is preferred that the particles of the
aluminum-based neutralizing agent substance be reduced in
size to less than lO0 microns using a conventional milling
method such as airjet or fragmentation milling prior to
using the milling method of this invention.
The coarse aluminum-based neutralizing agent
selected can then be added to a liquid medium in which it
is essentially insoluble to form a premix. The
concentration of the aluminum-based neutralizing agent in
the liquid medium can vary from about O.l - 60~, and
preferably is from 5 -30% (w/w). It is preferred, but not
essential, that the surface modifier be present in the
premix. The concentration of the surface modifier can vary
from about O.l to about 90%, and preferably is l - 75%,
more preferably 20 - 60%, by weight based on the total
combined weight of the aluminum-based neutralizing agent
and surface modifier. The apparent viscosity of the premix
suspension is preferably less than about lO00 centipoise.
The premix can be used directly by subjecting it to
mechanical means to reduce the average particle size in the
dispersion to less than 3 microns. It is preferred that
the premix be used directly when a ball mill is used for
attrition. Alternatively, the aluminum-based neutralizing
agent and, optionally, the surface modifier, can be
dispersed in the liquid medium using suitable agitation,
?~ 8 - PCT~S93/11720
e.g., a roller mill or a Cowles type mixer, until a
homogeneous dispersion is observed in which there are no
large agglomerates visible to the naked eye. It is
preferred that the premix be subjected to such a premilling
dispersion step when a recirculating media mill is used for
attrition.
The mechanical means applied to reduce the particle
size of the aluminum-based neutralizing agent conveniently
can take the form of a dispersion mill. Suitable
dispersion mills include a ball mill, an attritor mill, a
vibratory mill and media mills such as a sand mill and a
bead mill. A media mill is preferred due to the relatively
shorter milling time required to provide the intended
result, i.e., the desired reduction in particle size. For
media milling, the apparent viscosity of the premix
preferably is from about l00 to l000 centipoise. For ball
milling, the apparent viscosity of the premix preferably is
from about l up to about l00 centipoise. Such ranges tend
to afford an optimal balance between efficient particle
fragmentation and media erosion.
The grinding media for the particle size reduction
step can be selected from rigid media preferably spherical
or particulate in form having an average size less than
about 3 mm and more preferably, less than about l mm. Such
media desirably can provide the particles of the invention
with shorter processing times and impart less wear to the
milling equipment. The selection of material for the
grinding media is not believed to be critical. It has been
found that zirconium oxide, such as 95% ZrO stabilized with
magnesia, provide particles having levels of contamination
which are believed to be acceptable for the preparation of
pharmaceutical compositions. However, other media, such as
zirconium silicate, glass, stainless steel, titania,
alumina and 95% ZrO stabilized with yttrium, are expected
to be useful. Preferred media have a density greater than
about 3 g/cm3.
WO94/13304 21~ 0 3 8 3 PCT~S93l11720
_ g _
The attrition time can vary widely and depends
primarily upon the particular mechanical means and
processing conditions selected. For ball mills, processing
times of up to five days or longer may be required. On the
other hand, processing times of less than 1 day (residence
times of one minute up to several hours) have provided the
desired results using a high shear media mill.
The particles must be reduced in size at a
temperature which does not significantly degrade the
aluminum-based neutralizing agent. Processing temperatures
of less than about 30 - 40C are ordinarily preferred.
If desired, the processing equipment can be cooled with
conventional cooling equipment. The method is conveniently
carried out under conditions of ambient temperature and at
processing pressures which are safe and effective for the
milling process. For example, ambient processing pressures
are typical of ball mills, attritor mills and vibratory
mills. Processing pressures up to about 20 psi (1.4
kg/cm2) are typical of media milling.
The surface modifier, if it was not present in the
premix, can be added to the dispersion after attrition in
an amount as described for the premix above. Thereafter,
the dispersion can be mixed, e.g., by shAk;ng vigorously.
Optionally, the dispersion can be subjected to a sonication
step, e.g., using an ultrasonic power supply. For example,
the dispersion can be subjected to ultrasonic energy having
a frequency of 20-80 kHz for a time of about 1 to 120
seconds.
The antacid composition of the invention preferably
comprises from 5 to 80 percent by weight particles.
The relative amount of aluminum-based neutralizing
agent and surface modifier can vary widely and the optimal
amount of the surface modifier can depend, for example,
upon the particular surface modifier selected or the
critical micelle concentration of the surface modifier, if
it forms micelles. The surface modifier preferably is
WO94/13304 Q PCT~S93/11720
5~3~ ~ ~
-- 10 --
present in an amount of about O.l-lO mg per square meter
surface area of the aluminum-based neutralizing agent. The
surface modifier can be present in an amount of 0.1-90%, ¢
preferably 20-60~ by weight based on the total weight of
5 the dry particle. r
A simple screening process has been developed
whereby compatible surface modifiers and aluminum- based
neutralization agents can be selected which provide stable
dispersions of the desired particles. First, coarse
lO particles of the aluminum-based neutralizing agent are
dispersed in a liquid in which the drug is essentially
insoluble, e.g., water at 5% (w/w) and milled for 60
minutes in a DYNO-MILL under the standard milling
conditions which are set forth in Example l which follows.
15 The milled material is then divided into aliquots and
surface modifiers are added at concentrations of 2, lO and
50% by weight based on the total combined weight of the
aluminum-based neutralizing agent and surface modifier.
The dispersions are then sonicated (l minute, 20 kHz) to
20 disperse agglomerates and subjected to particle size
analysis by eXAm;nAtion under an optical microscope (lO00 x
magnification). If a stable dispersion is observed, then
the process for preparing the aluminum-based neutralizing
agent surface modifier combination can be optimized in
25 accordance with the teachings above. By stable it is meant
that the dispersion exhibits no flocculation or particle
agglomeration visible to the naked eye at least 15 minutes,
and preferably, at least two days or longer after
preparation.
The resulting dispersion is stable and consists of
the liquid dispersion medium and the above-described
particles. The dispersion of aluminum-based neutralizing
agent optionally with a surface modifier can be used in a
liquid form, in the form of tablets, and other forms
prepared by techniques well known in the art.
WO94/13304 21~ Q ~ ~ 3 PCT~S93111720
-- 11 --
Antacid compositions according to this invention
include the particles described above and a
pharmaceutically acceptable carrier therefor. Suitable
7 pharmaceutically acceptable carriers are well known to
5 those skilled in the art and include aqueous solutions, and
other non-toxic physiologically acceptable carriers,
adjuvants or vehicles for oral administration in solid or
liquid form and the like. Suitable carriers and excipients
are described in the ~n~hook ~f Ph~rm~el-tir~l ~x~;~;ents,
10 cited above.
The antacid composition of this inventions can
include natural and/or synthetic flavorants, such as cocoa,
chocolate, mint chocolate, butter, milk, cream, vanilla,
butter fat egg or egg white. The flavorant can be employed
in amounts within the range of from about 0.05 to about 75%
by weight of the composition.
The antacid composition of the invention can also
include pharmaceutically acceptable excipients, such as
sweetening agents, including sugars, sugar alcohols, and
synthetic sweeteners such as sorbitol, xylitol, saccharin
salts, etc. as well as coloring agents and other flavoring
agents.
Additionally, the composition can include various
inactive ingredients such as cellulose, hydroxypropyl
cellulose, paraffins, such as butyl paraffin, and the like.
The following example further illustrates the
invention.
3 0 F:XZ~r`qPT.F.
Approximately 300 ml of 1.1 mm diameter ZrO2 beads
were placed into a 500 ml, polyethylene wide-mouth bottle
together with approximately 6 oz (100 ml) of Mylanta~
antacid suspension. The antacid suspension comprised
35 particles of aluminum hydroxide and magnesium hydroxide,
simethicone, hydroxypropyl cellulose, cellulose and
WO94/133~ -- PCT~S93/11720
~5~3~ 12 -
butyl paraffin. The bottle was capped and rolled on a US
Stoneware roller mill at 1~7 rpm for l week to reduce the
particle size of the s~spension. The initial particle size
was measured using a Hiac Royko model 4100 particle sizer
coupled to a model 3000 sample module and found to be
between lO and 20 microns. After milling, the particle
size was again measured on the Hiac Royko and found to be
less than 2 microns in diameter.
The effect of ~;m~n;shed particle size was estimated
using a modified FDA test to determine the rate and extent
of neutralization of simulated stomach acid (i.e., O.l N
HCl) by the unmilled and milled samples. Fifteen
milliliters (15 ml) of O.l N HCl was placed into a lO0 ml
beaker with approximately 35 ml of deionized water. A
glass pH electrode was used to monitor the progress of the
neutralization reaction after addition of equal amounts (l
ml) of either milled or unmilled aluminum-based suspensions
to the test acid. In each case, the neutralization
reaction reached the same neutral pH value indicating equal
neutralization capacity. However, as indicated in the
Figure, the milled formulation unexpectedly achieved this
level within about 2 minutes while the unmilled formulation
required in excess of 20 minutes to reach the final pH
value.
The invention has been described in detail with
particular reference to preferred embodiments thereof, but
it will be understood that variations and modifications can
be effected within the spirit and scope of the invention.