Note: Descriptions are shown in the official language in which they were submitted.
21S0529
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
"METHOD AND APPARATUS FOR CONTINUOUSLY
SEPARATING MERCURY FROM FLOWING GASES"
Reference to related patent and appllcation, the dlsclosures
of whlch are hereby lncorporated by reference,
U.S. 4,729,882, Ide et al.;
Internatlonal Appllcatlon PCT/EP 92/01557, publlshed WO
93/02773, U.S. designated, Kurzlnger et al.
Reference to related patent 8:
European 0 289 810 Al, Vogg;
German 41 40 969 Cl, Korner et al.
* * * * * * *
FIELD OF THE INVENTION.
The present lnventlon relates to an apparatus and
method for contlnuously separatlng mercury from flowlng gases,
ln partlcular from exhaust gases of thermal processes. Such
gases arlse durlng operatlon of a glass meltlng lnstallatlon
for fragmented mercury-contalnlng llghtbulbs, e.g. fluorescent
lamps or metal hallde lamps.
BACKGROUND.
Conventlonally, ~ome mercury separatlon methods
employ actlvated charcoal for blndlng the mercury. For
example, the actlvated charcoal may be ln the form of fllters
or screens, and
27813-39
21~0S29
.
possibly used for pre-cleaning (for example Wo 93/02773,
Kurzinger et al.). Since activated charcoal is highly flammable,
its employment is limited to cleaning gases at an appropriately
low temperature.
The following methods are suitable for higher gas
temperatures. Lime, to which green coke has been added, is used
as the reaction agent for binding mercury, see EP 0 289 810. The
mercury-green coke-adsorbate being generated is separated in a
precipitator and stored. A large amount of special waste is
generated, which is disadvantageous for this type of method.
Another method involves bringing the mercury- containing
gases into contact with precious metal halides. In such a
method, the precious metals are reduced and the corresponding
-mercury halide is separated. However, high costs are incurred
because of the use of precious metals.
Some amalgam-forming methods are known. In the simplest
case, a sheet metal plate forming an amalgam is disposed in the
gas flow.~ The relatively small surface and the resultant short
service times are disadvantageous. To avoid this problem, it is
proposed in the German Patent Disclosure Document DE-OS 41 40 969
to apply amalgam-forming metals, for example gold, silver,
copper, tin, zinc, palladium, iridium, or rhodium, on a substrate
with a large inner specific surface (up to approximately
90 m2/g). However, the cost of these metallized substrates is
high.
A method for cleaning mercury-containing gases from
municipal waste incineration plants is disclosed in US Patent No.
4,729,882, Ide et al. In a first method step the mercury is
converted to water-soluble mercury chloride by the addition of
chlorine gas. The mercury is washed out of the gas in a
subsequent wet-chemical method step by means of a washing
solution. However, the high chemical aggressiveness of chlorine
is disadvantageous. Furthermore, wet washing causes an
additional pressure loss in the gas flow which is equivalent to
an increased energy outlay.
2150529
-
THE INVENTION.
It is an object of the invention to provide an efficient
method and apparatus for separating mercury (Hg) from gases on a
large industrial scale and so that the threshold values for
mercury concentrations in exhaust air fall below those prescribed
in the relevant regulations, while overcoming the previously
mentioned disadvantages.
Briefly, at least one halogen X, in particular iodine
and/or bromine, is admixed directly and continuously at a
controllable feed rate to mercury-containing gas in order to
thereby trigger a quantitative reaction of Hg + X2 - > HgX2. By
means of this reaction the concentration of elementary mercury in
the gas flow is directly reduced. The mercury halide(s) HgX2
generated in the course of the reaction is or are subsequently
separated in the solid state from the gas flow, possibly together
with further mercury compounds present in the gas flow.
Iodine is a preferred reactant, because it reacts well
with mercury to form mercury iodide. It is furthermore
advantageous in that as compared to chlorine and fluorine, iodine
is clearly less chemically aggressive. In addition, mercury
iodide has a lesser water solubility (approximately 0.006%) than
mercury bromide (approximately 0.62%), mercury chloride
(approximately 6.9%) and mercury fluoride (dissociates), as well
as a relatively high evaporation temperature (approximately
354C). Because of this, the humidity and temperature of
mercury-containing exhaust gases have a lesser effect on the
separation of the mercury iodide than with the other mercury
halides mentioned. In spite of this, however, it is possible in
principle to employ halogens other than iodine as reactants,
provided a reduced efficiency of the separation is acceptable.
It is also possible to use two or more different types of
halogens simultaneously as reactants.
In a first embodiment of the invention, the halogen is
2150529
-
.
continuously supplied in manually preset amounts. Optionally,
the feed rate of the halogen(s) is increased to such a degree
that the gas flow shows an excess of halogen. In this manner, the
elemental mercury can be completely converted to the greatest
extent into mercury halide(s) and separated from the gas flow.
The excess halogen contained in the gas flow after the reaction
is separated in a further method step.
In a second embodiment of the method, the mercury
concentration is selectively and continuously measured following
the reaction. The feed rate of the halogen(s) is readjusted as a
function of the measured value in such a way that a preselectable
threshold value of the mercury concentration occurs, particularly
less than 0.2 mg mercury per m3 of exhaust gas. In a preferred
embodiment of the method, the mercury concentration is adjusted
to a value which, although lying below a prescribed threshold
value, lies above zero. Overdosing with halogen is prevented in
this manner, even with fluctuating mercury concentrations in the
inflowing~gas. Accordingly, in this embodiment it is possible to
omit the additional method step for separating excess halogen,
which is generally required with the uncontrolled method. A
further advantage is the flexible adaptation of the supplied
amount of halogen to fluctuating mercury concentrations. Halogen
use is reduced to a minimum as well. This embodiment is
particularly efficient.
A basic apparatus which carries out the method in accordance
with the invention has a reaction vessel open on both ends,
wherein the mercury-containing gases flow in through its first
open end and flow out through its second open end. The flow can
be created by thermal convection or by means of a blower. The
reaction vessel is preferably pipe-shaped and the cross section
can be arbitrary, for example circular or polygonal, e.g.
rectangular. The reaction vessel has a supply or feed device for
supplying at least one halogen or for placing the halogen into
2150529
the reaction vessel.
In addition, the apparatus provides a separating device, in
which the mercury halide(s) can be separated from the gas flow in
the solid state, possibly together with further mercury compounds
present in the gas flow. In the process, the mercury halide
either (a) cools to a temperature below the evaporation
temperature in the separating device or (b) flows at an already
appropriate temperature into the separating device. When
employing two or more halogens, cooling is performed to below the
lowest evaporation temperature of the mercury halides used. For
example, for mercury iodide and for mercury bromide the
evaporation temperatures are approximately 354C and 319C,
respectively. Thus, if both halogens are used, the temperature
must be lower than 319C.
In a preferred form of the first embodiment, a heat
exchanger is used as the separating device. The advantage here
is that by this step the energy balance is improved at the same
time and in this way the efficiency of the entire system
increased. In conventional installations the heat exchanger is
primarily used in a manner known per se for the partial return of
the process heat contained in the exhaust gas. For this purpose
the heat exchanger has a large effective surface cooled by a
fluid. The mercury halide(s) is (are) deposited thereon.
Because of this, the effective flow cross section of the heat
exchanger is increasingly reduced and as a consequence the flow
resistance is increased. If it exceeds a threshold value, the
heat exchanger must be cleaned. The time when a change is due is
determined by means of two pressure probes, one in the inflow and
the other in the outflow of the heat exchanger. With the flow
speed being constant, the pressure difference between the two
probes, i.e., the pressure loss caused by the heat exchanger, is
a direct measurement of the flow resistance.
In the second embodiment, a filter is used as the separating
2150529
-
unit, for example an electrostatic filter (E-filter) or a
mechanical-one. The advantage of the E-filter is a reduced
pressure loss and conse~uently a reduced additional energy
outlay. However, a mechanical filter, for example a conventional
fibrous or textile filter, is cheaper.
To assure intimate mixing and reaction of mercury and
halogens, a minimum distance must be maintained between the
region where the halogen(s) enter(s) and where the corresponding
mercury halides are separated from the gas flow. In individual
cases this depends on the respective flow conditions and the type
of admixture of the halogen(s). In a conventional exhaust gas
installation the minimum distance is six times the hydraulic
diameter of the exhaust gas conduit. It may be advantageous to
assist admixing by suitable elements for the generation of flow
eddies disposed in the interior of the reaction vessel.
The apparatus may have a second separating device. By
means of this second separating device, halogen, which may be in
excess after the reaction, is separated from the gas chamber, for
example by means of an adsorption agent.
Preliminary tests with iodine have shown that aluminum chips
are well suited as an adsorption medium. With dry gases it was
possible to achieve an iodine absorption of 60 percent by weight,
and with moist gases, such as is the general case with fossil-
fired thermal processes, an iodine absorption of 40 percent by
weight, at least, related to the adsorption medium. In
comparison therewith, a clearly lesser iodine absorption was
measured in the case of zinc chips (with dry gases, only
15 percent by weight). Aluminum chips, such as occur as waste
products in machining operations, for example, are suitable.
However, prior to their use as an adsorption agent, it is
necessary to remove adhering lubricants which are often present.
In contrast, aluminum needles have not proven themselves
2150529
useful because of the high apparent weight. Also, the air
resistance of a corresponding filter is too great and the needles
tend to adhere together, particularly with moist gases.
An apparatus for realizing the method in accordance with the
invention with controlled halogen metering additionally has an
Hg-measuring device for measuring the concentration of elemental
mercury, as well as a control and regulating unit connected with
the Hg-measuring device and acting on the feed device for the
halogen. The measuring sensor of the Hg-measuring device is
disposed at a distance downstream from the place where the
halogen is added; the distance corresponds to at least six times
the hydraulic diameter of the reaction vessel. This assures that
at the place of measuring, the previously metered and supplied
halogen has reacted essentially completely with the mercury and
that therefore the actual remaining concentration of elemental
mercury is measured.
The second separating device for separating excess halogen
in the controlled apparatus may be omitted. The control unit
preferably is adjusted in such a way that although the remaining
concentration of mercury in the exhaust gas lies below a
prescribed threshold value, it still lies above zero. In this
way overdosing with halogen is impossible, i.e., the outflowing
gas is sufficiently free of halogen even without an appropriate
separating device.
DRAWINGS:
The invention will be explained below by means of some
exemplary embodiments.
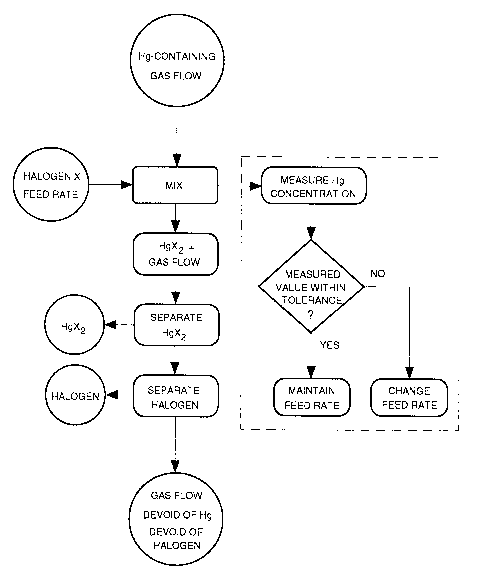
Fig. 1 is a schematic flow diagram of the method for the
continuous separation of mercury from flowing gases in accordance
with the invention. The additional method steps required for a
controlled operation (second embodiment) are those framed by
dashed lines.
Fig. 2 is schematic representation of an apparatus for
21~052~
executlng the directed embodlment of the method of Flg. 1.
Flg. 3 ls a schematlc representatlon of an apparatus
for executlng the controlled embodlment of the method of
Flg.l.
Flg. 4 ls a schematlc fragmentary view illustratlng
the separatlng devlce in form of a heat exchanger.
DETAILED DESCRIPTION
Flg. 2 shows an apparatus 8 for performing the method
of the flrst embodlment of the invention. The apparatus uses
iodine as the reactant for the mercury and comprises the
following components a tube-shaped reaction vessel 2 that ls
open at both ends and has a circular cross sectlon, and a feed
devlce 3-4-5 for lntroduclng the iodine lnto the reaction
vessel. The feed device lncludes at least one reservoir 3,
which contalns at least one halogen such as solld lodlne and
whlch ls connected to a halogen holder 4, e.g. an evaporatlon
trough, for charglng the holder 4 with the halogen. The
holder is displaceable (e.g., in a dlrectlon transverse to
that of the gas flow) vla a dlsplacement unlt 5 drlven by a
motor M. The apparatus also lncludes wlthin the reaction
vessel 2 a separation device 6, such as a flbrous or textlle
fllter or an electrostatlc fllter 6a (Flg. 3), and an
adsorptlon agent 7.
The feed device lntroduces at least one halogen lnto
the reactlon vessel 2, so that a predetermlned amount of the
approprlate halogen vapour ls admlxed wlth the gas flow. The
evaporatlon holder 4 ls introduced into vessel 2 by drlvlng
-- 8
27813-39
- 21S052~
the dlsplacement unlt 5, e.g. a supply belt, wlth the motor M.
The reservolr 3 ls external to the reactlon vessel 2; the
temperature of the halogen wlthln the reservolr 3 may be
approxlmately 30C whlch ls less than the temperature of the
gas flow wlthln the reactlon vessel 2.
The reactlon vessel 2 ls-placed lnto an exhaust alr
condult of a glass meltlng lnstallatlon (not shown) for
mercury-contalnlng fragments of llght bulbs. Mercury-
contalnlng gases flow through lt ln the dlrectlon of the
arrow, whereln the gases flow ln through the flrst open end of
the reactlon vessel 2 and, after havlng passed through the
ad~oining reaction area 8, flow out through the second open
end of the reactlon vessel, essentlally free of mercury.
The evaporatlon holder 4 of the feed devlce ls
located ln the area of the first end of the reactlon vessel 2
and ls charged wlth solld iodlne from the reservolr 3. The
feeding rate of gaseous lodlne to supply the gas flow
corresponds to the evaporatlon rate of the lodlne and can
therefore be affected by the temperature of the evaporation
holder 4 and by the lodlne surface exposed lnslde the reactlon
vessel, i.e., by the manually preselectable length of the
partial area of the evaporatlon holder 4 lntroduced lnto the
reactlon vessel 2. A heat exchanger 25 can be located
upstream of the feed device lf the temperature of the Hg-laden
gases entering vessel 2 ls excesslvely hlgh.
Because of the gas flow, the lndlvldual gas
components are mlxed wlth each other relatlvely qulckly ln the
ad~olnlng reactlon reglon 8 and the lodlne comblnes wlth the
g
27813-39
2 1 ~
mercury to form mercury lodlde.
Wlthln the reactlon area 8, whose length L
corresponds to approxlmately slx tlmes the dlameter D of the
reactlon vessel 2, the reactlon vessel 2 may have a slmple and
therefore cost-effectlve separatlon devlce 6 ln the form of a
flbrous or textlle fllter, whlch fllters out the mercury
lodlde. The lnstallatlon posltlon of the apparatus 1 ln
relatlon to an exhaust gas condult (not shown) and the
connectlon posltlon of the upstream heat exchanger 25, brlng
the mercury lodlde flowlng through the vessel 2 and then
through the flbrous or textlle fllter 6 to a temperature below
the evaporatlon temperature of the lodlde (approxlmately
354C). The downstream-connected adsorptlon agent 7 ls made
of alumlnum chlps at whose surfaces posslble excess lodlne ls
adsorbed.
- 9a -
27813-39
21S0529
The particular advantage of this apparatus lies in its
simple and cost-efficient design. However, the evaporation rate
and therefore also the metering of the iodine is possibly
affected by the temperature of the gas flowing around the
evaporation trough 4. In that case it is not possible to use
very small metered amounts and it may be necessary to accept
overdosing.
In a particularly simply designed and therefore cost-
effective embodiment, the supply or feed device consists of a
wide-meshed metal net. It extends preferably over the entire
cross section of the reaction vessel and is partially covered
with solid iodine. The gases flow around the iodine through the
uncovered mesh of the metal net, heat it and generate the desired
vapor pressure. This embodiment is suitable for uses which do
not require the exact control of the feed rate of halogen.
Fig. 3 schematically illustrates an apparatus 9 for
carrying out the method in accordance with the second embodiment
of the invention. The basic design is similar to that in Fig. 2.
The flow direction has again been marked by arrows. The
difference with respect to the embodiment of Fig. 2 lies in a
changed feed device for the iodine, an additional regulating
device and the omission of an adsorption agent.
The feed device uses a thermostat- controlled reservoir 11
of solid iodine 12, a suction line 13 provided with a blowback
safety valve 14 and drying cartridge 15. The suction line 13
terminates in the reservoir 11 via a controllable pressure pump
16 and a continuous pressure line 17. A supply pipe 18 connects
the reservoir 11 with the interior of the reaction vessel 10.
The pressure pump 16 sucks ambient air through the drying
cartridge 15 and the suction line 13 and introduces it via the
pressure line 17 into the reservoir 11. The reservoir 11 is
located inside a thermostat-controlled housing 19 which keeps the
iodine 12 at a temperature of approximately 30C. A suitably
2150~29
high vapor pressure of the iodine in the reservoir 11 is thereby
created. The compressed air flows over the surface of the iodine
12 and is enriched with iodine vapor. The iodine-air mixture
subsequently reaches the reaction vessel 10 through the supply
pipe 18. The feed rate of the mixture can be controlled by means
of controlling the flowing volume of the compressed air as well
as the temperature and therefore the vapor pressure of the
iodine 12.
The regulating device essentially includes an Hg-
measuring device 20 for the selective measurement of the mercury
concentration and a regulating device 21 connected therewith and
controlling the controllable pressure pump 16. A measuring probe
22 of the Hg-measuring device 20 is disposed inside the reaction
vessel 10 downstream of the supply pipe 18 at a distance A which
approximately corresponds to six times the diameter D of the
reaction vessel 10. A separation device 6 such as a fibrous or
textile filter 6 (Fig. 2) or an electrostatic filter 6a (Fig. 3)
is placed~downstream of the measuring probe 22.
Preliminary tests have shown that this embodiment can react
rapidly to fluctuations in the mercury content of the gas flow,
so that with a suitable setting of the control 20, 21, no iodine
overdosing occurs. For this reason an adsorption agent for
iodine was omitted here, in contrast to Fig. 2.
Fig. 3 also shows, highly schematically, the separating
device in form of an electrostatic precipitator 6a, connected to
a voltage source 6b, the other terminal of which is connected to
the vessel 10.
Fig. 4 shows, highly schematically, the separating device in
form of a heat exchanger 26, located in the stream of gas flow,
for use if the temperature of the gas and/or of the mercury
halide goes above the evaporation temperature of the halogen
being used. Heat recovered can be re-used. Cooling fluid is
connected to couplings 27, 28.
2160529
Varlous changes and modlflcatlons may be made, and
any features descrlbed hereln ln connectlon wlth any one of
the embodlments may be used wlth any of the others, wlthln the
scope of the lnventlve concept.
Example of an oPeratlve embodlment:
Inslde dlameter D of the reactlon vessel 2, 10:
1.10 m temperature of ga~es at lnlet end of reactlon vessel 2,
lO: 350C gas flow rate through the reactlon vessel: about
4.2 m3/sec temperature of gases ln reglon of lntroductlon of
halogen (Flg. 2: 4I Flg. 3: 18): 150C rate of supply of
halogen vapour from condult 18 (Fig. 3) ls such that the
remalnlng concentratlon of mercury wlll be about 10 ~g/m3
temperature of gase~ wlth mercury halldes at separator 6, 6a,
26: 140C.
- 12 -
27813-39