Note: Descriptions are shown in the official language in which they were submitted.
~ 2151~0~
~P 94/13148 PCTICA93/00518
PROCE8~ FOR r~L1UCING AN IJNDENZ~TU~D ~5Y ~KI~ CO~ ~ATE
Re1ate~ A~P1iCatiOn!~
Thi~ application i8 a continuation-in-part o~ U.S.
Serial No. 929,347 filed August 13, 1992, which was a division
and continuation-in-part of U.S. Serial No. 417,246 filed October
4, 1989 which was a continuation-in-part of U.S. Serial No.
289,971 filed December 23, 1988, which was a continuation-in-part
of U.SO Serial No. 188,271 filed April 28, 1988 and is a
continuation-in-part of U.s. Serial No. 866,756 filed April 10,
1992 which is a continuation-in-part of U.S. Serial No. 563,794
filed May 3, 1990 which in turn is a continuation-in-part of said
U.S. Serial No. 289,971. The contents of said applications are
hereby inco.~o ~ted by reference in their entirety.
B~c~v~ of th~ Tnv~tio~
As early as 1982 RYlno~ et al (1) showed ~hat d.~tary
whey protein ~-ol..e..L~ate (W.P.C.) im~ ed the active systemic
humoral immune l~pQ~re in a mammal, as measured by sheep red
blood cell injections. It was h~ v~ found that the use of high
temperature pasteurization of milk in 1988 and ~h-?quent years
following a Salmon~llQ~i~ epidemic in E~ greatly reA~c~ ~he
effectiveness of commercially available whey protein ro~centrates
in improving the immune ~ e.
In ~aid related applications the discovery that
undenatured whey protein ~"~--..L~te had an enhanced
immunological effect was ~.re~ted. It was furthermore
expl A; n~ that in the ~ol~v~l~Lional high temperature
pasteurization of milk the thermc~?~citive proteins serum Alh~lmin
and im~unoglobulin were partially heat denatured and hence
precipitated in the curd. Said related applications de~cribe
experiments where W.P.C. was prepared using the lowest level of
heat treatment of milk compatible with safety st~nAArds, so as
to obtain a whey protein distribution having a high content of
the thermolabile serum albumin and immunoglobin. It was found
that the presence in the serum ~lh~ (B.S.A.) of 6
glutamylcysteine (Glu-Cys) group/molecule (substrate for
glutathione (G.S.H.) synthesis) and the specific intramolecular
disulfide bond related to the undenatured conformation of the
WO94/131~ 2 1 5 1 ~ O G PCT/CA93/00518
molecule, were a key factor in the G.S.H. promoting activity of
W-P-C. ~nh~nc~ment of G.S.H. levels in tissues are believed to
represent the common denominator underlying the beneficial effect
of W.P.C.
Dietary W.P.C. produced with low level pasteurization
improves systemic humoral immune response, increases the
resistance of target cells against the carcinogenic effect of
chemical carcinogens such as dimethylhydrazine, improves
resistance to pneumococcAl infection, and provides a sustained
increase of tissue glutathione.
The extent of denaturation proAl~c~ during whey
concentrate production is normally assessed by the loss of
solubility at pH 4.6. Clearly the two most important factors
of protein denaturation are temperature and pH whereas lower pH
values usually ~nhAnc~ the denaturing effect of high temperature.
This is why throughout our entire procedure, we have maintAine~
a rather high pH ()6) ~h~nkc to a reduction of pH lowering
bacterial contamination through microfiltration with 1.4 ~ pores.
With a denaturation temperature of 64C. at pH 6, ~erum albumin
appears to be the most easily denatured serum protein since its
denaturation i8 not as reversible as that of alpha-lactalbumin
(4). Even a temperature as low as 55C. causes unfolding of the
B.S.A. molecule over a period of time. A pH of at least about
6 is therefore preferably maint~ine~ throughout the process.
The thermosensitivity of the immunoglobulins is very
similar to that of BSA (2.4). Although the amino acid sequence
of most immunoglQhlll inc in cow's milk is not known, we know that
these large molecules are very rich in Cysteine hence they may
contain Glu-Cys groups. Therefore they might exert upon
digestion and absorption an activity similar to that of BSA.
The major drawback with low temperature milk
pasteurization are:
a) Some degree of heat denaturation, h~nce precipitation
of the thermosensitive proteins;
b) The possibility of insufficien_ bacterial reduction;
c) The difficulty in maintaining the delicate hAlA~ce
between adequate pasteurization and the avoidance of protein
~094/131~ 215 I S a 6 PCT/CA93/00518
-- 3
denaturation.~This is likely to cause problems of control on a
factory scale;
d) The cost of low temperature pasteurization.
The object of this invention is to provide an improved
process for preparing a whey protein concentrate that has
adequate bacterial reduction without protein denaturation. It
is a particular object to provide a process that will achieve a
whey protein concentrate having a serum albumin of at least 10%
and adequate bacterial reduction. This approximately 10% level
of serum albumin was found in our studies to be important for the
achievement of sustained increase of tissue glutathione for the
properties such as im~u~ed systemic humoral immune response
described above (References 2 and 3).
We propose to utilize techn;ques of microfiltration to
achieve the objectives of this invention. Microfiltration using
ceramic membranes is a membrane based procedure which allows the
separation of particles ranging in size between O.l and lO
microns.
Microfiltration with membranes having a l.4 micron
porus size has been recently utilized to remove bacteria and
other particles from milk in order to obtain a commercially
"sterile" milk (5). With this method a 2 to 3 Log reduction of
bacterial count in milk was achieved; hence a bactericidal effect
greater than what is obtAineA by traditional milk pasteurization
(72-78C / 15 sec.: 98% bacterial reduction) (6). The industrial
methods of separation of the cA~ei n~ are ba~ed on the
destabilization of the~e proteins either by lowering the pH of
milk to the isoelectric point (pH 4.6) at 20C or by enzymatic
(rennet) hydrolysis of the Kappa casein which stabilizes the
micelles. The first prore~e is not suitable for the recovery
of native whey proteins because pasteurization at low pH of the
whey-concentrate would entail a substantial denaturation of the
proteins. The second procedure is less economic because the
caseins recovered are less functional.
The casein in raw milk occurs in the form of a
colloidal dispersion. The particles of this dispersion range
from 20 to 200 nm in diameter and are generally referred to as
WO94/131~ 21~ 1~ 0 6 PCT/CA93/00518
- 4 -
casein micelles. A recent study (7) has shown that a
microfiltration membrane cont~;n;ng pores with average diameter
of 0.2 micron will selectively retain from skim milk the casein
micelles. However this study concentrated on the separation of
casein. The milk that was used was skim milk that had been
pasteurized at a temperature of 72C. which would have resulted
in denaturation. Although the study does not indicate the serum
albumin content of the permeate, it is certain that it would be
a low value.
The Invention
In accordance with this invention, a process is provided for
producing anlln~en~tured serum albumin content of at least about
10% comprising the following steps, each of which is conducted
at a temperature not greater than about 50C (< 50):
l) Microfiltration of skim raw milk that has been skimmed
at a temperature not in eyc~cs of about 50C. to provide a first
permeate having a bacterial count at least as good as the
stA~A~rd applicable to pasteurized milk;
2) Microfiltration of the first permeate to separate most
of the casein as a retentate from a second permeate;
3) Ultrafiltration of the second permeate to give a whey
protein concentrate having a ~erum albumin content of at least
10%.
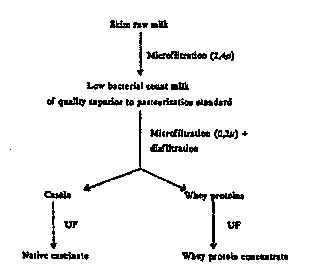
Brief DescriDt~on of drawings
Figure l is a schematic representation of the process
of this invention for producing a whey protein concentrate with
immunoenhancing properties.
Detaile~ De3criPtion of the Invention
Material and Methods
Individual whey proteins were measured by
polyacrylamide gel ele~Llu~horesis. Samples of concentrated w~ey
were applied on 16% polyacrylamide at p~ 8 ~Laemmli- buffer -
system) after the samples were reduced with 10%
2-mercaptoethanol. Samples were applied so that each slot
~ 094/13148 2~ `Q 6 PCT/CAg3/00518
-- 5 --
received 10-20 ~g of protein. Electrophoresis was performed at
200 volts for 70 minutes.
Extent of protein denaturation by the process was
determined in triplicate by the nitrogen solubility index (NSI)
at pH 4.6 and 3000 g. (AOCS 1985 ref.8) The method utilized in
these experiments differs from that described in Reference 2 and
represent a more accurate reflection of the undenatured state of
protein.
Protein content (N x 6.38) and total lipids of samples
were determined in duplicate respectively by the st~n~Ard method
of Kjeldahl and the method of Mojonnier.
Moisture content was determined in duplicate by the
AOCS method (9).
Total coliforms count was determined following
incubation at 37C for 18 h in brilliant green using the most
probable number method. Total bacteria count (aerobic
mesophiles) was determined following incubation at 32C for 48
h in P~A medium. Both methods are ap~oved by the International
Dairy Federation and American Public Health Association.
Referring now to Figure 1 of the drawings, both the
microfiltration of the raw milk ~nd the microfiltration to
separate the casein were carried out on a microfiltration system
with ool.L~olled transmembrane pressure as set up by Alfa-Laval
filtration system Denmark (MFS-7 system with 1.4 sqm membrane
surface). Ceramic membranes were obtA~nP~ from Membralox (pore
size 0~1 and 1.4 micron).
Table 1 below shows the operating conditions during
microfiltration of skim raw bovine milk using a ceramic membrane,
as previously described having a pore size of 1.4 microns.
WO94/131~ ~ l S 1 S 0 ~ - 6 - PCT/CA93/00518
TAB~ 1
Operating conditions during microfiltration for bacterial count
reduction (1.4~).
Temperature S 50
TMP O,5 bar
Tangential >6.6 m/s
velocity
CF 20
Flux 950 L/h/m2
TMP = transmembrane pressure
CF = concentration factor
Raw milk should be used that has been skimmed at a
temperature not in eYc~ss of about 50C. The best temperature
for skimming to a level of 0.05~ of fat is 45C to 50C.
The temperature used during microfiltration of the raw
milk should be in the range about 40C to 50C. As previously
noted denaturation of serum albumin does not begin to occur until
about 55C.
Table 2 shows the microbial counts of the milk
following microfiltration. We have noted a 2-3 log reduction in
bacterial counts in accordance with the literature (5). This
confirms the efficacy of the method. As expected, the lipids are
in the retentate. The protein content of the permeate is only
marginally different from that of the retentate and there is no
difference between permeate and retentate in the type of proteins
as measured by electrophoresis. Hence no selective retention of
proteins is noted over a 1.4~ membrane.
As indicated in Figure 1 microfiltration is followed
by diafiltration which involves adding distilled water to the
retentate. 1 volume of retentate to 1 volume of water is used
and this procedure is performed two or three times.
~O94/131~ _ 7 _
TAB~ 2.
Microbial counts (per/g) expressed in log.
E~cpen- Raw miL~ PerrnP~t10. R~ chon
ment
number To~ Co~fonms T C T C b~çt~
~ (C) coun~
1 4.40 -- 6.78 2.37 -- 2.03
2 6.05 4.25 6.644.23 3.070.48 2.98
3 3.93 1.80 4.661.29 1.76 0 2.17
4 5.72 3.08 6.333.18 3.350.3 2.37
5.49 3.67 6.32 ~ 2.53 -- 2.96
6 5.03 3.08 7.20 - 2.89 2.14
7 6.03 4.60 7.51 ~ 4.032.41 2.00
8 6.gO 5.32 8.457.51 4.272.48 2.63
9 6.52 4.00 7.805.78 3.761.80 2.76
Reduction in log from raw milk to permeate.
The microbial counts of the pèrmeate compare favourably
with st~n~rds applicable to conventional pasteurization.
These stA~rds differ in each jurisdiction. As an
example, the Province of Quebec, CA~A~A ~ requires that total
bacteria count (aerobic mesophiles (32C)) be maintA;n~ below
50,000 (log 4.69), both in the factory and in the final product,
in the case of powdered milk products. Coliforms are to be
below 10. The Province of Quebec has a st~n~Ard of a bacteria
count of 25,000 (log 4.39) and a coliform count of 5 in the
factory for milk products that have not been pasteurized or
fermented.
The second stage involved the separation of the casein
micelles with a 0.1 ~ membrane. The parameters are illustrated
in Table 3. It should be noted that these values may vary
throughout the procedure depending on conditions ~uch as clogging
of membrane pores, increased viscosity of the retentate etc. The
results of the process are given in Table 4. Bovine serum
WO94/131~ 2 i 51~ ~ ~ PCT/CA93/00518
albumin passes through the membrane. The protein composition of
the final product in powder form after concentration by
ultrafiltration and lyophylization (Table 4) meets the
requirements previously identified by us as essential for the
development of immunoenhancing activity and tissue GSH promotion:
serum albumin concentration around 10% and minimal degree of
denaturation. In Table 5 and Table 6 are presented for
comparison the concentrations of serum albumin in current
commercially available W.P.C.'s and the nitrogen solubility index
determined by De Wit in some W.P.C. products.
The temperature and other conditions during the second
stage are as shown in Table 3.
TA8LE 3
Operating conditions during microfiltration for milk
fractionation (O.l~).
Temperature s50C
TMP 0,5 bar
Tangential ~7 m/s
Velocity
CF 3X and DF 3X
Flux >60 L/h/m2
TMP = transmembrane pressure
CF = concentration factor
DF = diafiltration
The retentate from the second stage was subjected to
ultrafiltration on a pilot scale using a Romicon cartridge (l.5
~qm) with a cut off of 50,000 Dalton to provide a powder. The
temperature during ultrafiltration was <50C.
~O94/131~ ~Sl so~ PCT/CA93/00518
_ g _
Table 4 shows the results obtained by the process.
~AB~E 4
Composition of milk retentate and permeate (0,1~).
Example 1
Eroduct NSII % pH nM~ru 1 BSA ¦ C~n ¦ B-lg2 ¦ ~-~3
% oftot~ pro~n
powder 7.14 18.96 10.18 11.02 56.24 04.63
~trate 92.7 -- 25.13 09.67 09.01 51.83 04.37
atpH
4.6
lNSI = Nitrogen solubility index.
2B-lg = beta-lactoglobulin
3~-la = alpha-lactalbumin
MF = microfiltration
BSA - bovine serum albumin
IMMU - Immunoglobulin
TA~LE S
PRODUCT BOVINE SERUM ALBUMIN
in % of Total Whey Protein
Promod 4 + 1
Alacen 855 4 + 1
Lacprodan-80 4.8 + 2
Sapro 4.8 + O.1
Sa~o~lo-75 4 + 1
Bioisolate 5 + 1
Promix 4.3 + 1
Mean + SD
WO94/131~ 2151~ Q - lo - PCT/CA93/00518
- TAB~ 6
WHEY PROTEIN NSI at PH 4.6
CONCENTRATE (~)
Normal UF WPC 83
Neutral UF-DF-WPC 78
Acid UF-DF-WPC 42
De-fatted UF WPC 9l
Sphérosil 'OMA' WPC79
Sph~rosil 'S' WPC 35
Vistec WPC 35
Demin. de-lact. WPC72
From De Wit J.N. et al
Neth. Milk Dairy J. 37 (1983) pp. 37-49
The process of this invention therefore provides a practical
procedure for making undenatured whey protein concentrate.
Furthermore it has the advantage of permitting separate recovery
of the casein. This is a useful by-product that contributes to
the practical economies of the process.
094/131~ ~ S~6 PCT/CA93/00518
- R~F~RENCE8
(which are incorporated by reference in their entirety)
1. Bounous G., Kongshavn P.A.C. "Influence of Dietary Proteins
on the Immune System of Mice". J.Nutr. 112, 1747, 1982.
J
2. Bounous G., Gold P. "The Biological Activity of Undenatured
Dietary Whey Proteins: Role of Glutathione" Clin.Invest.
Med. 14: 296-309, 1991.
3. Bounous G., Batist. G; Gold P. "Immunoenhancing Property of
Dietary Whey Protein in Mice: Role of Glutathione". Clin.
Invest. Med. 12: 154-61, 1989.
4. Brown R.T. "Milk Coagulation and Protein Denaturation in
'Fundamentals of Dairy Chemistry',3rd Edition, N.P. Wong
(Ed) Van Nostrand Reynold C. (Publ.) New York 1988,
pp.583-607
5. Olesen, N. and F. Jensen, 1989. "Microfiltration. The
Influence of Operation Parameters on the Process".
Michwissenschaft 44(8~, p. 476, 1989.
6. Pedersen P.J., in "New Applications of Membrane Process"
Publ. by I.D.F. chapt. 4, p.33, 1991.
7. Fauquant, J.;, Maubois, J.L.; Pierre, A. "Microfiltration
du lait sur Membrane Minerale" Tech.Lait 1028, 21-23,
1988.
8. AOAC 1980, "Official Methods of Analysis" 13 Edition
Association of Official Analytical Chemists, WA~hington,
DoC~
9. AOCA 1985, "Official and Tentative Methods of the Ame~ica.
Oil Chemist Society, Official Methods", Ball65, Revised
Edition.