Note: Descriptions are shown in the official language in which they were submitted.
~ WO 94115666 ~ ' PCT/US94/00506
LIGHT EMITTING DIODE SOURCE FOR PHOTODYNAMIC THERAPY
1 BACKGROUND OF THE INVENTION
2 1. Field of the Invention
3 This invention relates to photodynamic therapy and more
4 specifically to a light source for photodynamic therapy.
2. Acknowledgement
This invention was made with Government support under
7 Grant No. 1R43CA55446-1 awarded by the Department of Health and
8 Human Services. The Government has certain rights in the
9 invention.
3. Prior Art
11 Photodynamic therapy (PDT) is presently undergoing
12 extensive basic pre-clinical and clinical testing and development
13 both domestically and internationally. The general method of
14 performing PDT is now well known and described, for example, in
U.S. Patents 4,968,715; 4,932, 934; and 5,028,621 to Dougherty, et
16 al.; and 5,002,962 to Pandey, et al. In PDT, photosensitizing
17 drugs such as hematoporphyrin derivatives are introduced into and
18 retained by the hyperproliferating cells or tissue such as
19 cancerous tissue and atheromas. With the exposure to suitable
wavelengths of light the photochemical reaction of the
21 photosensitizes can lead to selective destruction of
22 photosensitizes-associated cells or tissue. PDT also holds
23 potential for a number of possible applications other than cancer
24 treatment such as for treating microvascular lesions and blood
., 25 purging. To obtain the desired therapeutic response, all of these
26 applications require the delivery of sufficient light of
27 appropriate wavelength to the photosensitizes in vivo. The
1
WO 94115666 21 ~ ~ 3 .~ ~ ~ PCT/US94/005P'
z:.
1 activating light must be sufficiently intense at wavelengths
2 matching the absorption spectrum of the photosensitizer to initiate
3 the photochemical reaction. Secondly, these wavelengths need to
4 penetrate the host tissue to permit activation of the therapeutic
reaction at the desired depth. Additionally, the light must be
6 able to be delivered to the treatment area in sufficient quantities
7 to permit treatment on a reasonable and effective time scale.
8 Prior art sources of illumination have been primarily
9 lasers. The reasons for this are the efficient deliverability of
the laser light through flexible single optical fibers, the single
11 wavelength nature of the laser, the tunability of certain lasers,
12 and the ability to deliver sufficient effective power to permit
13 reasonable treatment times. All of these properties together have
14 permitted PDT to be administered endoscopically with the
interstitial delivery of the light for the treatment of otherwise
16 inaccessible or large thick lesions. The use of lasers has not
17 been without drawbacks. These negative qualities of the laser
18 include high cost, low reliability, large size, complex operating
19 procedures and constant attention to the safety issues required
when dealing with laser light.
21 Puliafito, et al. (Arch. Ophthalmology, Vol 105, March,
22 1987) disclose using laser diodes for Photodynamic therapy. There
23 are significant differences between LED's and laser diodes. A
24 Light Emitting Diode (LED) is a solid state electronic device
capable of emitting light when an electric current is passed
26 through the device. LED-derived light is relatively broad band
27 (20-40nm) and is emitted in a wide output distribution pattern, and
2
_ . WO 94/15666 ~ ' PCT/US94/00506 ,
1 lacks coherence. The light is produced at very low current levels
2 (20ma). All of these characteristics of LEDs serve to technically
3 differentiate them from laser diodes. The major advantage gained
4 by using a laser for PDT is the ability to couple significant light
power into flexible optical waveguides. This is necessary for
6 applications requiring interstitial or endoscopic delivery of
7 treatment light for PDT. Laser diode systems which include a large
8 power supply and cooling system are very expensive.
9 There are a significant number of applications for PDT
that do not require the use of a laser light source or the delivery
1l of light through light guides. In fact, the majority of the basic
12 pre-clinical and original trials of PDT using hematoporphyrin
13 derivative were done using non-laser light sources. For example
14 the treatment of cutaneous and subcutaneous skin lesions less than
1.0 cm thick can be treated using non-laser light sources. Skin
16 cancer incidence in the United States of America is over 550,000
17 new cases per year and rising. Even though a majority of these
18 cases can be easily treated with local resection or other methods,
19 there are a significant number involving multiple and/or recurrent
lesions that could be more conveniently treated using PDT. The
21 clinical use of PDT in many of these cases would be limited, in
22 part, due to the need to use lasers. This is due to the high cost
23 and lack of availability of suitable lasers. There is truly a need
24 for a low cost non-laser light source for use in PDT.
There are a number of non-laser light sources that could
26 potentially be used in certain PDT applications. The major
27 properties of these light sources that determine their
3
WO 94/15666 ' PCT/US94/0050~
i.1 ~t ~ '~
1 applicability in PDT are: a) output spectrum; b) brightness or
2 intensity at a suitable wavelength; c) deliverability; d) size;
3 and e) cost. These non-laser light sources include arc lamps,
4 incandescent lamps, fluorescent lamps and light emitting diodes
(LEDs). The lamp sources have a broad emission spectrum ranging
6 from ultraviolet to infrared. These broad spectrum sources require
7 the use of optical filtering to remove the undesired wavelengths,
8 particularly the ultraviolet and infrared, due to the potential of
9 carcinogenic effects and heating respectively. In addition, the
low brightness of these light sources at suitable wavelengths,
11 compared to lasers, make them all poor candidates for transmitting
12 sufficient power through small (less than 600 micron core),
13 flexible light guide to effect PDT. The best of these light
14 sources for brightness is the arc lamp due to the relatively high
intensity and small size of the discharge arc. Even though such
16 technology shows promise for certain medical applications,
17 including PDT, it still suffers from problems such as the need for
18 extensive filtering, limitations on its use for large area
19 exposure, and the requirement for high voltage and the concomitant
potential for arc lamp explosion.
21 LED technology, unlike the other non-laser light source
22 outlined above, has the advantage of small size, typically 0.3 mm
23 by 0.3 mm, limited emission spectrum band, typically 20 nm to 40
24 nm, high efficiency and low cost. The light power emitted from a
single diode is relatively low however (approximately 4 milliwatts
26 to 5 milliwatts for the brightest red LEDs using the specified
27 driving currents) but its emission angle is low when compared, for
4
WO 94115666 ' PCT/US94100506
~~,~:~s5333 7
<: .
1 example, to the arc lamp so that its actual brightness is
2 reasonably good. The small size of the LED along with its high
3 efficiency give the potential of using an array consisting of
4 multiple LEDs in a single device to significantly increase
deliverable power density over a large area. The low power output
6 has, however, delayed the acceptance of LED arrays as a suitable
7 light source for PDT. The intensity can be increased by over-
8 driving the LEDs in the array. Such over-driving results in
9 heating which shortens the lifetime of the LED and causes a
spectral shift in the output. LEDs are available in variety of
11 discrete packages as well as several one and two-dimensional array
12 packages. As used herein, an LED array means multiple LED's
13 integrally mounted in a single device. Commercially available
14 arrays, from manufacturers such as Mitsubishi, Hewlett Packard or
Stanley Electric, combine a few LEDs in a single package but not
16 in high enough packing density or in geometrics suitable for PDT.
17 None of these prior art devices can provide sufficient power
18 density for effective PDT treatments, nor can they be easily
19 configured in the geometries necessary for the wide range of
applications for surface illumination and PDT. It is desirable to
21 have a multiple integrated LED array with a power output suitable
22 for use in PDT.
2'3 SUMMARY OF THE INVENTION
~24 It is an object of this invention to provide an array of
multiple integrated LEDs useful for photodynamic therapy.
26 It is another object of the invention to provide an
27 inexpensive light source useful for photodynamic therapy.
5
75975-5 CA 02153337 2000-02-is
6
It is still another object of this invention to be
able to provide an LED array for photodynamic therapy that is
capable of illuminating the surface of various types of
tissues.
It is yet a further object of this invention to
provide an LED array for photodynamic therapy which enables
accurate wavelength and exposure control and permits accurate
dosimetry. .
It is another object of this invention to provide an
illuminating system for photodynamic therapy' that is safe to
both the physician and the patient.
According to the invention there is provided an
incoherent light source suitable for administering
illumination for photodynamic therapy, said incoherent light
source comprising, in combination: a) an LED array driver; b)
an LED array; and c) a cooling means.
The LED light source of the present invention is
novel because it teaches how to use the characteristics of the
LED to an advantage over the laser diode for applications of
PDT which do not require interstitial or endoscopic light
delivery. The wide output distribution pattern, small size,
and minimal cooling requirements of the LED allow large arrays
of the devices to be constructed which cumulatively are
capable of producing a total output light power exceeding that
of laser diodes. This opens up applications for large surface
area illumination (such as is needed in dermatology) for which
laser diode systems are inadequate.
These and other objects of the invention will soon
become apparent as we turn now to a brief description of the
drawings.
75975-5 CA 02153337 2000-02-18
6a
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic representation of an LED
system suitable for illumination of surfaces for photodynamic
therapy.
WO 94/15666 ~ PCT/US94100506
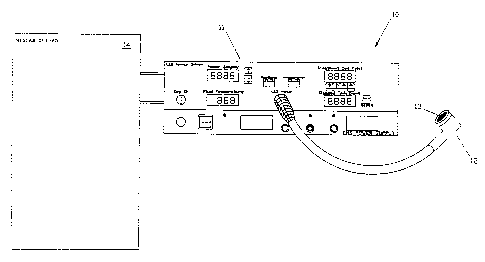
1 Figure 2 schematic diagram of the front panel of the LED
2 array driver showing the displays for controls for exposure power
3 and coolant temperature display.
'4 Figure 3 is a cross-sectional view of the LED handpiece
configured for flat surface illumination.
6 Figure 4 is a top view of the LED puck configured for
7 flat surface illumination.
8 Figure 5, which is a detailed top view of the area shown
9 in Figure 4 enlarged for ease of viewing, shows the top surface of
to the LED puck showing the machine holes and indicating the LED die.
11 Figure 6 is a cross-sectional view of the LED handpiece
12 for illumination of cylindrical surfaces.
13 Figure 7 shows the LED sleeve for cylindrical surface
14 illumination.
Figure 8 is a schematic diagram of a preferred embodiment
16 of the light output and wavelength detector.
17 DESCRIPTION OF THE PREFERRED EMBODIMENT
18 It is the combination of small size and high efficiency
19 that make the LED a potentially useful light source for PDT. The
small size of the LED allows them to be fabricated in high density
21 into applicators of various shapes for the direct contact treatment
22 of cutaneous lesions. The shape may be circular, rectangular (or
23 any curvilinear surface) for treating skin lesions or cylindrical
~24 for the treatment of cervical cancer. Planar arrays of LED~s may
be bent or folded to form various curvilinear surfaces to conform
26 to the surface being treated. To be useful, the LED's must be
27 overdriven to produce useful power outputs. The heat generated
7
WO 94/15666 ' PCT/US941005P'
1 during over-driving must be removed by cooling the LED in order to
2 control the wavelength and increase the lifetime of the LED.
3 Turning now to Figure 1, we see a schematic view of the LED system
4 configured for flat surface illumination and generally indicated
at the numeral 10. The system consists of the LED array driver 11,
6 the flat surfaced LED handpiece 12, the flat surfaced LED puck 13
7 and the closed loop chiller 14. The detailed controls of the front
8 panel of the system are shown in Figure 2 of the array driver 11,
9 and shows the displays for the controls of exposure 21, power 22,
the coolant temperature display 23 and the power supply 24.
11 An LED handpiece configured for flat surface illumination
12 12 is shown in cross section in Figure 3. The stainless steel
13 housing 31 and threaded retaining ring 32 are connected to the
14 system ground 33 and provide one electrical connection to the LED
puck 13. The heat sink 34 is connected to the LED supply voltage
16 35. This provides the second electrical connections to the LED
17 puck as well as removing the heat generated in the puck. The heat
18 sink is electrically insulated from the housing by the DELRIN~
19 insulator 36. The coolant tubes 37 provide a flow of cooling water
from the chiller to the heat sink. The light output power and
21 wavelength detector 38 (shown in greater detail in Figure 8)
22 detects the amount of light being delivered to the patient by
23 sensing the light through the light sense channel 39.
24 An LED puck configured for flat surface illumination is
shown in Figure 4. The puck, generally indicated at 13, comprises
26 a gold plated insulated copper and fiberglass laminate sheet 41
27 bonded to a flat copper substrate 42. Holes are machined through
8
WO 94115666 ~ PCT/US94100506
1 the copper laminate to the surface of the copper substrate. The
2 LED puck is coated with a clear epoxy potting material 43 to
3 protect the LED device and provide a smooth clean surface for
'4 patient contact.
Figure 5, shown as detail A of Figure 4, is an enlarged
6 view of the top surface of the LED puck showing the machined holes
7 and indicating the LED die 51 bonded to the copper substrate 42
8 with electrically and, thermally conductive epoxy 52. The figure
9 also shows the gold bonding wire 53 attached between the top
contact of the LED die and the surface of the copper laminate 41
11 using common integrated circuit assembly techniques.
12 Figure 6 shows a cross sectional view of the LED
13 handpiece for illumination of cylindrical surfaces, generally
14 indicated by 60. The stainless steel housing 31, threaded
retaining ring 32, coolant tubes 37, the photodiode detector 34 and
16 the insulator 36 function the same as in the flat surface
17 illuminating handpiece. The heat sink 61, the light sense channel
18 62 and the LED sleeve 63 are now shaped appropriately for insertion
19 into the cervical canal or rectum.
Figure 7 shows an LED sleeve configured for cylindrical
21 surface illumination 63. The copper laminate 71, copper substrate
22 72 and LED 73 are assembled in a similar manner to the flat surface
23 LED puck except the geometry is out of a tube instead of a disk.
24 ~ The light output power and wavelength detector is shown
in greater detail in Figure 8. The light transmitted through the
26 light sense channel 39 (Figure 3) is focused by the collimating
27 lens 81 and split into two equal light beams by the beamsplitter
9
WO 94/15666 ' PCT/US94/OOSP'
~2~~333~
1 82. The light power in one beam path is filtered by a filter 83,
2 and measured by the photodiode 85. The unfiltered photodiode 84
3 measures the light power in the other light beam path. Assuming
4 that proper calibration is done to compensate for the different
optical losses in each path, the total optical power and
6 verification of the wavelength can be accomplished with this
7 technique. It is clear that this device could also be configured
8 with a flexible light guide (not shown) built into the handpiece
9 which would then deliver the sampled light energy to the light
power output and wavelength detector shown in Figure 8 which could
11 conveniently be installed in the LED array driver 11.
12 In summary, it has been shown that an LED array can be
13 configured to provide power and wavelength outputs suitable for
14 PDT. In order to achieve the required power levels, it is
necessary to over-drive the LED's. The additional current required
16 for over-driving generates heat at the diode junction which results
17 in: (a) a red-shift and broadening of the output light; and (b) a
18 shorter lifetime. To overcome these problems, the LED array is
19 mounted on a puck enabling the LED array to be cooled to control
the bandwidth and wavelength of the output light and increase the
21 lifetime of the array. In practice, the output wavelength depends
22 on the diode s junction temperature. Monitoring the wavelength
23 permits adjustment of the coolant .temperature and flow rate to
24~ maintain the junction at the desired temperature.
The foregoing preferred embodiment of the LED system for
26 photodynamic therapy provides a low cost, high power excitation
27 source for PDT which can be produced in a variety of shapes used
W 94/15 6 ' PCT/US94/00506
T - ~ ; ~21 ~~.,~ ~3 ~
1 in a wide variety of applications. This device will allow PDT to
2 become viable treatment modality for many more cancer patients
3 inasmuch as it will now be cost effective for the physician's
4 office or small clinic. Although the invention has been described
in terms of particular embodiments and applications, one of
6 ordinary skill in the art in the light of this teaching, can
7 generate additional embodiments and modifications without departing
8 from the spirit of or exceeding the scope of the claimed invention.
9 For example, single LED chips may be fabricated into an array by
depositing them directly onto a chilled substrate by techniques
11 currently used in hybrid circuit fabrication. Accordingly, it is
12 to be understood that the drawings and descriptions herein are
13 preferred by way of example to facilitate comprehension of the
14 invention and should not be construed to limit the scope thereof.
16
17
18
19
21
22
23
24
26
27
11