Note: Descriptions are shown in the official language in which they were submitted.
s .
_2154313
...
TS 0249 CAN
PROCESS FOR PRODUCING A HYDROWAX
The present invention relates to a process for
producing a hydrowax which is very useful as a feedstock
for a thermal steam cracker to produce lower olefins and
as a feedstock for producing lubricating base oils.
Thermal steam cracking is a known method for
producing lower olefins, particularly ethene and to a
somewhat lesser extent propene. It is a strongly
endothermic process and basically involves heating a
hydrocarbon oil feed to a sufficiently high temperature
for cracking reactions to occur followed by rapid cooling
of the reactor effluent and fractionation of this
effluent into the different products. A steam cracker,
also commonly referred to as an ethene cracker, usually
consists of a hot section and a cold section. The hot
section consists of cracking furnaces, a cooling section
and a primary fractionator for separating the effluent in
a cracked residue, pan oil, cracked gas oil and cracked
gas. Steam is introduced into the cracking furnace to
dilute the feed. This is favourable for the final olefin
yield, while the added steam also suppresses the
deposition of coke in said furnace. In the cold section
the cracked gas is further separated into the various end
products among which are pure ethene and propene. In
general, this separation is achieved by first compressing
the cracked gas from the primary fractionator to a
pressure of about 30-40 bar followed by cooling the
compressed gas to temperatures below -100 °C to enable
separation into the various pure end products. The
removal of gases such as carbon dioxide and hydrogen
sulphide also takes place in the cold section of the
steam cracker. Because of the strong endothermic nature
_215431
_ 2 _
of the steam cracking process adequate and efficient heat
recovery is very important in order to make the process
economically viable.
A well known feedstock for the steam cracker is the
naphtha fraction produced in the processing of crude oil.
Atmospheric gas oils are also known as suitable cracker
feedstocks. For instance, in British patent specification
No. 1,537,822 a process for the production of lower
olefins is disclosed involving the steam cracking of a
hydrogenated gas oil feedstock, which is formed by the
subsequent steps of thermal cracking of a hydrocarbon oil
residue -suitably an atmospheric residue-, recovering a
gas oil fraction by distillation from the thermal
cracking effluent and catalytic hydrogenation of said gas
oil fraction. In German offenlegungsschrift No. 1,922,665
a steam cracking process for preparing olefins is
disclosed, wherein a dearomatised gas oil is used as the
feedstock. Important considerations in respect of the
choice of the feedstock are, beside the potential ethene
yield, chemical factors like the H/C ratio of the feed
as well as economic factors such as the price of the
feedstock and the stability of this price on the market.
Other factors such as availability of the feedstock,
synergy potential of a steam cracker and a refinery and
investments involved with integration of a steam cracker
in a refinery also play an important role in choosing the
appropriate feedstock.
Since the early eighties another feedstock has been
used on a commercial scale. In the publication of A.G.
Goossens, Hydrocarbon Processing, November 1986, pp. 84-
86 it is described that the hydrowax, i.e. the
hydrogenated residue, as produced by single-stage
hydrocracking of flashed distillates is suitable as a
feedstock for a steam cracker to produce ethene. The term
"flashed distillates" as used in this context refers to
2154313
- 3 -
the distillate fractions obtained in the vacuum flash
distillation of atmospheric residue. It is described that
hydrowax is an excellent steam cracker feedstock, inter
alia because it has an attractive and relatively constant
price, olefin yields are close to those for naphtha
feedstocks and it offers excellent possibilities for
integrating a steam cracker with a refinery. In said
publication it is furthermore described that fouling of
the transfer line heat exchanger or TLE, where high
enthalpy heat is recovered from the hot effluent of the
furnace for producing high pressure steam, is the major
factor determining the run length of the steam cracker.
This fouling is stated to be caused by the content of
aromatic compounds in the hydrowax and by the tars formed
during the pyrolysis in the cracking furnace. Since
hydrowax tends to cause more TLE-fouling than the
conventionally used naphtha feeds, specific measures have
to be taken to cope with this fouling so that it does not
. seriously limit the run time. Quench pipe diameters in
the TLE and maximum design and operating TLE outlet
temperature are important variables in this respect.
The present invention aims to provide a process for
the preparation of a hydrowax from hydrocarbon oil
fractions heavier than flashed distillates, which
hydrowax can be suitably applied as a feedstock in known
steam cracking processes for producing lower olefins,
particularly ethene, at a commercially attractive yield.
Processes for producing lubricating base oils from
hydrowax are known in the art. In EP-A-0,272,729, for
instance, a process is disclosed wherein a hydrocarbon
feedstock containing flashed distillate produced in a
residue conversion process is hydrocracked, after which
the bottom fraction of the cracked effluent, i.e. the
hydrowax, is dewaxed. The dewaxing treatment may be
preceded or followed by a hydrotreatment step in order to
2154313
. _
- 4 -
hydrogenate any unsaturated components present. The
preferred order is first dewaxing and then hydrotreating
the dewaxed material. The dewaxing step can be both
solvent dewaxing and catalytic dewaxing. The final
hydrogenation step increases the amount of saturated
components present, whereby the hydrogenation of olefins
into paraffinic and isoparaffinic components is
favourable for the viscosity index (VI) of the base oil
finally obtained.
In EP-A-0,280,476 another suitable process for
producing lubricating base oils from a hydrowax is
disclosed. According to this process, the hydrowax is
hydroprocessed over a hydroprocessing catalyst comprising
zeolite beta as an acidic component and a hydrogenation-
dehydrogenation component, suitably platinum. The
hydroprocessed product is subsequently separated into a
lower boiling fraction and a lubricating base oil
raffinate, the latter being advantageously subjected to
. an aromatics extraction step, optionally followed by a
hydrofinishing step, to yield the lubricating base oil.
The present invention also aims to provide a process
wherein a hydrowax is prepared which can be very suitably
used as a feedstock for producing lubricating base oils.
Accordingly, the present invention aims to provide a
process, wherein the hydrowax prepared is a suitable
feedstock for a dewaxing unit or a hydroprocessing unit.
Accordingly, the present invention relates to a
process for producing a hydrowax comprising the steps of
(a) hydrocracking a blend obtained by blending at least
one distillate fraction and a deasphalted oil (DAO):
(b) separating from the hydrocracker effluent a fraction
of which at least 90~ by weight, preferably at least 95~
by weight, has a boiling point of 370 °C or higher (the
370+ fraction); and
21513.
- 5 -
(c) separating the 370+ fraction in a top-fraction and a
bottom-fraction at an effective cutpoint below 600 °C,
suitably below 580 °C, thus yielding the hydrowax as the
top-fraction.
Because of the negative impact heavy metals usually
have on the activity of hydrocracking catalysts, the
blend of DAO and distillate fractions) should have a
sufficiently low heavy metal content. It is therefore
preferred that the heavy metal content of the
hydrocracker feedstock has been reduced prior to
hydrocracking. This implies that either the DAO is
hydrodemetallised before being blended with the
distillate fractions) or the blend of DAO and distillate
fractions) is hydrodemetallised prior to being subjected
to hydrocracking. In practice the first option, i.e.
hydrodemetallisation of the DAO will be preferred,
because all or almost all of the heavy metal present in
the blend of DAO and distillate fractions) was
originally contained in the DAO anyway, so that it is
economically more effective to hydrodemetallise the DAO.
In deciding whether or not a hydrodemetallisation step is
actually required, there are two major factors. Firstly,
the type of crude oil from which the DAO has been derived
and secondly the depth at which the deasphalting
treatment has taken place, i.e. the extent to which
asphaltenic components are removed. In case the DAO has
been derived from a crude oil in which heavy metals are
naturally occurring in relatively high amounts, the DAO
is likely to have a high heavy metal content as well.
Additionally, if the deasphalting depth in the
deasphalting treatment is high, i.e. only the most heavy
asphaltenic components are removed, then the DAO will
still contain substantial amounts of heavy metals as
compared with the deasphalting feed. If, on the other
hand, said depth is relatively low, i.e. beside the heavy
21543,
.~..
- 6 -
asphaltenic components some lighter asphaltenes have been
removed as well, then the heavy metals content in the DAO
will be significantly lower than the heavy metals content
of the deasphalting feed and a separate demetallisation
step can be dispensed with.
It is very surprising that a relatively heavy
fraction such as a hydrodemetallised deasphalted oil can
be used for preparing a hydrowax which is a suitable
feedstock for a steam cracker. Up to now it was generally
believed that the hydrowax should be produced from
flashed distillate fractions only, since the use of
heavier fractions would cause too much fouling in the
TLE, thus imposing an economically unacceptable run time
constraint on the steam cracker. It has now been found
that by flashing the 370+ fraction obtained from
hydrocracking a blend of flashed distillate and
optionally demetallised DAO at an effective cutpoint in
the range of from 400 to 600 °C, the hydrowax obtained as
the top-fraction can very suitably be used as the
feedstock for a steam cracker in order to produce ethene
and propene or as a feedstock for a dewaxing or
hydroprocessing unit for producing lubricating base oils.
The blending or weight ratio of distillate
fractions) to DAO, which optionally has been
hydrodemetallised, is not particularly critical and is
primarily determined by hydrocracker constraints. Thus,
this weight ratio is suitably in the range of from 20/80
to 80/20 and preferably from 40/60 to 70/30.
Hydrocracking of the blend of distillate fractions)
and optionally hydrodemetallised DAO may be performed in
any suitable way known in the art. Generally,
hydrocracking is carried out in the presence of hydrogen
and a suitable hydrocracking catalyst at elevated
temperature and pressure. Hydrocracking catalysts usually
consist of one or more metals from nickel, tungsten,
,."w
_ 7 -
cobalt and molybdenum in elemental, oxidic or sulphidic
form on a suitable carrier such as alumina, silica,
silica-alumina or a zeolite. There are many commercially
available hydrocracking catalysts, which can be suitably
applied in the process according to the present
invention. For the purpose of the present invention the
hydrocracking process can be a single- or a multiple-
staged process, whereby a single-staged process is
preferred because multiple-staged hydrocracking causes
deeper hydrogenation of polyaromatics, thus producing
more polynaphthenes. Such polynaphthenes produce more
fuels and tar and less olefins than a hydrowax resulting
from a single-stage hydrocracking process. In the case of
a single-staged process, a stacked bed of a hydro-
denitrification/first-stage hydrocracking catalyst on top
of a conversion catalyst can suitably be used.
Particularly suitable hydrodenitrification/first-stage
hydrocracking catalysts are NiMo/alumina and CoMo/-
~alumina, optionally promoted with phosphorus and/or
fluorine. Suitable conversion catalysts include those
based on a Group VIB metal, a Group VIII metal and an
acidic carrier. A promoter in the form of phosphorus (P)
may be present as well. Concrete examples of such
catalysts are NiW/zeolite, NiW/silica-alumina and
NiW/zeolite/silica-alumina. Common hydrocracking
conditions are an operating pressure of 80-250 bar,
suitably 100-200 bar, and an operating temperature of
300-500 °C, suitably 350-475 °C.
The distillate fractions useful in the process of the
present invention may be either heavy gas oil fractions
obtained from the atmospheric distillation of a crude oil
or flashed distillate fractions obtained from the vacuum
flash distillation or vacuum distillation of an
atmospheric hydrocarbon oil residue. For the purpose of
the present invention it is not strictly required to use
215 ~3~.~
_8_
sharply defined distillate fractions (such as obtained in
vacuum distillation) and therefore it is preferred that
the distillate fractions are produced by the less
expensive vacuum flash distillation of an atmospheric
hydrocarbon oil residue.
The DAO used is suitably obtained by deasphalting a
residual hydrocarbon oil, preferably a vacuum residue.
The deasphalting may be carried out in any conventional
manner. A well known and suitable deasphalting method is
solvent deasphalting, which involves the countercurrent
treatment of the residual hydrocarbon oil feed with an
extracting solvent. This extracting solvent usually is a
light hydrocarbon solvent containing paraffinic compounds
having 3 to 8 carbon atoms, such as propane, butane,
isobutane, pentane, isopentane, hexane and mixtures of
two or more of these. Preferred paraffinic hydrocarbons
are those having 3 to 5 carbon atoms with butane, pentane
and mixtures thereof being most preferred. The solvent
deasphalting treatment is conveniently carried out in a
rotating disc contactor or a plate column with the
residual hydrocarbon oil feed entering at the top and the
extracting solvent entering at the bottom. The lighter
hydrocarbons present in the residual hydrocarbon oil
dissolve in the extracting solvent and are withdrawn at
the top of the apparatus. From this top-fraction, the DAO
is obtained after recovery of the extracting solvent. The
asphaltenes, which are insoluble in the extracting
solvent, are withdrawn at the bottom of the apparatus.
The conditions under which deasphalting takes place are
known in the art. Suitably, deasphalting is carried out
at a total extracting solvent to residual hydrocarbon oil
ratio of 1.5 to 8 wt/wt, a pressure of 1 to 50 bar and a
temperature of 160 to 230 °C.
Hydrodemetallisation of either the DAO or the blend
thereof with distillate fractions) can be achieved by
2154313
- g _
any hydrodemetallisation method known in the art.
Usually, such method involves passing the feed to be
treated in an upward, downward or radial direction
through one or more vertically disposed reactors
containing a fixed or moving bed of hydrodemetallisation
catalyst particles at an elevated temperature and
pressure in the presence of hydrogen. Well known
demetallisation operations are the bunker flow operation,
the fixed bed operation, the fixed bed swing operation
and the movable bed operation. Suitable catalysts usually
consist of oxidic carriers such as alumina, silica or
silica-alumina, on which one or more metals of Group VIB
or VIII of the Periodic Table of Elements may be
deposited either in elemental form or as a metal
compound. Such hydrodemetallisation catalysts are
commercially available from many catalyst suppliers.
Particularly suitable catalysts are those having as the
active agent one of the combinations NiMo or CoMo,
optionally promoted with phosphorus (P), on an alumina
carrier. It is well known that the type of catalysts
described hereinbefore in practice will also exhibit some
upgrading activity in terms of hydrodenitrification
and/or hydrodesulphurization, removal of heavy hydro-
carbons and conversion of hydrocarbons having a boiling
point above 520 °C into components having a lower boiling
point. Hydrodemetallisation is usually carried out at a
hydrogen partial pressure of 20-250 bar, a temperature of
300-470 °C, preferably 310-440 °C, and a space velocity
of 0.1-10 1/l.hr, preferably 0.2-7 1/l.hr.
For the purpose of the present invention, it is
preferred that the DAO used is produced by deasphalting a
vacuum hydrocarbon oil residue, optionally followed by
hydrodemetallisation. Generally, a vacuum hydrocarbon oil
residue is obtained as the residual fraction of the
vacuum distillation of an atmospheric hydrocarbon oil
~r
_215~3I3
- 10 -
residue. As already described above, the distillate
fractions) used in the process according to the present
invention are preferably obtained from the vacuum
distillation of an atmospheric hydrocarbon oil residue as
well. In a further preferred embodiment of the present
invention said vacuum hydrocarbon oil residue from which
the DAO is produced, is derived from the same atmospheric
hydrocarbon oil residue as said distillate fraction(s).
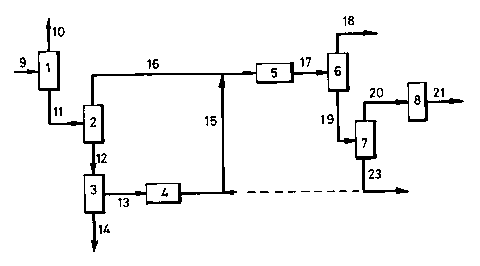
This preferred line-up is illustrated in Figure 1 and
clearly shows that the process of the present invention
for producing hydrowax offers a high synergy potential
between a refinery and a steam cracker, when said
hydrowax is used as a steam cracker feed. Similarly, a
high synergy potential can be recognised when integrating
the present process for producing hydrowax with a process
line-up for producing lubricating base oils.
Separation of the 370+ fraction from the hydrocracker
effluent (step (b)) can be achieved by means of
fractionation devices commonly applied in hydrocracker
units. The separation in step (c) can also be performed
by any method known in the art for separating a
hydrocarbon oil feed into two or more different fractions
based on the boiling points of the various components
present in said hydrocarbon oil feed. Examples of
suitable separation methods include distillation at
atmospheric or reduced pressure, such as conducted in a
mid vacuum flasher or a high vacuum distillation unit.
The only important parameter in this respect for the
purpose of the present invention is the effective
cutpoint, i.e. the temperature indicating the boiling
point of the highest boiling components of the top-
fraction and the lowest boiling components of the bottom-
fraction. In practice this will mean that at least 85$ by
weight, preferably at least 90~ by weight and most
preferably at least 95$ by weight, of the components
2154313
-11-
constituting the top-fraction has a boiling point below
the effective cutpoint, while at least 70~ by weight of
the components constituting the bottom-fraction has a
boiling point above the effective cutpoint. As already
indicated above the effective cutpoint must be below 600
°C and preferably below 580 °C. Preferably, the effective
cutpoint is above 400 °C and more preferably above 450
°C. From a yield perspective, it will usually be even
more preferred to use an effective cutpoint above 500 °C
and most preferably above 550 °C, because -as will be
generally appreciated- a lower effective cutpoint goes at
the expense of the hydrowax yield. On the other hand, if
it is the aim to have as little heavy hydrocarbonaceous
components as possible in the hydrowax, a lower effective
cutpoint should be chosen.
The hydrowax is eventually obtained in step (c) as
the top-fraction and can be used directly as feed for a
steam cracker or as feed for a process line-up for
producing lubricating base oils. The bottom-fraction
contains many heavy hydrocarbonaceous compounds, partly
polyaromatic ring-structures. In order to increase the
efficiency of the hydrowax production, at least a part of
the bottom-fraction obtained in step (c) may be blended
with the optionally hydrodemetallised DAO prior to
hydrocracking. In this way an optimum hydrowax yield can
be realised. Additional efficiency increasing measures
include for instance recycling at least a part of the
cracked residue and/or the cracked gas oil fraction
obtained from the steam cracker to the inlet of the
deasphalter and/or to the inlet of the
hydrodemetallisation reactor, if the DAO is
hydrodemetallised.
The present invention also relates to the hydrowax
obtainable by the process according to the present
invention and to the use of this hydrowax both as the
i i . r.. ~ ~ il , i -! ~i . . ~I.
CA 02154313 2004-12-02
- 12 -
feed in thermal steam cracking for producing lower olefins
and as the feed in the production of lubricating base oils.
In an apparatus aspect of the invention, there is
provided a hydrocracker refinery integrated with a process
s line-up for producing lubricating base oils, wherein the
hydrocracker refinery is capable of operating according to
the process of the invention, and a lubricating base oil
production facility capable of receiving hydrowax as feed
and producing lubricating base oil.
to In another apparatus aspect of the invention, there is
provided a hydrocracker refinery integrated with a steam
cracker, wherein the hydrocracker refinery is capable of
operating according to the process of the invention; and a
steam cracker capable of receiving hydrowax as feed and
15 producing lower olefins.
In both of the aforementioned apparatus aspects of the
invention, the refinery comprises an atmospheric distil-
lation unit capable of receiving a crude oil and separating
it into an atmospheric residue and distillate fractions, a
2o vacuum flash distillation unit adapted to receive the
atmospheric residue and to separate it into one or more
flashed distillate fractions and a vacuum residue, a
deasphalting zone capable of deasphalting the vacuum
residue and producing an asphaltic fraction and a DAO, a
25 hydrodemetallization unit capable of demetallizing the DAO,
a hydrocracker adapted to receive a blend of hydro-
demetallized DAO and at least one distillate fraction, a
fractionator capable of separating hydrocracker effluent
into a top fraction and a 370+ bottom fraction, a vacuum
3o distillation unit capable of separating the 370+ fraction
into a hydrowax and a bottom fraction.
i ~ ... . . il i . .~ n w . ~
CA 02154313 2004-12-02
- 13 -
Figure 1 illustrates how a preferred embodiment of the
process according to the present invention can be
integrated in a refinery-steam cracker line-up or in a
refinery having lubricating base oil production facilities.
s A crude oil (9) is passed into atmospheric distil-
lation unit (1), where it is separated into atmospheric
residue (11) and distillate fractions (10). The
atmospheric residue (11) is subjected to vacuum flash
distillation in vacuum flash distillation unit (2) and
to separated into one or more (vacuum) flashed distillate
fractions (16) and vacuum residue (12). Vacuum residue
(12) is subsequently passed into deasphalting zone (3)
resulting in asphaltic fraction (14) and DAO (13), which is
hydrodemetallised in hydrodemetallisation unit (4). The
15 hydrodemetallised DAO (15) is blended with distillate
fractions) (16) and the resulting blend stream is
subjected to hydrocracking in hydrocracker (5). The
hydrocracker effluent (17) is separated in fractionator (6)
into top-fraction (18) and 370+ bottom-fraction (19). This
20 370+ fraction is separated in (high) vacuum distillation
unit (7) into hydrowax (20) and bottom-fraction (23), part
of which may be blended with hydrodemetallised DAO (15).
This is indicated with a dotted line. The hydrowax (20) is
used as the feed for a unit (8) for producing a product
25 stream (21). Unit (8) may be steam cracker, in which case
product stream (21) may be ethene; or unit (8) may be a
lubricating base oil production facility, in which case the
product stream (21) is lubricating base oil.
The invention is further illustrated by the following
3o example.
CA 02154313 2004-12-02
- 14 -
Example
A flashed distillate FD having the properties as
listed in Table I was blended with a hydrodemetallised
DAO (DAO+) in a weight ratio of FD:DAO+ of 55:45. The
DAO+ was obtained by passing a DAO (obtained by
subjecting vacuum residue to butane deasphalting at
70 cwt yields properties listed in Table I) over a
conventional hydrodemetallisation catalyst (NiMoP on
alumina) under the conditions specified in Table II. The
resulting FD/DAO+ blend was subjected to hydrocracking
over a stacked bed of a conventional first stage
hydrocracking catalyst (NiMoP on alumina) on top of a
dedicated second stage hydrocracking catalyst (NiW on
amorphous silica-alumina) under the conditions listed in
Table II. The hydrocracker effluent was separated in a
fractionator into a top-fraction and a 370+ bottom-
fraction. This 370+ fraction was then separated in a
vacuum flasher at an effective cutpoint of 576 °C into a
hydrowax (the top fraction) and a bottom fraction. The
properties of the hydrowax are also given Table I.
The hydrowax was subsequently passed into a
steamcracking unit. The steamcracking unit was operated
at a temperature of 820 °C, an outlet pressure of 2.15
bar, a feed flow of 49.6 g/h and a gas flow of 43.8 N1/h.
Ethene yield was 28.0 cwt and propene yield was 13.8 $wt,
both weight percentages being based on weight of feed.
CA 02154313 2004-12-02
is
TABLE I Properties of FD, DAO and hydrowax
FD DAO Hydrowax
Carbon ($wt) 86.1 86.4 85.8
Hydrogen 12.4 12.2 14.2
( $wt)
Sulphur 1.1 1.4 0.02
(cwt)
Nitrogen 1200 4200 18.9
(mg/kg)
IBP (C) 298 396 346
10 $wt (C) 362 516 402
30 $wt (C) 408 580 427
50 cwt (C) 439 634 451
70 cwt (C) 465 692 483
90 $wt (C) 528 736 540
96 cwt (C) - - 570
FBP (C) >620 >740 616
TABLE II Process conditions
HDM HCU
Total Pressure 171.3 171.3
(bar)
WHSV (kg/llh) 0.6 0.6
Gas rate (N1 1000 2000
H2/kg)
Temperature (C) 350 389