Note: Descriptions are shown in the official language in which they were submitted.
WO 94/17853 PCT/US94/01152
-1-
ACTIVE DRUG DELIVERY DEVICE, ELECTRODE,
AND METHOD FOR I~Z1KING SAME
FIELD OF THE INVENTION
The present invention generally relates to
active devices for delivering a medicament to a
patient transdermally, i.e., through the skin, and
more specifically relates to an active flexible
electrode which can be used in an iontophoretic
device and a method for making same.
BACKGROUND OF THE INVENTION
Transdermal administration provides a method by
which a medicament can be delivered in a controlled
manner to target tissues either for localized effect
or for systemic absorption. The transdermal
administration of a medicament offers the advantage
of being a noninvasive procedure which does not
require percutaneous puncture devices, and further,
avoids placing stability requirements on the
medicament if it is to be introduced through the
gastrointestinal tract. Additionally, transdermal
administration is ideally suited for sustained
delivery of a medicament instead of the bolus dosage
characteristic of most other modes.
There are two general types of transdermal drug
delivery techniques i.e., "Passive" and "Active".
CA 02155107 1999-09-09
WO 94/I78~3~ PCTlUS94lO1~i52
-2-
The passive technique is traceable to biblical times
when healing oils and medicaments were applied to
the skin of a patient. Passive transdermal drug
delivery has its basis in natural physical phenomena
such as osmosis, diffusion and differential
solubility.
Active transdermal delivery was first reported
in 1908 when it was demonstrated that ions could be
driven across the skin by means of an electric
l0 current. The active technicrues are termed
iontophoresis, electro-osmosis and electrophoresis
and are co:Llectively referred to here simply as
iontophoresis.
Presently, passive transdermal systems are most
effective and used in the delivery o~ unionized
lipophilic moieties which are active at Iow
concentrations, e.g., nitroglycerin, nicotine,
estrogen anc others. Iontophoresis enables ionized
solutes to be delivered transdermally and further
allows control of delivery rate and duration of
delivery.
Conventional iontophoretic devices, such as
those described in U.S. Patent Nos. 4,820,263
( Spevak et al.), 4,927,408 (Hack et al.), and
5,084,006 (Lew et al.), are for actively
delivering a medicament transdermally. Basically,
these devices consist of two electrodes, i.e.; a
cathode and an anode. Both of these electrodes are
disposed so as to be in electrical contact with some
~
CA 02155107 1999-09-09
WO 94/178'3 . PCT/US94/01152
-3-
portion of the skin or other area of the patient,
such as a :mucous membrane. One electrode called the
donor electrode, is the electrode from which the
ionic substance, medicament, drug precursor or drug
is delivered into the body by iontophoresis. The
other electrode, called the counter or return
electrode, serves to close the electrical circuit
through the body. In conjunction with the patient's
skin contacted by the electrodes, the circuit is
completed by connection of the electrodes to a
source of electrical energy, e.g., a battery. In
the case where the ionic substance to be delivered
into the body is positively charged, i.~e., a cation,
then the anode will be the donor electrode and the
cathode will serve to complete the circuit. If the
ionic substance to be delivered is negatively
charged, i.e., an anion, then the cathode will be
the donor electrode and the anode will be the
counter electrode. Further, it may be possible to
deliver two drugs simultaneously by providing a
cationic drug at the anode and an anionic drug at
the cathode.
Iontophoresis should be suitable for
noninvasively delivering a medicament over a
.sustained period. It is often desirable to maintain
a certain constant level of medicaments in the
patient's system instead of periodically injecting a
bolus dosage. In many current iontophoretic
systems, such sustained delivery is not practical
because of the danger of electrical and chemical
burns. U. S. Patent 4,752,285 (Petelenz et al.),
CA 02155107 1999-09-09
WO 94/17853 PCT/~1594/01.;52
-4-
teaches these burns may stem from two
sources, galvanic where the electrical current
itself causes burns, and chemical where extremes in
pH (which develop during the iontophoretic process)
act in conjunction with electric current to cause
chemical burins.
Galvanic burns can be minimized or reduced by
keeping the current density per unit area of skin
below threshold values at which burning begins. Low
1o current densities can be achieved by attention to
design so as to maintain uniform electrode contact
with the skin. Avoidance of folds, wrinkles and
partial contact of the device electrode surf ace with
the patient's skin, all help to eliminate high
curxent density which induces galvanic burns.
U. S. Patent No. 4,883,457 (Sibalis),
teaches a multi-Iayer electrophoretic or
electro-osmotic dispensing device with multiple
reservoirs using conductors from electroconductive
graphite paint. There is no suggestion that these
conductors be active electrodes. The multiple
layers can be fabricated by a mufti-step silk screen
printing or t=ransfer process .
In addition, it has been suggested by the art
that multiple electrodes and highly flexible devices
may be useful in ensuring uniform contact with the
patient's s~:in to avoid galvanic burns. Highly
flexible devices are less likely to partially lift
off the area of the patient's skin where they are
WO 94/17853 PCT/US94/01152
-5-
placed, thus avoiding spots of high current
density. The use of multiple small electrodes each
with its own reservoir allow each electrode area to
be maintained in uniform contact with the placement
area on the patient's skin.
While galvanic burns may be substantially
controlled by the device design the control of pH
and the resulting burns caused by extremes in the
alkalinity or acidity of the medicament solution
1o during passage of electric current requires an
understanding of the electron transfer processes
which occur during active transdermal delivery. As
the current passes between the electrode and the
medium containing medicament, at a voltage greater
15 than 1.23 relative to the standard hydrogen
electrode (SHE) at the positive pole, the voltage
necessary for electrolysis of water, there is
increased production of hydrogen ions (H+) or at
the negative pole, 0.83 volts (SHE) for the
2o Production of hydroxide ions (OH ). When the
iontophoresis electrode is nonreactive, an increase
in H+ and OH ion concentration is caused by the
exchange of charge through the electrolysis of water.
Since the H+ and OH ions which result from
25 the electrolysis of water are extremely mobile, they
migrate rapidly through the electrolyte solution
away from the electrode and toward the skin of the
patient. Thus, an area of extreme pH is ultimately
created directly adjacent to the skin. This area of
3o extreme pH is clearly undesirable and serious burns
have been observed when these ions are actively
transported~~through the skin.
WO 94/17853 PCT/US94/01152
. '
-6-
The problem of electrolysis of water with its
concomitant generation of H+ and OH ions can be
addressed by keeping the electrode potential below
1.23 volts relative to (SHE), or by the use of an
electrode which is capable of reacting with the
complementary ion of the medicament to form an
insoluble precipitate at a voltage potential below
the voltage potential for the electrolysis of
water. The incorporation of a reactive electrode
to system into an iontophoretic device substantially
eliminates the generation of H+ and OH ions,
thus avoiding attack of the skin while maintaining
the medicament at a desired pH. The use of an
active electrode system with a counter ion which
forms a precipitate with the electrode ion further
precludes competition for transport between the
counter ion and the medicament. A electrode formed
from silver in conjunction with a chloride counter
ion for the medicament fulfills the requirements for
2o a reactive electrode system. While silver has been
shown to perform satisfactorily in an iontophoretic
system, cost constrains its use in commercial
devices.
The problem of burning a patient's skin during
iontophoresis thus can be substantially reduced by
ensuring intimate and uniform contact between an
iontophoretic delivery device and the skin and
incorporation of a reactive electrode into the ,
device.
As ~ described above, fulfillment of the
requirement for intimate and uniform contact is
WO 94/17853 PCT/US94/01152
facilitated by highly flexible devices and multiple
electrodes. Nowhere in the art has there been a
recognition that these design features could be
coupled with a reactive electrode such as silver,
copper or molybdenum thereby providing a significant
improvement in the art of iontophoresis. Further,
there has also been a need for a way to couple these
design features with the ability to readily
manufacture the device, while providing flexibility
and minimizing the amount of silver used to improve
the feasibility of widespread use of iontophoresis,
thereby enhancing benefits to patients. Such
methods and apparatus are disclosed and claimed
herein.
S'~ OF THE INVENTION
In contrast to the prior devices discussed
above, it has been found that an iontophoretic
device particularly suitable for providing uniform
contact with the skin, substantially eliminating
burning of patient's skin while improving delivery
efficiency by greatly reducing competitive ion
transport can be constituted in accordance with the
present invention.
The invention includes an iontophoretic
delivery device for delivering at least one
medicament to an applied area of a patient. The
device includes an electrode assembly for driving a
medicament for absorption into the applied area to
be absorbed by a patient's body. The electrode
WO 94/17853 PCT/US94/01152
_g_
assembly includes a flexible backing member. The
backing member has an inside surface and an outside
surface. The inside surface has a first area which
has at least one flexible active electrode of a
preselected pattern bonded to it, while a second
area of the inside surface of the flexible backing
member remains exposed.
The iontophoretic delivery device further
includes at least one reservoir for containing the
l0 medicament to be delivered situated in electrically
conductive relation to the flexible electrode
assembly. The device has a holder for holding the
electrode assembly. The holder also maintains
electrical communication between the electrode
assembly and the reservoir.
A flexible active electrode assembly for an
active drug delivery device of the present invention
includes a flexible backing member with an inside
surf ace and an outside surface. The flexible
backing member has an active metal member with a
preselected pattern bonded to a first area of the
backing member leaving a second area of the backing
member exposed. The invention further includes a
method for making a flexible active electrode for an
active drug delivery device. The method includes
providing a flexible backing member with an inside
surface and an outside surface. The inside surface
has two areas, a first area and a second area. An
electroconductive material is applied in a
Preselected pattern to the first area of the inside
surface while leaving the second area of the inside
WO 94/17853 PCT/US94/01152
-g_
' surface of the backing member exposed.
The flexible active electrode assembly may be
formed from at least one electrically conductive ink
applied to the backing member or alternatively from
a multi-layer active electrode including a layer of
silver applied to an inert ink circuit rendered
conductive by the inclusion. of graphite or an
intermediate metal conductive circuit layer bonded
to the surface of the backing member. Alternative-
l0 ly. the electroconductive ink may form the active
electrode directly by including a first ink
containing a metal selected from the group including
silver, copper and molybdenum and a second electro-
conductive ink containing an insoluble halide salt
of the metal. Alternatively, an active electrode
can be formed from an ink rendered conductive by
inclusion of graphite which incorporates a metal
selected from the group including silver, copper and
molybdenum. The active electrode may further
include an insoluble halide salt of the metal
selected.
Alternative embodiments may include a plurality
of reservoirs, with a plurality of active elec-
trodes. The active electrode assembly may include a
system for activating the plurality of electrodes
independently.
An alternative method of assembly may include
the backing member as a portion of a continuous web,
with a plurality of individual electrodes being
3o formed onto the web, a cutting step may be used to
WO 94/17853 PCT/US94/01152
,
-10- ,
release the individual electrodes from the web for
subsequent assembly into devices. A plurality of
iontophoretic devices may be formed on the web by '
adding reservoirs and holders to a plurality of the
flexible active electrode assemblies formed on
portions of the continuous web, a cutting step then
being used to release the devices from the web.
BRIEF DESCRIPTION OF THE DRAWINGS
The various features, objects, benefits, and
l0 advantages of the present invention will become more
apparent upon reading the following detailed
description of the preferred embodiments along with
the appended claims in conjunction with the
drawings, wherein like reference numerals identify
corresponding components, and:
Fig. 1 is a cross-sectional view of a preferred
embodiment of an iontophoretic device of the present
invention having a flexible active electrode;
Fig . 2 is a top plan view of the flexible electrode
of the present invention;
Figs. 3A-3E are a sequence of perspective views of
stages in a preferred method for manufacture of a
flexible electrode of the present invention;
Figs. 4A and 4B are enlarged fragmentary,
cross-sectional views of a flexible electrode of
preferred embodiments of the present invention
having a plurality of active metal members wherein
the members are formed as (4A) a layered structure
and (4B) as an electrically conductive ink;
WO 94/17853 PCT/LTS94/01152
-11-
Fig. 4C is a top plan view of a flexible electrode
of the present invention having a plurality of
electrodes and electrode circuits; and
Fig. 5 is a top plan view of a plurality of the
flexible electrodes of the present invention formed
with a flexible backing member as a continuous web.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The active drug delivery device of the present
invention is illustrated in Figs. 1-5 and is
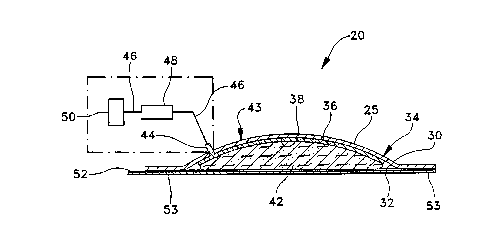
l0 generally designated as 20. Referring to Figs. 1
and 2, the active drug delivery device of the
present invention, preferably an iontophoretic
device 20, includes a flexible active electrode
assembly 25 and at least one reservoir 42 for
containing a medicament.
The electrode assembly 25 includes a flexible
backing member 30 with an inside surface 32 and an
outside surface 34. The electrode assembly further
includes an active metal member 36 bonded to a first
area 38 of inside surface 32 in a preselected
pattern 40 leaving a second area 41 of surface 32
exposed.
Reservoir 42 is held in electrically conductive
relation to the electrode assembly by a container or
holder 43 which also serves to hold the flexible
electrode assembly and maintain the electrode
assembly and reservoir in electrical communication.
The holder may include a connector 44 and conductors
WO 94/1853 PCT/US94/01152
-12-
46. Device 20 may further include a driver assembly
48 and a counter electrode 50 connected by con=
ductors 46 to complete the electrical circuit.
Device 20 may further include a protective
release covering 52 held to the device by a layer of
adhesive 53. The layer of adhesive 53 may also
serve for mounting the device to an area of a
patient to which the medicament is to be applied.
The preferred device 20 may have flexible
l0 backing member 30 serve as the backing member for
the reservoir, with inside surface 32 .also~serving
as the inside surface of reservoir 42 and outside
surface 34 serving as part of holder 43 for the
device.
Materials suitable for use as flexible backing
member 30 include, but are not limited to, flexible
films formed from polyethylene, polyethylene
terephthalate, polyvinyl chloride and polyvinylidene
fluoride. The particular material is not essential
to the present invention as long as it provides
flexibility, sufficient support for the active metal
member and is compatible with the medicament
system.
The method of making the electrode assembly of
the present invention is generally shown in Figs. 3A
through 3E. The active electrode assembly 25 may be
prepared with active metal member 36 bonded to
inside surface 32 of flexible backing member 30. A
flexible conductive metal layer, preferrably a
WO 94/17853 PCT/US94/01152
-13-
flexible foil, 6o forms an electrode circuit 61
which then has a layer of metallic silver 62 bonded'
thereto to form the active metal member 36, with
conductive foil 60 thereby intermediate silver layer
62 and backing member 36. An alternative for
formation of active metal member 36 would be to form
electrode circuit 61 from an ink rendered conductive
by the incorporation of graphite and the like, then
bonding metallic silver as a layer thereto.
l0 In a preferred embodiment of this method, as
shown in Figs. 3A and 3B, forming the preferred
active metal electrode assembly includes bonding a
layer of conductive metallic foil 60, preferrably a
layer of copper, to inside surface 32 of flexible
15 backing member 30. Preselected pattern 40, a
photoresist or the like, as shown in Fig. 3C, is
then formed on a surface 63 of foil 60. One skilled
in the art will appreciate that the preselected
pattern may be simple, as shown in Fig. 2, or
20 complex, with multiple _segments and the like. A
portion of foil 60 is removed by, for example.
etching the surface, with a portion 64 of the
preferred foil not included in pattern 40 being
removed, thereby forming electrode circuit 61 and
25 exposing second area 41 of surface 32 (Fig. 3D).
The etch process may be an acidic dissolution
process, an electrochemical enhanced milling
process, an ion milling, a laser milling process and
the like for removal of portion 64 of the foil to
30 leave electrode circuit 61 as shown in Fig. 3D. The
particular process is not material to the present
invention and is merely a matter of choice.
WO 94/17853 PCT/US94/01152
-14-
In a preferred embodiment, metallic silver
layer 62 is then bonded to at least a portion of the
electrode circuit 61 forming an active metal member
36, as shown in Fig. 3E. Metallic silver layer 62
may be applied by an electroplating process, vacuum
sputtering or any other method which results in a
uniform layer of metallic silver being formed over
the metallic foil electrode circuit. The preferred
embodiment of the instant invention of forming a
layer of metallic silver over the metallic foil
electrode circuit instead of simply using silver for
the entire electrode circuit serves to minimize the
amount of the more expensive silver while maximizing
the surface contact area of the silver with the
reservoir, thereby enhancing the transfer of silver
ions to the counter ion. In addition, by applying
the silver after etching the foil to form the
electrode circuit, waste of the silver is also
further eliminated. In addition, it should be
appreciated that the amount of silver applied can
vary depending upon the intended application. For
example, a thicker layer of silver may be applied
when the device is to be used for an extended period
of time for delivery of larger quantities of
medicament. Further, areas of an electrode circuit
external to the reservoir may be left free of silver
by masking during the silver application. The
metallic silver portion may be treated to form w
silver chloride on its surf ace. A preferred method
~of treatment to form the silver chloride would be to
treat the silver layer with aqueous hydrochloric
acid while applying an electric current to' the
electrode.
WO 94/17853 PCT/US94/01152
-15-
The preferred embodiment shown in Fig. 2 may
have active metal member 36 formed from an
electrically conductive ink containing a metal
selected from the group including silver, copper and
molybdenum, alh of which form insoluble halide
salts, with silver being preferred. The active
metal member 36 may be formed from at least one
electrically conductive ink applied in preselected
pattern 40 on inside surface 32 (Figs. 3A and 3D).
to The electroconductive ink may further include
graphite and an insoluble halide salt of the
selected metal. In addition, to provide a counter
electrode, a second electroconductive ink containing
an insoluble halogen salt of the metal may be
applied in a preselected pattern. A more preferred
embodiment of the ink includes metallic silver with
silver chloride. The techniques for application of
ink 54 may include, but are not limited to,
impression, lithography, offset, gravure, jet
2o application, silkscreen and the like. One skilled
in the art of application of electroconductive inks
will recognize that the electroconductive inks
containing one of the metals selected from the group
silver, copper and molybdenum with or without the
insoluble halide salt of the selected metal may be
applied so as to maximize the surface exposure of
the included metal and halide, thereby ensuring
maximum utilization of the metal and function of the
electrode. The aforementioned ink application
3o techniques may easily be utilized to apply
preselected patterns of more than one electro-
conductive ink for more than one circuit to a
device, analogous to multicolor printing processes.
WO 94/17853 PCT/US94/01152
. ..
~:~5~ ~.~~
-16-
The active electrode embodiment with a silver
anode is preferred for cationic drugs having a
chloride anion because silver chloride is very
insoluble in aqueous systems and further, many
cationic drugs are readily available and stable as
the hydrochloride salts. An iontophoretic delivery
of such a drug with a silver anode, i.e., an active
silver electrode, will result in the oxidation of
silver to silver ion at the anode surface. The
silver ion will react with the available chloride
ion from the reservoir and precipitate near the
electrode. Consequently, the drug cation will
migrate from the reservoir into the patient with
greater efficiency than in the case where the drug
cation had to compete with the silver cation. This
reaction of silver ion with chloride ion is enhanced
when silver chloride is incorporated into the
electrode as described above. Further because
production of H+ ion is substantially eliminated,
pH changes are minimized in the reservoir.
Figs. 4A and 4B show enlarged cross sections of
the preferred embodiments of active metal member 36
bonded to inside surface 32 of flexible backing
member 30 in preselected pattern 40 leaving second
Portion 41 of inside surface 32 exposed. Fig. 4A
shows the layered embodiment of active metal member
36, i.e. where the active metal, preferably silver, ,
is layered over the electrode circuit formed from a
bonded metal layer or from inert electroconductive
ink as is shown in Fig. 3D. Fig. 4B shows the
embodiment wherein the active metal member is
WO 94/17853 PCT/US94/01152
2~~~~0~
' electroconductive ink which directly incorporates
the active metal and/or the insoluble chloride salt
of the metal.
As shown in Fig. 4C, any of the preferred
embodiments of active metal member 36 may be used to
provide a plurality of flexible electrodes 36 which
may be formed on a single flexible backing member
30. The plurality of electrodes 36 may be attached
to either a first conductor 66 or a second conductor
l0 68. This arrangement of electrodes on two
conductors allows the groups of electrodes to be
activated independently or simultaneously. This
concept may be expanded to a plurality of conductors
coupled with a plurality of electrodes to allow
electrodes to be activated independently or together
by the electrode assembly driver. Alternatively,
this ability to form independent electrodes
separately in a preselected pattern allows for the
formation of a first active electrode circuit
containing a metal selected from the group including
silver, copper and molybdenum and a second active
electrode circuit containing an insoluble halide
salt of the metal. The first electrode may include
both the active metal and an insoluble halide salt
of the active metal. In addition, by allowing for a
plurality of electrodes on flexible backing member
30, the instant invention allows more than one
medicament to be contained in a plurality of
reservoirs associated with the plurality of
electrodes. The ability to replicate the electrodes
allows a design for the device to provide optimum
flexibility to conform to the application site on
WO 94/17853 PCT/US94101152
-18-
the patient thereby minimizing galvanic type burns '
caused by partial contact, creases and folds.
As shown in Fig. 5, the simple and easily
repeated construction of the flexible electrode of
the instant invention lends itself to an assembly
line process. For example, flexible backing member
30 may be a continuous web 31 and a plurality of
flexible electrode assemblies 25 may be applied to
the web. Each device may be formed on a portion of
to the web, to be further assembled into iontophoretic
devices. The finished devices could then be
released from the web in a .final cutting step. An
alternative to the completion of an iontophoretic
device on the web would be completion of a plurality
of flexible electrode assemblies, each on a portion
of the web. The individual electrodes would be
released from the web by a cutting step followed by
subsequent assembly steps to form completed devices.
Thus, while preferred embodiments of the
present invention have been described so as to
enable one skilled in the art to practice the
device, electrode and method of the present
invention, it is to be understood that variations
and modifications may be employed without departing
from the concept and intent of the present invention
as defined in the following claims. Accordingly,
the preceding description is intended to be ,
exemplary and should not be used to limit the scope
of the invention. The scope of the invention should .
3o be determined only by reference to the following
claims.