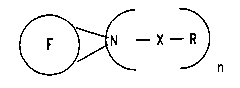Note: Descriptions are shown in the official language in which they were submitted.
21ss~273
HOECHST AKTIENGESELLSCHAFT HOE 94/F 221R Dr. DR/wo
Description
Azafullerene derivatives, processes for their preparation
and their use
Fullerene~ are cage-like carbon allotropes of the formula
(C20~2m), where m is a natural number. They contain twelve
five-membered rings and any number of, but at least two,
six-membered rings of carbon atoms. Although this class
of compound was discovered only in 1985 by Kroto and
Smalley (Nature, 1985, 318, 162) and Kratschmer and
Huffman only reported the preparation of macroscopic
amounts of C60 in 1990 (Nature 1990, 347, 354), such
compounds have very quickly attracted wide interest and
within a very ~hort time have become the ~ubject of
numerous re~earch studies (see, for example,
G.S. H G ond, V.J. Kuck (Editors), Fullerenes, American
Chemical Society, W~h;ngton DC 1992 and Accounts of
Chemical Research, March edition 1992).
Since a high potential i~ expected of this class of
substances, for example in the field of optoelectronics
and research on active compounds, comprehensive studies
have already been carried out on targeted formation of
derivative~, in particular C60 and C70 (see, for example,
R. Taylor, D.R.M. Walton, Nature 1993, 363, 685 and
A. Hir~ch, Angew. Chem. 1993, 105, 1189). In variou~
experiment~ on derivative formation, it was po~ible to
i~olate defined - o~nct~ of C60.
Cyclopropane derivatives were obt~;ne~, for example, by
reaction of fullerenes in 1,3 dipolar cycloadditions with
diazomethane derivatives with elimination of nitrogen
(see, for example, F. Wudl et al., Acc. Chem. Res. 1992,
25, 157 and F. Diederich et al., Helv. Chem. Acta 1993,
76, 1231), in [2+1~ carbene additions using nucleophilic
glycosylidene carbenes (see, for example, A. Vasella
21 SS2 73
_ - 2 -
et al., Angew. Chem. 1992, 104, 1383) and by reaction
with stabilized ~-halocarbanions (e.g. C. Bingel, Chem.
Ber. 1993, 126, 1957).
First examples of the nitrogen heterocycles correspo~;ng
S to the cyclopropane derivatives have been described.
Thus, 5-6 ring azafulleroids are obtained in the therm-
ally activated reaction of C60 with benzylazides and
alkoxymethylenazides (M. Prato, Q. Chan Li, F. Wudl,
J. Am. Chem. Soc. 1993, 115, 1148). 6-6-ring fulleren-
aziridines having a carbamate structure have beenprepared by the thermally activated reaction of C60 with
azidoformic esters (T. Ishida, R. Tanaka, T. Nogami,
Chem. Lett. 1994, 561; N.R. Banks et al., J. Chem. Soc.,
Chem. Commun. 1994, 1365).
While Ishida et al. recover the 6-6-ring fullerenazirid-
ine derivative having a carbamate structure and prepared
by them after 8 hours in boiling ortho-dichlorobenzene
without changes, Banks et al. describe that the 6-6-ring
fullerenaziridine having a carbamate structure studied by
them is isomerized by refluxing in tetrachloroethane to
give a fulleren-4,5-oxazole.
It is an object of the present invention to synthesize
defined fullerene derivatives cont~;n;ng structural units
having those functional groups which, on the one hand,
improve the physical properties such as the solubility
and polarity and, on the other hand, make possible
further chemical reactions to give new fullerene deri-
vatives.
It has surprisingly been found that fullerenaziridines
having an acid amide function can be prepared, for
example, from fullerene and acylnitrenes, and that the
reaction of N-haloacid-amide anions with fullerenes like-
wise gives fullerenaziridines.
The invention provides a fullerene derivative having an
2ls5273
_ - 3 -
acid amide function of the formula I
X--~ ( I )
where the ~ymbols and indices have the following
meaningQ:
F: a fullerene radical of the formula C20~2~ where m = 2
to 100;
X: C=O, SO2, SO, P(O)R, P(S)R or PR;
R: a straight-chain or branched Cl-C20-alkyl group, a
C6-Cl4-aryl-C1-Cl0-alkyl group or a C6-Cl4-aryl group,
where in the alkyl group one or more non-adjacent
CH2 group~ can be replaced by -C_C-, -CH=CH-, -O-,
-S-, -COO-, -SiR2- and/or -CO-, alkyl and aryl can,
independently of one another, be monosubstituted or
polysubstituted by identical or different groups OH,
ORl, COOR, OCOR, F, Cl, Br, NO2, CN, NHCORl or NRlCORl
and aryl can be substituted by Rl where Rl i~ a
Cl- C6 - alkyl group;
n: a natural number from 1 to 10+m where m = 2 to 100.
Preference iR given to compound~ of the formula I in
which the symbol~ and indice~ have the following
me~n;ng~
F: a fullerene radical of the formula C20~2~ where
m = 20, 25, 28 and/or 29;
X: C=O ~ S2 or SO;
n: a natural number from 1 to 10+m where m = 20, 25, 28
and/or 29 and R is as defined above.
Particular preference i8 given to compounds of the
formula I in which the RymbolR and indices have the
following me~n;ngs
F: C60 and/or C70;
X: C=O or S2;
R: a straight-chain or branched Cl-Cl0-alkyl group, a
21 552 73
- 4 -
phenyl-C1-Cc-alkyl group or a phenyl group, where
alkyl and phenyl can be, independently of one
another, monosubstituted to trisubstituted by iden-
tical or different groups OH, OR1, COOR, OCOR, F,
Cl, Br, NO2, CN, NHCOR1 or NR1COR1, and phenyl can be
substituted by R1 where R1 i8 a C1-C6-alkyl group;
n: a natural number from 1 to 6.
Very particular preference iB given to compounds of the
formula I in which the symbols and indices have the
following me~n;n~s:
F: C60;
X: C=O or S2;
R: a straight-chain or branched C1-C6-alkyl group or a
phenyl group, where alkyl and phenyl can be,
independently of one another, monosubstituted to
trisubstituted by identical or different groups OH,
oR2, COOR2, OCOR2, F, Cl, Br, NO2 or CN and phenyl
can be substituted by R2 where R2 is methyl or ethyl;
n: 1 or 2.
The formation of aziridines from olefins using various
reagents, such as the direct nitrene addition to olefins
or the 1,3-dipolar cycloaddition of azides to olefins,
followed by decomposition of the intermediate triazole,
is known (J.E.G. Remp, Comprehensive Organic Synthesis,
Vol. 7, Editor: B.M. Trost, I. Fleming, Pergamon Press,
Oxford, 1991, 469).
The invention further provides two processe~ for prepar-
ing fullerene derivatives of the formula I.
In the first process, a fullerene of the formula C20+2~,
where m = 2 to 100, is reacted with an acid azide of the
formula II,
R-X-N3 (II),
where R and X are a~ defined above, in an inert solvent
21 552 73
at a temperature of from -78C to 180C to give a com-
pound of the formula I.
The reaction i~ here preferably carried out in the
pre~ence of a light ~ource which emit~ light having a
wavelength of from 230 to 380 nm, preferably from 290 to
310 nm.
The 6-6-ring fullerenaziridine derivativeR are prepared,
for example, in a chlorinated solvent. Condition~ which
have been found to be advantageous are, for example,
1,1,2,2-tetrachloroethane and temperature~ of up to 40C.
The reaction in 1,1,2,2-tetrachloroethane gives higher
yieldQ than in methylene chloride. In contrast, for
example, when benzene i~ u~ed as nolvent under the
photochemical conditions ~mall amounts of the 5-6-ring
azafulleroids are formed.
In the ~econd proce~, fullerene of the formula C20~2m,
where m = 2 to 100, i6 reacted with a ntabilized nitrogen
anion of the formula III
R-X-Né (III),
cation
where R and X are an defined above,
Y i8 Cl, Br or I and
cation in alkali metal, tetraalkyl(aryl)ammonium,
tetraalkyl(aryl)phonphonium or heY~lkyl(aryl)-
guanidinium,
in an aprotic ~olvent such a~, for example, toluene,
chlorobenzene or CHaCl~ at a temperature of from -78C to
180C, preferably at from 0C to 110C, particularly
preferably at from 20C to 30C, to give a compound of
the formula I.
Preferred starting compound~ uned .in the preparation of
the compound~ of the formula I are fullerene~ of the
_ - 6 - 21 5~2 73
formula C20,2~ where m = 20, 25, 28 and/or 29, particularly
preferably where m = 20 and/or 25.
The fullerenes can be obtained as crude fullerene by
preparation of fullerene black in an electric arc process
with subsequent extraction using a nonpolar organic
solvent, as described in WO 92/09279. The further fine
separation can be carried out by column chromatography.
Some of the fullerenes used are also commercial products.
However, it is also possible to use any fullerene deriva-
tives such as, for example, monoadducts or polyadductswhich are obt~;n~hle by derivatization of fullerenes
(see, for example, R. Tayler, D.R.M. Walton, Nature 1993,
363, 685 and A. Hirsch, Angew. Chem. 1993, 105, 1189) and
which are stable and inert under the reaction conditions
described here.
The compounds of the formula II are known. They are
synthesized, for example, from the correspo~;ng acid
chloride and an azide:
- Organikum, 16th revised edition, 1986, p. 702
- Houben-Weyl, Methoden der Organischen Chemie IX,
Schwefel-, Selen-, Tellur-Verb;n~-~ngen, p. 653.
Some of the compounds of the formula III are commercial
products, or they can be prepared by known methods:
- Organikum, 16th revised edition, 1986, p. 700
Furthermore, thermal isomerization of the 6-6-ring
fullerenaziridines of the invention having the formula I
gives, inter alia, 6-6-ring fuller~noY~7oles, for example
by refluxing for a number of hours in 1,1,2,2-tetra-
chloroethane or simply by heating the solid for a number
of hours, preferably in the range from 140 to 200C.
The present invention accordingly further provides a
fullerene derivative of the formula IV
2l5s273
-- 7
~ . Z2 n ( I V )
where the symbols and indices have the following
me~ning8
F: a fullerene radical of the formula C20~2m where m = 2
to 100;
Z1: C, S, S=O or P;
Z2 or S;
R: a straight-chain or branched Cl-C20-alkyl group, a
C6-C14-aryl-C1-C10-alkyl group or a C6-C1~-aryl group,
where in the alkyl group one or more non-adjacent
CH2 groups can be replaced by -C=C-, -CH=CH-, -O-,
-S-, -COO-, -SiR2- and/or -CO-, alkyl and aryl can,
independently of one another, be monosubstituted or
polysubstituted by identical or different groups OH,
OR1, COOR, OCOR, F, Cl, Br, NO2, CN, NHCOR1 or NR1COR1
and aryl can be substituted by R1 where R1 is a
Cl - C6 - alkyl group;
n: a natural number from 1 to 10+m where m = 2 to 100.
Preference i8 given to compounds of the formula IV in
which the symbols and indices ha~e the following
meanings:
F: a fullerene radical of the formula C20~2~ where
m = 20, 25, 28 and/or 29;
Z1: C or S=O;
Z2: ;
n: a natural number from 1 to 10+m where m = 20, 25, 28
and/or 2~ and R is as defined above.
Particular preference is given to compounds of the
formula IV in which the symbols and indices ha~e the
following me~n;ngs:
F: C60 and/or C70;
Zl: C;
_ 21 SS2 73
-- 8
Z2: ;
R: a straight-chain or branched Cl-C10-alkyl group, a
phenyl-Cl-C6-alkyl group or a phenyl group, where
alkyl and phenyl can be, independently of one
another, monosubstituted to trisubstituted by iden-
tical or different groups OH, ORl, COOR, OCOR, F,
Cl, Br, N02, CN, NHCOR1 or NRlCORl, and phenyl can be
substituted by Rl where Rl is a Cl-C6-alkyl group;
n: a natural number from 1 to 6.
Very particular preference is given to compounds of the
formula IV in which the ~ymbols and indices have the
following me~n;ngs:
F: C60;
Zl: C;
Z2: ;
R: a straight-chain or branched Cl-C6-alkyl group or a
phenyl group, where alkyl and phenyl can be,
independently of one another, monosubstituted to
trisubstituted by identical or different groups OH,
oR2, COOR2, OCOR2, F, Cl, Br, N02 or CN and phenyl
can be substituted by R2 where R2 is methyl or ethyl;
n: 1 or 2.
The compounds of the invention having the formulae I and
IV are used, for example, in optoelectric components. The
invention is illustrated by the examples.
Example 1
162 mg (1.10 mmol) of benzoyl azide were added to a
solution of 145 mg (0.20 mmol) of C60 in 600 ml of
dichloromethane. The solution was placed in Pyrex glass
tubes, flushed with argon and irradiated at Ay~ = 300 nm
for 60 minutes in an RPR 100 Rayonet Photochemical
Chamber Reactor using RPR 3000 A lamps. During this
procedure, the color of the solution changed from violet
to brownish red. The solvent was removed by distillation
in vacuo at 40C. Unreacted C60 was separated off by
2155273
column chromatography (200 g of Merck silica gel 60,
particle eize from 63 to 200 ~m; n-hexane), and a wine-
red product fraction was sub~equently eluted with
toluene/n-hexane 50:50. After removing the solvent, 30 mg
(18%) of the 6,6-bridged compound of the formula
were obtained as a microcrystalline black-gray solid.
MS m/e (%)
DEI (70 kV): 839 (6; MH~), 838 (10; M'-H), 721 (46),
720 (100; M~-C7H5NO).
lH-NMR (360 MHz; CS2/acetone-d6 10:1)
~: 8.48 (m, 2H), 7.73 (m, lH), 7.64 (m, 2H) ppm.
3C-NMR (90.5 MHz; CS2/acetone-d6 10:1)
~: 169.77, 145.71, 145.61, 145.39, 145.30, 145.08,
145.04, 144.61, 144.32, 144.29, 143.66, 143.63,
143.33, 142.72, 142.64, 141.63, 140.60, 134.40,
131.51, 129.83, 129.72, 81.62 ppm.
FT-IR (KBr, v in cm~l)
1700 (8, C=O), 1448 (w), 1427 (m, TlUC60)~ 1395 (m),
1316 (w), 1264 (8), 1254 (8), 1182 (m, TlUC60), 1043 (m),
1021 (m), 692 (8), 576 (m, TlUC60)~ 527 (88~ TluC60)
US-Vis ~ in nm (~ in 1 mol~l cm~l)
n-Hexane: (c = 4.3 10-5): 208 (60000), 255 (42000),
322 (11000), 420 (400-500).
Toluene: 330, 425, 495.
2lss273
- 10 -
Example 2
The monoadduct characterized in Example 1 was also
prepared as follows.
A solution of 288 mg (0.40 mmol) of C60 and 252 mg of
benzoyl azide in 80 ml of 1,1,2,2-tetrachloroethane was
irradiated for 7.5 hours using a method similar to
Example 1. After precipitation of the fullerene compounds
with acetonitrile, isolation and w-~h;ng of the same with
acetonitrile, the residue was extracted with toluene and
the extract was evaporated under reduced pressure. The
residue was chromatographed on silica gel (particle size
from 63 to 200 ~m) using n-hexane and n-hexane/toluene
3:2 to 1:1. The first fraction contained 63 mg (22%) of
C60, the second fraction contained 133 mg (40%; 51~ based
on C60 reacted) of the monoadduct characterized in Example
1 and the third fraction contained 55 mg of polyadducts.
Example 3
197 mg (0.87 mmol) of p-bromobenzoyl azide were added to
a solution of 144 mg (0.20 mmol) of C60 in 600 ml of
dichloromethane. The solution was placed in Pyrex glass
tubes, flushed with argon and irradiated at A~ = 300 nm
for 60 minutes in an RPR 100 Rayonet Photochemical
Chamber Reactor using RPR 3000 A lamps. During this
procedure, the color of the eolution changed from violet
to brownish red. The solvent was removed by distillation
in vacuo at 40C. Unreacted C60 was separated off by
column chromatography (200 g of Merck silica gel 60,
particle size from 63 to 200 ~m; n-hexane), and a wine-
red product fraction was subsequently eluted with
toluene/n-h~YAne 50:50. The product fraction was
evaporated to 5 ml, the adduct was precipitated with 30
ml of acetonitrile and filtered off using an ultrafilter.
After drying in vacuo, 20 mg (11%) of the 6,6-bridged
compound of the formula
21 SS2 73
11
~ ;\l _ r ~\r ~ r
were obtained as a microcrystalline black-gray solid.
MS m/e 2 400 (%)
DEI (70 kV): 919 (5; MH~), 917 (3; MH~), 721 (73),
720 (100; M~-C7H4NOBr)
1H-NMR (300 MHz, CS2/acetone-d6 10:1)
~: 8.40 (m, 2H), 7.83 (m, 2H) ppm.
3C-NMR (90.5 MHz; CS2/acetone-d6 10:1)
~: 169.29, 145.80, 145.70, 145.50, 145.40, 144.63,
144.35, 144.08, 143.73, 143.71, 143.40, 142.77,
142.65, 141.72, 140.67, 133.18, 132.92, 131.80,
131.33, 81.48 ppm.
W-Vis A~ in nm (~ in 1 mol~l cm~l)
n-Hexane: (c = 6.5 10-5): 211 (48000), 255 (43000),
318 (13000), 421 (1300),
Toluene: 326, 425, 371
Example 4
The monoadduct characterized in Example 3 was also
prepared as follows.
A solution of 293 mg (0.41 mmol) of C60 and 368 mg
(1.63 mmol) of 4-bromobenzoyl azide in 80 ml of 1,1,2,2-
tetrachloroethane was irradiated for 5 hours using a
method similar to Example 3. After precipitation of the
fullerene compounds with 600 ml of acetonitrile, the
solid was isolated by filtration and washed with aceto-
nitrile. The residue was chromatographed on silica gel
215527~
- 12 -
(particle ~ize from 63 to 200 ~m) using n-hexane and n-
hexane/toluene 3: 2 to 1:1. The fir~t fraction contained
154 mg (52%) of C60, the second fraction contained 78 mg
(21%; 44% ba~ed on C60 reacted) of the monoadduct charac-
5 terized in Example 3 and the third fraction contained47 mg of diadduct.
Example 5
154 mg (1. 03 mmol) of p-methoxybenzoyl azide were added
to a solution of 148 mg (0.21 mmol) of C60 in 600 ml of
dichloromethane. The solution was placed in Pyrex glass
tubes, flu~hed with argon and irradiated at A~ = 300 nm
for 60 minute~ in an RPR 100 Rayonet Photochemical
Chamber Reactor using RPR 3000 A lamps. During this
procedure, the color of the solution changed from violet
to brownish red. The solvent was removed by distillation
in vacuo at 40 C and the reaction mixture was separated
by colllmn chromatography (200 g of Merck silica gel 60,
particle size from 63 to 200 ~m; toluene/n-hexane 50:50).
After removing the solvent, 19 mg (11%) of the 6,6-
20 bridged compound of the formula
~ C ~CMe
were obtained a~ a microcrystalline, black-gray solid.
MS m/e 2 400 (%)
DEI (70 kV): 869 (24; MH~), 722 (57), 721 (88), 720
(100; M -C8H7NO2)
lH-NMR (360 MHz; CS~/acetone-d6 10:1)
~: 8.44 (m, 2H), 7.13 (m, 2H), 3.96 (8, 3H) ppm.
21 ~52 7~
_
- 13 -
3C-NMR (90.5 MHz; CS2/acetone-d6 10:1)
~: 169.34, 145.85, 145.77, 145.68, 145.42, 145.36,
145.21, 145.11, 144.72, 144.67, 144.37, 143.71,
143.69, 143.41, 142.80, 142.75, 141.67, 140.66,
132.02, 123.76, 115.39, 81.82, 55.89 ppm.
FT-IR (RBr, v in cm-l)
1693 (8, C=O), 1602 (B), 1508 (m), 1459 (w), 1428
(m, T1UC60)~ 1396 (m), 1316 (w), 1265 (m), 1254 (8), 1182
(m, T1UC60)~ 1164 (8), 1092 (w), 1043 (m), 1026 (m), 843
(m), 793(m), 727 (m), 694 (m), 576 (m, T1uC60)~ 527 (88~
TluC60) -
W-Vis A~ in nm (~ in 1 mol~l cm~l)
n-Hexane: (c = 6.5 10-5): 211 (64000), 257 (54000),
318 (15000), 421 (1200).
Toluene: 330, 425, 493
Example 6
The monoadduct characterized in Example 5 was also
prepared as follows.
A solution of 434 mg (0.60 mmol) of C60 and 428 mg
(2.42 mmol) of 4-methoxybenzoyl azide in 120 ml of
1,1,2,2-tetrachloroethane was irradiated for 5 hours
using a method similar to Example 5 and worked up by a
method similar to Example 4. The residue was chromato-
graphed on silica gel (particle size from 63 to 200 ~m)
using n-hexane and n-hexane/toluene 1:1. The first
fraction contained 202 mg (47%) of C60 and the second
fraction contained 96 mg (18%; 34% based on C60 reacted)
of the monoadduct characterized in Example 5.
Example 7
A solution of 36 mg (0.05 mmol) of C60 and 26 mg
(0.15 mmol) of p-cyanobenzoyl azide in 140 ml of
dichloromethane was placed in Pyrex glass tubes, flushed
with argon and irradiated at A~ = 300 nm for 40 minutes
2155~73
- 14 -
in an RPR 100 Rayonet Photochemical Chamber Reactor using
RPR 3000 A lamps. During this procedure, the color of the
solution changed from violet to brownish red. The solvent
was distilled off in vacuo at 40C and the reaction
mixture was separated by column chromatography (200 g of
Merck silica gel 60, particle size from 63 to 200 ~m;
toluene/n-hexane 50:50). After removing the solvent, 5 mg
(12%) of the 6,6-bridged compound of the formula
I~N--C ~ C N
were obtained as a microcrystalline black-gray solid.
MS m/e (%)
DEI (70 kV): 865 (19), 864 (27; MH~), 720 (100;
M~~CaH4N2O)-
H-NMR (360 MHz; CS2/acetone-d6 10:1)
~ (ppm): 8.65 (m, 2H), 8.03 (m, 2H) ppm.
13C-NMR (90.5 MHz; CS2/acetone-d6 10:1)
~: 168.91, 145.84, 145.75, 145.57, 145.45, 145.13,
144.99, 144.59, 144.36, 143.76 (2x), 143.75, 143.43,
142.78, 142.60, 141.76, 140.69, 134.89, 133.59,
130.31, 118.36, 117.54, 81.32 ppm.
FT-IR (KBr, v in cm~1)
2228w, 1700B, 1696B, 1428m, 1400B, 1317w, 1296w, 1263B8,
1183w, 10938, 10428, 10168, 950w, 862w, 802sh, 79888,
727w, 691m, 578w, 573w, 565w, 555w, 544w, 52688, 525sh.
W-Vis A~ in nm
(Tolue~ne/n-hexane 50:50): 322, 425, 492.
21 S~2 73
-
- 15 -
Example 8
The monoadduct characterized in Example 7 was also
prepared as follows:
A solution of 143 mg (0.20 =ol) of C60 and 137 mg
(0.77 mmol) of 4-cyanobenzoyl azide in 40 ml of 1,1,2,2-
tetrachloroethane was irradiated for 4.5 hours using a
method similar to Example 7 and worked up using a method
similar to Example 2. The extract was chromatographed on
silica gel (particle size from 63 to 200 ~m) using
n-hexane/toluene 2:1 to 1:1. The first fraction contained
52 mg (36%) of C60 and the second fraction contained 47 mg
(27%; 42% based on C60 reacted) of the monoadduct charac-
terized in Example 7.
Example 9
A solution of 33.8 mg (0.04 mmol) of the 6-6-ring
fullerenaziridine from Example 1 in 40 ml of 1,1,2,2-
tetrachloroethane was refluxed for 18 hours with the
initially wine-red solution changing into a brownish red
solution. Addition of acetonitrile gave a precipitate
which was washed with acetonitrile and subsequently
eluted with trichloromethane. After removal of the
solvent, 30.4 mg (90%) of the 6,6-bridged compound of the
formula
~ ~C ~ H
were obtained.
DCl-MS (NH3), m/e (%): 842 (30), 841 (68), 840 (100; MH').
lH-NMR (360 MHz; CS2/acetone-d6 10:1):
21 552 73
- 16 -
= 8.46 (m, 2H), 7.71 (m, lH), 7.67 (m, 2H) ppm.
3C-NMR (90.5 MHz; CS2/acetone-d6 10:1):
= 165.73, 148.76 (2x), 148.35, 146.95, 146.94, 146.81,
146.66, 146.61, 146.31, 146.18, 146.04, 145.80,
145.68, 145.20, 145.16, 144.85, 144.43, 144.30,
143.37, 143.35, 143.26, 142.95, 142.94, 142.86,
142.71, 142.58, 141.01, 140.21, 138.42, 136.73,
133.13, 129.86, 129.44, 127.60, 97.92, 92.93 ppm.
FT-IR (RBr):
16428, 1511m, 1506sh, 1450w, 1326m, 12628, 1181w, 10928,
10268, 9838, 933w, 804br, 773w, 6908, 660w, 576w, 563m,
5278~.
W/Vi~ A~ in nm (~ in 1 mol~l cm~l)
in n-hexane: 212 (60000), 255 (49000), 315 (16000);
in toluene: 322 (28000).
Example 10
A solution of 14 mg (0.015 mmol) of the 6-6-ring
fullerenaziridine from Example 3 in 40 ml of 1,1,2,2-
tetrachloroethane was refluxed for 15.5 hours. After
work-up as in Example 9, 13.6 mg (97%) of the 6,6-bridged
compound of the formula
~;C--~ Br
were obtained as a brown powder.
DCl-MS (NH3), m/e (%): 923 (5), 922 (24), 921 (63),
920 (100; MX'), 919 (75), 918 (70), 722 (14), 721 (63),
720 (100).
21 SS2 73
- 17 -
H-NMR (360 MHz; CS2/acetone-d6 10:1):
= 8.36 (m, 2H), 7.82 (m, 2H) ppm.
3C-NMR (90.5 MHz; CS2/acetone-d6 10:1):
~ = 165.15, 148.79, 148.47, 148.38, 146.98 (2x), 146.85,
146.70, 146.64, 146.35, 146.12, 146.02, 145.83,
145.71, 145.36, 145.16, 144.86, 144.03, 143.41,
143.37, 143.30, 142.95, 142.93, 142.87, 142.73,
142.59, 142.56, 141.05, 140.25, 138.42, 136.75,
132.82, 131.36, 128.47, 126.59, 98.15, 92.88 ppm.
FT-IR (KBr):
1643s, 1632w, 1591w, 1485m, 1398m, 1384w, 1322m, 1261~,
1190w, 1181w, 10898, 10128, 9838, 931m, 798br, 723m,
657w, 603w, 563w, 52788.
W/Vis A~ in nm (~ in 1 mol~l cm~l)
in n-hexane: 210 (63000), 256 (36000), 315 (12000);
in toluene: 320 (31000).
Example 11
A solution of 17.4 mg (0.02 mmol) of the 6-6-ring
fullerenaziridine from Example 5 in 30 ml of 1,1,2,2-
tetrachloroethane was refluxed for 18 hours. Afteraddition of 200 ml of acetonitrile, the mixture was
cooled and subsequently filtered. The filter residue was
eluted with toluene and, after removing the solvent,
16 mg (92%) of the 6,6-bridged compound of the formula
~ ~C ~ OMe
were obtained.
21 5~2 73
- 18 -
DCl-MS (NH3), m/e (%): 872 (9), 871 (24), 871 (51; MH~),
720 (100).
H-NMR (360 MHz; CS2/acetone-d6 10:1):
~ = 8.37 (m, 2H), 7.15 (m, 2H), 3.99 (s, 3H) ppm.
13C-NMR (90.5 MHz; CS2/acetone-d6 10:1):
= 165.41, 163.66, 149.10,148.73, 148.32, 146.92,
146.89, 146.77, 146.63,146.57, 146.25, 146.18,
146.04, 145.76, 145.64,145.43, 145.16, 144.84,
144.57, 143.33, 143.32,143.22, 142.98, 142.91,
142.82, 142.67, 142.58,142.55, 140.96, 140.14,
138.41, 136.64, 131.64,119.75, 114.83, 97.76,
92.95, 55.78 ppm.
FT-IR (RBr):
1639B, 1607m, 1511B~ 1420w, 1320br, 1172m, 1140w, 1088m,
1028m, 983s, 931m, 837m, 727m, 604w, 578w, 563m, 527ss.
W/ViR A~ in nm (~ in 1 mol~l cm~l)
in n-hexane: 210 (83000), 257 (72000), 314 (25000);
in toluene: 322 (33000).
Example 12
A solution of 22.6 mg (0.026 mmol) of the 6-6-ring
fullerenaziridine from Example 7 in 30 ml of 1,1,2,2-
tetrachloroethane was refluxed for 3 hours. After work-up
as in Example 9, 20.5 mg (90%) of the 6,6-bridged com-
pound of the formula
~ ,C~ CN
were obtained as a brown powder.
21 552 73
-
- 19 -
DCl-MS (NH3), m/e (%): 868 (5), 867 (31), 866 (72), 865
(100, MH'), 721 (5), 720 (10).
'H-NMR (360 MHz; CS2/acetone-d6 10:1):
~ = 8.63 (m, 2H), 8.01 (m, 2H) ppm.
l3C-NMR (90.5 MHz; CS2/acetone-d6 10:1):
= 164.64, 148.80, 148.39, 148.04, 147.01, 147.00,
146.87, 146.71, 146.65, 146.39, 146.06, 145.97,
145.84, 145.73, 145.27, 145.13, 144.84, 144.42,
143.43, 143.38, 143.32, 142.95, 142.87 (2x), 142.73,
142.59, 142.50, 141.08, 140.28, 138.39, 136.77,
133.13, 131.35, 130.31, 117.81, 117.15, 98.38,
92.79 ppm.
FT-IR (KBr):
2230w, 16428, 1408w, 1326m, 12628, 10928, 10208, 982m,
929w, 849w, 803br, 658w, 603w, 577w, 563m, 5278, 525sh.
W/Vi8 A~ in nm (~ in l mol~' cm~')
in n-hexane: 210 (62000), 256 (50000), 314 (17000):
in toluene: 321 (37000).
Example 13
40 mg (0.176 mmol) of N-chloramine T and 2 mg of tetra-
butylA~mo~;um chloride as phase transfer catalyst were
added to a solution of 60 mg (0.082 mmol) of C60 in 25 ml
of toluene. The mixture was stirred at 25C. After
7 days, the reaction mixture was filtered, the ~olution
was evaporated to half its volume and chromatographed on
silica gel (SiO2, particle size from 63 to 200 ~m,
toluene/isoh~YAne 1:2 to pure toluene). Apart from
unreacted C60, 20 mg (= 27% of the theoretical yield) of
the 6,6-bridged compound of the formula
21 SS2 73
- 20 -
(~N--5 ~3 C H 3
o
were isolated.
Rf (SiO2/toluene) = 0.57
MS (FAB) = 889 (M, 10%), 720 (100%)
lH-NMR (360 MHz, CS2/CDCl3):
~ = 8.19 (m, 2H), 7.51 (d, 2H), 2.58 (E~, 3H).
3C-NMR (75 MHz, CS2/CDCl3):
= 145.17 (2C), 145.10, 144.93, 144.85, 144.78 (2C),
144.32, 143.97, 143.79 (2C), 143.71, 143.13, 142.96,
142.90, 142.59 (lC), 141.99, 141.68, 141.16, 140.72,
135.76 (lC), 129.90 (2C), 128.39 (2C), 79.70 (2C),
21.76 (lC).