Note: Descriptions are shown in the official language in which they were submitted.
WO 94/21641 215 5 ~ 7 3 PCTIUS94/01949
-1-
PROCESS FOR PRODUCTION OF AN IMIDAZOLE DERIYATIYE
BACKGROUND OF THE INVENTION
The present invention relates to a process for producing an imidazole derivative. More
particularly, the present invention relates to a process for producing an imidazole derivative
5 useful for hypertension, congestive heart failure, renal failure, glaucoma, hyperuricemia and the
like and an intermediate therefor.
The present inventors have aimed at angiotensin II antagonist as an agent for preventing
or treating hy~lellsion, congestive heart failure, renal failure, glaucoma, hyperuricemia and the
like, and studied intensively the drugs which have longer shelf life, higher activity, rapid
10 m~nife.st~tion of action upon intravenous injection, good absorbability into the body upon oral
~riminictration, lower toxicity and long-lasting action. As the result, we found the novel
imidazole derivatives having the hydl~ille cross-linking structure ,tp,~st;~lled by the formula:
R2 R3
15 N~N
R4 R5
and filed applications ~l~imjng the derivatives (JP-A 3-277537, JP-A 3-323474. JP-A 4-095191,
20 JP-A 4-216809 and published PCT application WO 93/08193).
In the process for producing the above imidazole co",puul-d, when the C ring double
bond of a colllpuulld produced by Diels Alder reaction is reduced (see the below Reaction
Scheme), in particular when R is a protecting group such as benzyl group and the like, the
reducing reaction is usually carried out in meth~nnl under hydrogen gas atmosphere using a
25 pa~ m hydroxide catalyst which makes possible a simultaneous deprotecting reaction such as
debenzylation and the like. However, in this reducing reaction, in particular in the case of the
co,l,pùulld (I) in which C ring represented by -P- forms bicyclo, 10 to 60% of a ring cleavaged
side product (3) is produced in addition to the end col.lpoulld (2) depending upon the kind and
size of R13 group and R17 group as well as the reaction conditions. Therefore, the purification
30 by column chromatography is indispensable. Such a reduction reaction is not s~ti~f~rtory, both
in terms of the yield and of the ecomonics of mass production-
21~5S73
WO 94/21641 ' PCT/US94/01949
R2 R3 R13 R2 R3 Rl3 R2 R3 R13
R1~ R1~ +R1~
R R4 Rs Rl7 R R4 R5 R17 RR4 R5 R17
(1) (2) (3)
R2 R3 R13 R2 R3 R13
N ~ N ~ ~ R1 ~ / ~ N
RR4 R5 R17 R4 R5 R17
(2) (4)
R2 R3 R13
N ~ N
R4 R5 R17
~ Tr
(5)
In addition, it is thought that there is the possibility that the formation of 7~-allyl-
25 palladium complex participates in this side reaction. However, the similar side reaction is also
observed when platinum oxide and p~ m carbon are used in place of p~ rlillm hydroxide.
Therefore, the detailed meçh~ni~m is not known. And it was found that this side reaction rarely
occurs in the case of Lindlar catalyst. However, in the case of this catalyst, the desired reaction
does not proceed in some cases, probably due to the impurities cont~in~.d in the raw materials
~0 upon mass production, and there is a problem with reprocl~-ct;~ility.
R2 R3 R
~4
-
WO 94/21641 215 ~ 5 7 3 PCT/US94/01949
-3-
INFORMATION DISCLOSI~RE
The prior route to the synthesis of the compounds produced by the process of this
invention is described in WO 93/08193.
' SUMMARY OF THE INVENTION
5 The Problems to be Solved by the Invention
The object of the present invention is to improve the selectivity on the reduction of C
ring double bond of the above imidazole derivative in order to achieve the high yield even in
mass production and exclude the necessity of cl mplic~t~d purific~tion steps.
The Means to Solve the Problems
In order to solve the above problems, the present inventors have studied intensively and,
as a result, found that C ring double bond can be reduced selectively without the C ring
cleavage reaction.
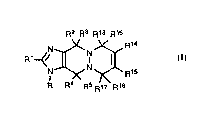
That is, the present invention provides a process for producing an imidazole derivative
represented by the formula (II):
R2 R3
17 R18
20 R R4 R5
wherein R R1 R2 R3 R4 R5 R13, R14, R15, R16, R17 and R18 are as defined below. or a
pharmacologically acceptable ester or salt thereof which compri~es reacting a compound
represented by the formula (I):
R2 R3
\ /R13 R16
wherein R1 is hydrogen atom, lower alkyl group, lower alkoxy group, lower alkylthio group,
lower alkylamino group, lower alkenyl group, -CF3 group, aryl group or aralkyl group;
R is hydrogen atom, or a group selected from the group co~ li"g of
215S~73 ~` ~
WO 94/21641 ~ PCT/US94/01949
--CH2 ~ --Ct 12 ~ --CH2 ~3
wherein X is -CH2-, -NR'-, oxygen atom or ~S(O)n~~ wherein R' is hydrogen atom or
lower alkyl group, wherein n is 0, 1 or 2;
- wherein X1, x2 and X3 are independently hydrogen atom, halogen atom. Iower alkyl
group, lower alkoxy group, nitro group, cyano group, lH-tetræol-5-yl group or alk~li metal salt
10 thereof, -Co2R7 group, -CONR'R" group, -CONHS02R8 group, amino group, -NHSO2CF3
group or -S03H group, or a group selected from the group con~ ing of
Co2R7 R10
--NH--C ~--NH--C--CH--NH--C--R6 --N~(
Y Y
Co2R7 R10
--O--CH~R11
R12
Y is lH-tetrazol-5-yl group or alkali metal salt thereof optionally ~.ubslilllled with cyano
group, benzyl group, tosyl group, methoxymethyl group, ethoxymethyl group,
methoxyethoxymethyl group or trirnethylsilylethoxymethyl group, -Co2R7 group, -CONR'R"
group, -CONHSO2R8 group, amino group, -NHSO2CF3 group or -S03H group;
R2, R3, R4 and R5 are independently hydrogen atom or lower alkyl group, or R2 and
R3, or R4 and R5 are taken together to form =O bond;
R6 is hydrogen atom, halogen atom, lower alkyl group, -CF3 group or -CF2CF3 group;
R7 is hydrogen atom, alkali metal atom or lower a~kyl group; ,,
R' and R" are independently hydrogen atom or lower alkyl group, or R' and R" aretaken together to form the alicyclic structure; !,
R8 is lower alkyl group, cycloalkyl group or aryl group;
R9 is lower ~lkyl group, lower alkoxy group, cycloalkyl group, cycloalkoxy group, aryl
~5 group or aryloxy group;
R10, R11 and R12 are independently hydrogen atom, halogen atom, lower a'kyl group,
~ 2~573
WO 94/21641 PCT/US94/01949
lower alkoxy group, nitro group, cyano group, -Co2R7 group or -CONR'R" group;
R13, R14, R15, R16, R17 and R18 are independently hydrogen atom, lower alkyl group,
lower fluoroalkyl group, -C(R')iR")-oR19 group, -(CH2)j-Co2R7 group, -(CH2)j-CN group, -
(CH2)j-C(=O)R' group, -(CH2)j-CONR'R'I group or -(CH2)j-Aryl group, wherein j is 0, 1 or 2,
5 wherein R16 and R18 may be taken together to form -(CH2)i- group, wherein i is 1, 2 or 3,
wherein Aryl is phenyl group, pyridyl group, pyrimidyl group, pyridazinyl group, furyl group,
thenyl group, pyrazolyl group, oxazolyl group, thiazolyl group, oxadiazolyl group or isooxazolyl
group optionally ~ubsliillled with halogen atom, lower alkyl group, hydroxy group, lower alkoxy
group, nitro group or cyano group;
R19 is hydrogen atom, or lower alkyl group optionally ~ul ~ llPd with hydroxy group
or ether group, with a diimide (HN=NH).
First, variable substituents used in the general formula of the compounds herein are
expl~inP,I
As the lower alkyl group represented by Rl, there are the alkyls having 1 to 8 carbon
15 atoms, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-butyl, n-pentyl,
isoamyl, n-hexyl, n-heptyl, n-octyl and the like. As the lower alkoxy group represented by R1,
there are methoxy, ethoxy, n-p~upo~y, isop.ul)o~y~ n-butoxy, isob,llo~y, t-butoxy, n-pentoxy,
isoamyloxy, n-hexyloxy, n-heptyloxy, n-octyloxy and the like. As the lower alkylthio group
,~p,~se~led by R1, there are methylthio, ethylthio, n-p,ul~ylLllio, isuulupyllllio, n-butylthio, n-
20 pentylthio, n-hexylthio, n-he~Lylil~io, n-octylthio and the like. As the lower alkylamino group
re~ ;sel,led by Rl, there are methylamino, dimethylamino, ethylamino, diethylamino, n-
propylamino, di(n-propyl)amino, isol,lu~ylamino, n-butylamino, n-pentylamino, pyrrolidino,
piperidino, ~ip~ o, morpholino and the like. As the lower alkenyl group represented by R1,
there are vinyl, I-~lupt;llyl, 2-~lu~tillyl, 2-methyl-1-~,upenyl, 1-butenyl, 2-butenyl, 1-pentenyl,
25 2-pellienyl, I-hexenyl, I-heptenyl, I-octenyl and the like. As the lower alkynyl group
,t;p,~sellled by Rl, there are acetylene group, l-pluL~yllyl, 2-Llrul~yllyl, l-butynyl, 2-butynyl, 1-
pentynyl, 2-pentynyl, 1-hexynyl, l-heptynyl, l-octynyl and the like. As the aryl group or
aralkyl group It;~ulcswlled by Rl, there are aryl or aralkyl group having 6 to 10 carbon atoms,
for example, phenyl, naphthyl, benzyl, phenethyl, 3-phellyllJIul)yl, 4-phenylbutyl and the like.
30 These aryl and aralkyl groups are optionally ~ub~ llPd with the sllbstitlle.nt(s) such as the above
described lower alkyl group, or lower alkoxy group, halogen atom, nitro group, cyano group and
the like.
When R is substill~tPd or Im~ubslil"led ph~llylmethyl group, as the halogen atom defined
hy X1, x2 and X3, there are fluorine atom, chlorine atom, bromine atom and iodine atom. As
35 the lower alkyl group and lower alkoxy group, there are above defined lower alkyl group and
alkoxy group. When X1, x2 and X3 are lH-tetrazol-5-yl group, as the alkali metal salt thereof,
21~5~7~ ~
~ . .. .
WO 94/21641 PCT/US94/01949
--6-
there are sodium salt, potassium salt and the like. When Xl, x2 and X3 are -Co2R7 group, as
the examples of R7 in the group, there are hydrogen atom, alkali metal atom such as lithium,
sodium, potassium and the like, alcohol ester of the above defined lower alkyl group. When
X1, x2 and X3 are -CONR'R", the examples of -NR'R" in the group, there are amino, r
5 methylamino, dimethylamino, ethylamino, diethylamino, n-propylamino, di(n-propyl)amino;
dii~uplupylamino, dibutylamino, pyrrolidyl, pi~ uillO, morpholino and the like. As the
examples of R8 in -CONHSO2R8 group, there are methyl, trifluoromethyl, ethyl, n-propyl, n-
butyl, isobutyl, t-butyl, cyclopentyl, cyclohexyl, phenyl and the like. When X1, x2 and X3 are
the group selected from the following:
Co2R7 R11
--NH--C ~ --NH--C--CH--NH--C--R6 --N~
Y Y
Co2R7 R10
--O--1H 4~
~=J R11
R12
, Y and R7 are the same as defined above. The lower alkyl group and lower alkoxy group
represented by R9 are as defined above. When R9 is cycloalkyl group or cycloalkoxy group,
the examples are 5 to 7 membered cyclic compounds such as cyclopentyl, cyclohexyl,
cyclopentyl and the like. As the e~mples of aryl group or aryloxy group represented by R9,
25 there are substit lt~d or ~ d phenyl coll-puunds such as phenyl, p-hydroxyphenyl, p-
carboxyphenyl, o-ll-lluph~llyl and the like. The examples of lower alkyl group, lower alkoxy
group, -Co2R7 group or -CONR'R" group represented by RlU, Rll or R12 are as defined
above. When R is s~lbstit--t~d biphenylmethyl group, as the examples of the aLkali metal salt of
substituent lH-tetrazol-5-yl, there are sodium salt, pol~iulll salt and the like. When Y is -
30 Co2R7 group, -CONR'R" group or -CONHS02R8 grûup~ the e~mp'~s ûf R7, -NR'R" and R8
in the groups are as defined above. When R is represented by the following formula:
--CH2~R6
X
Y~
2~5~7~
WO 94/21641 PCT/US94/01949
-7-
, the example of halogen and lower alkyl group l~pl~s~ ed by R6, the examples of alkali metal
salts of lH-tetrazol-5-yl represented by Y, and the examples of R7, NR'R" and R8 in -Co2R7
group, -CONR'R" group or -CONHSO2R8 group represented by Y are as defined above.When R2, R3, R4 and R5 are lower alkyl group, the examples of them are the alkylgroups having 1 to 8 carbon atoms, for ~x~mple, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, t-butyl, n-pentyl, isoamyl, n-hexyl, n-heptyl, n-octyl and the like.
When R13, R14, R15, R16, R17 and R18 are lower alkyl group, the example of them are
the alkyls having 1 to 8 carbon atoms, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, t-butyl, n-pentyl, isoamyl, n-hexyl, n-heptyl, n-octyl and the like. When R13, R14,
R15, R16, R17 and R18 are lower fluoroalkyl group, the examples of them are trifluoromethyl,
2,2,2,-trifluoroethyl, pentafluoroethyl and the like. When R13 R14 R15 R16 R17 and R18 are
-(CH2)j-Co2R7, -(CH2)j-C(=O)R', -C(R')(R")-OR19, or -(CH2)j-CONR'R", the examples of R7,
R' and R" are as defined above.
The diimide used in the process of the present invention can be generated by various
methorl~, for e~mrle, by oxid~tinn of hyd,a~u,e, decomposition of the dipotassium salt of
azodicarboxylic acid by an acid, the decomposition of arylsulLol,yd,~ide by a base, the
hydroxylamine-acetic acid ester method, and the like (Organic Reactions, 40, 91-155 (1991)).
Among them, the use of deco",po~ilion of p-tohlen~slllfohydrazide by sodium acetate is suitable
in view of ~implirity of the operations and high safety.
The reaction between the diimide and the colllLJuulld of the general formula (I) proceeds
effectively at room ~ ...e to 120 C. The reaction is carried out in a solvent. As the
solvent, there are the alcoholic solvents such as methanol, ethanol, propanol and the like, and
the etheric solvent such as lel~ ydlorul~l, dimetho~yt;~ le and the like. When p-
toluenesulfohydrazide is used to generate the diimide, it is desirable to react the diimide and the
25 compound of the formula (I) while g~,le,~illg the diimide in ~nu~illg dimethoxyethane.
According to the previous process, 10 to 60 % of ring opened products are produced as
side products. However, the above described reaction affords ~lu~ ely a product having
the reduced C ring double bond without side products.
In addition, when R is a protecting group such as benzyl group and the like, after
30 reduction of C ring double bond according to the present method, the reduced product is reacted
in methanol in the presence of the catalytic amount of 20 % p~ m hydroxide-carbon under
1 to 3 atmospheric l"~s~u~e hydrogen by a conventional method to easily effect the deprotection
such as debenzylation and the like. Therefore, a process of the present invention is also useful
for ~ ;paling an intermediate (4) for ~yllllle~is (see the above Reaction Scheme). Further, the
35 end imidazole derivative (5) can be obtained as crystals almost without side products by
ching the biphenyltetrazole part to the interm~ te according to the method described in JP-
21~S~73
WO 94121641 PCT/US94/01949
-8-
A 3-277537, JP-A 3-323474, JP-A 4-095191 and JP-A 4-216809 and WO 93/08193 (see the
above Reaction Scheme). Thereby, the purification by column chromatography which was
indispensable to the previous process becomes llnnPcec~ry, and it was found that the present
process can be applied to the mass production in the good reproductivity.
S If desired, the co"ll,uu"ds produced by the present process can be converted into the
ester or salt thereof by a conventional method. The esters or salts are pharmacologically
acceptable and non-toxic ones. Suitable esters include esters of lower alcohol having a straight
or branched chain such as methanol, ethanol and the like. Suitable salts include alkali metal
salts such as sodium salt, puLas~iulll salt and the like, and alkaline earth metal salts, hydrogen
halide salts such as hydrogen fluoride, hydrogen chloride and the like, inorganic acid salts such
as nitrate, sulfate and the like, lower alkylsulfonate salts such as meth~npslllfonate and the like,
organic acid salts such as maleate, fumarate and the like, and amino acid salts such as aspartate
and the like.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The following F.Y:lmplP~s further illustrate the present invention in detail.
EXAMPLE 1 Synthesis of 1-benzyl-2-n-butyl-5,8-dimethyl-5,8-ethano-5,6,7,8-tetrahydro-lH-
1,3,4a,8a-tetraæa-cyclop~ lPnP4,9-dione lc;~ s~llted by the formula:
o CH3
H3C ~/ ~N~
O CH3
1-Benzyl-2-n-butyl-5,8-dimethyl-5,8-ethano-5,8-dihydro-lH-1,3,4a,8a-tetraaza-
cyclopent~n~rhth~lene-4,9-dione (58 g, 0.14 mol) and p-toluPnPslllfonylhydrazide (187 g, 1.0
mol) were dissolved in dimethoxyethane (400 ml), and the mixture was heated to reflux. An
aqueous solution (400 ml) of sodium acetate (165 g, 2.0 mol) was added dropwise to this
30 solution over 4 hours. The mixture was cooled to room lelllpel~Lule, further cooled to 0 C, and
the precipit~tPd crystals were filtered. The crystals were dissolved in methylene chloride,
washed with water and brine, dried over ~ulhydluu~ sodium sulfate, and the solvent was distilled
off under reduced pressure. The residue combined with another residue (l-benzyl-2-n-butyl-5,8-
dimethyl-5,8-ethano-5,8-dihydro-1H-1,3,4a,8a-tetraaza-cyclop~ lprup4~8-dione~ 56.5 g)
~5 obtained by the similar procedures was purified by silica gel column chromatography (acetone:
hex~ne: methylene chloride=5:1:40) for the purpose of removing the impurities derived from the
215~57~
WO 94t21641 PCTIUS94101949
g
reagents to give the titled compound (105 g, 91%) as colorless solid. In this reduction of the
diimide, only the titled co~ )ou-ld can be obtained as a sole product and no side products which
were produced in the reduction by palladium hydroxide were recognized. The titled compound
has the following NMR spectrum.
S lH-NMR (CDC13) ~ppm: 0.84 (3H, t, J=7.3Hz), 1.25-1.38 (2H, m), 1.63-1.80 (6H, m),
1.82 (3H, s), 1.89 (3H, s), 2.13-2.24 (4H, m), 2.65 (2H, t, J=8.0Hz), 5.69 (2H, s), 7.09-7.15
(2H, m), 7.24-7.36 (3H, m)
EXAMPLE 2 Synthesis of 2-n-butyl-5,8-dimethyl-5,8-ethano-5,6,7,8-tetrahydro-lH-1,3,4a,8a-
tetraaza-cyclope, .l AnAl hll~AIPnP4~9-dione r~pl~sell~ed by the formula:
o CH3
H3C ~<~ 3~N~3
O CH3
1-Benzyl-2-n-butyl-5,8-dimethyl-5,8-ethano-5,6,7,8-letlal1ydlu-1H-1,3,4a,8a-tetraaza-
cyclope,~ Al l~ AlPnP-4,9-dione (53 g, 0.13 mol) was dissolved in a mixed solvent of mPthAn
(300 ml) and methylene chloride (80ml). 20% palladium hydroxide (cont~inin~ 50% water, 10
g) was added to this solution, and the mixture was stirred at 3 normal atmospheres at room
20 temperature for 4 hours under hydrogen atmosphere. The catalyst was filtered off, and the
solvent was distilled off under reduced pressure. The resulting residue was dissolved in a mixed
solvent of ethanol and toluene, and the solvent was distilled off under reduced pressure. These
procedures were repeated three times to give the titled colllpoulld (41.1 g, 100%) as colorless
solid. The titled cc~lllpoulld has the following NMR spectrum.
lH-NMR (CDC13+CD30D) oppm: 0.97 (3H, t, J=7.2Hz), 1.34-1.49 (2H, m), 1.80-1.94
(6H, m), 1.86 (6H, s), 2.10-2.21 (4H, m), 3.17 (2H, t, J=7.4Hz)
~ffects of the Invention
According to the present invention, there is provided a process for selectively reducing
the C ring double bond of an imidazole derivative having the hyd~ille cross-linking structure
30 and, thereby, there becomes possible the production of the useful imidazole derivative in high
yield even in the mass production and without complic~tPd p~lrific~tion steps.