Note: Descriptions are shown in the official language in which they were submitted.
20072/A 21 S ~ 2 3 1
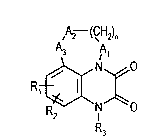
Oxa- or thia-aliphatically brid~ed quinoxaline-2,3-diones
The invention relates to novel oxa- or thia-aliphatically bridged quinoxaline-2,3-diones of the
general formula I
,A2 (CH2)n
R,~ ~ (I),
wherein
A1 is lower alkylidene or a group of the formula >CH-A4-R4 (la),
A2 is lower alkylidene or a group of the formula >CH-A4-R4 (la), ~C=O (Ib) or
>CH(OH)-A5-R4 (Ic),
A3 is oxy, optionally oxidised thio or a group >C(=O) (Ib),
A4 is lower alkylene,
As is lower alkylene or a direct bond,
n is 0 or 1 ,
R, and R2 are each independently of the other hydrogen, unsubstituted or lower alkyl-
and/or lower alkanoyl-substituted amino, nitro, lower alkanoyl, free or etherified or esterified
hydroxy, free or esterified or amidated carboxy, cyano, optionally halogenated lower alkyl or
halogen,
R3 is hydrogen or hydroxy, and
R4 is hydrogen, cyano, free or esterified or amidated carboxy, free or esterified phosphono
or 5-tetrazolyl,
and to the salts thereof, to pharmaceutical compositions comprising the novel compounds
and to the use thereof as medicinal active ingredients.
-2- 21~7231
Optionally oxidised thio is thio, sulfinyl or sulfonyl.
Unsubstituted or lower alkyl- and/or lower alkanoyl-substituted amino is, for example,
amino, lower alkylamino, lower alkanoylamino, di-lower alkylamino or N-lower alkanoyl-N-
lower alkylamino.
Free or etherified or esterified hydroxy is, for example, free hydroxy or hydroxy etherified by
a lower alkanol or esterified by a lower alkanoic acid, especially hydroxy, lower alkanoyloxy
or lower alkoxy, but may also be lower alkenyloxy or lower alkynyloxy.
Optionally halogenated lower alkyl is, for example, lower alkyl or polyhalo-lower alkyl,
especially trifluoromethyl.
Unsubstituted or lower alkanoyl-substituted amino is, for example, amino or lower
alkanoylamino.
Free or esterified or amidated carboxy is, for example, carboxy, lower alkoxycarbonyl,
carboxy-lower alkoxycarbonyl, lower alkoxycarbonyl-lower alkoxycarbonyl, lower
alkanoyloxy-lower alkoxycarbonyl; phenyloxycarbonyl, benzoyloxy-lower alkoxycarbonyl or
phenyl-lower alkoxycarbonyl, each of which is unsuhstitllted or substituted by lower alkyl,
lower alkoxy, hydroxy, halogen and/or by trifluoromethyl; carbamoyl, lower alkylcarbamoyl,
lower alkenylcarbamoyl, di-lower alkylcarbamoyl, di-lower alkylamino-lower alkylcarbamoyl,
amino-lower alkylamino-lower alkylcarbamoyl, 2-oxoimidazolidin-1-yl-lower alkylcarbamoyl,
unsubstituted or amino-lower alkylamino- or 2-oxoimidazolidin-1-yl-substituted lower
alkyleneamino-lower alkylcarbamoyl, such as amino-lower alkylamino-lower alkylene-
carbamoyl or 2-oxoimidazolidin-1-yl-lower alkylenecarbamoyl, oxa-lower alkyleneamino-
lower alkylcarbamoyl, unsubstituted or carboxy- or lower alkoxycarbonyl-substituted
cycloalkylcarbamoyl, cycloalkyl-lower alkylcarbamoyl, unsubstituted or oxo-substituted
azacycloalkylcarbamoyl, optionally benzo-fused lower alkylenecarbamoyl, unsubstituted or
lower alkyl-, lower alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-lower
alkylcarbamoyl, unsubstituted or lower alkyl-, lower alkoxy-, hydroxy-, halo-, amino, lower-
alkoxycarbonylamino-, nitro-, carboxy-, lower alkoxycarbonyl-, phenyl-, phenyloxy- and/or
trifluoromethyl-substituted N-phenyl- or N-lower alkyl-N-phenyl-carbamoyl, optionally
` - 21S723~
partially hydrogenated N-naphthylcarbamoyl, N-indanylcarbamoyl, N-heteroaryl- or N-
heteroaryl-lower alkyl-carbamoyl, mono- or dihydroxy-lower alkylcarbamoyl, mono- or di-
lower alkoxy-lower alkylcarbamoyl, polyhalogeno-lower alkylcarbamoyl, free or esterified
mono- or di-carboxy-lower alkylcarbamoyl, cyano-lower alkylcarbamoyl, free or esterified
carboxy-lower alkenylcarbamoyl or free or etherified N-hydroxycarba",oyl, such as N-
hydroxycarbamoyl, N-lower alkoxycarbamoyl, N-lower alkoxy-N-lower-alkylcarbamoyl, N-
lower alkenyloxycarbamoyl or unsubstituted or lower alkyl-, lower alkoxy-, hydroxy-, halo-
and/or trifluoromethyl-substituted N-phenyloxy-, N-phenyl-lower alkoxy- or N-phenyl-lower
alkenyloxy-carbamoyl .
Unsubstituted or carboxy- or lower alkoxy-substituted cycloalkylcarbamoyl is, for example,
C3-C7cycloalkylcarbamoyl, carboxy-C3-C7cycloalkylcarbamoyl or C,-C4alkoxycarbonyl-C3-
C7cycloalkylcarbamoyl, such as 1-carboxy-, 1-methoxycarbonyl- or 1-ethoxycarbonyl-
cyclopropylcarbamoyl.
Free or esterified phosphono may be fully or partially esterified and is, for example,
phosphono, lower alkylphosphono, di-lower alkylphosphono or tri-lower alkylphosphono.
Optionally benzo-fused lower alkylenecarbamoyl is, for example, azaridino- or 2-methyl-
azaridino-carbonyl, 2,3-dihydroindolin-1-ylcarbamoyl, pyrrolidinocarbonyl, piperidino-
carbonyl, morpholinocarbonyl, thiomorpholinocarbonyl, piperazinocarbonyl or N'-lower alkyl-
piperazinocarbonyl, such as N'-methylpiperazinocarbonyl.
Unsubstituted or oxo-substituted azacycloalkylcarbamoyl is, for example, 3-aza-2-oxo-
cycloheptylcarbamoyl .
Optionally partially hydrogenated naphthylcarbamoyl is, for example, naphthylcarbamoyl or
5,6,7,8-tetrahydronaphthylcarbamoyl.
Free or esterified carboxy-lower alkenylcarbamoyl is, for example, carboxy-loweralkenylcarbamoyl or lower alkoxycarbonyl-lower alkenylcarbamoyl.
21~Z~-
Free or esterified mono- or di-carboxy-lower alkylcarbamoyl is, for example, carboxy-lower
alkylcarbamoyl, lower alkoxycarbonyl-lower alkylcarbamoyl, dicarboxy-lower alkylcarbamoyl
or di-lower alkoxycarbonyl-lower alkylcarbamoyl.
Hereinbefore and hereinafter, lower radicals and compounds will be understood as being,
for example, those having up to and including 7, especially up to and including 4, carbon
atoms.
Di-lower alkylamino is, for example, N,N-di-C1-C7alkylamino, preferably N,N-di-C,-C4alkyl-
amino, such as especially dimethylamino, or, secondly, diethylamino, dipropylamino, diiso-
propylamino or dibutylamino.
Amino-lower alkylamino-lower alkylcarbamoyl is, for example, N-(amino-C2-C4alkylamino-C~-
C4alkyl)carbamoyl, such as N-[2-(2-aminoethylamino)ethyl]carbamoyl.
Amino-lower alkylamino-lower alkylenecarbamoyl is, for example, amino-C2-C4alkylamino-
piperidinocarbonyl, such as 4-(2-aminoethylamino)piperidinocarbonyl.
Carboxy-lower alkoxycarbonyl is, for example, carboxy-C1-C4-alkoxycarbonyl, such as carb-
oxymethoxycarbonyl, 2-carboxyethoxycarbonyl, 3-carboxypropyloxycarbonyl or 4-carb-
oxybutyloxycarbonyl .
Carboxy-lower alkenylcarbamoyl is, for example, carboxy-C2-C4alkenylcarbamoyl, such as
carboxyvinylcarbamoyl, 3-carboxyprop-2-en-2-ylcarbamoyl or 3-carboxyprop-2-en-1-yl-
carbamoyl.
Carboxy-lower alkylcarbamoyl is, for example, carboxy-C1-C4alkylcarbamoyl, such as carb-
oxymethylcarbamoyl, 2-carboxyethylcarbamoyl or 1-carboxy-2,2-dimethyl-propylcarbamoyl.
Cyano-lower alkylcarbamoyl is, for example, cyano-C,-C4alkylcarbamoyl, especially cyano-
methylcarbamoyl .
21572'.~ 1
- 5-
Cycloalkylcarbamoyl is, for example, N-C3-C6cycloalkylcarbamoyl, such as cyclopropyl-
carbamoyl, cyclobutylcarbamoyl, cyclopentylcarbamoyl or cyclohexylcarbamoyl, but may
also be polycyclic cycloalkylcarbamoyl, such as bicyclo[2.2.1]heptyl-, bicyclo[2.2.2]octyl- or
adamantyl-carbamoyl.
Cycloalkyl-lower alkylcarbamoyl is, for example, N-(C3-C6cycloalkyl)-C,-C4alkylcarbamoyl,
such as N-(cyclopropylmethyl)carbamoyl, N-(cyclobutylmethyl)carbamoyl, N-(cyclopentyl-
methyl)carbamoyl or N-(cyclohexylmethyl)carbamoyl.
Dihydroxy-lower alkyl is, for example"B,~-dihydroxy-C3-C4alkyl, such as 2,3-dihydroxypropyl.
Dihydroxy-lower alkylcarbamoyl is, for example, N,N-di(hydroxy-C2-C4-alkyl)carbamoyl,
such as N,N-di(2-hydroxymethyl)carbamoyl, N-(2-hydroxyethyl)-N-hydroxymethyl-carbamoyl
or N,N-di(2-hydroxyethyl)carbamoyl.
Dicarboxy-lower alkylcarbamoyl is, for example, dicarboxy-C1-C4alkylcarbamoyl, such as di-
carboxymethylcarbamoyl .
Di-lower alkoxy-lower alkylcarbamoyl is, for example, di-C,-C4alkoxy-C,-C4alkylcarbamoyl,
such as dimethoxymethoxycarbonyl or diethoxymethoxycarbonyl.
Di-lower alkylamino-lower alkylcarbamoyl is, for example, N,N-di-C,-C4alkylamino-C2-
C4alkylcarbamoyl, such as N-(2-dimethylaminoethyl)carbamoyl.
Di-loweralkylcarbamoyl is, forexample, N,N-di-C,-C7alkylca,La",oyl, preferdbly N,N-di-C,-
C4alkylcarbamoyl, such as especially dimethylcarbamoyl, or, secondly, diethylcarbamoyl,
dipropylcarbamoyl, diisopropylcarbamoyl or dibutylcarbamoyl.
Di-lower alkoxycarbonyl-lower alkylcarbamoyl is, for example, di-C,-C4alkoxycarbonyl-C,-
C4alkylcarbamoyl, such as dimethoxycarbonylmethylcarbamoyl or diethoxycarbonylmethyl-
carbamoyl.
6 21j~t2~Jl
Di-lower alkylphosphono is, for example, di-C,-C7alkylphosphono, preferably di-C,-C4alkyl-
phosphono, such as especially dimethylphosphono, or, secondly, diethylphosphono,dipropylphosphono, diisopropylphosphono or dibutylphosphono.
Halogen is, for example, halogen having an atomic number of up to and including 35, such
as chlorine or fluorine, also bromine.
Hydroxy-lower alkylcarbamoyl is, for example, N-(hydroxy-C2-C4alkyl)carbamoyl, such as
hydroxymethylcarbamoyl or 2-hydroxyethylcarbamoyl.
Heteroarylcarbamoyl is, for example, fur-2-ylcarbamoyl, thien-2-ylcarbamoyl,-thiazol-2-yl-
carbamoyl or N-benzthiazol-2-ylcarbamoyl.
Heteroaryl-lower alkylcarbamoyl is, for example, fur-2-yl-C,-C4alkylcarbamoyl, thien-2-yl-C,-
C4alkylcarbamoyl or thiazol-2-yl-C1-C4alkylcarbamoyl, such as fur-2-ylmethylcarbamoyl,
thien-2-ylmethylcarbamoyl, thiazol-2-ylmethylcarbamoyl or N-(3-oxo-2,3-dihydro-4H-1,4-
benzoxazin-7-yl)methylcarbamoyl.
N-Lower alkanoyl-N-lower alkyl-amino is, for example, N-C,-C7alkanoyl-N-C~-C4alkylamino,
especially N-C,-C4alkanoyl-N-C,-C4alkylamino, such as N-formyl-N-methylamino, N-acetyl-
N-methyl-amino, N-acetyl-N-ethyl-amino, N-ethyl-N-propionyl-amino, N-methyl-N-propionyl-
amino, N-butyryl-N-methyl-amino or N-isobutyryl-N-methyl-amino.
Lower alkanoyl is, for example, N-C1-C7alkanoyl, especially N-C~-C4alkanoyl, such as
formyl, acetyl, propionyl, butyryl or isobutyryl, but may also be Cs-C7alkanoyl, such as piva-
loyl.
Lower alkanoylamino is, for example, N-C,-C7alkanoylamino, especially N-C,-C4alkanoyl-
amino, such as formylamino, acetylamino, propionylamino, butyrylamino or isobutyrylamino,
but may also be C5-C7alkanoylamino, such as pivaloylamino.
215723~
-- - 7 -
Lower alkanoyloxy is, for example, N-C~-C7alkanoyloxy, especially N-C1-C4alkanoyloxy,
such as formyloxy, acetoxy, propionyloxy, butyryloxy or isobutyryloxy, but may also be Cs-
C7alkanoyloxy, such as pivaloyloxy.
Lower alkanoyloxy-lower alkoxycarbonyl is, for example, N-C,-C4alkanoyloxy-C,-C4alkoxy-
carbonyl, such as acetoxymethoxycarbonyl, propionyloxymethoxycarbonyl, tertiary butyryl-
oxymethoxycarbonyl or pivaloyloxymethoxycarbonyl.
Lower alkenylcarbamoyl is, for example, C2-C4alkenylcarbamoyl, such as allylcarbamoyl.
Lower alkoxycarbonyl-lower alkoxycarbonyl is, for example, C,-C4alkoxycarbonyl-C,-C4-
alkoxycarbonyl, such as methoxycarbonylmethoxycarbonyl, ethoxycarbonylmethoxy-
carbonyl or 2-methoxycarbonylethoxycarbonyl.
Lower alkoxycarbonyl-lower alkenylcarbamoyl is, for example, C,-C4alkoxycarbonyl-C2-C4-
alkenylcarbamoyl, such as methoxycarbonylvinylcarbamoyl, ethoxycarbonylvinylcarbamoyl,
3-methoxycarbonylprop-2-en-2-ylcarbamoyl, 3-ethoxycarbonylprop-2-en-2-ylcarbamoyl,
3-methoxycarbonylprop-2-en-1-ylcarbamoyl or 3-ethoxycarbonylprop-2-en-1-ylcarbamoyl.
Lower alkoxycarbonyl-lower alkylcarbamoyl is, for example, C,-C4alkoxycarbonyl-C,-C4alkyl-
carbamoyl, such as methoxycarbonylmethylcarbamoyl, ethoxycarbonylmethylcarbamoyl, 2-
methoxycarbonylethylcarbamoyl or 1-methoxycarbonyl-2,2-dimethyl-propylcarbamoyl.
Lower alkoxy-lower alkylcarbamoyl is, for example, C,-C4alkoxy-C,-C4alkylcarbamoyl, such
as methoxymethylca,L,a",oyl, 2-methoxyethylcarbamoyl or ethoxymethylcarbamoyl.
Lower alkenyloxy is, for example, C3-C4alkenyloxy, such as allyloxy or methallyloxy.
N-Lower alkenyloxycarl.an,oyl is, for example, N-C2-C4alkenyloxycarbamoyl, such as
N-vinyloxycarbamoyl, N-allyloxycarbamoyl or N-methallyloxycarbamoyl.
Lower alkynyloxy is, for example, C3-C4alkynyloxy, such as propargyloxy.
~1572~1
-- - 8 -
Lower alkoxy is, for example, C,-C7alkoxy, preferably C1-C4alkoxy, such as methoxy,
ethoxy, propyloxy, isopropyloxy or butyloxy, but may also be isobutyloxy, secondary butyl-
oxy, tertiary butyloxy or a C5-C7alkoxy group, such as a pentyloxy, hexyloxy or heptyloxy
group.
N-Lower alkenyloxycarbamoyl is, for example, N-C2-C4alkenyloxycarbamoyl, such asN-vinyloxycarbamoyl, N-allyloxycarbamoyl or N-methallyloxycarbamoyl.
Lower alkoxycarbonyl is, for example, C1-C7alkoxycarbonyl, preferably C,-C4alkoxycarbonyl,
such as methoxycarbonyl, ethoxycarbonyl, propyloxycarbonyl, isopropyloxycarbonyl or
butyloxycarbonyl, but may also be isobutyloxycarbonyl, secondary butyloxycarbonyl, tertiary
butyloxycarbonyl or a pentyloxycarbonyl, hexyloxycarbonyl or heptyloxycarbonyl group.
Lower alkyl is, for example, C,-C7alkyl, preferably C,-C4alkyl, such as especially methyl or,
secondly, ethyl, propyl, isopropyl or butyl, but may also be isobutyl, secondary butyl, tertiary
butyl or a Cs-C7alkyl group, such as a pentyl, hexyl or heptyl group.
Lower alkylamino is, for example, N-C,-C7alkylamino, preferably N-C,-C4alkylamino, such as
especially methylamino or, secondly, ethylamino, propylamino, isopropylamino or
butylamino, but may also be isobutylamino, secondary butylamino, tertiary butylamino or a
Cs-c7alkylamino group, such as a pentylamino, hexylamino or heptylamino group.
Lower alkylcarbamoyl is, for example, N-C,-C7alkylcarbamoyl, preferably N-C,-C4alkyl-
carbamoyl, such as especially methylcarbamoyl or, secondly, ethylcarbamoyl, propyl-
carbamoyl, isopropylcarbamoyl or butylcarbamoyl, but may also be isobutylcarbamoyl,
secondary butylcarbamoyl, tertiary butylcarbamoyl or a C5-C7alkylcarbamoyl group, such as
a pentylcarbamoyl, hexylcarbamoyl or heptylcarbamoyl group.
Lower alkylene may be straight-chained or branched and bonded in any desired position
and is, for example, straight-chained or branched C1-C7alkylene, preferably C1-C4alkylene,
such as methylene, 1,2-ethylene, 1,3- or 1,2-propylene, 1,4-, 1,3- or 2,3-butylene or,
secondly, 1,5-, 1,4- or 2,5-pentylene.
- 9~ 7 2 0~ 1
Lower alkylidene may be straight-chained or branched and geminally bonded in any desired
position and is, for example, straight-chained or branched C1-C7alkylene, preferably C,-
C4alkylene, such as methylene, 1,1-ethylidene, 1,1- or 2,2-propylidene, 1,1- or2,2-
butylidene or, secondly, 1,1- or 2,2-pentylidene.
N-Lower alkyl-N-phenyl-carbamoyl is, for example, N-C~-C4alkyl-N-phenyl-carbamoyl, such
as especially N-methyl-N-phenyl-carbamoyl or, secondly, N-ethyl-N-phenyl-carbamoyl,
N-propyl-N-phenyl-carbamoyl, N-isopropyl-N-phenyl-carbamoyl or N-butyl-N-phenyl-carbamoyl, but may also be N-isobutyl-N-phenyl-carbamoyl, N-sec-butyl-N-phenyl-
carbamoyl or N-tert-butyl-N-phenyl-carbamoyl.
Lower alkylphosphono is, for example, C,-C7alkylphosphono, preferably C,-C4alkyl-
phosphono, such as especially methylphosphono or, secondly, ethylphosphono, propyl-
phosphono, isopropylphosphono or butylphosphono, but may also be isobutylphosphono,
sec-butylphosphono, tert-butylphosphono or a Cs-C7alkylphosphono group, such as a
pentylphosphono, hexylphosphono or heptylphosphono group.
Oxa-lower alkyleneamino-lower alkylcarbamoyl is, for example, N-(morpholino-C2-C4alkyl)-
carbamoyl, such as especially N-(2-morpholinoethyl)ethylcarbamoyl.
2-Oxoimidazolidin-1-yl-lower alkylenecarbamoyl is, for example, N-(2-oxoimidazolidin-1-yl-
C4-Csalkylene)carbamoyll such as N-[4-(2-oxoimicl~ol d;n-1-yl)piperidinocarbonyl.
2-Oxoimidazolidin-1-yl-loweralkylcarbamoyl is, forexample, N-(2-oxo.."ida~olidin-1-yl-C2-
C5alkyl)carbamoyl, such as N-(2-oxoimidazolidin-1-yl)ethylcarbamoyl.
Phenyl-lower alkoxycarbamoyl is, for example, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-C~-C4alkoxycarbamoyl, such as
benzyloxycarbamoyl or 1-phenylethoxycarbamoyl.
Phenyloxy-lower alkoxycarbonyl is, for example, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- andlor trifluoromethyl-substituted phenyloxy-C,-C4alkoxycarbonyl, such as
phenyloxymethoxycarbonyl or 2-phenyloxyethoxycarbamoyl.
2~572~1
- 10-
N-Phenyl-lower alkenyloxycarbamoyl is, for example, N-phenyl-C2-C4alkenyloxycarbamoyl,
such as N-phenylvinyloxycarbamoyl or N-(3-phenylprop-2-enyloxy)carbamoyl.
Phenyl-lower alkoxycarbonyl is, for example, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-C,-C4alkoxycarbonyl, such as
benzyloxycarbonyl or 1-phenylethoxycarbonyl.
Phenyl-lower alkylcarbamoyl is, for example, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-C,-C4alkylcarbamoyl, such as
benzylcarbamoyl or 2-phenylethylcarbamoyl.
Tri-lower alkylphosphono is, for example, tri-C,-C7alkylphosphono, preferably tri-C1-C4alkyl-
phosphono, such as especially trimethylphosphono, or, secondly, triethylphosphono, tri-
propylphosphono, triisopropylphosphono or tributylphosphono.
Compounds of formula I having acidic groups, for example those compounds in which at
least one of the radicals R" R2 and R4 is or contains carboxy, phosphono or tet,a~olyl, may
form salts with bases. Compounds of formula I may also form acid addition salts.
Salts of compounds of formula I with bases are, for example, salts thereof with pharma-
ceutically acceptable bases, such as non-toxic metal salts derived from metals of groups la,
Ib, lla and llb, for example alkali metal salts, especially sodium or potassium salts, alkaline
earth metal salts, especially calcium or magnesium salts, and also ammonium salts with
ammonia or organic amines or quatemary ammonium bases, such as optionally C-hydroxyl-
ated aliphatic amines, especially mono-, di- or tri-lower alkylamines, for example methyl-,
ethyl- or diethyl-amine, mono-, di- or tri-(hydroxy-lower alkyl)amines, such as ethanol-,
diethanol- or triethanol-amine, tris(hydroxymethyl)methylamine or 2-hydroxy-tert-butylamine,
or N-(hydroxy-lower alkyl)-N,N-di-lower alkylamines or N-(polyhydroxy-lower alkyl)-N-lower
alkylamines, such as 2-(dimethylamino)ethanol or D-glucamine or choline, or quaternary
aliphatic ammonium hydroxides, for example tetrabutylammonium hydroxide.
~ ~723~
- 11 -
Acid addition salts of compounds of formula I are, for example, the pharmaceutically
acceptable salts thereof with suitable mineral acids, such as hydrohalic acids, sulfuric acid
or phosphoric acid, for example hydrochlorides, hydrobromides, sulfates, hydrogen sulfates
or phosphates, or salts with suitable aliphatic or aromatic sulfonic acids or N-substituted
sulfamic acids, for example methanesulfonates, benzenesulfonates, p-toluenesulfonates or
N-cyclohexylsulramales (cyclamates).
Also included are both total and partial salts, that is to say salts with 1, 2 or 3, preferably 2,
equivalents of base per mole of acid of formula I or salts with 1, 2 or 3 equivalents, prefer-
ably 1 equivalent, of acid per mole of base of formula 1.
For the purposes of isolation or purification it is also possible to use pharmaceutically
unacceptable salts. Only the pharmaceutically acceptable, non-toxic salts are used thera-
peutically, however, and they are therefore preferred.
The compounds of formula I have valuable pharmacological properties. They exhibit a
selective non-competitive antagonistic action with respect to N-methyl-D-aspartic-acid-
sensitive (NMDA-sensitive) excitatory amino acid receptors of warm-blooded animals. In
particular they are ~p~hle of binding to strychnine-insensitive glycine modulators of the
NMDA-receptor. The binding capacity of the compounds prepared according to the
invention and their salts to strychnine-insensitive glycine binding sites of the NMDA-receptor
can be determined in vitro, for example in the experimental procedure according to Baron
et al., Eur. J. Pharmacol., Molec. Pharmacol. Section 206, pages 149-154 (1991) and
Canton et al., J. Pharm. Pharmacol. 44, pages 812-816 (1992) on rat cortex membranes
and rat hippocampus membranes. In those experimental procedures it is determined to
what extent [3Hl-5,7-dichlorokynurenic acid (3H-DCKA) is displaced, there being determined
the percentage inhibition and optionally, by testing a number of concentrations, the concen-
tration (ICso) required for 50 % displacement. The concentration required for 50 % displace-
ment (IC50) lies in the nanomolar and lower millimolar range, that is to say at concentrations
of approximately from 0.01 to 10 ~mol.
By virtue of those properties the compounds of formula I and the pharmaceutically accept-
able salts thereof are excellently suitable for the prophylactic and therapeutic treatment of
~7~31
- 12-
pathological conditions that are responsive to the glycine-antagonistic blocking of NMDA-
sensitive receptors, for example neurodegenerative disorders, such as those arising from
stroke, hypoglycaemia, anoxia or symptoms of cerebral paralysis; cerebral ischaemic
disorders, such as cerebral ischaemia, cerebral ischaemia in cardiosurgery or cardiac
arrest, perinatal asphyxia, epileptic fits, Huntington's chorea, Alzheimer's disease and
Parkinson's disease, amyotrophic lateral sclerosis, spinal and cerebral trauma, and also
symptoms of poisoning resulting from neurotoxins or drug abuse; and ischaemic disorders
of the eyes; vascular and muscular spasms, such as migraine or local or general spasticity;
convulsions, such as epilepsy; and anxiety states and pain, such as trigeminal neuralgias.
The anticonvulsive properties of the compounds according to the invention can be deter-
mined in vivo, for example in mice with reference to their pronounced protective action with
respect to convulsions induced by electric shock or by metrazole, it being possible to use,
for example, the well-established electric shock mouse model or the mouse model for
mel,a~Gle induced convulsions according to Schmutz et al., Naunyn-Schmiedeberg's Arch.
Pharmacol. 342, 61-66 (1990).
The invention relates, for example, to compounds of formula I wherein
A~ is methylene or a group of the formula >CH-A4-R4 (la),
A2 is lower alkylidene and A3 is oxy, thio, sulfinyl, sulfonyl or carbonyl,
or
A2 is carbonyl and A3 is oxy, thio, sulfinyl or sulfonyl,
A4 is lower alkylene,
n isOor1,
R, and R2 are each independently of the other hydrogen, amino, lower alkylamino, lower
alkanoylamino, di-lower alkylamino, N-lower alkanoyl-N-lower alkylamino, hydroxy, lower
alkoxy, lower alkenyloxy, lower alkynyloxy, lower alkyl, polyhalo-lower alkyl or halogen,
R3 is hydrogen, and
R4 is hydrogen, carboxy, lower alkoxycarbonyl, unsubstituted or lower alkyl-, lower alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-lower alkoxycarbonyl, carbamoyl,
cyano or lower alkylcarbamoyl, unsubstituted or lower alkyl-, lower alkoxy-, hydroxy-, halo-
and/or trifluoromethyl-substituted phenylcarbamoyl or di-lower alkylcarbamoyl,
and to the salts thereof.
`- 21~3~-
- 13-
The invention relates, for example, also to compounds of formula I wherein
A1 is lower alkylidene or a group of the formula >CH-A4-R4 (la),
A2 is lower alkylidene or a group of the formula >CH-A4-R4 (la) or >C=O (Ib),
A3 is oxy or optionally oxidised thio,
A4 is lower alkylene,
n isOor1,
R1 and R2 are each independently of the other hydrogen, unsubstituted or lower alkyl-
and/or lower alkanoyl-substituted amino, free hydroxy or hydroxy etherified by a lower
alkanol, optionally halogenated lower alkyl or halogen,
R3 is hydrogen, hydroxy or unsubstituted or lower alkanoyl-substituted amino, and
R4 is hydrogen, cyano, tel,a~olyl or free or esterified or amidated carboxy,
such as those compounds wherein
A1 is a group of the formula ~CH-A4-R4 (la),
A2 is lower alkylidene,
A3 is oxy, optionally oxidised thio or carbonyl,
A4 is lower alkylene,
R1 and R2 are each independently of the other hydrogen, unsubstituted or lower alkyl-
and/or lower alkanoyl-substituted amino, free hydroxy or hydroxy etherified by a lower
alkanol, optionally halogenated lower alkyl or halogen,
R3 is hydrogen or hydroxy, and
R4 is hydrogen, cyano or free or esterified or amidated carboxy,
and to the salts thereof.
The invention relates especially to compounds of formula I wherein
A1 is lower alkylidene or a group of the formula >CH-A4-R4 (la),
A2 is lower alkylidene or a group of the formula >CH-A4-R4 (la), ~C=O (Ib) or
~CH(OH)-A5-R4 (Ic),
A3 is oxy, thio, sulfinyl or sulfonyl or a group ~C(=O) (Ib),
A4 is lower alkylene,
A5 is lower alkylene or a direct bond,
n isOor1,
- 21~7231
- 14-
R1 and R2 are each independently of the other hydrogen, amino, lower alkylamino, lower
alkanoylamino, di-lower alkylamino, N-lower alkanoyl-N-lower alkyl-amino, nitro, lower
alkanoyl, hydroxy, lower alkanoyloxy, lower alkoxy, lower alkenyloxy, lower alkynyloxy,
carboxy, lower alkoxycarbonyl, carbamoyl, cyano, lower alkyl, polyhalo-lower alkyl or halo-
gen,
R3 is hydrogen or hydroxy, and
R4 is hydrogen, cyano, carboxy, lower alkoxycarbonyl, carboxy-lower alkoxycarbonyl, lower
alkoxycarbonyl-lower alkoxycarbonyl, lower alkanoyloxy-lower alkoxycarbonyl, unsubstituted
or lower alkyl-, lower alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-
oxycarbonyl, benzoyloxy-lower alkoxycarbonyl or phenyl-lower alkoxycarbonyl, carbamoyl,
lower alkyloarl,al"oyl, lower alkenylcarbamoyl, di-lower alkylcarbamoyl, di-lower alkylamino-
lower alkylcarbamoyl, amino-lower alkylamino-lower alkylcarbamoyl, 2-oxoimidazolidin-1-yl-
lower alkyloa, L ar, loyl, amino-lower alkylamino-lower alkylenecarbamoyl, 2-oxGi" ,ida~olidin-
1-yl-lower alkylenecarbamoyl, oxa-lower alkyleneamino-lower alkylcarbamoyl, 3- to 7-
membered cycloalkylca,l,a",oyl, 3- to 7-membered carboxycycloalkylcarbamoyl, 3- to 7-
membered lower alkoxycarbonylcycloalkylcarbamoyl, 3- to 7-membered cycloalkyl-lower
alkylcarbamoyl, 3-aza-2-oxo-cycloheptylcarbamoyl, azaridinocarbonyl, 2-methylazaridino-
carbonyl, 2,3-dihydroindolin-1-ylcarbamoyl, pyrrolidinocarbonyl, piperidinocarbonyl, morpho-
linocarbonyl, thiomorpholinocarbonyl, piperazinocarbonyl, N'-lower alkylpiperazinocarbonyl,
unsubstituted or lower alkyl-, lower alkoxy-, hydroxy-, halo- and/or trifluoromethyl-
substituted phenyl-lower alkylcart,amoyl, unsubstitùted or lower alkyl-, lower alkoxy-,
hydroxy-, halo-, amino-, lower-alkoxycarbonylamino-, nitro-, carboxy-, lower alkoxycarbonyl-,
phenyl-, phenyloxy- and/or trifluoromethyl-substituted N-phenylcarbamoyl or N-lower alkyl-
N-phenylcarbamoyl, naphthylcarbamoyl, 5,6,7,8-tetrahydronaphthylcarbamoyl,, N-
indanylcarbamoyl, furyl-2-carbamoyl, thien-2-ylcarbamoyl, thiazol-2-ylcarbamoyl, N-
benzthiazol-2-ylcarbamoyl, fur-2-ylmethylcarbamoyl, thien-2-ylmethylcarbamoyl or thiazol-2-
ylmethylcarbamoyl, N-(3-oxo-2,3-dihydro-4H-1,4-benzoxazin-7-yl)methylcarbamoyl, mono-
or dihydroxy-lower alkylcarbamoyl, mono- or di-lower alkoxy-lower alkylcarbamoyl, polyhalo-
lower alkylcarbamoyl, carboxy-lower alkylcarbamoyl, lower alkoxycarbonyl-lower
alkylcarbamoyl, dicarboxy-lower alkylcarbamoyl, di-lower alkoxycarbonyl-lower
alkylcarbamoyl, cyano-lower alkylcarbamoyl, carboxy-lower alkenylcarbamoyl or lower
alkoxycarbonyl-lower alkenylcarbamoyl, N-hydroxycarbamoyl, N-lower alkoxycarbamoyl, N-
lower-alkoxy-N-lower-alkyl-carbamoyl, N-lower alkenyloxycarbamoyl or unsubstituted or
- ~1572~1
- 15-
lower alkyl-, lower alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted N-
phenyloxycarbamoyl, N-phenyl-lower alkoxycarbamoyl or N-phenyl-lower alkenyl-
oxycarbamoyl, phosphono, lower alkylphosphono, di-lower alkylphosphono or tri-lower alkyl-
phosphono or 5-tetrazolyl,
and to the salts thereof.
The invention relates above all to compounds of formula I wherein
A, is slraight-chained or branched C,-C7alkylene, preferably C1-C4alkylene, such as
methylene, 1,1-ethylidene, 1,1- or 2,2-propylidene, 1,1- or 2,2-butylidene, or a group of the
formula >CH-A4-R4 (la),
A2 is straight-chained or branched C,-C7alkylene, preferably C,-C4alkylene, such as
methylene, 1,1-ethylidene, 1,1- or 2,2-propylidene, 1,1- or 2,2-butylidene, or a group of the
formula >CH-A4-R4 (la) or ~CH(OH)-As-R4 (Ic),
A3 is oxy, thio, sulfinyl or sulfonyl,
A4 is straight-chained or branched C,-C7alkylene, preferably C,-C4alkylene, such as
methylene, 1,1-ethylidene, 1,1- or 2,2-propylidene or 1,1- or 2,2-butylidene,
As is C,-C4alkylene, such as methylene, 1 ,1-ethylidene, 1,1- or 2,2-propylidene, 1,1- or 2,2-
butylidene, or a direct bond,
n isOor1,
R, and R2 are each independently of the other hydrogen, amino, N-C,-C4alkylamino, such
as methylamino, ethylamino, propylamino, isopropylamino or butylamino, N-C~-C7alkanoyl-
amino, such as formylamino, acetylamino, propionylamino, butyrylamino or isobutyrylamino,
N,N-di-C~-C4alkylamino, such as dimethylamino, diethylamino, dipropylamino, diisopropyl-
amino or dibutylamino, N-C,-C7alkanoyl-N-C~-C4alkylamino, such as N-formyl-N-methyl-
amino, N-acetyl-N-methyl-amino, N-acetyl-N-ethyl-amino, N-ethyl-N-propionyl-amino, N-
methyl-N-propionyl-amino, N-butyryl-N-methyl-amino or N-isobutyryl-N-methyl-amino, nitro,
N-C,-C4alkanoyl, such as formyl, acetyl, propionyl, butyryl or isobutyryl, pivaloyl, hydroxy, N-
C,-C4alkanoyloxy, such as formyloxy, acetoxy, propionyloxy, butyryloxy or isobutyryloxy,
pivaloyloxy, C,-C4alkoxy, such as methoxy, ethoxy, propyloxy, isopropyloxy, butyloxy,
isobutyloxy, seconday butyloxy or tertiary butyloxy, C3-C4alkenyloxy, such as allyloxy or
methallyloxy, C3-C4alkynyloxy, such as propargyloxy, carboxy, C,-C4alkoxycarbonyl, such as
methoxycarbonyl, ethoxycarbonyl, propyloxycarbonyl, isopropyloxycarbonyl, butyloxy-
carbonyl, isobutyloxycarbonyl, sec-butyloxycarbonyl or tert-butyloxycarbonyl, carbamoyl,
21~7231
-
- 16-
cyano, C1-C4alkyl, such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, secondary butyl or
tertiary butyl, halogen or trifluoromethyl or halogen,
R3 is hydrogen or hydroxy, and
R4 is hydrogen, cyano, carboxy, C,-C~alkoxycarbonyl, such as methoxycarbonyl, ethoxy-
carbonyl, propyloxycarbonyl, isopropyloxycarbonyl, butyloxycarbonyl, isobutyloxycarbonyl,
sec-butyloxycarbonyl or tert-butyloxycarbonyl, carboxy-C1-C4alkoxycarbonyl, such as
carboxymethoxycarbonyl, 2-carboxyethoxycarbonyl, 3-carboxypropyloxycarbonyl or 4-
carboxybutyloxycarbonyl, C1-C4alkoxycarbonyl-C1-C4alkoxycarbonyl, such as methoxy-
carbonylmethoxycarbonyl, ethoxycarbonylmethoxycarbonyl or 2-methoxycarbonylethoxy-
carbonyl, N-C1-C4alkanoyloxy-C,-C4alkoxycarbonyl, such as acetoxymethoxycarbonyl,
propionyloxymethoxycarbonyl, tert-butyryloxymethoxycarbonyl or pivaloyloxymethoxy-
carbonyl, unsubstituted or C1-C4alkyl-, C1-C4alkoxy-, hydroxy-, halo- and/or trifluoromethyl-
substituted phenyloxycarbonyl, benzoyloxy-C1-C4alkoxycarbonyl or phenyl-C,-C4alkoxy-
carbonyl, such as benzyloxycarbonyl or benzoyloxymethoxycarbonyl, carbamoyl, N-C1-C4-
alkylcarbamoyl, such as methylcarbamoyl, ethylcarbamoyl, propylcarbamoyl, isopropyl-
carbamoyl, butylca,ba"loyl, isobutylcarbamoyl, sec-butylcarbamoyl or tert-butylcarbamoyl,
C2-C4alkenylcarbamoyl, such as allylcarbamoyl, N,N-di-C1-C4alkylcarbamoyl, such as
dimethylcarbamoyl, or, secondly, diethylcarbamoyl, dipropylcarbamoyl, diisopropyl-
carbamoyl or dibutylcarbamoyl, C3-C7cycloalkylcarbamoyl, such as cyclopropyl- orcyclohexyl-carbamoyl, carboxy-C3-C7cycloalkylcarbamoyl, such as 1-carboxycarbamoyl, C1-
C4alkoxycarbonyl-C3-C7cycloalkylcarbamoyl, such as 1-methoxycarbonyl- or 1-ethoxy-
carbonyl-cyclopropylcarbamoyl, 3- to 7-membered N-(C3-C6cycloalkyl)-C1-C4alkylcarbamoyl,
such as N-(cyclopropylmethyl)carbamoyl, N-(cyclobutylmethyl)carbamoyl, N-(cyclopentyl-
methyl)carbamoyl or N-(cyclohexylmethyl)carbamoyl, 3-aza-2-oxo-cycloheptylcarbamoyl,
azaridinocarbonyl, 2-methylazaridinocarbonyl, 2,3-dihydroindolin-1-ylcarbamoyl, pyrrolidino-
carbonyl, piperidinocarbonyl, morpholinocarbonyl, thiomorpholinocarbonyl, piperazino-
carbonyl, N'- C,-C4alkyl-, such as N'-methyl-piperazinocarbonyl, unsubstituted or C1-C4alkyl-,
C,-C4alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-C1-C4alkylcarbamoyl,
such as benzylcarbamoyl or 2-phenylethylcarbamoyl, unsubstituted or C1-C4alkyl-, C1-
C4alkoxy-, hydroxy-, halo-, trifluoromethyl-, amino, C1-C4alkoxycarbonylamino, such as
methoxy- or ethoxycarbonylamino, nitro-, carboxy-, C1-C4alkoxycarbonyl-, such as methoxy-
or ethoxy-carbonyl-, phenyl-, phenyloxy- and/or trifluoromethyl-substituted N-
phenylcarbamoyl or N-C1-C4alkyl-N-phenylcarbamoyl, such as N-methyl-N-phenyl-
~ 1 ~, 7 2 ~ ~
- 17-
carbamoyl, naphthylcarbamoyl, 5,6,7,8-tetrahydronaphthylcarbamoyl, indanylcarbamoyl,
furyl-2-carbamoyl, thien-2-ylcarbamoyl, thiazol-2-ylcarbamoyl, N-benzthiazol-2-ylcarbamoyl,
fur-2-ylmethylcarbamoyl, thien-2-ylmethylcarbamoyl or thiazol-2-ylmethylca,l,amoyl, N-(3-
oxo-2,3-dihydro-4H-1,4-benzoxa~in-7-yl)methylcarbamoyl, N-(hydroxy-C2-C4alkyl)carbam-
oyl, such as hydroxymethylcarbamoyl or 2-hydroxyethylcarbamoyl, N,N-di(hydroxy-C2-C4-
alkyl)carbamoyl, such as N,N-di(2-hydroxyethyl)carbamoyl, C,-C4alkoxy-C1-C4alkyl-
carbamoyl, such as methoxymethylcarbamoyl, 2-methoxyethylcarbamoyl or ethoxymethyl-
carbamoyl, di-C,-C4alkoxy-C,-C4alkylcarbamoyl, such as dimethoxymethoxycarbamoyl or
diethoxymethoxycarbamoyl, polyhalo-C2-C4alkylcarbamoyl, such as 2,2,2,-
trifluoroethylcarbamoyl, carboxy-C,-C4alkylcarbamoyl, such as carboxymethy!carbamoyl, 2-
carboxyethylcarbamoyl or 1-carboxy-2,2-dimethyl-propylcarbamoyl, C,-C4alkoxycarbonyl-C,-
C4alkylcarbamoyl, such as methoxycarbonylmethylcarbamoyl, ethoxycarbonylmethyl-
carbamoyl, 2-methoxycarbonylethylcarbamoyl or 1-methoxycarbonyl-2,2-dimethyl-
propylcarbamoyl, dicarboxy-C,-C4alkylcarbamoyl, such as dicarboxymethylcarbamoyl, or di-
C,-C4alkoxycarbonyl-C,-C4alkylcarbamoyl, such as dimethoxycarbonylmethylcarbamoyl or
diethoxycarbonylmethylcarbamoyl, cyano-C,-C4alkylcarbamoyl, especially cyanomethyl-
carbamoyl, carboxy-C2-C4alkenylcarbamoyl, such as carboxyvinylcarbamoyl, 3-carboxyprop-
2-en-2-ylcarbamoyl or 3-carboxyprop-2-en-1-ylcarbamoyl, C,-C4alkoxycarbonyl-C2-
C4alkenylcarbamoyl, such as methoxycarbonylvinylcarbamoyl, ethoxycarbonylvinyl-
carbamoyl, 3-methoxycarbonylprop-2-en-2-ylcarbamoyl, 3-ethoxycarbonylprop-2-en-2-
ylcarbamoyl, 3-methoxycarbonylprop-2-en-1-ylcarbamoyl or 3-ethoxycarbonylprop-2-en-1-
ylcarbamoyl, N-hydroxycarbamoyl, N-C,-C4alkoxycarbamoyl, such as methoxycarbamoyl,
ethoxyca,bamoyl, propyloxycarbamoyl, isopropyloxycarbamoyl, butyloxycarbamoyl orespecially tert-butyloxycarbamoyl, N-C2-C4alkenyloxycarbamoyl, such as N-vinyloxycarbam-
oyl, N-allyloxycarbamoyl or N-methallyloxycarbamoyl, or unsubstituted or C,-C4alkyl-, C,-
C4alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted N-phenyloxycarbamoyl, N-
phenyl-C,-C4alkoxycarbamoyl, such as benzyloxycarbamoyl or 1-phenylethoxycarbamoyl, or
N-phenyl-C2-C4alkenyloxycarbamoyl, such as N-phenylvinyloxycarbamoyl or N-(3-phenyl-
prop-2-enyloxy)carbamoyl, phosphono, C,-C4alkylphosphono, such as methylphosphono,
ethylphosphono, propylphosphono, isopropylphosphono or butylphosphono, di-C,-C4alkyl-
phosphono, such as especially dimethylphosphono, diethylphosphono, dipropylphosphono,
diisopropylphosphono or dibutylphosphono, tri-C1-C4alkylphosphono, such as especially
-18- 21~ 7 2 3 ~
trimethylphosphono, or, secondly, triethylphosphono, tripropylphosphono, triisopropyi-
phosphono or tributylphosphono, or 5-tetrazolyl,
and to the salts thereof.
The invention relates above all, for example, to compounds of formula I wherein
A, is methylene or a group of the formula ~CH-A4-R4 (la),
A2 is straight-chained or branched C,-C7alkylidene, such as methylene, 1,1-ethylidene, 1,1-
or 2,2-propylidene, 1,1- or 2,2-butylidene or, secondly, 1,1- or 2,2-pentylidene,
A3 is oxy, thio, sulfinyl or sulfonyl,
A4 is straight-chained or branched C1-C7alkylene, such methylene, 1,2-ethylene, 1,3- or
1,2-propylene, 1,4-, 1,3- or 2,3-butylene or 1,5-, 1,4- or 2,5-pentylene,
n isOor1,
R, and R2 are each independently of the other hydrogen, amino, N-C,-C7alkylamino, such
as methylamino, ethylamino, propylamino, isopropylamino or butylamino, also isobutyl-
amino, sec-butylamino, tert-butylamino or a C5-C7alkylamino group, such as a pentylamino,
hexylamino or heptylamino group, N-C,-C7alkanoylamino, such as formylamino, acetyl-
amino, propionylamino, butyrylamino or isobutyrylamino, also Cs-C7alkanoylamino, such as
pivaloylamino, N,N-di-C,-C7alkylamino, such as dimethylamino, diethylamino, dipropyl-
amino, diisopr~pylamino or dibutylamino, N-C,-C7alkanoyl-N-C,-C4alkylamino, such as N-
formyl-N-methyl-amino, N-acetyl-N-methyl-amino, N-acetyl-N-ethyl-amino, N-ethyl-N-
propionyl-amino, N-methyl-N-propionyl-amino, N-butyryl-N-methyl-amino or N-isobutyryl-N-
methyl-amino, hydroxy, C,-C7alkoxy, such as methoxy, ethoxy, propyloxy, isopropyloxy or
butyloxy, also isobutyloxy, secondary butyloxy or tertiary butyloxy, C3-C4alkenyloxy, such as
allyloxy or methallyloxy, C3-C4alkynyloxy, such as propargyloxy, C,-C7alkyl, such as methyl,
ethyl, propyl, isopropyl or butyl, also isobutyl, secondary butyl, tertiary butyl or a Cs-c7alk
group, such as a pentyl, hexyl or heptyl group, trifluoromethyl or halogen,
R3 is hydrogen or hydroxy, and
R4 is hydrogen, carboxy, C,-C7alkoxycarbonyl, such as methoxycarbonyl, ethoxycarbonyl,
propyloxycarbonyl, isopropyloxycarbonyl or butyloxycarbonyl, but may also be isobutyloxy-
carbonyl, sec-butyloxycarbonyl, tert-butyloxycarbonyl or a pentyloxycarbonyl, hexyloxy-
carbonyl or heptyloxycarbonyl group, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-, hydroxy-,
halo- and/or trifluoromethyl-substituted phenyl-C,-C4alkoxycarbonyl, carbamoyl, tetrazolyl,
cyano, C,-C4alkylcarbamoyl, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-, hydroxy-, halo-
~1~723~
-_ - 19-
and/or trifluoromethyl-substituted phenylcarbamoyl or N,N-di-C1-C7alkylcarbamoyl, such as
dimethylcarbamoyl, diethylcarbamoyl, dipropylcarbamoyl, diisopropylcarbamoyl or
dibutylcarbamoyl,
and to the salts thereof.
The invention relates especially to compounds of formula I wherein
one of the radicals A, and A2 is a group of the formula >CH-A4-R4 (la) and the other is
straight-chained or branched C,-C4alkylene, such as methylene, 1 ,1-ethylidene, 1,1- or 2,2-
propylidene, 1,1- or 2,2-butylidene,
A3 is oxy, thio, sulfinyl or sulfonyl,
A4 is straight-chained or branched C,-C4alkylene, such as methylene, 1 ,1-ethylidene, 1,1-
or 2,2-propylidene, 1,1- or 2,2-butylidene,
n isO,
R, and R2 are each independently of the other hydrogen, nitro, N-C,-C4alkanoyl, such as
formyl, acetyl, propionyl, butyryl or isobutyryl, pivaloyl, hydroxy, C,-C4alkoxy, such as
methoxy, carboxy, C,-C4alkoxycarbonyl, such as methoxycarbonyl or ethoxycarbonyl,
carbamoyl, cyano, C1-C4alkyl, such as methyl or ethyl, halogen, trifluoromethyl or halogen,
R3 is hydrogen and
R4 is hydrogen, carboxy, C,-C7alkoxycarbonyl, such as methoxycarbonyl or ethoxycarbonyl,
C,-C4alkoxycarbonyl-C,-C4alkoxycarbonyl, such as methoxycarbonylmethoxycarbonyl, 1-
ethoxycarbonylmethoxycarbonyl or 2-methoxycarbonylethoxycarbonyl, N-C~-C4alkanoyloxy-
C,-C4alkoxycarbonyl, such as tert-butyryloxymethoxycarbonyl; phenyloxycarbonyl,
benzoyloxy-C,-C4alkoxycarbonyl or phenyl-C,-C4alkoxycarbonyl, such as benzyloxycarbonyl
or benzoyloxymethoxycarbonyl, each of which is unsubstituted or substituted by C1-C4alkyl,
C,-C4alkoxy, hydroxy, halogen and/or by trifluoromethyl; carbamoyl, N-C,-C4alkylcarbamoyl,
such as methylcarbamoyl, butylcarbamoyl or tert-butylcarbamoyl, C2-C4alkenylcarbamoyl,
such as allylcarbamoyl, N,N-di-C,-C4alkylcarbamoyl, such as dimethylcarbamoyl,
diethylcarbamoyl or dibutylca,L,ar"oyl, C3-C7cycloalkylcarbamoyl, such as cyclo-propylcarbamoyl, C,-C4alkoxycarbonyl-C3-C7cycloalkylcarbamoyl, such as 1-methoxy-
carbonyl- or 1-ethoxycarbonyl-cyclopropylcarbamoyl, 3- to 7-membered N-(C3-C6cycloalkyl)-
C,-C4alkylcarbamoyl, such as N-(cyclopropylmethyl~carbamoyl, 3-aza-2-oxo-
cycloheptylcarbamoyl, azaridinocarbonyl, 2-methylazaridinocarbonyl, 2,3-dihydroindolin-1-
ylcarbamoyl, pyrrolidinocarbonyl, piperidinocarbonyl, unsubstituted or C,-C4alkyl-, C1-C4-
2 3 1
- 20 -
alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-C,-C4alkylcarbamoyl, such
as benzylcarbamoyl, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-, hydroxy-, halo- and/or
trifluoromethyl-, nitro-, carboxy-, C,-C4alkoxycarbonyl-, such as methoxy- or ethoxy-
carbonyl-, phenyl-, phenyloxy- and/or trifluoromethyl-substituted N-phenylcarbamoyl or
N-C,-C4alkyl-N-phenylcarbamoyl, such as N-methyl-N-phenyl-carbamoyl, naphthyl-
carbamoyl, 5,6,7,8-tetrahydronaphthylcarbamoyl, furyl-2-carbamoyl, thien-2-ylcarbamoyl,
thiazol-2-ylcarbamoyl, fur-2-ylmethylcarbamoyl, thien-2-ylmethylcarbamoyl or thiazol-2-yl-
methylcarbamoyl, C,-C4alkoxy-C,-C4alkylcarbamoyl, such as methoxymethylcarbamoyl,
2-methoxyethylcarbamoyl or ethoxymethylcarbamoyl, di-C,-C4alkoxy-C1-C4alkylcarbamoyl,
such as dimethoxymethoxycarbonyl or diethoxymethoxycarbonyl, carboxy-C1-C4alkyl-carbamoyl, such as carboxymethylcarbamoyl, 2-carboxyethylcarbamoyl or 1-carboxy-2,2-
dimethyl-propylcarbamoyl, C1-C4alkoxycarbonyl-C1-C4alkylcarbamoyl, such as methoxy-
carbonylmethylcarbamoyl, ethoxycarbonylmethylcarbamoyl, 2-methoxycarbonylethylcarb-
amoyl or 1-methoxycarbonyl-2,2-dimethyl-propylcarbamoyl, dicarboxy-C,-C4alkylcarbamoyl,
such as dicarboxymethylcarbamoyl, or di-C1-C4alkoxycarbonyl-C1-C4alkylcarbamoyl, such as
dimethoxycarbonylmethylcarbamoyl, carboxy-C2-C4alkenylcarbamoyl, such as 3-carboxy-
prop-2-en-2-ylcarbamoyl, C1-C4alkoxycarbonyl-C2-C4alkenylcarbamoyl, such as 3-methoxy-
carbonylprop-2-en-2-ylcarbamoyl, 3-ethoxycarbonylprop-2-en-2-ylcarbamoyl, 3-methoxy-
carbonylprop-2-en-1-ylcarbamoyl or 3-ethoxycarbonylprop-2-en-1-ylcarbamoyl, N-
hydroxycarbamoyl, N-C1-C4alkoxycarbamoyl, such as methoxycarbamoyl, ethoxycarbamoyl,
propyloxycarbamoyl, isopropyloxycarbamoyl, butyloxycarbamoyl or especially tert-butyloxycarbamoyl, N-C2-C4alkenyloxycarbamoyl, such as N-vinyloxycarbamoyl, N-allyl-
oxycarbamoyl or N-methallyloxycarbamoyl, or unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted N-phenyl-C1-C4alkoxycarbamoyl,- such as
benzyloxycarbamoyl or 1-phenylethoxycarbamoyl, N-phenyl-C2-C4alkenyloxycarbamoyl,
such as N-phenylvinyloxycarbamoyl or N-(3-phenylprop-2-enyloxy)carbamoyl, phosphono or
S-tetrazolyl,
and to the salts thereof.
The invention relates especially, for example, to compounds of formula I wherein
A1 is methylene or a group of the formula ~CH-A4-R4 (la),
- 21~7231
- 21 -
A2 is a group of the formula ~CH-A4-R4 (la) or, when A, is a group of the formula >CH-A4-R4
(la), A2 is straight-chained or branched C,-C4alkylene, such as methylene, 1,1-ethylidene,
1,1- or 2,2-propylidene, 1,1- or 2,2-butylidene,
A3 is oxy, thio, sulfinyl or sulfonyl,
A4 is straight-chained or branched C,-C4alkylene, such as methylene, 1,2-ethylene, 1,3- or
1,2-propylene or 1,4-, 1,3- or 2,3-butylene,
n isO,
R, and R2 are each independently of the other hydrogen, hydroxy, C,-C4alkoxy, such as
methoxy, C,-C4alkyl, such as methyl or ethyl, trifluoromethyl or halogen,
R3 is hydrogen or hydroxy, and
R4 is hydrogen, carboxy, C,-C4alkoxycarbonyl, such as methoxycarbonyl, ethoxycarbonyl,
propyloxycarbonyl, isopropyloxycarbonyl or butyloxycarbonyl, also isobutyloxycarbonyl, sec-
butyloxycarbonyl or tert-butyloxycarbonyl, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted phenyl-C,-C4alkoxycarbonyl, carbamoyl,
C,-C4alkylcarbamoyl, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-, hydroxy-, halo- and/or
trifluoromethyl-substituted phenylcarbamoyl, N,N-di-C,-C4alkylcarbamoyl, such as dimethyl-
carbamoyl, diethylcarbamoyl, dipropylcarbamoyl, diisopropylcarbamoyl or dibutylcarbamoyl,
or 5-tet, a~olyl,
and to the salts thereof.
The invention relates preferably to compounds of formula I wherein
one of the radicals A, and A2 is a group of the formula ~CH-A4-R4 (la) and the other is
methylene,
A3 is thio,
A4 is methylene,
n isO,
R, and R2 are each independently of the other hydrogen, C,-C4alkyl, such as methyl or
ethyl, trifluoromethyl or halogen,
R3 is hydrogen, and
R4 is hydrogen, carboxy, C,-C4alkoxycarbonyl, such as methoxycarbonyl, ethoxycarbonyl,
propyloxycarbonyl, isopropyloxycarbonyl or butyloxycarbonyl, unsubstituted or C,-C4alkyl-,
C,-C4alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted benzoyloxy-C,-C4alkoxy-
carbonyl, such as benzoyloxymethyloxycarbonyl, unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
21572~
- 22 -
hydroxy-, halo- and/or trifluoromethyl-, nitro-, carboxy-, C,-C4alkoxycarbonyl-, such as
methoxy- or ethoxy-carbonyl-, and/or trifluoromethyl-substituted N-phenylcarbamoyl,
naphthylcarbamoyl, 5,6,7,8-tetrahydronaphthylcarbamoyl, furyl-2-carbamoyl, thien-2-yl-
carbamoyl, thiazol-2-ylcarbamoyl, bicyclo[2.2.1]heptyl-, bicyclo[2.2.2]octyl- or adamantyl-
carbamoyl, 3-aza-2-oxo-cycloheptylcarbamoyl, N-C1-C4alkoxycarbamoyl, such as methoxy-
carbamoyl or tert-butyloxycarbamoyl, N-C2-C4alkenyloxycarbamoyl, such as N-allyloxy-
carbamoyl or N-methallyloxycarbamoyl, or unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted N-phenyl-C~-C4alkoxycarbamoyl, such as
benzyloxycarbamoyl or 1-phenylethoxycarbamoyl, N-phenyl-C2-C4alkenyloxycarbamoyl,
such as N-phenylvinyloxycarbamoyl or N-(3-phenylprop-2-enyloxy)carbamoyl, or 5-tetrazol-
yl,
and to the salts thereof.
The invention relates preferably, for example, to compounds of formula I whereinone of the radicals A, and A2 is methylene or a group of the formula >CH-A4-R4 (la) and the
other is methylene,
A3 is thio,
n isO,
R, and R2 are each independently of the other hydrogen, C,-C4alkyl, such as methyl or
ethyl, trifluoromethyl or halogen,
R3 is hydrogen, and
R4 is carboxy, C,-C4alkoxycarbonyl, such as methoxycarbonyl, ethoxycarbonyl, propyloxy-
carbonyl, isopropyloxycarbonyl or butyloxycarbonyl, 5-tetrazolyl or unsubstituted or C,-
C4alkyl-, C,-C4alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted phenylcarbamoyl,
and to the salts thereof.
The invention relates above all to compounds of formula I
~15723~
- 23 -
R4
S~
,~N~O (I')
~N O
wherein
R, and R2 are each independently of the other hydrogen, C,-C4alkyl, such as methyl or
ethyl, trifluoromethyl or halogen,
R3 is hydrogen, and
R4 is hydrogen, carboxy, C,-C4alkoxycarbonyl, such as methoxycarbonyl, ethoxycarbonyl,
propyloxycarbonyl, isopropyloxycarbonyl or butyloxycarbonyl, unsubstituted or C,-C4alkyl-,
C,-C4alkoxy-, hydroxy-, halo- and/or trifluoromethyl-substituted benzoyloxy-C,-C4alkoxy-
carbonyl, such as benzoyloxymethyloxycarbonyl, bicyclo[2.2.1]heptyl-, bicyclo[2.2.2]octyl- or
adamantyl-carbamoyl, 3-aza-2-oxo-cycloheptylcarbamoyl, unsubstituted or C,-C4alkyl-, C,-
C4alkoxy-, hydroxy-, halo- and/or trifluoromethyl-, nitro-, carboxy-, C,-C4alkoxycarbonyl-,
such as methoxy- or ethoxy-carbonyl-, and/or trifluoromethyl-substituted N-phenylcarbam-
oyl, naphthylcarbamoyl, 5,6,7,8-tetrahydronaphthylcarbamoyl, furyl-2-carbamoyl, thien-2-yl-
carbamoyl, thiazol-2-ylcarbamoyl, bicyclo[2.2.1lheptyl-, bicyclo[2.2.2]octyl- or adamantyl-
carbamoyl, 3-aza-2-oxo-cycloheptylcarbamoyl, N-C,-C4alkoxycarbamoyl, such as methoxy-
carbamoyl or tert-butyloxycarbamoyl, N-C2-C4alkenyloxycarbamoyl, such as N-allyloxy-
carbamoyl or N-methallyloxycarbamoyl, or unsubstituted or C,-C4alkyl-, C,-C4alkoxy-,
hydroxy-, halo- and/or trifluoromethyl-substituted N-phenyl-C~-C4alkoxycarbamoyl, such as
benzyloxycarbamoyl or 1-phenylethoxycarbamoyl, N-phenyl-C2-C4alkenyloxycarbamoyl,
such as N-phenylvinyloxycarbamoyl or N-(3-phenylprop-2-enyloxy)carbamoyl, or ~-tetrazol-
Yl.and to the salts thereof.
The invention relates specifically to the compounds of formula I mentioned in the Examples
and to the salts of those compounds, especially the pharmaceutically acceptable salts
thereof.
21~7~
- 24 -
The process for the preparation of the novel compounds of formula I is based on methods
known perse and is carried out, for example, as follows:
a) a compound of formula ll
~A2--(CH2)n
A3 A~
R~ Y, (Il),
R3
wherein
one of the radicals Y, and Y2 is a group of the formula -C(=O)-Y3 (lla) wherein Y3 iS a
functionally modified carboxy group, and the other is hydrogen,
or in each case a salt thereof, is cyclised intramolecularly, or
b) a compound of formula lll
A3 IX2
N~O (Ill),
R3
wherein X, is a group of the formula -A2-(CH2)n-CH(Y3)-A4-R4 (Illa) or-A2-(CH2)n-CH=A'4-R4
(Illb) wherein Y3 is a nucleofugal leaving group and A'4 is a lower alkanylylidene group
corresponding to the group A4, X2 is hydrogen and R1, R2, R3, A,, A2, A3 and A4 are as
defined, is cyclised intramolecularly
or
c) in a compound of formula IV
~A2--(CH2)n
A3 A'~
R,~ ~ (IV),
21S7231
- 25 -
wherein A'1 is a group of the formula >C=A'4-R4 (Illa) wherein A'4 is lower alkanylylidene, the
extracyclic double bond is reduced to a single bond,
and in each case, if desired, a resulting compound is converted into a different compound
of formula 1, a mixture of isomers obtainable in accordance with the process is separated
into the components and the preferred isomer is isolated, and/or a free compoundobtainable in accordance with the process is converted into a salt or a salt obtainable in
accordance with the process is converted into the corresponding free compound.
The intramolecular cyclisation of compounds of formula ll according to process variant a) is
effected in customary manner, if necessary in an inert organic solvent, such as acetone,
tetrahydrofuran, dioxane or dimethylror")anl.de, where appropriate in admixture with water
and/or in the presence of a basic condensation agent, such as a tertiary aliphatic amine,
such as triethylamine, or a tertiary aromatic nitrogen base, such as pyridine, or a metal
base, such as an alkali metal hydroxide, alkali metal carbonate or alkali metal amide, for
example sodium or potassium hydroxide, sodium or potassium carbonate or sodium or
potassium amide, advantageously with heating, for example in a temperature range of
approximately from 25 to 120, preferably from 50 to 100.
Starting materials of formula ll are preferably prepared in situ and cyclised intramolecularly
without being isolated. For example, compounds of formula ll wherein Y1 is a group of the
formula -C (=O) -Y3(11a) and Y2 is amino are obtained by condensing a compound of
formula llb
,A2--(CH2)n
A3 A~
~ H (llb),
R,~
R2
in customary manner, for example in the presence of a tertiary amine, such as
triethylamine, in dichloromethane, with a reactive oxalic acid derivative, for example with a
compound of the formula Y3-C(=O)-C(=O)-Y'3 (llc), wherein Y3 and Y'3 are identical or
different nucleophilic leaving groups and Y3 is preferably halogen and Y'3 is preferably
etherified hydroxy, such as lower alkoxy, nitrating the reaction product of formula lld
2157231
- 26 -
,A2--(CH2)n
A3 A O
(lld)
R2
in customary manner, for example with nitric acid and sulfuric acid, and then reducing the
product of the nitration. There is formed as intermediate a compound of formula lle
,A2--(CH2)n
~ (lle)~
which is cyclised according to the invention in situ under the conditions of its fo",)alion.
Intermediates of formula llb wherein A1 is a group of the formula ~CH-A4-R4 (la), A2 and A4
are methylene, A3is preferably thio, R1 and R2 are as defined, R3 is hydrogen, R4is an
esterified carboxy group and n is 0, are prepared, for example, by
condensing a compound of formula
~H
A3
Rl~NH2 (lle)
R2
in customary manner with an ~-haloacetoacetic acid ester of the formula Hal-A2-C(=O)-CH2-
R4(llf)orin a compound offormulallg
A,A2
~,HN (119)
R2
2157231
- 27 -
converting the carbonyl group into thiocarbonyl in customary manner, for example in
accordance with Lawesson, condensing the reaction product of formula llh
A2 S
A3~ ~f~
~,HN (llh)
R2
with a haloacetic acid ester of the formula Hal-CH2-COOR (lli; R = lower alkyl?, treating the
reaction product of formula llj
A--A2yS~COOR
~HN (llj)
R~
with triphenylphosphine, and in each case in the reaction product of formula llk
A~A2y~ R
~,HN (llk)
R2
reducing the extracyclic double bond to a single bond in customary manner, for example by
reaction with sodium cyanoborohydride or with a stereoselective homogeneous rhodium
catalyst.
In accordance with an alternative procedure, the intermediate of formula lle can be reacted
with a 3-haloprop-2-enoic acid ester directly to form the corresponding compound of
formula llb.
~1S7231
- 28 -
According to another modification of this process, the compound of the formula lle can be
reacted, in a manner known per se, for example in tetrahydrofuran, with (S)-
epichlorohydrine yielding a compound of the formula llv
HO
A3~""~CI
,~,NH2 (llv) (llv)
l ll
which is then cyclisised, in a manner known per se, for example by treatment with
potassium hydroxide in ethanol, to the corresponding compound of the llw
OH
A3~
~,NH (llw),
l ll
the hydroxy group of which is then according to known methods replaced by one of the
groups R4, for example, by cyano, optionally esterified or amidated carboxy, tel,d~olyl oor
optionally esterified phosphono.
Compounds of formula lle are in turn obtained, for example, by hydrolysing corresponding
compounds of formula llh
NH2
r~/N (llm)
Rl~No2
or by reducing, for example with sodium thiosulfate, corresponding disulfides of formula lln
2 1 ~ ~ 2
- 29 -
Rl~No2
NH2
A3~A3 (lln).
,~,NH2
R1t 11
R2 N2
In analogous manner, it is also possible to obtain intermediates of formula llb.wherein A,
and A2 are methylene and A3 is thio by reacting the compound of formula lle with an
aqueous haloacetic acid, for example with chloroacetic acid.
Compounds of formula ll wherein A, is a group >CH-A4-R4 (la), A2 is methylene, A3 is oxy,
A4 is methylene, n is O, Y, is a group of the formula -C (=O) -Y3 (lla) and Y2 is amino can
advantageously be prepared also by condensing a compound of formula llo
OH
~ NH (llo)
R,t 11
R2 N2
in customary manner with an ~-haloacetoacetic acid ester of the formula Hal-CH2-C(=O)-
CH2-R4 (llp, Hal = halogen) and removing the group Hal from the reaction product of formula
llq
Hal~
o~R4
,~NH (llq)
R~
R2 ~N2
in customary manner, for example by treatment with sodium hydride in tetrahydrofuran, with
the ring being extended, reducing the nitro group in the resulting product of formula llr
2157 2~ ~
- 30 -
O~R4
,NH (llr)
R NO2
to amino in customary manner, for example by catalytic hydrogenation with Raney nickel in
ethanol, and then reducing the extracyclic double bond to a single bond, for example with
sodium cyanoborohydride in ethanol, and condensing the resulting product of formula lls
O~R4
~,NH (lls)
R2 NH2
in custo",ary manner, for example in the presence of a tertiary amine, such as triethyl-
amine, in dichloromethane, with a reactive oxalic acid derivative, for example with a
compound of the formula Y3-C(=O)-C(=O)-Y'3 (llc), wherein Y3 and Y'3 are identical or
different nucleophilic leaving groups and Y3 is preferably halogen and Y'3 is preferably
etherified hydroxy, such as lower alkoxy. The corresponding compound of formula ll is
formed as intermediate and is cyclised according to the invention in situ under the
conditions of its fo""alion.
In analogous manner compounds of formula ll wherein A, is methylene or a group ~C(=O)
(Ib), A2 is methylene, A3 is oxy, n is 0, Y1 is a group of the formula -C(=O)-Y3 (lla) and Y2 is
amino can advantageously also be prepared by reacting a compound of formula llt
HO
Rl~NH2 (llt)
R2 N2
with an aqueous haloacetic acid, for example with chloroacetic acid; in a resulting
compound of formula llu
~l~723l
- 31 -
0~0
~,NH (llu)
R NO2
in customary manner reducing the nitro group to amino and, if desired, the carbonyl group
to methylene, and condensing the reaction product with a reactive oxalic acid derivative, for
example with a compound of the formula Y3-C(=O)-C(=O)-Y'3 (llc) wherein Y3 and Y'3 are
identical or different nucleophilic leaving groups and Y3 is preferably halogen and Y'3 is
pr~ferably etherified hydroxy, such as lower alkoxy. The corresponding compound of
formula ll is formed as intermediate and is cyclised according to the invention in situ under
the conditions of its formation.
For the preparation of compounds of formula ll wherein A3 is oxidised thio, i.e. sulfinyl or
sulfonyl, the thio group in one of the intermediates mentioned above wherein A3 is thio is
oxidised in customary manner, advantageously at the stage of the preparation of the
respective nitro compound in accordance with lld ----~ lle.
For the preparation of compounds of formula ll wherein R, and R2 are other than hydrogen
and R, is, for example, halogen bonded in the 3-position with respect to the A3group, those
substituents are introduced preferably at the stage of the intermediate of formula llb, lln or
lls.
In starting materials of formula lll according to process variant b) nucleophilic leaving groups
are, for example, reactive esterified hydroxy groups, such as hydroxy groups esterified by a
mineral acid or sulfonic acid, especially halogen atoms, for example chlorine, bromine or
iodine, or hydroxy groups esterified by an aliphatic or an unsubstituted or substituted
aromatic sulfonic acid, for example lower alkanesulfonyloxy, such as methanesulfonyloxy,
or unsubstituted or substituted benzenesulfonyloxy, such as benzenesulfonyloxy, bromo-
benzenesulfonyloxy or toluenesulfonyloxy, also tertiary amino groups, such as di-lower
alkylamino, or lower alkyleneamino that is optionally interrupted by oxygen, sulfur or by
nitrogen, for example pyrrolidino, piperidino, morpholino or thiomorpholino.
21572~
- 32 -
Functionally modified carboxy groups are, for example, free or esterified or anhydridised
carboxy groups, such as carboxy, lower alkoxycarbonyl, especially methoxy- or ethoxy-
carbonyl, isopropyloxycarbonyl or tert-butyloxycarbonyl, unsubstituted or lower alkyl-, lower
alkoxy-, halo- and/or nitro-substituted phenyloxycarbonyl, halocarbonyl, such aschlorocarbonyl, or lower alkanoyloxycarbonyl, especially formyloxycarbonyl, acetoxy-
carbonyl or pivaloyloxycarbonyl.
The intramolecular cyclisation of compounds of formula lll is effected in customary manner,
if necessary in an inert organic solvent, such as tetrahydrofuran, dioxane or dimethylform-
amide, and/or in the presence of a basic condensation agent, such as a tertiary aliphatic
amine, such as triethylamine, or a tertiary aromatic nitrogen base, such as pyridine, or a
metal base, such as an alkali metal hydroxide, alkali metal carbonate or alkali metal amide,
for example sodium or potassium hydroxide, sodium or potassium carbonate or sodium or
potassium amide, advantageously with heating, for example in a temperature range of
approximately from 25 to 120, preferably from 50 to 100.
The starting materials of formula lll can be prepared by methods known perse.
For example, compounds of formula lll wherein n is 0, A2 is lower alkylidene and A3 is oxy or
thio are obtained by condensing a compound of formula Illa
Hal
R1~J~NH2 (Illa)
wherein Hal is a halogen atom, such as a chlorine or bromine atom, with a reactive
derivative of carbonic acid, such as a haloformic acid lower alkyl ester of the formula
Hal-C(=O) -COOR (Illb; R= alkyl, unsubstituted or substituted phenyl), nitrating the reaction
product of formula Illc
Hal
Rl~ (Illc)
R NH C00R
2lr~2
- 33-
in the o-position with respect to Hal and to the amino group in customary manner, for
example with potassium nitrate and sulfuric acid, condensing the reaction product of
formula Illd
Hal
,~NO2
Rl~L (Illd)
HN
R2
O~COOR
with a compound of the formula H-A~A2~CH2)n-C(H)=A'4-R4, and in the resulting
compound of formula Ille
A3-A2~A'4`R
,~N2
R1~HN (Ille)
O~COOR
reducing the nitro group to amino in cuslo",ary manner, for example using tin(ll) chloride;
the reaction product cyclises spontaneously under the conditions of its formation to the
corresponding quinoxalinedione of formula lll.
The reduction of the extracyclic double bond in compounds of formula IV in accordance with
process variant c) is carried out in customary manner, for example by reaction with a di-light
metal hydride, such as sodium cyanoborohydride, or by catalytic hydrogenation, for
example in the presence of Raney nickel.
Starting materials of formula IV can be obtained, for example, by condensing a compound
of formula
~H
A3
R~NH2 (lle)
R2
2157 ~3 ~-
- 34 -
in customary manner with an cD-haloacetoacetic acid ester of the formula Hal-A2-C(=O)-CH2-
R4 (llf), condensing the reaction product of formula IVa
A,A2~`R
~,NH (IVa)
R~J
R2
in customary manner, for example in the presence of a tertiary amine, such as triethyl-
amine, in dichloromethane, with a reactive oxalic acid derivative, for example v~lith a
compound of the formula Y3-C(=0)-C(=0)-~3 (llc) wherein Y3 and Y'3 are identical or
different nucleophilic leaving groups and Y3 is pr~rer~bly halogen and Y'3 is preferably
etherified hydroxy, such as lower alkoxy, nitrating the reaction product of formula IVb
in customary manner, for example with nitric acid and sulfuric acid, and then reducing the
product of the nitration. A compound of formula IVc
R4
A,A2~ J
~NH2 (IVc)
is formed as intermediate and is cyclised in situ under the conditions of its formation to form
the corresponding compound of formula IV wherein n is 0 and A'4 is methine.
Compounds of formula lle are in tum obtained, for example, by hydrolysing corresponding
compounds of formula llh
NH2
A3~
r~/N
Rlt
R ~No2
2 (llh)
2 1 7 2 3 1
or by reducing corresponding disulfides of formula lli
R,~ N2
R1+ 11
~NH2
,~ NH2
Rl~No2
2 (lli),
for example with sodium thiosulfate.
For the pr~paralion of compounds of formula IV wherein A3 is oxy, A2 is methylene, A'4 is
methine and n is 0, it is also possible to condense a compound of formula llj
OH
R,~ NH (I Ij)
R2 NO2
in customary manner with an ~o-h~'cacetoacetic acid ester of the formula Hal-CH2-C(=O)-
CH2-R4 (llk, Hal = halogen), to remove the group Hal from the reaction product of formula llk
Hal
o~R4
~NH (llk)
R~NO2
in customary manner, for example by treatment with sodium hydride in tetrahydrofuran, with
the ring being extended, to reduce the nitro group in the resulting product of formula llm
- 36 2 1 ~ 7 2 3 ~
O~R4
~,NH (llm)
R2 N2
to amino in customary manner, for example by catalytic hydrogenation with Raney nickel in
ethanol, and to condense the reaction product in customary manner, for exa""~'e in the
presence of a tertiary amine, such as triethylamine, in dichloromethane, with a reactive
oxalic acid derivative, for example with a compound of the formula Y3-C(=O)-C(=O)-Y'3 (llc)
wherein Y3 and Y'3 are identical or different nucleophilic leaving groups and Y3 is preferably
halogen and Y'3 is pr~:ferdbly etherified hydroxy, such as lower alkoxy, there likewise being
formed as intermediate a compound of formula IVc which cyclises in situ under the
conditions of its formation to form the corresponding compound of formula IV wherein n is O
and A'4 is methine.
For the preparation of compounds of formula IV wherein A3 is oxy or especially thio, A2 is
methylene, A'4 is methine and n is 0, it is also possible to convert the carbonyl group in a
compound of formula 119
2 0
~3,HN (119)
R2
into thiocarbonyl in customary manner, for example in accordance with Lawesson, to
condense the reaction product of formula llh
~,A2~f~ S
I
~HN (llh)
R2
_ ~37~ 2157231
with a haloacetic acid ester of the formula Hal-CH2-COOR (lli; R = lower alkyl) and to treat
the reaction product of formula llj
A--A2~ S ~, COOR
_~HN (llj)
with triphenylphosphine.
Compounds oblai. ,able in accordance with the process can be converted in customary
manner into different compounds of formula 1.
For example, a compound of formula I wherein R4 is carboxy can be esterified to the
corresponding compound of formula I wherein R4 is esterified carboxy. Similarly, a
compound of formula I wherein R4 is free or esterified carboxy can be a",;dated to form the
corresponding compound of formula I wherein R4 is amidated carboxy. Conversely, a
compound of formula I wherein R4 is cyano or esterified or amidated carboxy can be
hydrolysed to form the corresponding compound of formula I wherein R4 and optionally R5
are carboxy. It is also possible to hydrolyse a cyano group R5 to carbamoyl.
Furthermore, in obtainable compounds of formula I wherein A3 is thio, the thio group can be
oxidised to sulfinyl or sulfonyl in customary manner, for example by reaction with a suitable
peroxy compound, such as m-chloroperbenzoic acid or permonophthalic acid.
Moreover, in a compound of formula I wherein R, and/or R2 are hydrogen, the hydrogen
atom can be replaced by a radical R1 and/or R2 that is other than hydrogen.
For example, lower alkanoyl can be introduced in customary manner, for example by
reaction with a reactive lower alkanoic acid derivative, such as a lower alkanoic acid
chloride or lower alkanoic acid nitrile, in the presence of aluminium trichloride, and in the
case of reaction with a lower alkanoic acid nitrile if necessary in the presence of boron
trichloride, preferably in a halogenated hydrocarbon, if necessary at boiling temperature.
38 21~72~1
Resulting salts can be converted into the free compounds in a manner known perse, for
example by treatment with a base, such as an alkali metal hydroxide, a metal carbonate or
metal hydrogen carbonate, or another of the salt-forming bases mentioned at the beginning,
or with an acid, such as a mineral acid, for example with hydrochloric acid, or another of the
salt-forming acids mentioned at the beginning.
Resulting salts can be converted into different salts in a manner known perse; acid addition
salts, for example, by treatment with a suitable metal salt, such as a sodium, barium or
silver salt, of a different acid in a suitable solvent in which an inorganic salt being formed is
insoluble and is therefore eliminated from the reaction equilibrium, and basic salts by
freeing of the free acid and conversion into a salt again.
The compounds of formula 1, including their salts, may also be obtained in the form of
hydrates or may include the solvent used for crystallisation.
As a result of the close relationship between the novel compounds in free form and in the
form of their salts, hereinabove and hereinbelow any reference to the free compounds and
their salts is to be understood as including also the corresponding salts and free
compounds, respectively, as appropriate and expedient.
Resulting diastereoisomeric mixtures and mixtures of racemates can be separated into the
pure diastereoisomers or racemates in known manner on the basis of the physico-chemical
differences between the constituents, for example by chromatography and/or fractional
crystallisation.
Resulting racemates can also be resolved into the optical antipodes in accordance with
known methods, for example by recrystallisation from an optically active solvent, with the
aid of microorganisms or by reaction of the resulting diastereoisomeric mixture or racemate
with an optically active auxiliary compound, for example depending on the acidic, basic or
functionally modifiable groups present in compounds of formula 1, with an optically active
acid, base or an optically active alcohol, to form mixtures of diastereoisomeric salts or
functional derivatives, such as esters, and separation thereof into the diastereoisomers from
7 2 ~ ~
- 39 -
which the desired enantiomer can be freed in the appropriate customary manner. Bases,
acids and alcohols suitable for that purpose are, for example, optically active alkaloid
bases, such as strychnine, cinchonine or brucine, or D- or L-(1-phenyl)ethylamine, 3-
pipecoline, ephedrine, amphetamine and similar synthetically obtainable bases, optically
active carboxylic or sulfonic acids, such as quinic acid or D- or L-tartaric acid, D- or L-di-o-
toluylld, laric acid, D- or L-malic acid, D- or L-mandelic acid or D- or L-camphorsulfonic acid,
and optically active alcohols, such as borneol or D- or L-(1-phenyl)ethanol.
The invention relates also to those forms of the process according to which a compound
obtainable as intermediate at any stage of the process is used as starting material and the
remaining steps are carried out or a starting material is used in the form of a salt or, espe-
cially, is formed under the reaction conditions.
The invention relates also to novel starting materials, which have been developed
specifically for the preparation of the compounds according to the invention, especially the
group of starting materials that result in the compounds of formula I described at the
beginning as being preferred, to the processes for their preparation and to their use as
intermediates.
The invention relates also to pha""aceutical compositions comprising the compounds
according to the invention or pharmaceutic^"y acceptable salts thereof as active ingre-
dients, and to processes for the preparation thereof.
The pharmaceutical compositions according to the invention, which comprise the compound
according to the invention or pharmaceutically acceptable salts thereof, are for enteral, such
as oral and also rectal, and parenteral administration to (a) warm-blooded animal(s), the
compositions comprising the pharmacological active ingredient alone or together with a
pharmaceutically acceptable carrier. The daily dose of the active ingredient depends upon
age and individual condition and upon the mode of administration.
The novel pharmaceutical compositions comprise, for example, from approximately 10 % to
approximately 80 %, preferably from approximately 20 % to approximately 60 %, active
ingredient. Pharmaceutical compositions according to the invention for enteral or parenteral
- 40 - 2 1 ~ 7 2 3 1
administration are, for example, those in unit dose forms, such as dragées, tablets,
capsules or suppositories, and also ampoules. They are prepared in a manner known per
se, for example by means of conventional mixing, granulating, confectioning, dissolving or
Iyophilising processes. For example, pharmaceutical compositions for oral administration
can be obtained by combining the active ingredient with solid carriers, optionally granulating
a resulting mixture, and processing the mixture or granules, if desired or necessary, after
the addition of suitable excipients, to form tablets or dragée cores.
Suitable carriers are especially fillers, such as sugars, for example lactose, saccharose,
mannitol or sorbitol, cellu'~se preparations and/or calcium phosphates, for example tri-
calcium phosphate or calcium hydrogen phosphate, and also binders, such as starch pastes
using, for example, corn, wheat, rice or potato starch, gelatin, tragacanth, methylcellulose
andlor polyvinylpyrrolidone, if desired disintegrators, such as the above-mentioned
starches, and also carboxymethyl starch, cross-linked polyvinylpyrrolidone, agar, alginic
acid or a salt thereof, such as sodium alginate. Excipients are especially flow agents, fiow-
conditioners and lul,ricants, for example silicic acid, talc, stearic acid or salts thereof, such
as magnesium or calcium stearate, and/or polyethylene glycol. Dragée cores are provided
with suitable, optionally enteric, coatings, there being used interalia concentrated sugar
solutions which may contain gum arabic, talc, polyvinylpyrrolidone, polyethylene glycol
andlor titanium dioxide, or coating solutions in suitable organic solvents or solvent mixtures
or, for the prep&rdlion of enteric coatings, solutions of suitable cellulose preparations, such
as acetylcellulose phthalate or hydroxypropylmethylcellulose phthalate. Dyes or pigments
may be added to the tablets or dragée coatings, for example for identification purposes or
to indicate different doses of active ingredient.
Other orally adrl,inisL,dble pharmaceutical compositions are hard gelatin capsules and also
soft, sealed capsules consisting of gelatin and a plasticiser, such as glycerol or sorbitol. The
hard gelatin capsules may comprise the active ingredient in the form of granules, for
example in admixture with fillers, such as lactose, binders, such as starches, and/or
glidants, such as talc or magnesium stearate, and if desired stabilisers. In soft capsules the
active ingredient is preferably dissolved or suspended in suitable liquids, such as fatty oils,
paraffin oil or liquid polyethylene glycols, it likewise being possible to add stabilisers.
-41- 2~S7231
Suitable rectally acl",inislrdble pharmaceutical compositions are, for example, suppositories
that consist of a combination of the active ingredient with a suppository base material.
Suitable suppository base materials are, for example, natural or synthetic triglycerides,
paraffin hydrocarbons, polyethylene glycols or higher alkanols. It is also possible to use
gelatin rectal capsules which comprise a combination of the active ingredient with a base
material. Suitable base materials are, for example, liquid triglycerides, polyethylene glycols
or paraffin hydrocarbons.
For parenteral administration there are suitable, especially, aqueous solutions of an active
ingredient in water-soluble form, for example in the form of a water-soluble salt, and also
suspensions of the active ingredient, such as corresponding oily injection suspensions,
there being used suitable lipophilic solvents or vehicles, such as fatty oils, for example
sesame oil, or synthetic fatty acid esters, for example ethyl oleate or triglycerides, or
aqueous injection suspensions which comprise viscosity-increasing substances, for
example sodium carboxymethylcellulose, sorbitol and/or dextran, and optionally also
stabilisers.
The dosage of the active ingredient depends upon the species of warm-blooded animal,
age and individual condition and also upon the mode of administration. In a normal case the
approximate daily dose for oral ad",inisL(dlion to a patient weighing about 75 kg is
estimated to be from approximately 10 mg to approximately 500 mg.
The following Examples serve to illustrate the invention; temperatures are given in degrees
Celsius, pressures in mbar. The ring atoms of 4,5-dioxy-2,3,5,6-tetrahydro-4H-1-X-3a,6-
diazaphenalenes according to the invention are numbered in accordance with the following
formula: 2
1x~l3
~N~O
Example 1: 3,3-Dihydro-6H-1-oxa-3a,6-diaza-phenalene-4,5-dione
- 42 - 2 1 ~ 7 2 ~ 1
1.03 9 (6.86 mmol) of 3,4-dihydro-2H-benzo[1,4]oxazin-5-ylamine are dissolved in 23 ml
(167.7 mmol) of diethyl oxalate and the solution is rotated on a rotary evaporator at 80 and
20 mbar for 22 hours. The resulting suspension is filtered and the residue is washed with
ether and dried under reduced pressure at 60. 1.05 9 (5.14 mmol = 75%) of the title
compound are obtained in the form of brown crystals; m.p. 288 after sublimation at 200
and 0.13 mbar;
H-NMR (D~DMSO, 300 MHz): 4.03 (t, J=4.8 Hz, 2H, H2C-N); 4.32 (t, J=5.0 Hz, 2H, H2C-O);
4.03 (t, J=4.8 Hz, 2H, H2C-N); 6.69-6.77 (m, 2H, H(arom.)C(4, 6)); 7.00-7.05 (m, 1H,
H(arom.)C(5)); 12.02 (s br,1H, HN); C-NMR (D~DMS0, 74 MHz): 40.35 (H2C-N); 63.61(H2C-O); 108.08 (HC); 110.66 (HC); 114.06 (C); 123.97 (HC); 126.62 (C); 143.88 (C(1));
153.29 (C=O); 154.22 (C=O).
Example 2: In a manner analogous to that described in Example 1 and in the description it
is also possible to prepare the following compounds of formula l:
8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid ethyl
ester;
8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid;
8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl-(N-phenYI)-
acetamide;
8-chloro-6H-1 -thia-3a,6-diaza-phenalene-3,4,5-trione;
8-chloro-1,4,5-trioxo-2,3,5,6-tetrahydro-1 H,4H-1 -thia-3a,6-diaza-phenalen-3-ylacetic acid
ethyl ester;
8-bromo-1,4,5-trioxo-2,3,5,6-tetrahydro-1 H,4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid
ethyl ester;
8-chloro-1,4,5-trioxo-2,3,5,6-tetrahydro-1H,4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid;
_, -43- 2157231
8-bromo-1,4,5-trioxo-2,3,5,6-tetrahydro-1H,4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid;
8-chloro-1 ,4,5-trioxo-2,3,5,6-tetrahydro-1 H,4H-1-thia-3a,6-diaza-phenalen-3-yl-(N-phenyl)-
acetamide;
8-bromo-1 ,4,5-trioxo-2,3,5,6-tetrahydro-1 H,4H-1-thia-3a,6-diaza-phenalen-3-Yl-(N-phenyl)
acetamide;
4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-ylacetic acid ethyl ester;
4,5-dioxo-2,3,5,6-tetrahydro4H-1-oxa-3a,6-diaza-phenalen-3-ylacetic acid;
4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl-(N-phenyl)acetal,l;de;
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-ylacetic acid ethyl
ester;
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-ylacetic acid and
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -oxa-3a,6-diaza-phenalen-3-yl-(N-phenyl)-
acetamide.
Example 3: In a manner analogous to that described in Example 1 and in the description it
is also possible to prepare the following compounds of formula l:
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-2-ylacetic acid ethyl
ester;
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-2-ylacetic acid;
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-2-ylacetic acid N-
phenylamide;
- 44 - 2 1~ 7 23 ~
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-2-ylacetamide;
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-2-ylacetonitrile;
8-bromo-2(1 H-tetrazol-5-ylmethyl)-2,3-dihydro-6H-1-thia-3a,6-diaza-phenalene-4,5-dione.
Example 4: 8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic
acid ethyl ester 4.58 9 (11 mmol) of 7-bromo-3,4-dihydro-2H-benzo[1,4]thiazin-3-yl-acetic
acid ethyl ester dissolved in 12 ml of concentrated sulfuric acid are nitrated at -10 with 12
ml of fuming nitric acid. The mixture is stirred overnight at 0 to complete the reaction,
poured into ice-water and extracted three times with ethyl acetate, and the organic phases
are washed with water, saturated sodium hydrogen carbonate solution and saturated
sodium chloride solution and dried over magnesium sulfate, and the solvent is removed.
The crude, nitrated intermediate is dissolved in 50 ml of acetone and at 0 slowly added
dropwise to a mixture of 140ml of 15 % TiCI3 in approximately 10 % hydrochloric acid, 75 ml
of water and 110 ml of acetone and the mixture is stirred at 0 for 18 hours. The resulting
white precipitate is filtered off, washed neutral with a large amount of water and dried under
a high vacuum at 60. 2.51 9 (6.52 mmol) = 59 % of 8-bromo4,5-dioxo-2,3,5,6-tetrahydro-
4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid ethyl ester are obtained in the form of a
beige powder; 1H-NMR (CDCI3, 300MHz): 1.25 (t, 3H); 2.69 (dd, 1H); 2.99 (dd,1H); 3.26
(dd, 1H); 3.38 (dd, 1H); 4.11 (q, 2H); 5.70 (m,1H); 7.11 (d,1H); 7.22 (d, 1H); 11.78 (sbr,
1H); 13C-NMR (CDCI3, 75MHz, APT): 15.2 (CH3); 28.7 (CH2); 36.6 (CH2); 48.1 (C); 62.2
(CH2); 117.8 (C); 117.9 (CH); 121.2 (C); 124.0 (C); 126.5 (CH); 127.9 (C); 155.0 (NC=O);
155.6 (NC=O); 171.0 (C=O); FD-MS: 284,286 (M+); analysis: C 43.98% (43.65); H 3.72%
(3.40); N 7.13% (7.27); TLC: (dichloromethane/methanol = 9:1) Rf = 0.53.
The preparation of the starting materials is described below:
a) 3,4-Dihydro-2H-benzo~1,41thiazin-3-yl-acetic acid ethyl ester
8 9 (34 mmol) of (4H-benzo[1,4]thiazin-3-ylidene)-acetic acid ethyl ester are dissolved in
50 ml of ethanol, and 0.5 ml of 0.1% ethanolic bromocresol green solution and 0.5 ml of 5N
HCI/ethanol are added. 2.14 9 (34 mmol) of sodium cyanoborohydride are added in
4 portions at room temperature and the reaction mixture is kept acidic by the dropwise
-45- ~1572~
addition of HCI/ethanol. After 2 hours' stirring at room temperature to complete the reaction
the solvent is removed on a rotary evaporator and the residue is taken up in ethyl acetate
and washed with water and saturated sodium chloride solution. The organic phases are
dried over magnesium sulfate, concentrated and purified on a column of silica gel (eluant:
petroleum ether/ethyl acetate = 8:2). 6.1 9 (25.6 mmol) = 75% of (3,4-dihydro-2H-benzo-
[1,4]thiazin-3-yl)-acetic acid ethyl ester are obtained in the form of a yellow oil; 1 H-NMR
(CDCI3, 200MHz): 1.28 (t, 3H); 2.51-2.89 (m, 3H); 3.05 (dd, 1H); 4.07 (m,1H); 4.17 (q, 2H);
4.60 (sbr, 1H, NH); 6.48 (dd,1H); 6.62 (m,1H); 6.85-7.02 (m, 2H); 13C-NMR (CDCI3,
50MHz): 15.3; 31.4; 41.6; 48.4; 61.8; 116.7 (2x); 119.1; 126.9; 128.6; 142.1; 172.8; FD-MS:
237 (M+); TLC: (petroleum ether/ethyl acetate = 9:1) Rf = 0.24.
b) 7-Bromo-3,4-dihydro-2H-benzo~1,41thiazin-3-yl-acetic acid ethyl ester
A solution of 3.7 9 (20.6 mmol) of N-bromosuccinimide in 45 ml of dimethylformamide is
added dropwise at 0 to 4.9 9 (20.6 mmol) of (3,4-dihydro-2H-benzo[1,4]thiazin-3-yl)-acetic
acid ethyl ester in 45 ml of dimethylformamide and the mixture is stirred at room tempera-
ture for 18 hours, then freed of solvent under a high vacuum at 50. The resulting oil is
taken up in dichloromethane, washed with water and saturated sodium chloride solution,
and the organic phases are dried over magnesium sulfate, concentrated and chromato-
graphed on silica gel (petroleum ether/ethyl acetate = 9:1). 6.3 9 (97%) of 7-bromo-3,4-
dihydro-2H-benzo[1,4]thiazin-3-yl-acetic acid ethyl ester are obtained in the form of a yellow
oil; 1H-NMR (CDCI3, 300MHz): 1.28 (t, 3H); 2.54-2.84 (m, 3H); 3.00 (dd, 1H); 4.06 (m, 1H);
4.18 (q, 2H); 4.68 (sbr, 1H, NH); 6.36 (d, 1H); 6.97 (dd, 1H); 7.11 (d, 1H); 13C-NMR (CDCI3,
75MHz): 15.2; 31.1; 41.5; 48.3; 61.9; 110.3; 117.9; 118.4; 129.6; 130.6; 141.1; 172.7;
FD-MS: 315, 317 (M~); TLC: (petroleum ether/ethyl acetate = 9:1) Rf = 0.09.
c) 2-(7-Bromo-3-ethoxycarbonylmethyl-2,3-dihydro-benzo~1,4]thiazin-4-yl)-2-oxo-acetic acid
ethyl ester
2.8 ml (25.27 mmol) of oxalic acid monoethyl ester chloride are added at 0 under argon to
6.66 9 (21.06 mmol) of (7-bromo-3,4-dihydro-2H-benzo[1,4]thiazin-3-yl-acetic acid ethyl
ester and 5.9 ml (42.12 mmol) of triethylamine in 5 ml of dichloromethane and the mixture is
stirred at room temperature for 2 hours, then diluted with 40 ml of dichloromethane and
washed with saturated sodium chloride solution, 2N hydrochloric acid, water and again with
saturated sodium chloride solution. The organic phases are dried over magnesium sulfate,
- 46 - 2 1 ~ 7 2 3 1
concentrated and chromatographed on silica gel (petroleum ether/ethyl acetate = 17:3).
8.1 9 (19.5 mmol) (96.4%) of (7-bromo-3-ethoxycarbonylmethyl-2,3-dihydro-benzo[1,4]-
thiazin-4-yl)-oxo-acetic acid ethyl ester are obtained in the form of a yellowish-orange oil;
1H-NMR (CDCI3, 200MHz): presumably mixture of rotamers: 1.10-1.30 (2xt, 3H); 2.55 (dbr,
2H); 3.00 (ddbr,1H); 3.49 (ddbr,1H); 4.02-4.18 (quart,m, 4H); 5.49 (m, 1H); 6.89 (d, 1H);
7.18 (dd, 1H); 7.40 (d,1H).
FD-MS: 415, 417 (M+); TLC: (petroleum ether/ethyl acetate = 7:3) Rf = 0.28.
Example 5: 8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic
acid
4.1 9 (10.64 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-
3-yl-acetic acid ethyl ester are stirred in 180 ml of methanol at room temperature for 4 hours
with 26.6 ml of 2N aqueous sodium hydroxide solution, then rendered acidic with 48 ml of
2N hydrochloric acid. The resulting precipitate is filtered off, washed neutral with a large
amount of water and dried under a high vacuum (60). 3.62 9 (10.12 mmol) = 95% of 8-
bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid are
obtained in the form of a white powder; melting point: >300; 1H-NMR (d6-DMSO, 300MHz):
2.55 (dd, 1H); 2.76 (dd, 1H); 3.27 (m, 2H); 5.48 (m, 1H); 7.06 (d,1H); 7.22 (d, 1H); 12.11
(sbr,1H); 12.59 (sbr, 1H); 13C-NMR (CDC13, 75MHz, APT): 28.0 (CH2); 36.5 (CH2); 46.9
(C); 62.2 (CH2); 115.6 (C); 115.7 (CH); 121.6 (C); 123.4 (C); 124.1 (CH); 129.0 (C); 154.1
(NC=O); 154.9 (NC=O); 172.4 (C=O); FD-MS: 356, 358 (M+); analysis: C 40.69% (40.35); H
2.67% (2.54); N 7.88% (7.84).
` - 215723~
- 47 -
Example 6: 8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic
acid sodium salt
5.6 ml of 0.1 N sodium hydrogenate carbonate solution are added to a suspension of
200 mg (560 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-
3-yl-acetic acid in 40 ml of water and the mixture is boiled under reflux for 1 hour, filtered
and Iyophilised.
Example 7: 8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic
acid potassium salt
Preparation analogous to Example 6.
Example 8: 8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic
acid triethylammonium salt
A suspension of 200 mg (560 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-
3a,6-diaza-phenalen-3-yl-acetic acid in 40 ml of 1 N triethylammonium hydrogen carbonate
is heated at 60 for 1 hour, filtered, concen(,~led on a rotary evaporator/under a high
vacuum and Iyophilised from 8 ml of water.
Example 9: 2-(8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-Yl)-N
phenyl-acetamide
274 ~l (3 mmol) of aniline are added at room temperature under argon to a solution of
714.4 mg (2 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-
3-yl-acetic acid, 540.5 mg (4 mmol) of 1-hydroxybenzol,ia~ole and 766.8 mg (4 mmol) of N-
ethyl-N'-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) in 10 ml of dimethyl-
formamide and the mixture is stirred for 24 hours. The mixture is poured into ice-water, and
the precipitate is filtered off, washed with a large amount of water and dried under a high
vacuum (60). 741.5 mg (1.72 mmol) = 86% of 2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-
1-thia-3a,6-diaza-phenalen-3-yl)-N-phenyl-acetamide are obtained in the form of a beige
powder; m.p: 285-290; 1H-NMR (d6-DMSO,200MHz): 2.62 (dd, 1H); 2.90 (dd, 1H); 3.30
(m, 2H); 5.68 (m, 1H); 7.06 (m, 2H); 7.30 (m, 3H); 7.55 (m, 2H); 10.00 (sbr, 1H); 12.10 (sbr,
1 H); El-MS: 433, 431 (M+); 313, 311; 299, 297; analysis: contains 0.41% water: C 50.00%
(49.80); H 3.35% (3.30); N 9.49% (9.68).
- 48 ~ 2 lS~ 23 1
Example 10: In a manner analogous to that described in Example 9 it is also possible to
prepare the following amides with the correponding free amines (primary, secondary,
anilines) as coupling partners.
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-methyl-N-
phenyl-acetan,ide; m.p.: 148-152; 1H-NMR (d6-DMSO,200MHz): 2.27-2.85 (m, approx.
2H); 3.10-3.40 (m, approx. 2H); 3.18 (s, 3H); 5.55 (m,1H); 7.00-7.48 (m, 7H); 12.05
(sbr, 1H);
FD-MS: 445, 447 (M+); analysis: C 49.69% (51.13); H 3.78% (3.61); N 10.43% (9.41);
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-thiazol-2-yl-
acetamide; yield: 92%; m.p.: 260-261; 1H-NMR (d6-DMSO, 300MHz): 2.72 (dd, 1H); 3.05
(dd, 1 H); 3.27 (s, 2H); 5.67 (m, 1 H); 7.09 (d, 1 H); 7.22 (d,1 H); 7.28 (d, 1 H); 7.46 (d,1 H);
12.14 (sbr, 1H) 12.26 (sbr, 1H); FAB-MS: 439, 414 (M+); analysis: C 39.03% (41.01);
H 3.19% (2.52); N 12.00% (12.75);
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-cyclopropyl-
methyl-acetamide; yield: 82%; m.p.: 250-253; 1H-NMR (d6-DMSO, 300MHz): 0.12-0.14 (m,
2H); 0.36-0.40 (m, 2H); 0.84-0.89 (m, 1H); 2.35-2.68 (m, 2H); 2.89-2.95 (m, 2H); 3.19-3.29
(m, 2H); 5.55 (m,1H); 7.07 (d, 1H); 7.25 (d,1H); 8.04-8.08 (m, 1H); 12.11 (sbr, 1H); FD-MS:
409, 411 (M+); analysis: C 45.19% (46.84); H 4.00% (3.93); N 9.78% (10.24);
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-cyclopropyl-
acetamide; yield: 60%; m.p.: 180-185; 1H-NMR (d6-DMSO, 300MHz): 0.34-0.39 (m, 2H);
0.55-0.61 (m, 2H); 0.84-0.89 (m, 1H); 2.29-2.61 (m, approx. 3H); 3.20 (sbr, 2H); 5.53 (m,
1H); 7.07 (d, 1H); 7.24 (d, 1H); 8.04 (d,1H) 12.01 (sbr, 1H); FD-MS: 395, 397 (M+);
2-~2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetyl-
aminol-3,3-dimethyl-butyric acid tert-butyl ester; yield: 96%; m.p.: 149-154; 1H-NMR
(d6 DMSO, 300MHz): 0.92 (d, 9H); 1.40 (d, 9H); 2.80-2.92 (m, 1H); 3.13-3.20 (m, 2H); 4.01
-49- 215~231
(dd, 1H); 5.55 (m, 1H); 7.07 (m, 1H); 7.26 (m, 1H); 8.08 (dd, 1H); 12.09 (sbr, 1H); FAB-MS:
527, 529 (M++1); 492, 494 (M-tBu); analysis: C 50.19% (49.32); H 3.35% (5.36); N 7.36%
(7.98); TLC: (dichloromethane/methanol = 9:1) Rf = 0.26;
N-adamantan-1 -yl-2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-
3-yl)-acetamide; m.p.: 210-215>; FD-MS: 489, 491 (M+); analysis: C 53.51% (53.88);
H 5.77% (4.93); N 10.00% (8.57).
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N,N-dibutYl-
acetamide; yield: 77%; 1H-NMR (d6-DMSO, 300MHz): 0.81-0.91 (m, 6H); 1.17-1.27 (m, 4H);
1.39-1.43 (m, 4H); 2.44-2.86 (m, 2H); 3.15-3.30 (m, 6H); 5.50 (m, 1H); 7.08 (m, 1H); 7.25
(m, 1H); 12.13 (sbr, 1H); FAB-MS: 468, 470 (M++1); analysis: C 50.67% (51.28); H 5.44%
(5.59); N 9.07% (8.97).
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-YI)-N-(2-oxo-
azepan-3-yl)-acetan,.~E; yield: 45%; m.p.: 305-307; 1H-NMR (d6-DMSO, 300MHz): 1.17-
1.89 (m, 6H); 2.18-3.30 (m, 6H);4.38 (m, 1H); 5.53 (m, 1H); 7.07 (m, 1H); 7.25 (m, 1H); 7.77
(m, 1H); 7.97 (m, 1H); 12.11 (sbr, 1H); FAB-MS: 467, 469 (M++1); analysis: C 50.26%
(50.67); H 4.62% (4.48); N 9.86% (9.33);
N-allyl-2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-
ace~a",ide; yield: 49%; m.p.: 150-153; 1H-NMR (d6-DMSO, 300MHz): 2.42 (dd, 1H); 2.58
(dd, 1H); 3.21 (d, 2H); 3.69 (m, 2H); 5.03-5.15 (m, 2H); 5.56 (m, 1H); 5.74-5.84 (m, 1H);
7.07 (m, 1H); 7.24 (m, 1H); 7.77 (m, 1H); 8.16 (t, 1H); 12.11 (sbr, 1H).; El-MS: 395, 397
(M+); analysis: C 44.83% (45.47); H 3.69% (3.56); N 10.33% (10.60);
8-bromo-3-~2-(2,3-dihydro-indol-1 -yl)-2-oxo-ethyll-2,3-dihydro-6H-1 -thia-3a,6-diaza-phen-
alene-4,5-dione; yield: 99% (beige crystals); m.p.: 270-273; 1H-NMR (d6-DMSO, 300MHz):
3.00-3.62 (m, approx. 6H); 4.02 (d, 2H); 5.64 (m, 1H); 7.00-7.28 (m, 5H); 8.08 (d, 1H); 12.15
(sbr, 1H); FD-MS: 457, 459 (M+); analysis: C 51.20% (52.41); H 4.18% (3.52); N 10.20%
(9.71 );
-50- 21372~
8-bromo-3-(2-oxo-2-piperidin-1 -yl-ethyl)-2,3-dihydro-6H-1 -thia-3a,6-diaza-phenalene-4,5-
dione; yield: 89% beige crystals; m.p.: 170-172; 1H-NMR (d6-DMSO, 300MHz): 1.40-1.60
(m, 8H); 2.30-3.60 (m, 6H); 5.52 (m,1H); 7.07 (d, 1H); 7.24 (d, 1H); 12.10 (sbr, 1H); FD-MS:
423, 425 (M+); analysis: C 47.60% (48.12); H 4.36% (4.28); N 9.80% (9.90);
8-bromo-3-~2-(2-methyl-aziridin-1 -yl)-2-oxo-ethyll-2,3-dihydro-6H-1 -thia-3a,6-diaza-
phenalene-4,5-dione; yield: 62% (beige crystals); m.p.: 193-195
1H-NMR (d6-DMSO, 300MHz): 1.08 (m, 3H); 2.62 (m, 2H); 3.21-3.72 (m, approx. 5H); 3.90-
4.41 (m,1H); 5.57 (m,1H); 7.07 (d,1H); 7.24 (d, 1H); 12.10 (sbr,1H); FD-MS: 395,397 (M+); analysis: C 43.81 % (45.47); H 3.77% (3.56); N 11.66% (11.60).
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(2,2-di-
methoxy-ethyl)-acetar"ide; yield: 69% (beige crystals); m.p.: 190-192; 1H-NMR (d6-DMSO,
300MHz): 2.28-2.72 (m, 2H); 3.10-3.20 (m, 4H); 3.21 (2xs, 6H); 4.32 (t,1H); 5.58 (m, 1H);
7.07 (d,1H); 7.24 (d, 1H); 8.09 (t, 1H); 12.10 (sbr, 1H); FD-MS: 443, 445 (M+); analysis:
C 41.74% (43.25); H 4.06% (4.08); N 9.10% (9.46);
4-~2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetyl-
aminol-benzoic acid methyl ester; yield: 59% beige crystals; m.p.: 145-147; 1H-NMR
(d6 DMSO, 300MHz): 2.60-2.95 (m, 2H); 3.22 (m, 2H); 3.80 (s, 3H); 5.68 (m, 1H); 7.07 (d,
1H); 7.23 (d, 1H); 7.70 (d, 2H); 7.91 (d, 2H); 10.20 (s,1H); 12.10 (sbr, 1H); FD-MS: 489,
491 (M+);
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(5,6,7,8-
tetrahydro-naphthalen-1-yl)-acetamide; yield: 97% beige crystals; m.p.: 323-325; 1H-NMR
(d6-DMSO, 300MHz): 1.62-1.73 (m, 4H); 2.60-2.95 (m, 6H); 3.22 (m, 2H); 5.65 (m, 1H);
6.90-7.30 (m, 5H); 9.25 (s, 1H); 12.15 (sbr,1H); FD-MS: 485, 487 (M+); analysis: C 54.28%
(54.33); H 4.18% (4.14); N 9.51% (8.64);
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-furan-2-yl-
methyl-acetamide; yield: 82% beige crystals; m.p.: 202-203
-51 - 21~723 1
1H-NMR (d6-DMSO, 300MHz): 2.20-2.71 (m, 2H); 3.21 (m, 2H); 4.23 (m, 2H); 5.68 (m,1H);
6.22 (d, 1H); 6.39 (d, 1H); 7.07 (d, 1H); 7.22 (d, 1H); 7.58 (d, 1H); 8.48 (t, 1H); 12.10 (sbr,
1H); FD-MS: 435, 437 (M+); analysis: C 46.31% (46.80); H 3.33% (3.23); N 9.66% (9.63).
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(2-methoxy-
ethyl)-acetamide; yield: 38% beige crystals; m.p.: 135-137
1H-NMR (d6-DMSO, 300MHz): 2.20-2.71 (m, 2H); 3.21 (m, 6H); 3.23 (s, 3H); 5.68 (m, 1H);
7.07 (d, 1H); 7.22 (d, 1H); 8.08 (m,1H); 12.12 (sbr, 1H); FD-MS: 413, 415 (M+); analysis:
C 42.96% (43.49); H 3.92% (3.89); N 10.01% (10.14).
Example 11: 1-~2-(8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-
yl)-acetylaminol-cyclopropane-carboxylic acid methyl ester
165.2 mg (1090 mmol) of 1-aminocyclopropane-1-carboxylic acid methyl ester hydrochloride
are added at room temperature under argon to a solution of 259.6 mg (727 mmol) of
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid,
196.4 mg (1454 mmol) of 1-hydroxybenzotriazole, 279 mg (1454 mmol) of N-ethyl-N'-(3-
dimethylaminopropyl)-carbodiimide hydrochloride (EDC) and 153 ~11 (1090 mmol) oftriethylamine in 10 ml of dimethylformamide and the mixture is stirred for 48 hours. The
mixture is then poured into acidified ice-water and the precipitate is filtered off, washed with
a large amount of water and dried under a high vacuum (60). 220.3 mg (485 mmol) = 67%
of 1-[2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-acetyl-
amino]-cyclopropane-carboxylic acid methyl ester are obtained in the form of a beige
powder;
m.p: 186-188; 1H-NMR (d6-DMSO, 300MHz): 1.02 (m, 2H); 1.34 (m, 2H); 2.37-2.59 (m,
approx. 3H); 3.21 (sbr, 2H); 3.58 (s,3H); 5.54 (m, 1H); 7.07 (m,1H); 7.24 (m, 1H); 8.65 (s,
1H); 12.11 (sbr, 1H), FAB-MS: 454, 456 (M+); analysis: C 44.95% (44.23); H 3.82% (3.55);
N 9.24% (9.25).
Example 12: In a manner analogous to Example 11, it is also possible to prepare the
following amides with amine hydrochlorides as coupling partners:
-52- 21572~J~ ~
N-adamantan-2-yl-2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-
3-yl)-acetamide; yield: 91%; m.p.: 183-185; 1H-NMR (d6-DMSO, 300MHz): 1.40-2.00 (m,
approx. 15H); 2.74 (m, 1H); 3.19 (m, 2H); 3.82 (m, 1H); 5.57 (m, 1H); 7.07 (d, 1H); 7.26 (d,
1H); 7.84 (d, 1H); 12.10 (sbr, 1H); FAB-MS: 490, 492 (M++1).
analysis: C 53.58% (53.88); H 5.08% (4.93); N 8.67% (8.57);
N-bicyclo~2.2.11hept-2-yl-2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-
phenalen-3-yl)-acetamide; yield: 88%; m.p.: 215-222; 1H-NMR (d6-DMSO, 300MHz): 0.8-
1.8 (m, approx. 7H); 2.00-2.40 (m, approx. 3H); 2.65 (m, 1H); 3.16 (m, 2H); 3.87 (m, 1H);
5.54 (m, 1H); 7.07 (d, 1H); 7.25 (d, 1H); 7.91 (m, 1H); 12.10 (sbr, 1H); FAB-MS: 450, 452
(M++1); analysis: C 45.56% (46.26); H 4.26% (4.10); N 11.88% (11.99);
2-(8-bromo-4,~dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-tert-butoxy-
acetamide; yield: 29%; m.p.: 175-180; 1H-NMR (d6-DMSO, 300MHz): 1.14 (s, 9H); 2.25-
3.40 (m, 4H); 5.55 (m, 1H); 7.07 (m, 1H); 7.27 (m, 1H); 10.45 (sbr, 1H); 12.11 (sbr, 1H);
FAB-MS: 428, 430 (M++1);
N-benzyloxy-2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-
acetamide; yield: 93%; m.p.: 140-148; 1H-NMR (d6-DMSO, 300MHz): 2.28-2.45 (m, 2H);
3.12-3.23 (m, 2H); 4.77 (q, 2H); 5.55 (m, 1H); 7.07 (m, 1H); 7.24 (m, 1H); 7.38 (sbr, 5H);
11.14 (sbr, 1H); 12.12 (sbr, 1H); FAB-MS: 462, 464 (M++1); analysis: C 49.14% (49.36);
H 3.77% (3.49); N 9.72% (9.09).
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-prop-2-ynyl-
acetam.de; yield: 73%; m.p.: 181-182; 1H-NMR (d6-DMSO, 300MHz): 2.40 (dd, 1H); 2.68
(dd, 1H); 3.13 (s, 1H); 3.20 (d, 2H); 3.85 (m, 2H); 5.54 (m, 1H); 7.07 (m, 1H); 7.24 (m, 1H);
8.46 (m, 1H); 12.11 (sbr, 1H); FAB-MS: 394, 396 (M++1).
Analysis: C 44.92% (45.70); H 3.33% (3.07); N 10.43% (10.66);
2-~2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetyl-
aminol-malonic acid dimethyl ester; yield: 51% beige crystals; m.p.: 143-145; 1H-NMR
~15723~
- 53 -
(d6 DMSO, 300MHz): 2.38 (m, 1H); 2.80 (m, 1H); 3.20 (m, 2H); 3.72 (s, 6H); 5.17 (d, 1H);
5.57 (m, 1H); 7.07 (m, 1H); 7.24 (m,1H); 9.01 (t, 1H); 12.11 (sbr, 1H); FD-MS: 485,
487 (M+); analysis: C 40.68% (41.99); H 3.47% (3.32); N 8.64% (8.64).
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-cyano-
methyl-acelan,ide; yield: 54% beige crystals; m.p.: 212-214
1 H-NMR (d6-DMSO, 300MHz): 2.40-2.50 (m,1 H); 2.70 (m, 1H); 3.20 (m, 2H); 3.72 (s, 6H);
4.13 (d,1H); 5.57 (m, 1H); 7.07 (m, 1H); 7.24 (m,1H); 8.75 (t,1H); 12.11 (sbr, 1H); FD-MS:
394, 396 (M+);
3-~2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetyl-
aminol-propionic acid methyl ester; yield: 43% brown crystals;
m.p.: 240-242; 1H-NMR (d6-DMSO, 300MHz): 2.25-2.75 (m, approx. 4H); 3.18 (s, 2H);
3.28 (t, 2H); 3.60 (s, 3H); 5.57 (m, 1H); 7.07 (m, 1H); 7.23 (m, 1H); 8.13 (t, 1H); 12.11 (sbr,
1H).
FD-MS: 441, 443 (M+);
N-allyloxy-2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-
acetamide; yield: 77% beige crystals; m.p.: 162-165
1H-NMR (d6-DMSO, 300MHz): 2.23-2.40 (m, 2H); 3.18 (m, 2H); 4.23 (m,2H); 5.25 (2xd,
2H); 5.57 (m, 1H); 5.90 (m, 1H); 7.07 (m,1H); 7.24 (m, 1H); 11.09 (sbr, 1H); 12.11 (sbr,1H);
FD-MS: 411, 413 (M+); analysis: C 43.53% (43.70); H 3.72% (3.42); N 10.47% (10.19);
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-methoxy-
acetamide; yield: 90% beige crystals; m.p.: 182-185
1H-NMR (d6-DMSO, 300MHz): 2.23-2.40 (m, 2H); 3.19 (m, 2H); 3.58 (s, 3H); 5.57 (m, 1H);
5.90 (m, 1H); 7.07 (m,1H); 7.24 (m, 1H); 11.14 (sbr, 1H); 12.10 (sbr, 1H); FD-MS: 385,
387 (M+); analysis: C 39.03% (40.43); H 3.50% (3.13); N 10.24% (10.88);
-54- 21Y~72~l
Example 13: 2-~2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-
yl)-acetylaminol-benzoic acid methyl ester
A solution of 754.5 mg (2.11 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-
diaza-phenalen-3-yl-acetic acid, 590.6 mg (2.32 mmol) of N,N-bis(2-oxo-3-oxazolidinyl)-
phosphinic acid chloride (BOP-CI), 705.3 ~l of triethylamine and 300.5 ~ll of anthranilic acid
methyl ester in 10 ml of dimethylformamide is heated under argon at 60 for 3 hours, then
590.6 mg (2.32 mmol) of BOP-CI (= bis(oxo-3-oxazolidinyl~phosphinic acid chloride) and
705 3 tll of triethylamine are added and the mixture is stirred for a further 2 hours at 60 and
for 15 hours at room temperature. The mixture is then poured into acidified ice-water and
the resulting precipitate is filtered, washed neutral with a large amount of water and dried
under a high vacuum (60). 987.2 mg (2.01 mmol) = 95% of 2-[2-(8-bromo-4,5-dioxo-
2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-acetylamino]-benzoic acid methyl
ester are obtained in the form of a beige powder; m.p.: 165-175; 1 H-NMR (d6-DMSO,
300MHz): 2.72-2.97 (m, 2H); 3.24-3.33 (m, 2H); 3.83 (s, 3H); 5.63 (m, 1H); 7.09 (d, 1H);
7.22 (t,1H); 7.28 (d, 1H); 7.61 (t, 1H); 7.89 (d, 1H); 8.12 (d, 1H); 10.58 (sbr, 1H) 12.13 (sbr,
1H); FAB-MS: 490, 492 (M++1); analysis: C 46.98% (48.99); H 3.51% (3.29); N 8.70%
(8.57).
Example 14: Analogously to Example 13 it is also possible to prepare 3-~2-(8-bromo-4,5-
dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-acetylaminol-but-2-enoic acid
methyl ester; yield: 47% beige powder; m.p.: 160-167; FD-MS: 453, 455 (M+); analysis:
C 45.38% (44.95); H 3.80% (3.55); N 9.35% (9.25).
Example 15: 2-~2-(8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-
yl)-acetYlaminol-benzoic acid
5 ml of 2N sodium hydroxide solution are added to a slurry of 149 mg (303.9 mmol) of 2-[2-
(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-acetylamino]-
benzoic acid methyl ester in 80 ml of methanol and the reaction mixture is stirred at room
temperature for 4.5 hours. 8 ml of 2N hydrochloric acid are added, the methanol is removed
on a rotary evaporator, and the resulting precipitate is filtered off, washed neutral with a
large amount of water and dried under a high vacuum (60). 105 mg (220 mmol) = 73% of
2-[2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetyl-
~ 1 ~ 7 2 3 1
- 55 -
amino]-benzoic acid are obtained in the form of a beige powder; m.p.: 205-212; 1 H-NMR
(d6-DMSO, 300MHz): 2.72-2.97 (m, 2H); 3.23-3.33 (m, 2H); 5.63 (m,1H); 7.09 (d, 1H); 7.17
(t, 1H); 7.27 (d, 1H); 7.60 (t,1H); 7.86 (d, 1H); 8.39 (d, 1H); 11.21 (sbr,1H) 12.12 (sbr, 1H);
FD-MS: 475, 477 (M+); analysis: C 45.04% (47.91); H 3.03% (2.96); N 8.35% (8.82).
Example 16: In a manner analogous to that described in Example 15, it is also possible to
prepare the following compounds:
3-~2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-acetyl-
aminol-propionic acid; yield: 79% beige powder;
m.p.: 284-286; 1H-NMR (d6-DMSO, 300MHz): 2.25-2.75 (m, approx. 4H); 3.18-3.28 (m,
approx. 4H); 5.59 (m, 1H); 7.07 (d,1H); 7.23 (d,1H); 8.08 (t, 1H); 12.15 (sbr, 2H); FD-MS:
427, 429 (M+);
~2-(8-bromo-4,5-dioxo-2,3.5.6-tetrahYdro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetylaminol-
acetic acid; (with decarboxylation); yield: 42% white crystals, m.p.: 193-195; 1H-NMR
(d6 DMSO, 300MHz): 2.20-3.75 (m, approx. 6H); 5.57 (m, 1H); 7.07 (d, 1H); 7.24 (d, 1H);
8.18 (t, 0.5H); 8.67 (d, 0.5H); 12.13 (sbr, 1H).
FD-MS: 413, 415 (M+);
Example 17: 1-r2-(8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-
yl)-acetylaminol-cyclopropane-carboxylic acid
10 ml of 2N hydrochloric acid are added to a solution of 84.3 mg (186 mmol) of 1-[2-(8-
bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetylamino]-
cyclopropane-carboxylic acid methyl ester in 40 ml of dioxane and the mixture is stirred at
room temperature for 18 hours. 20 ml of hydrochloric acid are added and the mixture is
stirred at room temperature for 24 hours and concentrated on a rotary evaporator. The
resulting precipitate is washed neutral with a large amount of water and dried under a high
vacuum (60). 53.2 mg (121 mmol) = 65% of 1-[2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-
1-thia-3a,6-diaza-phenalen-3-yl)-acetylamino]-cyclopropane-carboxylic acid are obtained in
the form of a beige powder; m.p.: 300-302; 1H-NMR (d6-DMSO, 300MHz): 0.97 (m, 2H);
-56- 21~723~
1.30 (m, 2H); 2.36-2.63 (m, approx. 3H); 3.21 (sbr, 2H); 5.52 (m, 1H); 7.07 (m, 1H); 7.24 (m,
1H); 8.55 (s, 1H); 12.09 (sbr, 1H); 12.36 (sbr, 1H); FAB-MS: 440, 442 (M+).
Example 18: 2-~2-(8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-
yl)-acetylaminol-3,3-dimethyl-butyric acid
2 ml of trifluoroacetic acid are added to a solution of 113 mg (214.6 mmol) of 2-[2-(8-bromo-
4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-acetylamino]-3,3-dimethyl-
butyric acid tert-butyl ester in 5 ml of dichloromethane and the mixture is stirred at room
temperature for 5 hours. The solvents are removed on a rotary evaporalor and the residue
is dissolved in 1 ml of dimethylformamide and stirred into 50 ml of ice-water. The product
that precipitates is filtered off, washed with a large amount of water and dried under a high
vacuum (60). 32.8 mg (70 mmol) = 33% of 2-[2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-
1-thia-3a,6-diaza-phenalen-3-yl)-acetylamino]-3,3-dimethyl-butyric acid are obtained in the
form of a white powder, m.p.: 195-198; 1H-NMR (d6-DMSO, 300MHz): 0.93 (d, 9H); 2.85-
2.96 (m, 1H); 3.15-3.19 (m, 2H); 4.10 (dd, 1H); 5.55 (m, 1H); 7.07 (m, 1H); 7.26 (m, 1H);
8.10 (dd, 1H); 12.12 (sbr, 1H); 12.55 (sbr, 1H); FAB-MS: 470, 472 (M++1); TLC: (dichloro-
ethane/ethanol = 9:1) Rf = 0.07.
Example 19: 2,2-Dimethyl-propionic acid (8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-
3a,6-diaza-phenalen-3-yl)-acetoxymethyl ester
A mixture of 201.4 mg (563.9 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-
3a,6-diaza-phenalen-3-yl-acetic acid, 42.9 mg (310.1 mmol) of K2C03 and 98.3 ~lg(676.6 mmol) of pivalic acid chloromethyl ester in 5 ml of dimethylformamide is stirred at
room temperature overnight. The mixture is poured into ice-water and concentrated slightly
at 50, and the product that precipitates is filtered off, washed with water and dried under a
high vacuum (60). 56.5 mg (120 mmol) = 21% of 2,2-dimethyl-propionic acid (8-bromo-4,5-
dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-acetoxymethyl ester are
obtained in the form of a white powder, m.p.: 163-167; 1H-NMR (d6-DMSO, 300MHz):
1.07-1.24 (m, 9H); 2.65-2.93 (m, 2H); 3.20 (m, 2H); 5.48 (m, 1H); 5.70 (m, 2H); 7.06 (d, 1H);
7.25 (d, 1H); 12.14 (sbr, 1H); FD-MS: 470, 472 (M+).
Example 20: In a manner analogous to that described in Example 19, it is also possible to
prepare benzoic acid (8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-
~_ 57 ~l57231
3-yl)-acetoxymethyl ester; yieid: 71%; m.p.: 140-148; 1H-NMR (d6-DMSO, 300MHz): 2.65-
2.98 (m, 2H); 3.20 (m, 2H); 5.52 (m,1H); 5.96 (m, 2H); 7.07 (d,1H); 7.24 (m, 1H); 7.57 (m,
2H); 7.70 (m, 1H); 8.00 (m, 2H); 12.11 (sbr,1H); FD-MS: 490, 492 (M+); analysis: C 48.89%
(48.89); H 3.23% (3.08); N 5.96% (5.70).
Example 21: In a manner analogous to that described in Example 9, it is also possible to
prepare 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic
acid methoxyca,l,onyl methyl ester; yield: 56%.; m.p.: 185-191; 1H-NMR (d6-DMSO,
300MHz): 2.65-2.98 (m, 2H); 3.24 (m, 2H); 3.62 (s, 3H); 4.73 (s, 2H); 5.53 (m,1 H); 7.08 (d,
1H); 7.26 (d, 1H); 12.13 (sbr,1H); FD-MS: 428, 430 (M+); analysis: C 41.16% (41.97);
H .14% (3.05); N 7.69% (6.53).
Example 22: 2-(8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-
acetamide
In a screw-threaded bomb tube, 1.0 9 (2.6 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-
4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid ethyl ester in 30 ml of 4N methanolic
ammonia solution is heated at 100 for 8 hours. The product that precipitates is filtered off,
washed with ethanol and dried under a high vacuum (60). 370 mg (1.04 mmol) = 40% of 2-
(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-acetamide are
obtained in the form of a grey powder; m.p. ~320; 1 H-NMR (d6-DMSO, 300MHz): 2.32-
2.70 (m, 2H); 3.17-3.30 (m, 2H); 5.52 (m, 1H); 6.99 (sbr); 7.06 (d, 1H); 7.24 (d,1H); 7.45
(sbr, 1H); 11.58 (sbr,1H); FD-MS: 355, 357 (M~); analysis: C 40.55% (40.46); H 3.19%
(2.83); N 11.40% (11.80); TLC: (dichloromethane/methanol = 9:1) Rf = 0.07.
Example 23: 8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-
acetonitrile
396 ~11 (4.32 mmol) of phosphorus oxychloride are squirted under argon into a solution,
heated to 90, of 770 mg (2.16 mmol) of 2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-
3a,6-diaza-phenalen-3-yl)-acetamide in 20 ml of dimethylformamide and the mixture is
stirred for 2 hours. The mixture is then poured into ice-water and the resulting precipitate is
filtered off, washed with a large amount of water and dried under a high vacuum (60). The
resulting intermediate (imine chloride) is filtered over a small amount of silica gel with
dichloromethane, dried, dissolved in 30 ml of dioxane and hydrolysed at room temperature
-58- ~.57231
with 3.16 ml of 1 N sodium hydroxide solution for 2 hours. The mixture is again poured into
ice-water and then acidified with a small amount of hydrochloric acid. The resulting
precipitate is filtered off, washed with water and dried under a high vacuum (60). 421.5 mg
(1.25 mmol) = 58% of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-
3-yl-acetonitrile are obtained in the form of an ochre-yellow powder, m.p.: 235-250; 1H-
NMR (d6-DMSO, 300MHz): 2.95-2.99 (m, 2H); 3.27 (m, 2H); 5.55 (m, 1 H); 7.09 (d, 1H); 7.29
(d,1H); 12.19 (s,1H); FD-MS: 337, 339 (M+).
Analysis: (contains approx.1 equiv. water): C 40.53% (42.62); H 2.67% (2.38); N 11.52%
(12.43); TLC: (dichloromethane/methanol = 19:1) Rf = 0.11.
Example 24: 8-Bromo-3-(2H-tetrazol-5-ylmethyl)-2,3-dihydro-6H-1-thia-3a,6-diaza-phenalene-4,5-dione
A solution of 200 mg (591 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-
diaza-phenalen-3-yl-acetonitrile,115.3 mg (1.774 mmol) of sodium azide and 122.1 mg
(887 mmol) of triethylamine hydrochloride in 5 ml of N-methyl-2-pyrrolidone is heated at
150 under N2 for 4 hours. The mixture is allowed to cool, and then poured into 100 ml of
ice-water, acidified with hydrochloric acid and extracted twice with 150 ml of ethyl acetate
each time. The organic phases are then washed with saturated sodium chloride solution
and dried over magnesium sulfate. The solvent is removed on a rotary evaporator and the
residue is dried under a high vacuum (60). The resulting oil is taken up in 5 ml of dimethyl-
fo""anlide and the product is precipitated by being stirred into 100 ml of acidified ice-water.
The precipitate is washed with water and dried under a high vacuum.101.3 mg
(266.4 mmol) = 45% of 8-bromo-3-(2H-tetrazol-5-ylmethyl)-2,3-dihydro-6H-1-thia-3a,6-
diaza-phenalene-4,5-dione are obtained in the form of a brown powder; m.p.: 238-242;
1H-NMR (d6-DMSO, 300MHz) : 3.20-3.40 (m, 4H); 5.58 (m, 1H); 7.10 (d, 1H); 7.30 (d,1H);
12.14 (s, 1H) 16.20 (sbr, 1H); FD-MS: 380, 382 (M+).
Example 25: 3(S)-8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-
acetic acid ethyl ester
Enantiomerically pure 3(S)-8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-
phenalen-3-yl-acetic acid ethyl ester is obtained by HPL-chromatographic separation of the
racemate. Column: Chiracel OJ 10x50cm; hexane/ethanol = 7:3; 150 ml/min; approx.45 bar; det.: 220 nm. Subsequent purification of the enantiomerically pure fractions
` - 21S723~
59
(removal of phthalates) by flash column chromatography: silica gel, d;:hloromethane/-
methanol = 9:1.; [a]D = + 104.4 (c = 0.25, dimethylformamide); 1H-NMR (CDCI3, 300MHz):
identical to racemate (Example 4); El-MS: 384, 386 (M+); analysis: C 44.42% (43.65);
H 3.73% (3.40); N 7.06% t7.27).
Example 26: 3(R)-8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-Phenalen-3
acetic acid ethyl ester
Preparation analogous to racemate cleavage according to Example 25 [a]D = - 111.2 (c =
0.25, dimethylro"l~a"..de); 1H-NMR (CDCI3, 300MHz): identical to racemate (Example 1).
El-MS: 384, 386 (M+); analysis: C 43.61% (43.65); H 3.59% (3.40); N 6.93% ~7.27).
Example 27: 3(S)-8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-
acetic acid
Preparation analogous to Example 5 from enantiomerically pure 3(S)-8-bromo-4,5-dioxo-
2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid ethyl ester; [a]D = + 94.4
(c = 0.25, dimethylformamide); 1 H-NMR (CDC13, 300MHz): identical to racemate
(Example 2); El-MS: 356, 358 (M+); analysis: C 40.38% (40.35); H 3.15% (2.54); N 7.36%
(7.84).
Example 28: 3(R)-8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-Y
acetic acid
Preparation analogous to Example 5 from enantiomerically pure 3(R)-8-bromo-4,5-dioxo-
2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid ethyl ester; [a]D = - 87.6
(c = 0.25, dimethylro"~amide); 1 H-NMR (CDC13, 300MHz): identical to racemate
(Example 2); El-MS: 356, 358 (M+); analysis: C 40.35% (40.35); H 3.09% (2.54); N 7.23%
(7.84).
Example 29: 2-(3(S)-8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-
3-yl)-N-phenyl-acetamide
Preparation analogous to Example 5 from enantiomerically pure 3(S)-8-bromo-4,5-dioxo-
2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid; [a]D = + 128.0 (c = 0.25,
215~
- 60 -
dimethylformamide); 1H-NMR (CDCI3, 300MHz): identical to racemate (Example 5); El-MS:
431, 433 (M+); analysis: C 49.95% (50.01); H 3.64% (3.26); N 9.19% (9.72).
Example 30: 2-(3(R)-8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-
3-yl)-N-phenyl-acetamide
Preparation analogous to Example 5 from enantiomerically pure 3(R)-8-bromo-4,5-dioxo-
2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid; [a]D = - 124.4 (c = 0.25,
dimethylror"~an.:de; 1 H-NMR (CDCI3, 300MHz): identical to racemate (Example 5); El-MS:
431, 433 (M+); analysis: C 49.96% (50.01); H 3.69% (3.26); N 9.36% (9.72).
Example 31: 8-Chloro-2,3,5,6-dihydro-6H-1-thia-3a,6-diaza-phenalene-4,5-dione
Prepared from (7-chloro-2,3-dihydro-benzo[1,4lthiazin-4-yl)-oxo-acetic acid ethyl ester
analogously to Example 4. Yield: 24%. A solution of 1.0 9 ( 2.6 mmol) of 8-bromo-4,5-dioxo-
2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid ethyl ester in 30 ml of
glacial acetic acid and 25 ml of 30 % water2 is heated at 100 for 18 hours. The solution is
then concentrated almost to dryness and 300 ml of ice-water are added thereto. The
product that precipitates is filtered off, washed neutral with water and dried under a high
vacuum (60). 670 mg (1.61 mmol) = 62 % of 8-bromo-1,1,4,5-tetraoxo-2,3,5,6-tetrahydro-
1 H,4H-6-thia-3a,6-diaza-phenalen-3-yl-acetic acid ethyl ester are obtained in the form of a
white powder, m.p.: >330o; 1 H-NMR (d6-DMSO, 300MHz): 3.20-3.23 (m, 2H); 4.25-4.29 (m,
2H); 6.93 (d, 1H); 7.12 (d, 1H); 12.11 (sbr, 1H); El-MS: 254, 256 (M++1); analysis:
C 47.15% (47.16); H 2.77% (2.81); N 10.92% (1 1.00); TLC: (dichloromethane/methanol
= 19:1) Rf = 0.32.
The preparation of the starting material is described below:
a) (7-Chloro-2,3-dihydro-benzo~1,41thiazin-4-yl)-oxo-acetic acid ethyl ester
Prepared from 7-chloro-3,4-dihydro-2H-benzo[1,41thiazine (CAN: 106016-84-6) analogously
to Example 4b; 1H-NMR (CDC13, 300MHz): 1.14 (t, 3H); 3.21 (t, 2H); 4.03 (t, 2H); 4.16
(quart, 2H); 6.97 (m, 2H); 7.22 (d, 1 H); FD-MS: 285, 287 (M+); TLC: (dichloromethane/-
petroleum ether = 1:1 ) Rf = 0.10.
~1~7~ ~
- 61 -
Example 32: 8-Chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-
acetic acid ethyl ester
Prepared analogously to Example 4; yield: 33%; m.p.: 222-226; 1H-NMR (CDCI3,
200MHz): 1.25 (t, 3H); 2.69 (dd, 1H); 3.00 (dd, 1H); 3.26 (dd, 1H); 3.93 (dd, 1H); 4.18 (q,
2H); 5.78 (m, 1H); 7.08 (dd, 1H); 7.12 (dd, 1H); 12.65 (sbr, 1H); FD-MS: 340, 342 (M+)
TLC: (dichloromethane/methanol = 9:1) Rf = 0.56.
The preparation of the starting materials is described below:
a) (7-chloro-3,4-dihydro-2H-benzo~1,41thiazin-3-yl)-acetic acid ethyl ester
Prepared analogously to Example 4a starting from (7-chloro-4H-benzo[1,4]-3-ylidene)-acetic
acid ethyl ester; yield: 78%; 1H-NMR (CDC13, 300MHz): 1.28 (t, 3H); 2.57 (dd, 1H); 2.70
(dd, 1H); 2.81 (dd, 1H); 3.01 (dd, 1H); 4.06 (m, 1H); 4.18 (q, 2H); 4.67 (sbr, 1H, NH); 6.40
(d, 1H); 6.84 (dd, 1H); 6.98 (dd, 2H); 13C-NMR (CDCI3, 75MHz): 15.2; 31.2; 41.5; 48.3;
61.9; 117.5, 117.9; 123.4; 126.7; 127.8; 140.6; 172.7; TLC: (petroleum ether/ethyl acetate
= 9:1) Rf = 0.12.
b) (7-chloro-3-ethoxycarbonylmethyl-2,3-dihydro-benzo~1,41thiazin-4-yl)-oxo-acetic acid
ethyl ester
Preparation analogous to Example 4c; 1 H-NMR (CDCI3, 300MHz): presumably a mixture of
rotamers: 1.09-1.25 (2xt, 3H); 2.56 (m, 2H); 3.00 (ddbr, 1H); 3.48 (ddbr, 1H); 4.11 (quart,
4H); 5.49 (m, 1H); 6.94 (d, 1H); 7.02 (dd, 1H); 7.25 (d, 1H); FD-MS: 371, 373 (M+).
TLC: (hexane/ethyl acetate = 4:1) Rf = 0.21.
Example 33: 8-Chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-
acetic acid
Preparation analogous to Example 5; yield: 82%; melting point ~300o; 1 H-NMR (d6-DMSO,
300MHz): 2.56 (dd, 1H); 2.77 (dd, 1H); 3.28 (m, 2H); 5.50 (m, 1H); 6.95 (d, 1H); 7.15 (d,
1H); 12.13 (sbr, 1H); 12.58 (sbr, 1H); FD-MS: 312, 314 (M+).
~15723~
- 62 -
Example 34: 2-(8-Chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl)-
N-phenyl-acetamide
Preparation analogous to Example 9; yield: 64%; melting point: >300; 1H-NMR (d6-DMSO,
300MHz): 2.62 (dd, 1 H); 2.90 (dd, 1 H); 3.28 (m, 2H); 5.67 (m, 1H); 6.96 (d, 1H); 7.04 (m,
1H); 7.17 (d, 1H); 7.30 tm, 2H); 7.55 (d, 2H); 10.04 (s, 1H); 12.15 (sbr, 1H).
FD-MS: 387, 389 (M+); analysis: C 55.61% (55.74); H 3.90% (3.64); N 10.94% (10.83).
Example 35: 8-Bromo-1,1,4,5-tetraoxo-2,3,5,6-tetrahydro-1H,4H-6-thia-3a,6-diaza-phenalen-3-yl-acetic acid ethyl ester
A solution of 1.0 g (2.6 mmol) of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-
phenalen-3-ylacetic acid ethyl ester in 30 ml of glacial acetic acid and 25 ml of 30% water2
is heated at 100 for 18 hours and then concentrated almost to dryness. 300 ml of ice-water
are added. The product that precipitates is filtered off, washed neutral with water and dried
under a high vacuum (60). 670 mg (1.61 mmol) = 62% of 8-bromo-1,1,4,5-tetraoxo-2,3,5,6-
tetrahydro-1 H,4H-6-thia-3a,6-diaza-phenalen-3-ylacetic acid ethyl ester are obtained in the
form of a white powder; m.p.: 257-261; 1H-NMR (d6-DMSO, 300MHz): 1.20 (t, 3H); 2.68-
2.90 (m, 1H); 3.08-3.25 (m, 1H); 4.05 (m, 2H); 4.11 (q, 2H); 5.62 (m, 1H); 7.50 (d, 1H); 7.73
(d, 1H); 12.50 (sbr, 1H); FD-MS: 416, 418 (M+); analysis: C 38.51% (40.30); H 3.09%
(3.14); N 6.84% (6.71); TLC: (dichloromethane/hexane/methanol = 6:3:1) Rf = 0.21.
Example 36: 8-Bromo-1, 1 ,4,5-tetraoxo-2,3,5,6-tetrahydro-1 H,4H-6-thia-3a,6-diaza-
phenalen-3-yl-acetic acid
Preparation analogous to Example 5; yield: 83%.; melting point: 281-284; 1H-NMR(d6 DMSO, 300MHz): 2.50 (dd, 1H); 3.18 (dd, 1H); 4.05 (m, 2H); 5.58 (m, 1H); 7.50 (d, 1H);
7.72 (d, 1H); 12.50 (sbr, 2H); FD-MS: 388, 390 (M+); analysis: C 37.26% (37.03); H 2.49%
(2.33); N 7.19% (7.20).
Example 37: 2-(8-Bromo-1, 1 ,4,5-tetraoxo-2,3,5,6-tetrahydro-1 H,4H-6-thia-3a,6-diaza-
phenalen-3-yl)-N-phenyl-acetamide
Preparation analogous to Example 5; yield: 88%; melting point: 185-190; 1H-NMR
(d6 DMSO, 200MHz): 2.75-2.90 (m, 1H); 3.15-3.25 (m, 1H); 4.05 (m, 2H); 5.80 (m, 1H);
7.08 (m, 1H); 7.30 (m, 2H); 7.56 (m, 3H); 7.77 (m, 1H); 10.09 (s, 1H); 12.50 (sbr, 1H);
21572~1
- 63 -
FD-MS: 463, 465 (M+); analysis: C 46.48% (46.56); H 3.47% (3.04); N 9.87% (9.05); TLC:
(dichioromethane/methanol = 9:1) Rf = 0.20.
Example 38: 8-Ghloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-Phenalen-3acetic acid ethyl ester
A solution of 4.2 g (15.51 mmol) of 5-amino-7-chloro-3,4-dihydro-2H-benzo[1,4]oxazin-3-yl-
acetic acid ethyl ester in 52 ml of oxalic acid diethyl ester is rotated on a rotary evaporator
at 80 and 60 mbar for 18 hours. The resulting solid is suspended in Et2O, filtered, washed
with 250 ml of Et2O and dried under a high vacuum (80). 3.35 9 ( 10.31 mmol) = 66% of
8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-ylacetic acid ethyl
ester are obtained in the form of white crystals, m.p.: 265: 1 H-NMR (d6-DMSO, 300MHz):
1.16 (t, 3H); 2.62 (m, 2H); 4.07 (q, 2H); 4.12 (m, 1H); 4.48 (d, 1H); 4.97 (m, 1H); 6.79 (d,
1H); 6.88 (d, 1H); 12.14 (sbr, 1H); FD-MS: 324, 326 (M+).
The preparation of the starting materials is described below:
a) 2-(2-chloromethyl-4-nitro-2,3-dihydro-benzo-oxazol-2-yl)-acetic acid ethyl ester
A mixture of 30 g (194.6 mmol) of 2-hydroxy-6-nitroaniline and 26.5 ml of 4-chloro-aceto-
acetic acid ethyl ester in 130 ml of benzene is boiled in a water separator for 12 hours. The
solvent is removed and the brown, semi-crystalline residue is subjected to flash chromato-
graphy on silica gel (eluant: petroleum ether/ethyl acetate = 9:1). 49.7 g (165.3 mmol) =
85% of (2-chloromethyl-4-nitro-2,3-dihydro-benzo-oxazol-2-yl)-acetic acid ethyl ester are
obtained in the form of fluorescent-orange crystals; m.p.: 90-91; 1H-NMR (CDCI3,
300MHz): 1.24 (t, 3H); 3.09 (d, 1H); 3.28 (d, 1H); 3.92 (dd, 2H); 4.19 (q, 2H); 6.63 (t, 1H);
6.82 (d, 1H); 7.09 (sbr, 1H, NH); 7.48 (d, 1H); 13C-NMR (CDC13, 50MHz): 14.5; 41.6; 48.1;
62.1; 100.6; 112.2; 116.4; 118.7; 135.8; 151 5; 169.2; FD-MS: 300, 302 (M+); TLC:
(petroleum ether/ethyl acetate = 9:1) Rf = 0.23
b) 2-(6-chloro-2-chloromethyl-4-nitro-2,3-dihydro-benzooxazol-2-yl)-acetic acid ethyl ester
A solution of 45 g (149.7 mmol) of (2-chloromethyl-4-nitro-2,3-dihydro-benzo-oxazol-2-yl)-
acetic acid ethyl ester and 24 g (179.6 mmol) of N-chlorosuccinimide in 500 ml of
dimethylformamide is heated at 60 for 18 hours. The solution is concentrated on a rotary
~15723~
- 64 -
evaporator/under a high vacuum, and the brownish-orange residue is taken up in
dichloromethane, washed twice with water and saturated sodium chloride solution, dried
over magnesium sulfate and concentrated on a rotary evaporator (50). 58.2 g (~100%) of
(6-chloro-2-chloromethyl-4-nitro-2,3-dihydro-benzooxazol-2-yl)-acetic acid ethyl ester are
obtained in the form of a reddish-orange oil that is pure according to NMR; 1 H-NMR
(CDCI3, 200MHz): 1.26 (t, 3H); 3.18 (AB-syst., 2H); 3.90 (dd, 1H); 4.18 (q, 2H); 6.69 (dd,
1H); 7.12 (sbr, 1H, NH); 7.48 (d, 1H); TLC: (dichloromethane/hexane = 7:3) Rf = 0.27.
c) (7-chloro-5-nitro-4H-benzo~1,41Oxazin-3-ylidene)-acetic acid ethyl ester
A solution of 30 g (89.51 mmol) of 6-chloro-2-chloromethyl-4-nitro-2,3-dihydro-benzooxazol-
2-yl)-acetic acid ethyl ester in 150 ml of THF is added dropwise at 0-5 to a suspension of
4.7 g (107.4 mmol) of sodium hydride (in the form of a 55% suspension in oil) in 150 ml of
THF. The mixture is stirred for 30 minutes and then heated to room temperature and stirred
for 1 hour. The solvent is removed on a rotary evaporator (50) and the semi-crystalline,
black oil is chromatographed on silica gel (dichloromethane/hexane = 7:3) . After crystal-
lisation from ether/hexane, 20.3 g (68 mmol) (76%) of (7-chloro-5-nitro-4H-benzo[1,4]-
oxazin-3-ylidene)-acetic acid ethyl ester are obtained in the form of orange crystals; m.p.:
162-163; 1H-NMR (CDCI3, 300MHz): 1.33 (t, 3H); 4.28 (q, 2H); 4.67 (d, 2H); 4.98 (s, 1H);
7.18 (dd, 1H); 7.89 (d, 1H); 12.27 (sbr, 1H); TLC: (dichloromethane/hexane = 7:3) Rf =
0.40.
d) 5-amino-7-chloro-4H-benzo~1,41Oxazin-3-ylideneacetic acid ethyl ester
7.0 g (23.44 mmol) of 7-chloro-5-nitro-4H-benzo[1,4]oxazin-3-ylideneacetic acid ethyl ester
are hydrogenated in 300 ml of ethanol with 2 g of Raney nickel at room temperature and
under normal pressure for 6 hours. The reaction mixture is filtered and concentrated and
the residue is chromatographed on silica gel (dichloromethane/hexane = 7:3) . After
crystallisation from ethanollhexane, 5.67 g (21.1 mmol) (90%) of 5-amino-7-chloro-4H-
benzo[1,4]oxazin-3-ylideneacetic acid ethyl ester are obtained in the form of fine pink
needles; m.p.: 158-159
1H-NMR (CDCI3, 200MHz): 1.28 (t, 3H); 3.55 (sbr, 2H, NH2); 4.17 (q, 2H); 4.51 (s, 2H);
4.72 (s, 1H); 6.42 (d, 1H); 6.45 (d, 1H); 9.95 (sbr, 1H, NH); FD-MS: 268, 270 (M+); TLC:
(dichloromethane/hexane = 7:3) Rf = 0.21.
-65- ~1572~1
e) 5-amino-7-chloro-3,4-dihydro-2H-benzo~1,41oxazin-3-ylacetic acid ethyl ester
Preparation analogous to Example 4a; yield 94% yellowish oil; 1H-NMR (CDC13, 300MHz):
1.27 (t, 3H); 2.55 (d, 2H); 3.38 (sbr, 3H, NH, NH2); 3.89-3.95 (m, 2H); 4.14-4.23 (m, 3H);
4.17 (q, 2H); 6.33 (dd, 1H); 6.38 (dd, 1H); 13C-NMR (CDC13, 50MHz): 14.7; 37.2; 47.3;
61.4; 68.8; 108.8; 109.5; 119.7; 125.0; 137.2; 145.8; 171.9; TLC: (dichloromethane) Rf =
0.13.
Example 39: 8-Chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl-
acetic acid
P,eparalion analogous to Example 5; yield: 98% whitish-beige crystals; melting point: 236-
240; 1H-NMR (d6-DMSO, 200MHz): 2.52 (t, 3H); 4.12 (dd, 1H); 4.50 (d, 2H); 4.94 (td, 1H);
6.83 (d, 1H); 6.88 (d, 1H); 12.13 (s, 1H); 12.65 (sbr, 1H); FD-MS: 296, 298 (M+); analysis:
C 44.31% (46.36); H 3.43% (3.43); N 8.57% (9.01); Cl 4.56% (4.58).
Example 40: 8-Chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl-
acetic acid sodium salt
Preparation analogous to Example 2a.
Example 41: 2-(8-Chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl)-
N-phenyl-acetamide
Preparation analogous to Example 9; yield: 93% pale beige crystals; m.p.: 263-265;
1H-NMR (d6-DMSO, 200MHz): 2.60-2.73 (m, 2H); 4.15 (dd, 1H); 4.50 (d, 2H); 5.10 (m, 1H);
6.82 (d, 1H); 6.88 (d, 1H); 7.05 (t, 1H); 7.30 (t, 2H); 7.55 (d, 2H); 10.05 (s, 1H); 12.10 (sbr,
1H); FD-MS: 371, 373 (M+); analysis: C 55.11% (58.15); H 4.03% (3.80); N 11.11% (11.30);
Cl 9 94% (9 54).
Example 42: In a manner analogous to that described in Example 41 it is also possible to
prepare 2-(8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl)-N-
methyl-N-phenyl-acetamide; yield: 82% beige crystals; 1H-NMR (d6-DMSO, 300MHz): 2.32
(d, 2H); 3.15 (s, 3H); 4.07 (d, 1H); 4.45 (d, 1H); 4.97 (m, 1H); 6.70 (d, 1H); 6.78 (d, 1H);
-66 215~31
7.28-7.45 (m, 5H); FD-MS: 385, 387 (M+); analysis: C 58.08% (59.15); H 4.34% (4.18);
N 12.00% (10.89); TLC: (dichloromethane/methanol/NH3 = 700:50:1) Rf = 0.37.
Example 43: N-Adamantan-1-yl-2-(8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-
diaza-phenalen-3-yl)-acetamide
Preparation analogous to Example 9. Beige powder; m.p.: 235-238; 1H-NMR (d3-DMSO
200MHz): 1.60-2.05 (m, approx.15H); 2.32-2.46 (m, 2H); 4.10 (dd, 1H); 4.38 (d, 1H); 4.11
(q, 2H); 4.97 (m, 1H); 6.78 (d, 1H); 6.88 (d, 1H); 7.50 (sbr, 1H); 12.10 (sbr, 1H); FD-MS:
429, 431 (M+).
Example 44: 2-(8-Ghloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl)-
N, N-bis(2-hydroxy-ethyl)acetamide
Preparation analogous to Example 9; yield: 15% white crystals; m.p.: 220-222; 1H-NMR
(d3-DMSO 300MHz): 1.60-1.82 (m, 2H); 3.42 (m, 6H); 4.12 (d, 1H); 4.25 (d, 1H); 4.72 (dbr,
2H); 4.97 (d, 1H); 6.80 (d, 1H); 6.88 (d, 1H); 12.10 (sbr, 1H); FD-MS: 383, 385 (M+)
Example 46: 2,3-Dihydro-6H-1-oxa-3a,6-diaza-phenalene-4,5-dione
Preparation analogous to Example 38; yield 75% yellow crystals; m.p.: 291; 1H-NMR
(d6 DMSO, 300MHz): 4.03 (t, 2H); 4.32 (t, 2H,); 4.03 (t, 2H); 6.69-6.77 (m, 2H); 7.00-7.05
(m, 1H); 12.02 (s br, 1H, HN); 13C-NMR (d6-DMSO, 74MHz): 40.35; 63.61; 108.08; 110.66;
114.06; 123.97; 126.62; 143.88; 153.29; 154.22; FD-MS: 204 (M+); analysis: C 58.68%
(58.82); H 4.04% (3.95); N 13.33% (13.72).
- 67 ~15 ~ '~ 3 i
Example 46: 4,5-Dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl-acetic acid
ethyl ester
Analogous to Example 38; yield: 79% white crystals; m.p.: 256-257; 1H-NMR (d6-DMSO,
300MHz): 1.71 (t, 3H); 2.66 (m, 2H); 4.09 (q, 2H); 4.15 (m,1H); 4.48 (d, 1H); 5.00 (tbr, 1H);
6.74-6.83 (m, 2H); 7.07 (t, 1H); 12.08 (sbr,1H); 13C-NMR (d6-DMSO, 50MHz): 14.1; 33.9;
46.7; 60.8; 66.1; 108.5; 110.7; 113.3; 124.1; 126.8; 143.3; 153.0; 154.2; 170.2;
El-MS: 290 (M+); analysis: C 57.79% (57.93); H 5.03% (4.86); N 9.70% (9.65).
The preparation of the starting materials is described below:
a) 5-nitro-4H-benzor1,41Oxazin-3-ylideneacetic acid ethyl ester
Preparation analogous to Example 37c; yield: 97% yellow crystals; m.p.: 142; 1 H-NMR
(CDCI3, 300MHz): 1.33 (t, 3H); 4.28 (q, 2H); 4.66 (s, 2H); 4.94 (s, 1H); 7.16 (t, 1H); 7.19 (d,
1H); 7.89 (dd, 1H); 12.25 (sbr, 1H); FD-MS: 264 (M+); TLC: (petroleum ether/ethyl acetate
=9:1) Rf=0.43.
b) 5-amino-3,4-dihydro-2H-benzo~1,41oxazin-3-ylacetic acid ethyl ester
1.0 9 (3.78 mmol) of 5-nitro-4H-benzo[1,4]oxazin-3-ylideneacetic acid ethyl ester is
hydrogenated at from room temperature to 50 in 50 ml of ethanol with 0.2 9 of 10% Pd/C
under normal pressure for 50 hours. The reaction mixture is filtered and concentrated and
the residue is chromatographed on silica gel (petroleum ether/ethyl acetate = 8:2). 753 mg
(3.19 mmol) = 84% of 5-amino-3,4-dihydro-2H-benzo[1,4]oxazin-3-yl-acetic acid ethyl ester
are obtained in the form of an orange oil; 1 H-NMR (CDCI3, 200MHz): 1.25 (t, 3H); 2.55 (d,
2H); 3.40 (sbr, 3H); 3.80-3.97 (m, 2H); 4.05-4.15 (m,1H); 4.18 (q, 2H); 6.31-6.40 (m, 2H);
6.60 (t,1 H); FD-MS: 236 (M+); TLC: (ethyl acetate/petroleum ether = 7:3) Rf = 0.17.
Example 47: 7,9-Dibromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl-
acetic acid ethyl ester
Preparation analogous to Example 4b with 2 equivalents of N-bromosuccinimide. Yield:
92% white crystals; m.p.: 285-290; 1H-NMR (d3-DMSO 300MHz): 1.18 (t, 3H); 2.61 (d, 2H);
4.08 (q, 2H); 4.15 (d, 1H); 4.60 (d,1H); 5.02 (m,1H); 7.68 (s, 1H); 11.28 (sbr, 1H); FD-MS:
446, 448, 450 (M+).
` 21~23~
- 68 -
Example 48: 8-Bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl-
acetic acid ethyl ester
Preparation analogous to Example 38; yield 15% beige crystals; m.p.: 222-227; 1H-NMR
(d6-DMSO, 200MHz): 1.17 (t, 3H); 2.62 (m, 2H); 4.08 (q, 2H); 4.12 (m, 1H); 4.50 (d,1H);
4.97 (m, 1H); 6.95 (d, 1H); 7.00 (d, 1H); 12.11 (sbr,1H); FD-MS: 368, 370 (M+).
The preparation of the starting materials is described below:
a) 5-nitro-3,4-dihydro-2H-benzo~1,4loxazin-3-ylacetic acid ethyl ester
A solution of 1.0 9 (3.33 mmol) of (2-chloromethyl-4-nitro-2,3-dihydro-benzo-oxazol-2-yl)-
acetic acid ethyl ester in 5 ml of THF is added at 0-5 to 145 mg (3.33 mmol) of 55%
sodium hydride dispersion in 5 ml of THF. The mixture is stirred for 15 minutes at 0 and for
1 hour at room temperature. 2.1 9 (33.26 mmol) of sodium cyanoborohydride are then
added and the mixture is cooled to 0 and 15 ml of 5N ethanolic hydrochloric acid are
added dropwise. The mixture is concentrated on a rotary evaporator and the residue is
taken up in dichloromethane and washed with water and saturated sodium chloride solution.
The organic phases are dried over magnesium sulfate and concentrated and the orange
crude product is subjected to flash chromatography. 389 mg (1.46 mmol) = 44% of (5-nitro-
3,4-dihydro-2H-benzo[1,4]oxazin-3-yl)-acetic acid ethyl ester are obtained in the form of an
orange oil; 1 H-NMR (CDCI3, 200MHz): 1.28 (t, 3H); 2.63 (m, 2H); 3.96-4.20 (m, 3H); 4.21
(q, 2H); 6.56 (t, 1H); 7.00 (d, 1H); 7.78 (d, 1H); 8.21 (sbr, 1H, NH); FD-MS: 266 (M+); TLC:
(dichloromethane/hexane = 8:2) Rf = 0.28.
b) 7-bromo-5-nitro-3,4-dihydro-2H-benzo~1,41oxazin-3-ylacetic acid ethyl ester
Preparation analogous to Example 4b; yield: 53% orange oil; 1H-NMR (CDC13, 200MHz):
1.29 (t, 3H); 2.61 (d, 2H); 3.98-4.20 (m, 3H); 4.21 (q, 2H); 7.10 (d, 1H); 7.92 (d, 1H); 8.26
(sbr, 1 H, NH); TLC: (dichloromethane/hexane = 8:2) Rf = 0.38
c) 5-amino-7-bromo-3,4-dihydro-2H-benzo~1,41oxazin-3-ylacetic acid ethyl ester
811 mg (3.6 mmol) of tin(ll) chloride dihydrate are added to a solution of 248 mg
(0.72 mmol) of (7-bromo-5-nitro-3,4-dihydro-2H-benzo[1,4]oxazin-3-yl)-acetic acid ethyl
ester in 10 ml of ethanol and the mixture is stirred at 80 for 5 hours. The solvent is
-69 2 15~ 2 3 ~
removed and the residue is taken up in dichloromethane and washed twice with water. The
organic phases are dried over magnesium sulfate and concentrated. 225 mg of (5-amino-7-
bromo-3,4-dihydro-2H-benzo[1,4]oxazin-3-yl)-acetic acid ethyl ester are obtained in the form
of a brown oil which is used further without being purified.
Example 49: 8-Chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-Phenalen-3acetic acid butyl ester
In a water separator, 3.0 9 (10.11 mmol) of 8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -oxa-
3a,6-diaza-phenalen-3-yl-acetic acid are heated under reflux overnight in approx. 100 ml of
CCI4, 1.02 ml (11.12 mmol) of 1-butanol and 27.1 1ll of concentrated sulfuric acid. A further
15 ml of 1-butanol and 100 ~l of concentrated sulfuric acid are added and the mixture is
heated for 6 hours. The solvent is removed and the residue is taken up in dichloromethane
and washed with water, saturated sodium hydrogen carbonate solution and saturated
sodium chloride solution and dried over magnesium sulfate. The solvent is removed and the
residue is crystallised from dich'oromethane/petroleum ether. 2.59 9 (7.34 mmol) = 73% of
8-chloro-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,6-diaza-phenalen-3-yl-acetic acid butyl
ester are obtained in the form of brownish-beige crystals; 1 H-NMR (d6-DMSO, 200MHz):
0.87 (t, 3H); 1.30 (m, 2H); 1.50 (m, 2H); 2.65 (m, 2H); 4.00 (t, 2H); 4.15 (dd, 1H); 4.50 (dbr,
1H); 4.97 (t(m), 1H); 6.81 (d,1H); 6.88 (d,1H); 12.15 (sbr,1H); FD-MS: 352, 354 (M+); TLC:
(dichloromethane/methanol = 19:1) Rf = 0.33.
Example 50: 8-Methyl-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-
acetic acid ethyl ester
Preparation analogous to Example 4.
The preparation of the starting materials is described below:
a) (7-methyl-3,4-dihydro-2H-benzo~1,41thiazin-3-yl)-acetic acid ethyl ester
Prepared from (7-methyl-4H-benzo[1,4]-3-ylidene)-acetic acid ethyl ester analogously to
Example 4a; 1H-NMR (CDCI3, 300MHz): 1.28 (t, 3H); 2.57 (dd,1H); 2.70 (dd, 1H); 2.81 (dd,
1H); 3.01 (dd, 1H); 4.06 (m,1H); 4.18 (q, 2H); 4.67 (sbr, 1H, NH); 6.40 (d,1H); 6.84 (dd,
1H); 6.98 (dd, 2H); TLC: (petroleum ether/ethyl acetate = 9:1) Rf = 0.12.
70 215~
b) 2-(7-methyl-3-ethoxycarbonylmethyl-2,3-dihydro-benzo[1,41thiazin-4-yl)-2-oxo-acetic acid
ethyl ester
Prepared analogously to Example 4c; 1H-NMR (CDCI3, 300MHz): presumably a mixture of
rotamers: 1.09-1.25 (2xt, 3H); 2.56 (m, 2H); 3.00 (ddbr,1H); 3.48 (ddbr, 1H); 4.11 (quart,
4H); 5.49 (m, 1 H); 6.94 (d, 1H); 7.02 (dd,1 H); 7.25 (d,1 H); FD-MS: 371, 373 (M+);
TLC: (hexane/ethyl acetate = 4:1) Rf = 0.21.
Example 52: In a manner analogous to that described in Examples 4 to 48, it is also
possible to prepare 8-methyl-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-
yl-acetic acid.
Example 53: In an analogous manner as described in 1 to 52, also the following compounds
can be manufactured: beschrieben kann man auch folgende Verbindungen der Formel I
herstellen:
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-ethoxy-
acelam.de, melting point 202-204;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-hydroxy-
acetamide, melting point 220-221 ;
2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(2,2,2-
trifluoroethyl)-acetamide, melting point 270-271;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-methoxy-N-
methyl-acetamide, melting point 201-202;
2-[2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-
yl)acetylamino)-acetic acid ethyl ester, melting point 200-201;
2-[2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H -1 -thia-3a ,6-diaza-phenalen-3-
yl)acetylamino)-propionic acid methyl ester, melting point 283-285;
21572~1
- 71 -
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(2,3,4,5,6-
pentafluorobenzyloxy)-acetamide, melting point 147-148;
2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-phenoxy-
acetamide, melting point 192-193;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N -(3-oxo-3,4-
dihydro-2H-benzo[1,4]oxazin-7-ylmethyl)-acetamide, melting point 300-301;
2-[2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-
yl)acetylamino)-5-(tertiary-butyloxycarbonylaminomethyl)benzoic acid methyl ester,
melting point 94-95;
2-[2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-
yl)acetylamino)-5-(tertiary-butyloxycarbonylaminomethyl) benzoic acid, melting
point ~ 300;
4-[2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-
yl)acetylamino)-benzoic acid, melting point 298-300;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(3,5-
bistrifluoromethylphenyl)-acetamide, melting point > 250;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(2-chlor-5-
trifluoromethylphenyl)-acetamide, melting point 192-198;
2-~8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(2-
methoxyphenyl)-acetamide, melting point > 250;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(2-
trifluoromethoxyphenyl)-acetamide, melting point 14Z-162;
2157~31
- 72 -
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(4-
methoxyphenyl)-acetamide, melting point ~ 250;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(2,5-
dimethoxyphenyl)-acetamide, melting point > 250;
2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(2-fluoro-5-
trifluoromethyl-phenyl)-acetamide, melting point 163-170;
2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(4-
phenoxyphenyl)-acetamide, melting point 137-145;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(4-(4-
chlorphenoxy)phenyl]-acetamide, melting point 104-120;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(3,4,5-
trimethoxyphenyl)-aceldr"ide, melting point 240-246;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(4-fluoro-2-
trifluoromethyl-phenyl)-acetamide, melting point 198-205;
2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(3-
phenoxyphenyl)-acetamide, melting point 120-128;
2-(8-bromo4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a,6-diaza-phenalen-3-yl)-N-(1 -cyano-1 -
methylethyl)-acetamide, melting point.~ 250;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(2-methoxy-
5-methyl-phenyl)-acetamide, melting point.~ 250;
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H- 1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(3-methoxy-
phenyl)-acetamide, melting point 98-110;
~1S723~
- 73 -
2-(8-bromo-4,5-dioxo-2,3,5,6-tetrahydro4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(3,5-
dimethoxy-phenyl)-acetamide, melting point 130-138;
8-Methyl-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid,
melting point ~ 250;
2-(8-methyl-4,5-dioxo-2,3,5,6-tetrahydro-4H-1 -thia-3a ,6-diaza-phenalen-3-yl)-N-phenyl-
acetamide, melting point > 250;
2-(8-methyl-4,5-dioxo-2,3,5,6-tetrahydro-4H -1 -thia-3a ,6-diaza-phenalen-3-yl)-N-(tertiary-
butyloxy)-acetamide, melting point ~ 250.
Example 54: Tablets, each comprising 50 mg of active ingredient, for example 8-bromo-4,5-
dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-yl-acetic acid, or a salt thereof,
can be prepared as follows:
Composition (10 000 tablets)
active ingredient 500.0 g
lactose 500 0 g
potato starch 352.0 g
gelatin 8.0 9
talc 60.0 g
magnesium stearate 10.0 9
silicon dioxide (highly dispersed) 20.0 9
ethanol q.s.
The active ingredient is mixed with the lactose and 292 g of the potato starch, and the
mixture is moistened with an ethanolic solution of the gelatin and granulated through a
sieve. After drying, the remainder of the potato starch, the magnesium stearate, the talc and
the silicon dioxide are mixed in and the mixture is compressed to form tablets each
weighing 145.0 mg and comprising 50 mg of active ingredient; if desired, the tablets may be
provided with dividing notches for finer adaptation of the dose.
~ 74 21572~ 1
Example 55: A sterile-filtered aqueous gelatin solution, containing 20 % cyclodextrins as
solubiliser, comprising 3 mg of 8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-
phenalen-3-yl-acetic acid or a salt, for example the sodium salt, thereof as active ingredient,
is so mixed, with heating and under aseptic conditions, with a sterile gelatin solution
containing phenol as preservative, that 1.0 ml of solution has the following composition:
active ingredient 3 mg
gelatin 150.0 mg
phenol 4.7 mg
dist. water with 20 % cyclodextrins as solubiliser 1.0 ml
Example 56: For the preparation of a sterile dry substance for injection, comprising 5 mg of
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid or a
salt, for example the sodium salt, thereof, 5 mg of one of the compounds of formula I
mentioned in the preceding Examples as active ingredient are dissolved in 1 ml of an
aqueous solution containing 20 mg of mannitol and 20 % cyclodextrins as solubiliser. The
solution is sterile-filtered and, under aseptic conditions, introduced into a 2 ml ampoule,
frozen and Iyophilised. Before use, the Iyophilisate is dissolved in 1 ml of dis tilled water or
1 ml of physiological saline. The solution is administered intramuscularly or intravenously.
This formulation can also be introduced into double-chamber disposable syringes.
Example 57: For the preparation of 10 000 film-coated tablets, each comprising 100 mg of
8-bromo-4,5-dioxo-2,3,5,6-tetrahydro-4H-1-thia-3a,6-diaza-phenalen-3-ylacetic acid or a
salt, for example the sodium salt, thereof, the method of preparation is as follows:
active ingredient 1000 9
corn starch 680 9
colloidal silicic acid 200 9
magnesium stearate 20 9
stearic acid 50 9
sodium carboxymethyl starch 250 9
water q. s.
2 l~7 231
- 75-
A mixture of one of the compounds of formula I mentioned in the preceding Examples, as
active ingredient, 50 9 of corn starch and the colloidal silicic acid is processed to form a
moist mass with a starch paste consisting of 250 9 of corn starch and 2.2 kg of demineral-
ised water. The mass is forced through a sieve of 3 mm mesh size and dried in a fluidised-
bed drier at 45 for 30 minutes. The dried granules are then pressed through a sieve of
1 mm mesh size, mixed with a previously sieved mixture (1 mm sieve) of 330 9 of corn
starch, the magnesium stearate, the stearic acid and the sodium carboxymethyl starch and
compressed to form slightly biconvex tablets.
Example 58: In a manner analogous to that described in Examples 54 to 57 it is also
possible to prepare pharmaceutical compositions comprising another compound according
to any one of Examples 1 to 53.