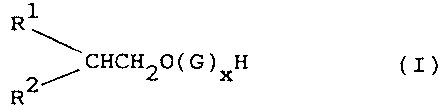Note: Descriptions are shown in the official language in which they were submitted.
21x7285
ALKYL GLYCOSIDE AND USE THEREOF
The present invention relates to a new alkyl glyco-
side presenting an advantageous combination of good clean-
s ing power and low foaming, which renders it particularly
suitable for cleaning hard surfaces.
In recent years, attention has focused on alkyl gly-
cosides, since these have proved to be more easily bio-
degradable than other non-ionic surfactants, such as ethy-
lene oxide adducts of fatty alcohols. US Patent Specifica-
tion 3,839,318 thus describes the production of alkyl glu-
cosides and alkyl oligosaccharides, such as n-octyl gluco-
side, n-hexyl glucoside, n-decyl glucoside, n-dodecyl glu-
coside, isodecyl glucoside, isoundecyl glucoside, isotri-
decyl glucoside and the corresponding oligosaccharides.
The United States Stationary Invention Registration H171
states that alkyl glycosides of formulae R(OG) and R(OG)x
are excellent surfactants. In these formulae, R is an
alkyl or alkenyl group which is branched at the second
carbon atom or at a higher carbon atom, the branch being
selected from the group methyl, ethyl, isopropyl, n-pro-
pyl, butyl, pentyl, hexyl and mixtures thereof, provided
that R contains from about 7 to about 30 carbon atoms;
G is a saccharide group selected from the group glucose,
fructose, mannose, galactose, talose, allose, altrose,
idose, arabinose, xylose, lyxose, ribose and mixtures
thereof; and x is 2 or more. Example 1 contains a descrip-
tion of the production of two product mixtures substan-
tially made up of 2-ethylhexyl glycoside and isooctyl gly-
coside, respectively.
DE 20 36 472, EP 306 650, EP 306 651 and EP 366 652,
inter alia, also describe alkyl glycosides.
Even though alkyl glycosides generally are easily
biodegradable, they are only used to a limited extent in
many ranges of application, such as the cleaning of hard
surfaces, since they are too high-foaming and/ or have a
poor cleaning power. Also, alkyl glycoside products con-
'A
2
taining branched alkyl groups often have a disagreeable
smell. It is therefore a desideratum to provide non-ionic
surfactants which are about as easily biodegradable, but
which have a better cleaning power and/or are more low-
s foaming than known alkyl glycosides.
According to the invention, it has now surprisingly
been found that an alkyl glycoside of the general formula
R1
~CHCH20(G)xH
R2
wherein R1 is an alkyl group having 2-5 carbon atoms,
preferably 2-4 carbon atoms; R2 is an alkyl group having
4-7 carbon atoms, preferably 5 or 6 carbon atoms, the sum
of the carbon atoms in R1 and R2 being 7-11, preferably
7-9, G is a monosaccharide residue, and x is 1-4, prefer-
ably 1 or 2, has good cleaning and wetting properties and
is low-foaming compared with other alkyl glycosides of
approximately the same chain length. Compounds of formula
(I) in which R1 is an alkyl group having 3 carbon atoms,
R2 is an alkyl group having 5 carbon atoms, and G is a
glucose residue, are especially preferred. The glucosides
according to the invention do not have any disagreeable
smell. In addition, they have been found to be easily
degradable and have low biotoxicity. Tests have not shown
any skin irritations caused by the alkyl glycosides.
The inventive compounds can be produced in conven-
tional manner by reacting an alcohol of formula
R
~CHCH20H (I1)
R2
wherein R1 and R2 are as indicated above, with a monosac-
charide, the molar ratio of the alcohol to the monosaccha-
ride being 2:1-80:1, in the presence of an acid catalyst.
A
215725
3
The catalyst may be an inorganic or organic acid. The
reaction is carried out under vacuum at 90-120C for about
1-4 h. Conveniently, the resulting reaction mixture is
first filtered and then neutralised with an organic and/or
an inorganic base. Finally, excess alcohol is carefully
removed, e.g. by distillation, if so desired.
The alcohols of formula (II) can be obtained by a
Guerbet reaction starting from n-pentanol, n-hexanol or
mixtures of n-pentanol and n-hexanol, n-pentanol and n-
-butanol, n-hexanol and n-butanol, and n-hexanol and n-
-pentanol, or by an aldol condensation of the correspond-
ing aldehydes. Preferably, the alkanol of formula (II) is
2-propyl heptanol. The monosaccharide used as reactant
suitably is pentose and hexose. Specific examples of mono-
saccharides used in the production of the inventive gluco-
sides are glucose, mannose, galactose, talose, allose,
altrose, idose, arabinose, xylose, ribose and lyxose. Glu-
cose is usually preferred for commercial reasons.
The inventive alkyl glycosides are suitably used
as surfactants in cleaning compositions, e.g. for degreas-
ing hard surfaces or washing up. Excellent results are
obtained in the degreasing of lacquered or unlacquered
metal surfaces. Apart from the inventive alkyl glycoside,
these compositions preferably contain a water-soluble
solubiliser and suitably contain a complexing agent.
Examples of solubilisers are alkyl ether polyalky-
lene glycol, such as monobutyl diethylene glycol; glycols,
such as diethylene glycol, dipropylene glycol and propy-
lene glycol; alcohols, such as ethanol, propanol and iso-
propanol; alkyl glycosides in which the alkyl group has
4-18 carbon atoms; and/or tertiary or quaternary amine
alkoxylates, in which the alkyl group, which may be
straight or branched, saturated or unsaturated, has 8-20
carbon atoms, and 6-30 mol of alkylene oxide is added per
mol of amine. Preferably, 50-100 mol per cent of the added
alkylene oxide consists of ethylene oxide, while the
remaining amount preferably consists of propylene oxide
or
2~~~2~~
4
a mixture of propylene oxide and butylene oxide. The dif-
ferent alkylene oxides can be added randomly or in blocks.
If the cleaning composition should be low-foaming, the
alkylene oxide chain suitably ends with an addition of
1-5 mol of propylene oxide and/or butylene oxide. Usually,
the ratio of solubiliser to the inventive alkyl glycoside
is 1:10-5:1, preferably 1:3-3:1.
The complexing agent may be a conventional inorganic
or organic complexing agent, such as an inorganic phos-
phate or NTA, EDTA, citric acid or a polycarboxylate. The
amount added may vary from nothing at all to 300$ by
weight of the inventive alkyl glycoside. Preferably, the
quantity ratio of the complexing agent to the alkyl glyco-
side is 1:10-2:1.
The cleaning compositions may further contain other
additives, such as pH-adjusting agents, antifoaming
agents, enzymes, other surfactants and scents. The compo-
sitions are usually aqueous and in the form of emulsions,
microemulsions or solutions.
The invention will be further illustrated by the fol-
lowing Examples.
Example 1
An alkyl glycoside was produced by reacting 3 mol of
2-propylheptanol with 0.45 mol of glucose in the presence
of 0.015 mol of sulphuric acid as catalyst at 110°C and
70 mbar. The reaction was interrupted after 65 min. The
resulting product mixture was treated by distilling off
excess alcohol under vacuum. The yield was 50 g, consist-
ing of 74$ of 2-propylheptyl monoglucoside, 15$ of 2-pro-
pylheptyl diglucoside and a residue of higher oligomers.
The glucosides had an average degree of polymerisation
(DP) of about 1.3. The structure was determined by gas
chromatography, mass spectrometry and NMR.
Example 2
As in Example 1, 2-butyloctanol was reacted with glu-
cose. The reaction temperature was 112°C, and the reaction
time was 90 min. The average DP was 1.5.
'A
2157285
Example 3
Here, 20 ml of each of the cleaning compositions
below, diluted with 10 parts by weight of water per part
by weight of the composition, was applied to a vertically
arranged iron sheet soiled with mineral oils, soot, salts
and clay. After application, the coated surface was rinsed
with water without any mechanical treatment.
Components Composition,
$
by
weight
1 2 3 4 A B C D E
Glucoside (Example 1) 5 5 5
Glucoside (Example 2) 5
Glucoside A 8.5 5
Glucoside B 5
Glucoside C 5
Glucoside D 5
Butyldiethylene glycol 11 11 11 11 11 11 11
Quaternary ethoxy-
lated fatty amine
(Berol 555) 4
NTA 5 5 3 3 3 3 3 3 5
Water 86 79 83.5 81 81 81 81 81 84
Glucoside A = 2-ethylhexyl-O(G)xH
Glucoside B = isooctyl-O(G)xH
Glucoside C = n-dodecyl/n-tetradecyl glycoside (Plantaren,
APG-600, Henkel)
Glucoside D = n-decyl glucoside (Lutensol GD-70, BASF)
G = glucoside residue and x = 1.5 (average value).
The resulting cleaning effect was assessed with
respect to the area of the cleaned surface, as well as
the actual cleanness of this surface, the figure 1 indi-
cating no improvement and the figure 10 indicating a per-
fectly clean surface. The following results were obtained.
WO 94/21655 PCT/SE94/00199
21572s5
6
-,-
Composition Cleaned surface, cm2 Cleanness
1 88 g
2 120 g
128 g
4 112 8
A 0 1
H 80 4
C 48 6
D 72 6
0 1
The foaming of the different ready-to-use solutions
was measured according to Ross-Miles ASTM D 1173-53. The
following results were obtained.
Foam height, mm
Composition
Instantaneously After 5 min
1 19 3
2 23 5
3 8 5
4 30 7
A 7 0
H 20 3
C 67 63
D 46 45
21~728~
These results show that the alkyl glycosides accord-
ing to the invention have an excellent cleaning power and
are clearly superior to alkyl glycosides having a straight
carbon chain with 10-14 carbon atoms, while at the same
time showing an acceptable degree of foaming. The compo-
sition containing alkyl glycosides having an alkyl group
with 8 carbon atoms shows an unsatisfactory cleaning
power.
15
25
35
A