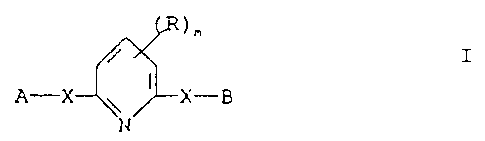Note: Descriptions are shown in the official language in which they were submitted.
CA 02158981 2005-03-09
-1-
HERBICIDAL HETEROCYCLIC-SUBSTITUTED PYRIDINES
The present invention is concerned with certain 2,6-substituted
pyridines, their preparation and use as herbicides.
Pyridines and pyridine derivatives have many uses, for example, as
pharmaceuticals, pesticides (herbicides, acaricides, anthelmintics, bird
repellants), feed supplements, solvents, reagents, intermediates, and
chemicals for the polymer and textile industry. Various 2,6-diaryloxy-
or -diarylmethoxy-pyridine derivatives have been investigated for such uses,
the compounds often having additional substitution of the central pyridine
ring.
The dibenzyloxy analogue of 2,6-diphenoxy-pyridine, in addition to
being mentioned in JP-A-63250324, EP-A-180188 and J. Med. Chem, 10(2),
pages 320 to 325, all medical research publications, was prepared as an
example of the herbicidal compounds proposed in US-A-3 535 328 and the
divisional published as US-A-3 687 959. Both texts are predominantly directed
to the herbicidal, fungicidal, and, for the compounds claimed in US-A-3 535
328, nematocidal and insecticidal activity, of amido or aminothioethoxy
derivatives. No herbicidal activity data is given for the disclosed 2,6-
dibenzyloxypyridine.
Applicant's European Patent 572093 describes how certain substituted
forms of 2,6-diphenoxypyridine and 2,6-dibenzyloxypyridine, and also the
related asymmetric 2-phenoxy-6-benzyloxy pyridines, have a spectrum of
herbicidal activity significantly different from the unsubstituted analogues
in
possessing post-emergence action against both grass-type plants and also
broadleaf plants. Certain of those compounds are known per se, and said
application claims their herbicidal application.
It has now been found, and forms the subject of the
present invention, that good herbicidal activity is also present
in related, novel pyridine derivatives having nitrogen-containing
WO 94/22833 PCT/EP94/00969
- 2 -
h~teroaromatic substituents. In one embodiment, the present
invention therefore provides 2,6-substituted pyridines of the
formula I:-
S
/ I
A-X ~ ~ X-B
t :-,
wherein X represents an oxygen or sulphur atom; A represents
;. , .,
an optionally substituted S or 6 membered nitrogen- ..-a~-
containing heteroaromatic group; B represents an optionally
substituted 5 or 6 membered cyclic hydrocarbon, alkyl, alkenyl,
alkynyl, aryl or aralkyl group, or one of the meanings for A;
R represents a halogen atom or an alkyl, haloalkyl, alkoxy,
alkylthio or dialkylamino group; and m represents 0, 1 or 2.
Group A is suitably pyridyl, especially 4-pyridyl or
pyrazolyl;-especially 5-pyrazolyl,~ and group B is suitably one of
such groups, or phenyl or benzyl. The groups A and B may be the
same or different, and are preferably substituted, in particular
having a substituent at the meta position (except for B-benzyl).
The substituent groups may be any of those customarily employed in
the modification and/or development of pesticidal compounds and are
especially substituents that maintain or enhance the herbicidal
activity associated with the compounds of the present invention, or
influence persistence of action, soil or plant penetration, or any
other desirable property of such herbicidal compounds. There may
be one or more of the same or different substituents present in
each radical.
Optional substituents in groups A and/or B include halogen
atoms and vitro, cyano, alkyl, haloalkyl, alkoxy, alkylthio and
aryl groups. Preferred substituents are a halogen, especially
chlorine, atom, or an alkyl or haloalkyl group of 1 to 6 carbon
atoms.
An alkyl group, either as a substituent on one or other of
. "
groups.A and B, or as group R, may have a straight or branched
SUBSTITUTE SHEET (RULE 26~
WO 94/22833 ~ PCT/EP94/00969
- 3 -
chain, suitably containing up to 12, preferably 1 to 6, carbon
atoms. In a haloalkyl group, whether as a substituent on one or
other of groups A and B, or as group R, the halogen atom may be one
or more fluorine, chlorine, bromine or iodine atoms, with fluorine
being preferred and trifluoromethyl the preferred haloalkyl group.
Preferred compounds are those wherein each X represents an
oxygen atom and m is 0 or 1.-
The compound of formula I wherein X is oxygen may be prepared
by appropriate adaptation of conventional methods of obtaining
substituted pyridine compounds, the basic technique being, for
example, reaction of a metal salt of the appropriate alcohol with
an appropriate 2,6-dihalopyridine, in a solvent and suitably at
elevated temperature, ideally at reflux. For the preparation of
symmetrical pyridine compounds, the reaction can be carried out in
one step by using a molar ratio of alcohol salt to pyridine of at
least 2:1., For asymetrical compounds of formula I, separate
introduction of the two substituents is required in a two-stage
process.
The present invention therefore provides, in a further
embodiment, a process for the preparation of a compound of formula
I wherein A and B are the same, which comprises reacting a
2,6-dihalopyridine of formula II-
~m
Hal ~ ( Hal
with at least a twofold molar excess of a metal salt of an alcohol
or thiol of formula AXH, the symbols A, X, R and m being as defined
in relation to formula I and Hal denoting a halogen, preferably
chlorine or bromine, atom.
When group B in formula I is different from group A, the
2,6-dihalopyridine of formula II above is first reacted with at
most an equimolar amount of a metal salt of an alcohol or thiol of
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 PCT/EP94/00969
~~,~898~-
- 4 -
formula AXH, followed by further reaction with at least an
equimolar amount of a metal salt of an alcohol or thiol of formula
BXH.
An alternative, and preferred, process for the preparation of
compounds of formula I where B is different from A comprises
reacting a halopyridone of formula III
( w
( I I I~)'r:.~.
Hal
with a halide of formula BHal, wherein each Hal denotes a halogen,
suitably bromine, atom, followed by reaction with a metal salt of
an alcohol or thiol of formula AXH. The first stage of this
reaction is conveniently carried out by reacting the pyridone of
formula III with an alkali metal hydroxide to form the pyridoyxlate
salt, which.is then reacted with the halide BHal, preferably in a
solvent such as dimethylformamide.
Either of the above reactions is conveniently carried out in
an organic solvent, the selection of which is dependent on the'
precise nature of the reactants involved. Generally any polar
organic solvent is suitable, for example dimethylformamide and
tetrahydrofuran.
~e metal salt of the alcohol or thiol is preferably an alkali
metal salt, for example a sodium or potassium salt,~and is
conveniently generated by reaction of the alcohol with a suitable
metal base, for example a metal carbonate or hydride.
The prepared compounds of formula I may, if desired, be
isolated and purified using conventional techniques.
Compounds of general forrula II, and the alcohols/thiols bf
formula BXH are generally known and/or are easily preparable by
r
standard techniques.
Compounds of the general formula I have been found to have
interesting activity as herbicides having a wide range of pre- and
SUBSTITUTE SHEET (RULE 2fi)
WO 94/22833 PCT/EP94/00969
- 5 -
post-emergence activity against undesirable species.
The present invention therefore provides a herbicidal .
composition which comprises a compound of the present invention in
association with a carrier.
The present invention additionally encompasses the preparation
' of such a herbicidal composition by the process of bringing a
carrier into association with a compound of the present invention.
Preferably there are at least two carriers in a composition of
the present invention, at least one of which is a surface-active
agent.
The present invention further provides the use of a compound
according to the invention as a herbicide.
Further, in accordance with the invention there is provided a
method of combating undesired plant growth at a locus by treating
the locus with a composition or compound according to the
invention. The locus may, for example, be the soil or plants in a
crop area. Application to the locus may be pre-emergence or
post-emergence. The dosage of active ingredient used may, for
example, be from 0.01 to lOkg/ha, preferably 0.01 to 4kg/ha.
A carrier in a composition according to the invention is any
material with which the active ingredient is formulated to
facilitate application to the locus to be treated, which may for
example be a plant, seed or soil, or to facilitate storage,
transport or handling. A carrier may be a solid or a liquid,
including a material which is normally gaseous but which has been
compressed to form a liquid, and any of the carriers normally used
in formulating herbicidal compositions may be used. Preferably
composition' according to the invention contain 0.5 to 958 by
weight of active ingredient.
Suitable solid carriers include natural and synthetic clays
and silicates, for example natural silicas such as diatomaceous
earths; magnesium silicates, for example tales; magnesium aluminium
silicates, for example attapulgites and vermiculites; aluminium
silicates, for example kaolinites, montmorillonites and micas;
calcium carbonate; calcium sulphate;.ammoni.um sulphate; synthetic
SUBSTITUTE SHEET (RULE 26)
W094/22833 ~~~~ PCT/EP94/00969
- 6 -
hydrated silicon oxides and synthetic calcium or aluminium
silicates; elements, for example carbon and sulphur; natural and
synthetic resins, for example coumarone resins, polyvinyl chloride,
and styrene polyaers and copolymers; solid polychlorophenols;
bitumen; waxes; and solid fertilisers, for example superphosphates.
Suitable liquid carriers include water; alcohols, for example
isopropanol and glycols; ketanes, for example acetone, methyl ethyl
ketone, methyl isobutyl ketone and cyclohexanoneether; aromatic
or araliphatic hydrocarbons, for example benzene,:~toluene and
xylene; petroleum fractions, for example kerosine and light mineral
oils; chlorinated hydrocarbons, for example carbon tetrachloride,
perchloroethylene and trichloroethane. Mixtures of different
liquids are often suitable.
Agricultural compositions are often formulated and transported
in a concentrated form which is subsequently diluted by the user
before application. The presence of small amounts of a carrier
which is a surface-active agent facilitates this process of
' dilution. Thus preferably at Least one carrier in a composition
according to the invention is a surface-active agent. For example,
the composition may contain at least two carriers, at least one of
which is a surface-active agent.
A surface-active agent may be an emulsifying agent, a
dispersing agent or a wetting agent; it may be nonionic or ionic.
Examples of suitable surface-active agents include the sodium or
calcium salts of polyacrylic acids and lignin sulphonic acids; the
condensation of fatty acids or aliphatic amines or amides
containing at least 12 carbon atoms in the molecule with ethylene
oxide and/or propylene oxide; fatty acid esters of glycerol,
sorbitol, sucrose or pentaerythritol; condensates of these with
ethylene oxide and/or propylene oxide; condensation products of
fatty zicohol or alkyl phenols, for example p-octylphenol or '
p-octylcresol, with ethylene oxide and/or propylene oxide;
sulphates or sulphonates of these condensation products; alkali or
. alkaline earth metal salts, preferably sodium salts, of sulphuric
or sulphonic acid esters containing at least 10 carbon atoms in the
SUBSTITUTE SHEET (RULE 2B)
WO 94/22833 - PCT/EP94/00969
- 7 -
molecule, for example sodium lauryl sulphate, sodium secondary
alkyl sulphates, sodium salts of sulphonated castor oil, and sodium
alkylaryl sulphonates such as dodecylbenzene sulphonate; and
polymers of ethylene oxide and copolymers of ethylene oxide and
propylene oxide.
The herbicidal composition of the invention nay also contain
other biologically active ingredients, for example compounds
possessing herbicidal, insecticidal or fungicidal properties.
The following Examples illustrate the invention. The
structures of the compounds of the invention prepared in the
following Examples were confirmed by mass spectrometry and NMR.
Example 1
Preparation of 2,6-di-(2'-chloropyrid-4'-yloxy)pyridine
A mixture of 11.8g 2,6-dibromopyridine, 13 g 2-chloro-4-
hydroxypyridine and 13.8 g'potassium carbonate in 20 ml
N,N-dimethylformamide was heated to reflux for 12 hours. After
cooling, 150 ml of water was added and the aqueous layer extracted
three times each with 100 ml ethyl acetate. The combined extracts
were dried with anhydrous magnesium sulphate. The solvent was
evaporated and the resulting brown oil purified by flash silica gel
column chromatography using hexane/ethyl acetate 7/3. The title
compound was obtained as a pale brown solid, mp 95'C.
Analysis. Calc. C 53.9; H 2.7; N 12.6%
Found: C 53.7; H 3.0; N 12.4%
Example 2
A) Preparation of 2-bromo-6-(1'-methyl-3'-trifluoromethyl-
pyrazol-5'-yloxy)-pyridine
A mixture of 14.3 g 2,6-dibromopyridine, 10 g 1-methyl-3-tri-
fh;aromethyl-5-hydroxypyrazole and 8.3 g potassium carbonate in
20 ml N,N-dimethylformamide was refluxed for 4 hours. The reaction
mixture then was allowed to cool to ambient temperature and 150 ml
of water was added. The aqueous layer was extracted three times
each with 100 ml ethyl acetate. The combined extracts were dried
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 PCT/EP94/00969
_ g _
with anhydrous magnesium sulphate and the solvent evaporated in
vacuo. Purification of 2-bromo-6-(1'-methyl-3'-trifluoromethyl-
pyrazol-5'-yloxy)pyridine by flash silica gel column chromatography
using hexane/ethyl acetate 8/2 gave a white solid, mp 53'C. '
B) Preparation of 2-(1'-methyl-3'-trifluoromethylpyrazol-5'- '
yloxy)-6-(3 " -trifluoromethylphenoxy)pyridine
1.6 g of the monosubstituted intermediate, prepared as
described above, 0.6 ml of 3-hydroxybenzotrifluoride and 0.7 g
potassium carbonate were mixed with 2 ml N,N-dimethylformamide and
heated to reflex for 12 hours. After cooling 50 ml water was added
and the mixture extracted three times each with 50 ml ethyl
acetate. The combined extracts were dried with anhydrous
magenesium sulphate, the solvent evaporated and the crude product
purified by flash silica gel column chromatography using
hexane/ethyl acetate 9/1. The title compound was obtained as a
pale yellow oil. Analysis: Calc.~C 50.6; H 2.7; N 10.4%
Found: C 50.8; H 2.7; N 10.6%.
Example 3
A) Preparation of 2-bromo-6-benzyloxypyridine
2.9 ml benzyl alcohol was dissolved in 10 ml N,N-dimethyl-
formamide and 0.7g sodium hydride (purity 95%) was slowly added.
After gas evolution has ceased, 6 g 2,6-dibromopyridine was added
and the mixture heated to reflex for 3 hours. After cooling, the
reaction mixture was filtered through a silica gel column using
hexane/ethyl acetate 1/1. The solvent was remSved in vacuo and the
crude product purified by flash silica gel column chromatography.
~e title compound was obtained as a colourless oil.
B) Preparation of 2-bromo-6-(4'-fluorobenzyloxy)pyridine
(alternative route)
A solution of 4.6 g 6-bromo-2-pyridone (the preparation of
this compound is described in Synthesis, 707 (1974)) and 1.56 g
SUBSTITUTE SHEET .(RULE 26~
WO 94/22833 PCT/EP94/00969
~~.~~98 I
potassium hydroxide in 20 ml methanol was prepared. The solvent
was evaporated in vacuo after toluene was added to give~'the
anhydrous potassium pyridoxylate. This was dissolved in 10 ml
N,N-dimethylformamide, 3.7 ml 4-fluorobenzyl bromide was added and
the mixture heated to reflex for 2 hours. After cooling, 100 ml of
water.was added and the aqueous layer extracted three times with
ethyl acetate. The combined extracts were dried with anhydrous
magnesium sulphate and the solvent removed in vacuo. The residue
was purified by flash silica gel column chromatography using
hexane/ethyl acetate 95/5. The title.compound was obtained as a
white solid (3.3g, 44%) of melting point 82'C.
C) Preparation of 2-(2'-chloropyrid-4'-yloxy)-6-benzyloxypyridine
To a solution of 0.65 g 2-chloro-4-hydroxypyridine in 10 ml
N,N-dimethylformamide, 0.13 g sodium hydride was added slowly.
After gas evolution had ceased, 1.2 g 2-bromo-6-benzyloxypyridine
(prepared as described in A) above) was added and the mixture
heated to reflex for 30 hours. After cooling, the reaction mixture
was filtered through a silica gel column using hexane/ethyl acetate
4/1. The solvent was evaporated in vacuo and the crude product
purified by flash silica gel column chromatography using
hexane/ethyl acetate 9/1. The title compound was obtained as a
yellow oil. Analysis. Calc: C 65.3; H 4.2; N 9.08
Found. C 65.2; H 3.9; N 9.1%.
Example 4-8
Following procedures analogous to one or other of those
described in Examples 1-3, further compounds of the invention were
prepared whose details are given below in Table I. In that Table,
the structure of each .compound is identified by reference to the
substituents in formula IV below.
(IV)
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 ~ PCT/EP94/00969
~1~898
,. - 10
Table I
Analysis
x. M.Pt. Calc/Found ,
o. A B 'C C H N,
~ . .
...t ~
L..
w
4 CH3 <, ~ Oil 58 . 4 4 . 0 12 . 0
\N-N -CH2Phenyl ~ (nmr) 58.0 3.7 11.9
\ ~~ CF3
C\ C\ 111 44 . 2 2 . 7 17 . 2
N-N N-N , 44.3 2.8 16.7
' \ I! F \ ~ CF
! 3 3
i
6 ' 1 F3 Oil 55.7 2.7 7.6
~ \N ~ ~ (nmr) 55.7 2.5 7.3
i
i
i
~ 7 ~ C\3 ' C1 Oil 48.6 2.7 15.1
N-N ~ N (nmr) 48.7 2.8 14.9
\ ~~ CF3
I
8 / \ Phenyl oil 64.3 3.7 9.4
(nmr) 64.2 3.7 9.2
For those compounds of formula I described above which were
obtained as oils, the relevant nmr data (ppm in CDC13 solution) are
listed below in Table II.
SUBSTITUTE SHEET (RULE ~6)
WO 94!22833 ~ PCT/EP94/00969
- 11 -
Table II
Compound
of Ex. No. Nmr characteristics
2 3:68(s,CH3 group); 5.84(s,lH); 6.73(d,lH);
6.80(d,lH); 7.22-7.34(m,2H); 7.46-7.51(m,2H);
7.79(t,lH).
3 5.19(s,2H-benzylic); 6.62(dd,lH); 6.88(dd,lH);
7.05(d,lH); 7.23-7.37(m,6H); 7.65(t,lH);
8.22(d,lH).
4 3.67(s,CH3-group); 5.18(s,2H-benzylic); 6.20(s,lH);
6.56(d,lH); 6.62(d,lH); 7.23-7.40(m,SH);
7.66(t,lH).
6 6.75(d,lH); 6.89(dd,lH); 7.02(d,lH); 7.29(m,2H);
7.36(s,lH); 7.83(t,lH); 8.19(d,lH).
7 3.72(s,CH3-group); 6.12(s,lH); 6.81-6.89(m,2H),
6.92-6.97(m,lH); 7.08(d,lH); 7.88(t,lH);~
8.29(d,lH).
8 ' 6.66(d,lH); 6.69(d,lH); 6.93(dd,lH); 7.08(d,2H);
7.12-7.22(m,2H); 7.31-7.39(m,~2H); 7.75(t,lH);
8.18(d,lH).
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 ~ ~ ~ g 9 g 1 PCT/EP94/00969
12
Examt~le 9
Preparation of 2-Fluoro-6-l1'-methyl-3'-trifluoromethyl-
pyrazol-5'-~loxy)pyridine
To a mixture of 4.54 ml (0.05 mol) 2,6-difluoropyridine
in 100 ml of anhydrous N,N-dimethylformamide and 7.6 g
(0.055 mol) potassium carbonate was added 9.3 g '
(0.05 mol) of 1-methyl-3-trifluoromethyl-5'~hydroxy-
pyrazole in one gram portions while hewing the mixture
to reflux for 4 hours. The time intervals between the
portions were 15 minutes.
After cooling the solvent was removed in vacuo and the
residue suspended in 200 ml ethyl acetate. The organic
layer was washed with 100 ml water, separated, and the
solvent was removed in vacuo.
Flash silica gel column chromatography using hexane/ethyl
acetate 8/2 afforded 5 g (380) of a yellow oil.
Examples 10 to 16:
By methods analogous to that of Example 9, the following
intermediates of the general formula XX have been
prepared (table II):
Table II
HAL N R~
Example
No R~
1',3'-dimethylpyrazol-5'-yloxy
11 1'-methyl-3'-tert.-butylp«razol-5'-yloxy
12 1'-methyl-3'-ethylpyrazol-5'-yloxy
13 1'-methyl-3'-isopropylpyrazol-5'-yloxy '
14 1'-ethyl-3'-trifluoromethylpyrazol-5'-yloxy
2'-chloropyrid-4'-yloxy
16 3'chloropyrid-5'-yloxy
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 ~ PCT/EP94/00969
13
Example 17:
Preparation of 2-(1'-methyl-3'-trifluoromethylpyrazol-5'-
yloxy)-6-phenvoxypyridine
A mixture of 1.31 g (0.005 mol) 2-Fluoro-6-(1'-methyl-3'-
trifluoromethylpyrazol-5'-yloxy)pyridine (prepared as
described in Example 9), 0.51 g phenol (0.0055 mol) and
0.76 g (0.0055 mol) potassium carbonate in 3 ml
N,N-dimethylformamide was heated to reflux for 1 hour.
After cooling, the mixture was poured in 20 ml water, and
the aqueous layer was extracted three times with each
20 ml ethyl acetate. The combined extracts were dried
with anhydrous magnesium sulphate. The solvent was
evaporated and the residue purified by a flash silica gel
column chromatography using hexane/ethyl acetate 8/2.
The title compound was obtained as a white solid (1.1 g,
66%) of melting point 78°.
Examples 18 to 56
By methods analogous to that of Example 17, the compounds
of the general formula XX listed in table IIIa were
prepared starting from intermediataes of the general
formula XX prepared as described in Examples 9 to 16.
Table IIIa
z
R N R
Exam le
No. p R' Rz mp yield
(°C) (-s)
18 1'-Me-3'-CF3-pyrazol-
5'-yloxy cyclopentyloxy oil 36
19 2'-chloropyrid-4'-yl phenyloxy oil 14
20 2' -chloropyrid-4' yl 3 "-CF3-4 "-
fluorophenyloxy 71 29
21 2'chloropyrid-4'-yl 2 " -fluoro-
phenyloxy 79 32
SUBSTITUTE SHEET (RULE 28)
WO 94/22833 ~ . . PCT/EP94/00969
14
22 1'-Me-3'-CF3- 4 "-fluoro-
pyrazol-5'-yloxy phenyloxy 95 25
23 1'-Me-3'-CF3- 2 "-fluoro-
pyrazol-5'-yloxy phenyloxy 104 54
24 1'-Me-3'-CF3- 2" , 4 "-difluoro-
pyrazol-5'-yloxy phenyloxy ; 98 58
25 1' -Me-3' -CF3- 2 "-methyl-
pyrazol-5'-yloxy phenyloxy oil 40
2 1' -Me-3 ' -CF3- 2 " -pyridyl oxy
6
pyrazol-5'-yloxy 135 42
27 1' -Me-3' -CF3- 3 "-chloropyrid-
pyrazol-5'-yloxy 5 " -yloxy oil 32
28 1' -Me-3' -CF3- 2 "-chloropyrid-
pyrazol-5'-yloxy 6 " -yloxy oil 27
29 1' -Me-3 ' -CF3 3 "-f luoro-
pyrazol-5'-yloxy phenyloxy 66 51
30 1'-Me-3'-CH3 3"-CF4-4 "-fluoro-
pyrazol-5'-yloxy phenyloxy oil 29
31 1'-Me-3'-Me- 1'-Me-3'-Me-
pyrazol-5'-yloxy pyrazol-5'-yloxy oil 49
32 1'-Me-3'-CF3- 1"-Me'-3 "-
pyrazol-5'-yloxy pyrazol-5 " -yloxy 85 73
33 1'-Me-3'-t-Butyl- 1"-Me-3 "-Me-
pyrazol-5'-yloxy pyrazol-5 " -yloxy oil 50
34 1'-Me'3'-t-Butyl- 1 " -Me-3 " -i-propyl-
pyrazol-5'-yloxy pyrazol-5 " -yloxy oil 90
3 1' -Me-3 ' -CF3- 1 " -Me-3 " -t-Butyl-
pyrazol-5'-yloxy pyrazol-5 " -yloxy oil 85
36 1'-Me-3'-Me- 1 " -Me-3 " -i-propyl-
pyrazol-5'-yloxy pyrazol-5 " -yloxy oil 36
SUBSTITUTE SHEET (R(JLE 26)
WO 94/22833 PCT/EP94/00969
37 1'-Me-3'-Ethyl- 1'-Me-3'-Ethyl-
pyrazolyl-5'-yloxy pyrazolyl-5'-yloxy oil 18
3 1' Me-3' -CF3-pyrazol 1 "-Me-3 " ' -i-propyl-
8
5'-yloxy pyrazol-5 " -yloxy oil 42
39 1'-Me-3'--i-propyl- 1'-Me-3'-i-propyl-
pyrazol-5'-yloxy pyrazol-5'-yloxy oil 44
40 1'-Me-3'-t-Butyl- 1'-Me-3'-t-Butyl-
pyrazol-5'-yloxy pyrazol-5'-yloxy oil 33
41 1'-Me-3'-t-Butyl- 2 " -chloropyrid-
pyrazol-5'-yloxy 4 " -yloxy oil 78
42 1'-Me-3'-t-Butyl- 4 " -fluorophenyloxy
pyrazol-5'-yloxy oil 82
43 1'-Me-3'-t-Butyl- 3 " -fluorophenyloxy
pyrazol-5'-yloxy oil 82
44 1'-Me-3'-t-Butyl- 2 " -fluorophenyloxy
pyrazol-5'-yloxy oil 53
45 2'-chloropyrid- 2 " -methylphenyloxy
4'-yloxy oil 32
46 2'-chloropyrid- 4 " -fluorophenyloxy
4'-yloxy oil 35
47 2'-chloropyrid- 3 " -fluorophenyloxy
4'-yloxy oil 36
48 3'-chloropyrid- 3'-chloropyrid-
5'-yloxy 5'-yloxy 62 54
49 1'-Me-3'-CF3- 3" , 4 "-dimethyl-
pyrazol-5'-yloxy phenyloxy 88 70
50 1'-Me-3' -CFA- 3 "-methyl-4 "-
pyrazol-5'-yloxy chlorophenyloxy 52 41
51 1 ' -Me' 3 ' -CF3- 3 " -methy 1-4 " -
pyrazol-5'-yloxy nitrophenyloxy 103 55
SUBSTITUTE SHEET (#3ULE 26)
WO 22833 ~ ~ ~ PCT 94/0096
94/ EP
/ 9
i
16
52 1'-Me-3'-CF3- 3 "-4"-dichloro-
pyrazol-5'-yloxy phenyloxy 92 27
53 1'-Me-3'-CF3- 3"-CF3-4 "-chloro-
pyrazol-5'-yloxy phenyloxy 70 68 ,
54 1'-Me-3'-CF3- 3 " -cyanophenyloxy
pyrazol-5'-yloxy 91 71 -
55 1' -Me-3 ' -CF3- 3 ' ' -chl oro-4~'~
,' -
pyrazol-5'-yloxy cyanophenyloxy 95 16
56 1'-Me-3'-CF3- 3 "-chloro-4 "-
pyrazol-5'-yloxy fluorophenyloxy 76 65
Abbreviations: Me = methyl, t-Butyl = tertiary butyl,
i-propyl = isopropyl, CF3 = trifluoromethyl
Examples 57 to 85
Following procedures analagous to Examples 17 to 48, the
following compounds of the general formula XX listed in
table IIIb were prepared.
Table IIIb
i ~ z
R N R
Example mp yield
No Ft~ R2 ( 'C)
57 1'-Me-3'-CH3- 3 " -fluorobenzyloxy
pyrazol-5'-yloxy oil 68
58 1'-Me-3'-CF3- 4 " -fluorobenzyloxy
pyrazol-5'-yloxy oil 44
59 1'-Me-3'-CF3- 2 " -fluorobenzyloxy
pyrazol-5'-yloxy oil ~ 33
60 1' -Me-3' -CF3- 4 "-CF3-benzyloxy
pyrazol-5'-yloxy oil 43
SUBSTITUTE SHEET (RULE 26)
2155~~~.
WO 94/22833 ~ PCT/EP94/00969
17
~ ___ _____~__..__.._~.__-___~~_~_.~_._ ..__.__~_____..______-_____,_-
~_..
61 1'-Me-3-CF,-pyra2ol-5~ylvxy 3 " -CF,-benzyloxy
oil 3e
_ __ ~
_ ___ _ _ _ ____ ___
' ~ 62 1'-Me-3'-CF,-pyrazol-5-yloxy4"-methylbenzyloxy-
oil ~ 39
. _-_ _.._-..__--~.-__-_--_-_~_~ _~
__.
_
__ ___~______-_-__-____.._____ ___
63 _ 2 '-mettiylbenzyloxy
1'-Me-3'-CF,-pyra2ol-5'-yloxy
oil 39
~64 1'-He-3'-CF,-pyrazol-5'-yloxy_ 4 " -tert.-butylbenzyloxy
oil 35
_-_ _________~________-__~._--~~_.____-___-~
__
_ ___
65 1'-Me-3'-CF,-pyrazol-5'-yloxy___-_____________-
3 " -chlorobenzyloxy
Oil 36
66 1'-Me-3'-CF,-pyrazol-5'-yloxy3" -methylbenzyloxy
oil 41
67 1'-Me-3'-CF,-pyrazol-5'-yloxy2 " ,6 " -dichloroben2yloxy
110 67
i68 1'-Me-3'-CF,-pyrazol-5~-yloxya-methylbenzyloxy
oil 50
69 1'-Me-3'-CF,-pyrazol-5'-yloxya " -chlorobenzyloxy
oil 63
70 1'-Me-3'-CF,-pyrazol-5'-yloxy,2 " -chlorobenzyloxy
oil 57
71 1'-Me-3'-CF,-pyrazol-5'-yloxy2 " ,4 " -difluorobenzyloxy
oil 20
72 1'-Me-3'-CF,-pyrazol-5'-yloxy2",4",5"-trifluorobenzyloxy
68 20
73 1'-Me-3'-CF,-pyrazol-5'-yloxy2" , 3" , 4 "-trif luorobenzyloxy
55 25
74 2'-chloropyrid-4'-yloxy 4 " -fluorobenzyloxy -- ~ -
Oll 6
75 1'-Me-3'-CF,-pyrazol-5~-yloxya-CF,-benzyloxy
i 75 67
______-_.._______..___________-____- ,___...._______-________-___..________
SUBSTITUTE SHEET (RULE 26)
WO 94/22833
PCT/EP94/00969
18
76 1'-Me-3'-t-butyl- 2 " -methylbenzyloxy
pyrazol-5'-yloxy oil 51
77 2'-chloropyrid- 2 " -fluorobenzyloxy
4'-yloxy oil 27 y
78 2'-chloropyrid- 3 " -fluorobenzyloxy
4'-yloxy oil 55 -
79 2'-chloropyrid- 2 " -methylbenzyloxy
4'-yloxy ' oil 52
.
..
__________-_______________' _____ _____
80 2'-chloropyrid- ,_
__________.~____________
2 " ,4'~=difluoro-
4'-yloxy benzyhoXy oil 34
81 1'-Me-3'-t-butyl- 4 " -fluorobenzyloxy
pyrazol-5'-yloxy oil 33
82 1'-Me-3'-t-butyl- 3 " -fluorobenxyloxy
pyrazol-5'-yloxy oil 41
83 1'-Me-3'-t-butyl- 2 " -fluorobenzyloxy
pyrazol-5'-yloxy 61 50
84 1'-Me-3'-t-butyl- 2 " ,4 " -difluoro-
pyrazol-5'-yloxy benzyloxy oil 22
85 1'-Me-3'-t-butyl- benzyloxy
pyrazol-5'-yloxy oil 29
Examples 86 to 88
By a method analogous to that of Example 17, further
compounds of the general formula XX were prepared and are
listed in~table IIIc.
Table IIIc
N R2
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 ~ 9 ~ ~2 ~ ~ ~ ~ PCT/EP94/00969
Example mp yield
No R~ . R2 ( °C) (%)
86 1'-Me-3'-CF3- 1 " -phenylethyloxy
pyrazol-5'-yloxy oil 39
87 1'-Me-3'-CF3- 2"-propinyloxy
pyrazol-5'-yloxy 57 19
88 1'-Me-3'-CF3- tert.-butyloxy
pyrazol-5'-yloxy oil 77
Example 89
Preparation of 2,6-Dibromo-4-methylpyridine
To a solution of 2-methyl-2-chloromethyloxirane
(50 g; 0.47 mol) in 23 ml of concentrated hydrochloric acid)
at ice-bath temperature was added a solution of sodium
cyanide (27.4 g: 0.56 mol) in 23 ml of hydrochloric acid.
After stirring for 10 hours at that temperature, the
reaction mixture was warmed up to 40°C.
To this solution of potassium cyanide (33.8 g; 0.52 mol) in
50 ml water was added. The reaction mixture then was warmed
to 50°C and stirred for 4 hours.
After cooling, the mixture was brought to pH 7 and extracted
three times with 150 ml ethyl acetate.
The combined extracts were direct with anhydrous magnesium
sulphate. Removal of the solvent in vacuo afforded 1,3-
dicyano-2-methyl-2-hydroxypropane (56 g, 96~).
The whole amount was added carefully to a 33~ solution of
hydrogren bromide in glacial acetic acid at ice-bath
temperature.
The reaction mixture was stirred for 3 days at ambient
temperature.
The solvent was removed in vacuo and the residual brown oil
_ brought to pH 12 with a 10 molar aqueous solution of sodium
hydroxide.
The alkaline solution was extracted 3 times with 100 ml
ethyl acetate. The combined extracts were dried with
anhydrous magnesium sulphate and the solvent was removed in
SUBSTITUTE SHEET (RULE 26~
WO 94/22833 ~ ~ ~ ~ 9 2~2 PCT/EP94/00969
vacuo to afford 6-amino-2-bromo-4-methylpyridine (56 g, 66~)
as colourless crystals. Melting point 99°C.
This compound was forwarded to the next step without further
purification. '
To a stirred solution of 6-amino-2-bromo-4-methylpyridine
J.
(45.2 g; 0.24 mol) in 100 ml water and 43 g concentrated
sulphuric acid at ice-bath temperature was given a solution
of sodium nitrite (13.2 g: O.l~~lmo1) in
SUBS11TUTE SHEET (S(~~E 26)
WO 94/22833 2~ 1 ~2~ ~ ~ ~ ~ ~ ~ PCT/EP94/00969
20 ml water. After 2 hours the reaction mixture was warmed
to 60°C and stirred for further 60 minutes.
After cooling, the mixture was extracted with 200 ml of
dichloromethane.
The solvent was removed in vacuo and 2-bromo-4-methyl-6-
hydroxypyridine (20 g, 56~) was obtained as colourless
crystals of melting point 152°C.
This was mixed with 24 g phosphorylbromide in 100 ml
bromoforme and heated to reflux for 3 hours.
After cooling the reaction mixture was hydrolyzed carefully
with a 50o aqueous solution of sodium hydroxide and
extracted 2 times with 100 ml dichloromethane. The solvent
was removed in vacuo and the crude product purified by flash
silica gel column chromatography using hexane/ethyl acetate
7/3. 2,6-Dibromo-4-methylpyridine (6.9 g, 25~) was obtained
as a colourless solid melting point 77°C.
Examples 90 to 99
Successive conversions according to procedures outlined in
Examples 9 and 17 to 2,6-dibromo-4-methylpyridine (prepared
as described in Example 89) gave compounds of the general
formula XX. These are listed in table IV.
Table IV
CH3
R~ u~ 2
R
SUBST)TUTE SHEET (RULE 26)
WO 94/22833 2p 2~ PCT/EP94/00969
2
Example ' mp yield
No R~ RZ (C) (~)
'90 1'-Me-3'-CF3- 1'-Me-3'-CF3-
(WL396407) pyrazol-5'-yloxy pyrazol-5'-yloxy 93 52
91 1'-Me-3'-CF3- 3 "-fluro-
(CL370022) pyrazol-5'-yloxy phenyloxy 68 22 '
92 1'-Me-3'-CF3- 2"-fluoro-
(CL3700223) pyrazol-5'-yloxy phenylox~ 67 27
,w
~~ _
93 1'-Me-3'-CF 2"
4~~~~'-difluoro-
(CL370024) 3 , 84 21
pyrazol-5'-yloxy phenyloxy
94 1'-Me-3'-CF3- 2"-methyl-
(CL370025) pyrazol-5'-yloxy phenyloxy 91 11
SUBSTITUTE SHEET (RULE 26~
WO 94/22833 w ~ PCT/EP94/00969
21
________.~___ __________~__~__.-. _.~_~____..
95 1'-He-3~-CF,-pyrazol-5'-yloxy 3~~-chloropyrid-5 " -yloxy
(CL370026)
64
_..____________ ~ ~_...._... _____ _~_ ~____-~,
96 1~-Me-3~-CFs pyra2ol-5'-yloxy phenyloxy
I (CL370029)
oil 37
_______~___~~________~..__~.__~_~__~~_~_~_____.
97 2'-chloropyrid-4~-y~.oxy phenyloxy
(CL370030)
oil 17
.._________~_________ ~__~____ ~~___..._____~_ _______
98 ~2'-ahloropyri.d-4~-yloxy 2~~-methylphenyloxy
(G2370031) oil 13
99 1~-Me-3'-CF,-pyrazol-5'-yloxy benzyloxy
(WL396406) oil 66
ANALYSIS
Example C(%) H(~) N(~)
No. calc. fon~~nd calo Zound calo. found
1 53.9 53.7 2.7 3.0 12.6 12.4
2A 37.3 36.8 2.2 2.0 13.0 13.2
2B 50.6 50.8 2.7 2.7 10.4 10.6
.3A 54.6 54.7 3.8 3.6 5.3 5.3
___ ______________________________________....___________________
___ 51.1 51.1 3.2 3.1 5.0 4.8
3B
3C 65.3 65.2 4.2 3.9 9.0 9.1
ex. 4 to 8 of Table I
4 58.4 58.0 9.0 3.7 12.0 11.9
44.2 44.3 2.7 2.8 17.2 16.7
' 6 55_7 55.7 2.7 2.5 7.6 7.3
7 48.6 48.7 2.7 2.8 15.1 14.9
8 64.3 64.2 3.7 3.7 9.4 9.2
SUBSTITUTE SHEET (RULE 26j
WO 94/22833 PCT/EP94/00969
22
~ple g
Example C(~) H(~) g
N~ CalC. fOL~ltd ~C810 nnnrl ~lr fnttn
9 46.0 45.7 2.7 2.5 16.1 16.0
0
=10 58.0 58.1 4.9 ' 4.7 20.3 20.4
.11 62.6 62_8 6.5 _ __-6.5 ____i6.g______i6.6
X12 59.7 59.3 5.5 __ ~ 19 0 - lg
5'3 ' 6
;13 61.3 61.2 6.0 6.0 17 9 17 8
______ __________..________________
;
. ____..________.,.____________.._
,14 48.0 47.7 .___ 3.1 15.'3 15.5
3.3
:15 53.5 53.4 2.7 2.4 ' 12.5 '12.1
'
,16 53.5 53.1 2.7 2.4 12.5 12.0
SUBSTITUTE SHEET (RULE 26)
WO 94122833 ~ ~ ~ PCT/EP94/00969
23
~XBmnle 17
Example C(t) H(~)
' ~ o. a a c.
. ~ 17 57.3 5?.1 3.6 3.8 12.5 12.3
~. 18 to 56 cf table IIIa
18 55.0 55.1 4.9 ' 4.9 '12.g _"___i2_6_
19 64.3 64.2 3.7 3.7 9.4 9.2
20 53.1 52.8 2.4 2.2 ~ 7.3 ?.4
21 60.7 60.4 3.2 3.4 8.8 4 '8.?'
22 54.4 54.2 3.1 3.4 11.9 ' 11.8
23 54.4 53.9 3.1 3.4 11.9 12.1
24 51.8 52,2 2.7 2.9 11.3 ' '11.I
25 58.4 58.2 4.0 3.9 12.0 ~ -12.0_
26 53.6 53.5 3.3 3.4 16.7 -16 6
27 48.6 48.9 2.7 2.7 15.1 "15 0-
28 48.6 48.5 2.7 2.8 ~ 15.1 15 1
29 54.4 54.1 3.1 3.0 ' '11.9 r 11.6
30 48_5 48.4 2.4 . 2.5 '_ _ '10.0 ___9.8
31 60.2 60.3 5.7 5.3 23 4 23.0
32 51.0 51.0 4.0 3.8 s ' 19.8 ___i9_9_
-_
33 63.6 63.2 6.8 6.5 20 5' ~ 20
2
34 65.0 64.8 7.4 7.4 -__ ___i9.0-___- 19.1
35 54.7 54.7 5.1 _-5.1 _~_ ___i7.7
_-
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 PCT/EP94/00969
24
__ __62_2__ ~_ _
6 6
g 5
_ ___ _
37 62.4 62.0 _ _ 21.4 21.3 '
6.5 ____
6.4
38 53.5 53.4 4.8 4.8 18.4 18.1
I 39 64.2 64.4 9.1 ~ :7.0 19.7 19.7
40 65.8 65.9 7.6 7.6 18.3 18.1
41 60.2 60.2 5.3W 5.4 15.6 15.6
42 66.8 66.5 5.9 6.0 12.3 12.2
43 66.8 66.7 5.9 5.8 12.3 12.4
_____.._ _______..._______. ______.___________
.
44 _______~_ _-..-______. __ 12.3 12.3
66.8 66.7 5.9 5.7
45 65.3 65.3 4.2 4.0 9.0 8.8
__ ___ _ .._ ___ ___ ___
__ ___ ___ ___ __
46 _60.7 __ __ _3.2 3.2 8.g 9.p
s0.g
47 60.7 60.9 3.2 3.3 8.8 8.8
48 53.9 53.7 2.7 2.9 12.6 12.5
49 59.5 59.5 4.4 4.3 11.6 11.6
50 53.2 52.9 3.4 3.2 10.9 10.6
51 56.4 56.5 3.6 3.6 15.5 15.2
52 47.5 47.6 2.5 2.3 10.4 10.4
53 46.6 46.6 2.3 2.4 9.6 9.4
_ ____ _ ___
__ ..
__ _______ _________ ~ ___________________._________
i _ 56.7 56.6 ~...._ 2.8 15.5 15.5
54 3.1
55 51.7 51.8 2.6 2.6 14.2 14.0
56 49.5 49.5 2.6 2.4 10.8 11.0
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 _ PCT/EP94/00969
~.~ ~8~g~
Ex. 5i to 85 cf table rrrb
' Example C(g) H(~)
es ~' found p 1 n mind oalc f~"nr~
-
57 55.6 55.9 3.6 3.6 11.4
I _...________..________...___ 11.2
- _ _
__
i 58 55.6 55.5 3.6 ,......_3.3-_ 11.4 _ __ll_0_
-_- -
59-_____ __55_6__~__g~_4__ __3_6__ ~_ 11.4 ___11.2
3
3
~ _ ~ ___
60 51.8 51.8 3.1 _ ..~____ 9.9
I6i________51,8 ____51.5 ___3.1 ____ 10.1 ____10.1
_ __ ~. ' 3.0 _.._io_i__
__ ____ ___ ___ _~ .____3.p
___
__
__________ ___________~~_____
162 59.5 59.4 4.4 4.6 11.6 11.5
63 59.5 59.5 4.4 - ____4.5 ___i1.6 ____i1.5
________ ____..___________..__________.___ _
.
___________________________.._
.64 62.2 62.3 5.5 5.3 10.4 10.4
I65______.._~3_2______53_2_ __3_4_ 3 11_ ___~0.8
5
66 59.5 59.4 __ ____ __ 11.3
67 48. 49. 2 4.4 _ __ ____l0-3
8 2. g _____ I1.6
~_ 4.4 __io_i__
~~__2_9_____
68 59.5 59.5 4.4 -~ r_--4.1 __ii_6______11.2
-___
_
69 53.2 53.0 3.4 '_-3.3 -_____ii_o_..---11
0
70 53.2 53.3 3.4 '~___g_4__-11 0_y ____10.8
r
71 53.0 52.7 3.1 ~__3.0 ______l~_9~~~__-11.0
w
__
72 50.6 50.6 2.7 ~~__2.g __i0.4 ___i0.3w
-_ ____ __
_
73 50.6 50.1 2.7 ___2.4 ______i0.4 ___iO___
~_____ .., ___ __ ____ __ ?
_ ___ __
___
74 ~i 61.7 3.7 3.4_____ __ ____ ______
7 8.5 _
e.l
75.._______51_8______51_6______3_1_______3_~_______I~_1______10.0
76V-______?1_$______?=_5______?..2______ 12 $
6 ~ 11
g
.
_ _r" _
77 61.7 61,.8 3.7 _ _ _
____________..________________________ ___ ___
..___ 8.5 ___8_4.,
3.7 _________
_____..______
SUBSTITUTE SHEET (RULE 26)
WO 94/22833 PCT/EP94/00969
.. 2 6
____ _____~___ _________~_~____~ _____..~ ______r.
7$ 61.7 61.7 3.7 3.7 __ 8.3
8.5
79 66.2 66.0 4.6 4.7 8.6~ ~ A8.6
80 58.5 58.0 3.2 2.8 8.0 y 8.3~
___..~_...___ ___ ____
__
, ___....~___._. r_______...___~_____.~_.-___
81 67.6 67.5 6.2 w 6.0 11.8 11.7
82 67 . 6 68 . 0 6. 2 6 . 2 ll . 8 11. 8
___________~.._____________: ~ ~_.
' __
____:: _
~~.
, .__ _____________~.~_
83 67.6 67.7 6.2 __ 11.8 11.8
6.3
84 64.3 64.3 5.7 5~.4 11.2 11.0
85 71.2 71.4 6.9 6.9 12.4 12.0
~ Example C(%) Fi(%) N(%)
No. aalc. fond talc found ca7.c Found
86 59.5 59.4 4.4 4.2 11.6 11.6
87 52.5 52.5 3.4 3.6 14.1 14 0
______ ___.,.~...._..__.._____________________
, _____ ___________________
~88 , 53.3 53.0 5.1 'S.2 13.3 13.3
,
SUg~[~-TUfE SHEET (RUL.E 26~
t~ WO 94122833 - ~ g ~, PCT/EP94/00969
27
EX9mDl~ $9
Example C(~) 8(~) N(~)
a a
89 v Z8.7 28.7 2.0 1.8 5.6 .5.8
EX. 90 to~99 aP, s~h~a Ty
___ww _r..~rrrrwww..~w _ r _wwrw.. _r._e.ww_______ ___
90 55.0 54.8 4.9 4.8 12.8 ~w_ZZ.8
_w__ ww.wrrw_w__w__________www w w
_.. __. w___rM __w___wr
91 '55.6 55.6 3.6 r.._ww__ 11.4 11.2
____ ___wwrrw___r..ww___________w 3..7
w_. ...__rw_____________~...~.w..~_--.
a2 55.6 55.3 3.6 3.4 11.4 11.3
www_r __rwrwwrw________r__r..r....ww_w
______w___r~.r____~.__________
g3____53.0 52.6 3.1 3.0 10.9 10.9
_..._....__~_____________ ~....__
______v_____ -..~_-~____
94 ____59.5 59.5 4.4 4.2 11.6 -__11.5
-_ __ _________________r
__ _....._.w_w_~_____~___________
~5-~~ _ 49.9 50.0 3.1 3.1 14.6 14.4
__w__w_r ________ __r__w_w
w_~________ ___~~.._r..________
96~r_ ____5g_5___58.1 4.0 12.0 11.9
3.8 __~_...____________
~7r__w' 65.3 ___________......_........r~_____~.___ 9.0
__rw______65.4 4.2 ~
3.9 ~
_______..__r.____r___w
_w_____~____..__________
98'_~_____b6_Z ___66_1___4.6 4.2 8.6 _
._ __ __
8.6
M.a.____ ______________r..
99__.....____595r_____5~_2_____ ___4.1 11.6 11.4
__4.4 W_M_____w ______r____r___.-
r______
Example 100
Herbicidal Activity
To evaluate their herbicidal activity, compounds according to
the invention were tested using as representative range of plants:
maize, Zea ways (Mz); rice, 0 za sativa (R); barnyard grass,
Echinochloa crus~alli (BG); oat, Avena sativa (0); linseed, Linum
usitatissimum (L); mustard, Sinapsis alba (M); sugar beet, Beta
vulEaris (SB) and Soya bean, Glycine max (S).
The tests fall into two categories, pre-emergence and post-
emergence. The pre-emergence tests involved spraying a liquid
formulation of the .compound onto the soil in which the seeds of the
plant specied mentioned above had recently been soc.-n. The
SUBSTITUTE SHEET (RULE 26)
CA 02158981 2005-03-09
-28-
post-emergence tests involved two types of test, viz., soil drench and foliar
spray tests. In the soil drench tests the soil in which the seedling plants of
the
above species were growing was drenched with a liquid formulation
containing a compound of the invention, and in the foliar spray tests the
seedling plants were sprayed with such a formulation.
The soil used in the tests was a prepared horticultural loam.
The formulations used in the tests were prepared from solutions of the
test compounds in acetone containing 0.4% by weight of an alkyl-
phenol/ethylene oxide condensate available under the trade mark TRITON~
X-155. These acetone solutions were diluted with water and the resulting
formulations applied at dosage levels corresponding to 5 kg or 1 kg of active
material per hectare in a volume equivalent to 600 litres per hectare in the
soil
spray and foliar spray test, and at a dosage of level equivalent to 10
kilograms
of active material per hectare in a volume equivalent to approximately 3,000
litres per hectare in the soil drench tests.
In the pre-emergence tests untreated sown soil and in the post-
emergence tests untreated soil bearing seedling plants were used as controls.
The herbicidal effects of the test compounds were assessed visually
twelve days after spraying the foliage and the soil, and thirteen days after
drenching the soil and were recorded on a 0-9 scale. A rating 0 indicates
growth as untreated control, a rating 9 indicates death. An increase of 1 unit
on the linear scale approximates to a 10% increase in the level of effect.
The results of the tests are set out in Table III below, in which the
compounds are identified by reference to the preceding examples. Absence of
a numeral in the Table indicates a zero rating, an asterisk indicates that no
result was obtained.
WO 94/22833 PCT/EP94/00969
'~ '
29
V W vD fw0 OO 1W N v0 O O
T ~!1 f~ D O ~1
N
N OO O, Ov Qv O~ W O~ O O
00 CD Ov Ov O D Op
v1
C) CO 00 Ov O~ CO tyT o0 O O
~," f~ CO Ov Ov o0 00
U
~ G
d
ep .~ n r~ o. o. u, ~ 0 0
a c~ n r~ m o. N .0
a
T d ~ ~ rwc ao ~ ~ .o 0 0
o o .o 00 ~ .-.~ u,
d
"
~. '
w o o. . o. o. oo ao 0 0
W m oo o. o. v~ en 00
o.
Gi O tf1 10 fw ~' f~ W O O
O N u1 V1 N N O
N
N
~ \O vD fW v0 1f1 V1 O O
N N vD D ,!1 N ~
V1 10 00 00 00 00 tW 00 N r-i
vC CO 00 00 OD D 00
04
tn 00 Ov O~ O~ O, Q' C~ OW
O~ Q\ O. O~ 00 Q' O~ O
OD OO Ov Ov CO 00 O~ 1W
CO OD O~ a' O~ 00 O~ C
CL
N OD O OO OO aO a0 t~ W f1
a IW ,t1 tp o0 o0 OJ t N
Sa
..1 V1 f~ n t~ f~ n a0 ~C
O N 1D \D I~ 1~ t~ f~ O
O
Lrr
C5
04 OO 00 CO OO 00 OO CD 00
~ OD n 00 OO 00 OD n
W
a
Gi N vD 1W fW f~ V1 0 n.-1
O ~ D D v1 y tf'1p
E
N
W T vD rw0 0o o wD rw0 N O
N ,t1 rw vo
d
CD
c0
m n n v~ w u~ n n wn
y w .-~ rr .--r .--m .-m -- r
O . .-
00
ox
v~ ~ ~ ,-r ...~ * o ~ o
a0
N n 00 ~D n * y0 Op
CD
x r~ ao r~ a. * v,
~
o0
0
a vD tf1 N vT * N vD
x O
U
G
~1 1~ ,f1 1~ * v0 fw
p
.d
O
r-1 00 OO OO o~ * n c0 t~'1
~0
.,.t
O
N
CL~ N ~D 1~ t~ * ~? n O,
' N
.n .o .D .o k r~ r~ o
,
v
C -
O
O W
Z
E
O X r-1 N C'~1 ~ tf1 ~p n
U W
SUBSTITUTE
SHEET
(RULE
2~)
WO 94/22833 ~ PCT/EP94/0 0969
~
~ 3 0 .
/
2
cn * 0 0 0 ~o ~ o o co ~ w o
al * N O O 01 01 O O 01 01 01 01
* l0 O O CO I~ O O 01 01 01 Q1
U
U a * 0 0 0 ~ ~ o o co co co c~
rn
N O * M O O I~ lflO O CO 00 01 CO
P
1 C7 * N o o co co o b o~ o~ o~ o~
a~ m '
:~i
pa ~,
Qi * e-I O O lf1 cr ~ O I~ N I~ In
O
N * r-i O O t0 ~O O O t0 tn I~ t0
* Lt1 N rl CO CO M O 00 CO CO CO
C'4 * lp 01 tC 01 01 l0 d' 01 01 O~ 01
~r ~. * CO I~ l0 O~ 01 CO 10 01 01 01 CO
f~
Qr a it d' tI~ N CO CO O O CO t0 I~ t~
O * d' ~O O I~ ~D O O I~ t0 I~ t~
ca
.-I
* 1D CO t~ CO CO O O CO CO 00 CO
U
v
L~' * N r-I O tf1 d' O O CO tf1t~
w ~ * 'd' N O I~ I~ O O CO t~ t0 I~
a
H
O
ax
cn o ~ o ~o
.C C~l * M t0 O 01 01
x
.~e.~' * d' tf1 O Q1 CO
O
a ,. * O M O I~ t~
U
~ O * o ~ o t~ co
N
L1 U' * M I~ O CO CO
O Qi * O cf' O 00 I~
N * O tn O lfl CO
b
O
owz
n~ o co o, o ~ t~ 00
rl r-1 N N lf1 ll7
o x
U w
SIJBSTITUTE SHEET (RULE 26)
WO 94/22833 _ PC T/EP94/00969
2
30 ~
2
tn In ~i'M N N O ~J'M t~ l0 N O
W a1 01 a1 O1 01 O1 Q1 d1 a1 p1 ltd V'
o~ o~ o~ o~ o~ o~ o~ ~ o~ h
~ a h ~ ~ ~r vo ~ h ~ m m o 0
CO I~ I~ ~O CO CO CO tn CO f~ N O
I C7 O1 O1 Q1 CD O1 I~ 01 O1 01 O1 f~ In
N Ga
L~
Q.i l0 d' O O O O O O l!7 d' O O
N l0 lf1d' M In d' tn Wit'l~ In O O
Cn t0 CO OD 00 00 l~ 00 h c0 CO l~ I~
L~7 01 Q1 d1 01 d1 01 p1 Q1 01 01 ~ 01
CO 00 O1 CO 01 CO CO h CO CO 01 CO
fCS
~a r r r r ~o h h r r r r r
~ o r .o r ~ ~o h r ~ ~o h r
~a
+; .'.,
~c~ ~ ~ ~ r ~ r ~ ~ ~ ~ o r
0
U
Q: t0 tf~~Y N lf7 d' ~O t17 l0 LIB N O
W N I~ 10 t~ l~ !~ 10 l~ tn !~ l0 I~ I~
a
as
H
0
ox
Cl~ M N N cr 1p N
rr Ol 1fl ~Q pp pp
~
x
m co co co co r
'
a ~o M . ~ h h
U
t~ Wit' l!7 l!7 er ~-I
Ca h to ~o ~ c m n
~
O Q', M O O p ~ p
N d' M
N M l~ N
O
owz
S~ 01 O v-I N M cr
O
O
X
U W
SUBSTITUTE SHEET (RULE 26)
WO PCT/EP94/00969
94/22833
d' O N O O O O O ~O l11tD lf1
01 CO 01 00 O O N O 01 01 01 01 01 O1
01 01 01 CO O O l0lf~ 01 01 01 01 CO CO
ti' N d' M O O O O t0 ltdh l~ In d'
lC In l0 In O O O O CO h h ~D ~O
01 00 01 CO O O Wit'N 01 01 01 ~1 01 CO
M O O O O O O O h tf1t0 lf~ ~ N
~ d' Ln N O O N O ~ ~O lf1 In
~
CO h h l0 In d' tf)cY CO h CD CO
01 01 O~ O~ 01 CO COCO ~ ~ 01 O Ov CO
01 a1 01 CO CD CD 01h 01 01 O~ 01 01 h
h h h h t0 d' l!'!~' CO CO h h CO h
l~ ~p h t0 d' ~ V'N h h (~ In h t!7
CO OD 00 h In t!7 tnN CO CO CO CO h l0
~D In d' N O O Incr h ~O ~O lf1 tn N
O h 10 lf1 lf1 tI1~!' O h h ~
~f'
N O O O N M *
01 c0 O O CO ~D *
h h O O CO h *
lf1 M O O In tn *
~
h ~ O O h Lf)
CO tn O O CO CO *
N O O O ~ ~t'1
~O ~ O O l~ ~ *
In 1fl h CO O1 O rl
t0 ~O 10 ~O h h '
SUBSTITUTE SHEET (RULE 26)
WO 94/22833
' ,~
~
PCT/EP94/00969
31 2/2
h 10 h Wit'N * M O 1D h h
tn
01 01 Q1 O 01 * CO h 01 O1 01
O1
01 CO CO 0~ CO * CO 00 O1 O1 O1
CO
CO CO h l0 tn * ~d' N CO t0 CD
h
01 CO CO h h * h d' h h CO t0
O~ CO 01 CO 01 * 01 CO 01 01 01
00
d' N tn * M O 1!7 tI7 tf1
O
1p \O ~D In tf1 * Wit'N Wit' l0 tD
M
CO CO CO CO CO * CO h 00 CO CO
CO
01 01 01 01 01 * 01 CO 01 01 01
01
01 01 G1 d1 01 * 01 CO CO CO CO
h
CO h CO h CO * h 10 CO CO h
h
t0 CO CO t0 h * CO h h h CO h
CO CO CO CO CD * CO CO 00 CO h
CO
tn d' N lf1 * vD 1!7 h In In tn
h h 10 tf1h * h h l0 h h
10
I ~ ~ I ~
h ~I' * cr M M
d' l0 * al tl~ h
lD In * CO h h
lf1 M * t0 d' " tn
h Wit' '~ d' h h
0~ h * h h CO
h tn * O tf1 Wit'
CO cr * 1D ~O ltd
h h h CO d1 p1
SUBSTITUTE SHEET (RULE 26)