Note: Descriptions are shown in the official language in which they were submitted.
n
. ~ ,~,,.t' .:: /L,.i..~,..' .1..~.~j- , :, .1. r' ~ _ / , ,,': ~ , j i -.. ~
( ( . 'r~ % < ; J i- ~ y
1
A SYSTEM FOR ULTRA-TRACE LEVEL ANALYSIS OF WATER
AND A TRAPPING MEDIUM THEREFOR
of the Invention
This invention relates to the analysis of water
to detect the presence of minute amounts of trace
05 contaminants - "ultra-trace level analysis" and to the
trapping of such contaminants. .More particularly, it
relates to procedures, materials and apparatus for the
trapping and concentration of anaiytes in order to
facilitate the analytic prooess. It also relates to
procedures and materials for trapping ultra-trace level
contaminants to effect their removal from water.
~ackgxound to the Invention
Ultra-trace level analysis, when applied to the
testing of water, entails endeavouring to identify and
measure the quantities of non-aqueous components of
water, "analytes", that are present at levels of
generally parts per billion or trillion, or less. Such
analytes are either-dissolved in water as molecules and
ions are adsorbed on extraneous particulates and/or
colloidal matter usually present in water or, in the
case of organic micro-organisms, are in suspension.
Thus, trace contaminants in water are distributed
between dissolved and non-dissolved components.
This invention relates to analyzing the non-
dissolved component of contaminants in fresh, or non-
saline water. (Further reference to water herein is
directed to non-saline water.) It also relates to
analyzing dissolved components in certain cases Where
CA 02160235 2004-04-29
WO 94124553 216 0 2, ~ ~ p~pA94100190
- 2 -
such components may be forced to precipitate from
solution.
In general, the most toxic contaminants for
chronic diseases present at ultra-trace levels in water
05 are in the non-dissolved form. If a complete analysis
of a water sample is required, the contaminants
remaining in the permeate, principally those dissolved
in the aqueous phase, may also be extracted by means of
adsorption on a resin column following standard
techniques. However, often it is sufficient to only
analyze for the presence of the non-dissolved fraction.
To carry-out ultra-trace level analysis on water
it is essential to sample sufficient volumes of water in
order to collect a detectable quantity of contaminants.
The cost of analysis increases when only smaller
quantities of samples are available for testing.
Therefore, the accumulation of large sample quantities
will reduce costs and increase the number of techniques
available to effect analysis. Accumulation of trace
samples of micro-organisms will also allow
identification through culturing samples to be effected
more reliably.
Difficulties arise in applying most normal
trapping techniques, such as filtration and adsorption,
to the ultra-trace analysis of the non-dissolved
components in surface or waste water. When suspended
matter is present it is typical that only a relatively
small volume of water can be made to flow readily
-- WO 94/24553 ~ j PCT/CA94/00190
- 3 -
through the normal sieve-type filter medium or the
barrier-type filters used in ultra-trace filtration.
This is because such filters have very fine pore
dimensions, F~lug-up easily, and rapidly develop a high
05 back-pressure:. These are not convenient characteristics
when it is intended to filter large volumes of water.
Depth-filters are less susceptible to blockage
than barrier filters. Known depth-filters, on the other
hand, are composed of random mats of polymeric materials
and rely on t:he density and thickness of the mats to
trap particle's. These filters are generally capable of
retaining larger quantities of particles within their
matrices and this makes filtration of large volumes of
fluids more F~ractical. However, such filters are not
efficient at trapping micron-level sized particles and
colloids. Moreover, commercially available depth-
filters usua7.ly contain contaminating binders that bleed
during extracaion of the analytes and thereby complicate
analytical procedures.
Both sieve and depth filters of conventional
design need t:o be of unwieldy size to be able to sample
large volume:a of turbid fluids.
Adsorption columns perform poorly as filters and
have limited capacity to trap analytes. The consequence
is that large: volumes of adsorbers must be used if
adsorption columns are to be used to collect significant
quantities oi' anal;ytes.
WO 94/24553 2 ~ ~ O 7 ) 5 PCT~CA94100190
- 4 -
A further concern with adsorbers is that an
extraction process is required to recover the analytes.
The adsorber then contributes contaminants to the
extract at levels which interfere with ultra-trace
05 analysis. This inherent contamination problem persists
in spite of extensive cleaning.
A previously unappreciated aspect of ultra-trace
analysis as applied to fresh water is that many ultra-
trace analytes have not been reliably quantified because
l0 they are entrained within metal hydroxyl, or ~~light
metal, colloids such as alumina silica colloids.
Colloids create a problem in filters in that they
readily block filter pores, thus limiting sample
quantities. Consequently, to collect convenient
15 quantities of colloidally-trapped analytes, large filter
areas are required. Larger filters increase
contamination.
In the case of adsorbers, while their poor
recovery ratios have been recognized, there has
20 apparently been little or no appreciation of the fact
that adsorbers must compete with the binding capacity of
colloids in order to accumulate trace analytes.
Accordingly, it is desirable to develop a
compact form of a binder-free analytical trapping medium
25 that will allow the extraction of ultra-trace level
quantities of non-dissolved analytes found in non-saline
water. Further, it is desirable to collect such
analytes in concentrations that will make analysis
WO 94/24553 ~ ~ ~ ~ ~ ~ PCT/CA94/00190
- 5 -
convenient. or simply to remove them as contaminants.
A further advantageous procedure when large
volumes of water are being sampled, would be to limit
the sampling time to only that required to produce a
05 level of accumulated analytes susceptible to convenient
detection.
It is on the basis of this background that the
present invention i.s directed to improving the procedure
by means of which ultra-trace analysis of large volumes
of water and 'the removal of ultra-trace contaminants may
be carried-out .
The invention in its general form will first be
described, and then its implementation in terms of
specific embodiments will be detailed with references to
the drawings :following hereafter. These embodiments are
intended to demonstrate the principle of the invention,
and the manner of its implementation. The invention
will then be :Further described, and defined, in each of
the individua:L claims which conclude this Specification.
Summary of thEa Invention
According to the invention in its broadest
sense, improvE:d analytical trapping media have been
devised by fo~.-ming .a three dimensional, depth-filter
matrix of micro-porous adsorbent material that will
provide substantially irreversible binding sites for the
entrapment of colloidal carriers and analytes present in
non-saline wager. After exposure of such media to water
WO 94/24553 ~ ~ ~ ~ ~ PC'TICA94100190
- 6 -
which is to be sampled, ultra-trace analysis may be
carried-out either directly on the analytes entrained
within the entrapped colloidal carriers (e.g. by
spectroscopy techniques), or by extraction.
05 The trapping media may comprise a variety of
microporous materials that present "active" hydroxyl
groups over the surface of such material. "Active"
hydroxyl groups are those capable of forming new bonds
with the hydroxyl-bridges found within the colloidal
carriers. This is effected through the release or
elimination of a hydrogen ion.
Such hydroxyl groups may be formed on the
surfaces of both organic and inorganic materials. An
inorganic example would be a micro-porous support coated
with freshly-prepared aluminum hydroxide. Suitable
supports include zeolites, kieselghur, fuller's or
diatomaceous earth, alumina and silica gel. A calcined
diatomaceous earth product produced by John Mansville
Corporation of the United States of America and sold
2o under the trade mark CELITE is directly effective in
this procedure as it contains a degree of active
hydroxyl groups in its natural form and has a high
internal surface area with voids that readily
accommodate colloidal material. CELITE, as with the
other referenced micro-porous inorganic materials, will
perform in a superior manner if treated to add hydroxyl
groups as described above.
-~ WO 94124553
PCT'ICA94/00190
An o~cganic example of a suitable trapping media
is the range of porous materials originating from
Pharmacia Incorporated of the State of New Jersey,
United State: of America, and sold under the trade mark
05 SEPHADEX. This material is a polymerized polysaccharide
in the form of beads. Specified pore-sizes can be
prepared as required, ranging from 100 to 1 million
Daltons. This material contains naturally "active"
hydroxyl groups as part of the sugar structure.
Trapping media provided with active hydroxyl
groups have t:he valuable feature that the colloidal
carriers become irreversibly bound in the media.
Without restricting the scope of the invention claimed
herein, it is. believe that this occurs due to a chemical
reconstruction process in which they become bound to the
hydroxyl groups. This is suggested by the fact that it
has been found that for each ion of the colloid which is
bound, a hydrogen ion is released. Under electron-
microscopy, the immobilized colloidal gel can actually
be seen accumulated within the pores of the trapping
media.
It appears, therefore, that the dissociation
constant for the colloidal gels, once adsorbed, has been
reduced by many orders of magnitude compared to trapping
on conventional adsorber materials such as AMBERLITE
(trade mark) resins.
The analytea collected on the basis of this
invention may be extracted from the trapping media by
WO 94124553 216 0 2 3 5 ~T~CA94100190
_ 8
known procedures. Once extracted, they may be analyzed
by known techniques to determine their character and
quantity. In this manner the ultra-trace analysis of
the original volume of water that passed through the
05 depth-filter may be completed. Because no binders are
employed in the preferred embodiment, such chemicals are
not present to interfere with the process of analysis.
The efficiency of the trapping of the heavy
metals within trapping media can be influenced by
l0 adjusting the pH of the water sample being fed to the
trapping media. The pH may be adjusted to the optimum
values for effecting the precipitation, as hydroxides,
of the metal, or groups of metals being isolated.
The method of the invention makes possible the
15 ultra-trace analysis of contaminants of greatest concern
to society, e.g. the detection of hydrophobic organic
substances and insoluble hydroxides of heavy metals.
Examples include polychlorinated biphenyls (PCB's),
dioxins, furans, polycyclic aromatic hydrocarbons
20 (PAH's), most metals such as lead, chromium, cadmium,
mercury, and others. The filter medium of the invention
will also accumulate and concentrate bacterial,
protozoa, diatoms and other microbiota. It does not,
however, substantially concentrate "volatiles" (i.e. low
25 boiling point organic liquids) such as chloroform,
acetone or methanal. Nor does it concentrate salts or
compounds of calcium, potassium or sodium.
~w WO 94/1A553 216 0 2 3 5 PCTICA94/00190
~n Y
-
A convenient method of analysis for the presence
of heavy metals in the trapping media is by X-ray
fluorescence. (XRF). This technique will identify both
an element and its quantity, while it is still entrained
05 within the trapping media. XRF analysis is convenient
because it is inexpensive and portable. The use of XRF
is made possible because of the relatively large
quantities of anal.ytes made available for analysis by
the preconcentrati.on method of the invention.
For XRF to be utilized, the trapping media must
be sufficiently X-ray transparent. It is ideally suited
where an organic trapping media is employed. Where less
transparent inorganic trapping media are used, analytes
and heavy metals can often still be detected using back-
scatter compensation.
A further advantage of the trapping media of
this invention arises from its efficiency in removing
suspended matter from the water. Subsequent analysis of
the permeate for dissolved material is simplified
because of the reduced incidence of contamination from
suspended matter.
The :foregoing summarizes the principal features
of the invention. The invention may be further
understood b~~ the description of the preferred
embodiments, in conjunction with the drawings, which now
follow.
WO 94!24553 216 0 2 3 5 ~'~CA94100190
- 10 -
The invention in its general form will first be
described, and then its implementation in terms of
specific embodiments will be detailed with reference to
the drawings following hereafter. These embodiments are
05 intended to demonstrate the principle of the invention
and the manner of its implementation. The invention
will then be further described, and defined, in each of
the individual claims which conclude this Specification.
Summary of the Figures
Figure 1 is a schematic depiction of a basic
continuous-flow system for trapping contaminants in
fresh water.
Figure 2 is a more versatile version of the
system of Figure 1.
Figure 3 is a schematic depiction of the
equipment layout for effecting X-ray fluorescence
analysis of a sample of trapping media containing an
analyte.
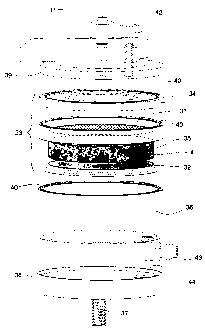
Figure 4 is a side view of an assembly for
carrying the trapping media in a cartridge form.
Figure 5 is an exploded side perspective view of
the assembly of Figure 5.
Figure 6 is a schematic depiction of the
entrapment of a colloid on the surface of the trapping
media of the invention.
Description of the Preferred Embodiments
In Figure 1 a flow of water 1 enters a cylinder
2 containing a column 3 of trapping media 4 supported on
WO 94/24553 ~ ~ ~ ~ ~ PCT/CA94/00190
- 11 -
filter paper 5. Above the media 4 is a glass fiber
matrix 6 for the removal of larger sized particulates.
In a preferred embodiment the trapping media 4 is CELITE
(trade mark) upon whose surfaces has been deposited a
05 thin layer oi: alum:inum hydroxide, preferably freshly-
prepared. Alternately, magnesium hydroxide, for example
could be deposited if it was desired to test for
aluminum comF>ounds as an analyte. A meter 15 registers
the flow of water :L.
The column 3 receives the flow of water 1 in the
manner of a depth filter. Colloids, typically aluminum
sulphate colloids, become bound to the surface and
within the pores of the trapping media 4. Analytes
within the colloids thereby become immobilized and
accumulate within t:he trapping media 4.
The flow of: water 1 is maintained until,
gradually, the accumulation of trapped analytes
reaches a concentration which will be convenient for
analysis. Analysis; is carried-out, in one variation, by
transportation of t:he trapping media 4 to a laboratory
where standard procedures are applied. Alternately, as
shown in Figure 3, x-ray fluorescence analysis may be
effected in-situ.
In Figure 2 a more complex double filtering
system is provided with two branches, a first combined
organic and i:norgan.ic extraction branch 7, and a second
inorganic extraction branch 8. Water may enter from
a self-pressurized source through feed valve 9, or from
WO 94/24553 PC'TICA94100190
- 12 -
an unpressurized source through a feed valve 10 wherein
a pump 11 provides the pressure. A bleed valve 12 may
be connected on the down-stream side of these valves to
release water from the system.
05 A pressure meter 13 may be provided and an auto-
shut-off pressure limiting valve 14 installed to protect
system components from over-pressure conditions. Branch
valves 15, 16 provide water access to the respective
branches.
In the first, combined analysis branch 7, a
mixer 17 may be included to incorporate "spiked" or
injected standard quantities of a calibration compound
into the water flow. A bypass valve 18 permits water to
bypass the subsequent filter components for flushing-out
the rest of the system and to allow for stabilization on
a continuous flow basis to be established.
In branch 7 a cylinder 2 with filter column 3
according to the invention is provided, followed by a
resin column 19. A check valve 20 passes the exiting
water through a flow meter 21 to enter the return
supply line 22. Up to the check valve 20 it is
preferable to form all conduits from non-organic matter
e.g. stainless steel, as branch 7 is intended to analyze
for organic analytes.
In the inorganic branch 8, the mixer 17A is
placed within a recirculating loop 23 powered by a
recirculating pump 24 and incorporating an alkali
reservoir 25 and pH sensor and controller 26. A
WO 94124553 216 0 2 3 5 ~T~CA94f00190
- 13 -
controllable bypass valve 18A permits diversion of flow
through a bypass 27, allowing a steady-state level of pH
to be established for the water arriving at the bypass
valve 18A. .A preferred pH level is in the range of l0
05 to 12. The ;pH level is established by control of the
flow through the reservoir 25 in response to a preset
target pH pr~wided to the sensor 26. Control is
effected thr~~ugh a link 50. Once the desired pH is
established, flow is diverted through the trapping media
4A. Water tlhen exits the branch 8 through check valve
20A, flow meter 21A and leaves the system via the return
supply line 22.
In the inorganic branch 8, organic conduits,
such as poly~aropylene piping may conveniently be used.
The 'trapping media 4 in the combined branch 7
helps to avoid exposure of the resin column 19 to
particulates. It also may be used to accumulate
particulates and to trap a portion of the organics at
the pH level of the infeed.
The strapping media 4A in the inorganic branch
may be removed and replaced with fresh media 4A as
different pH levels are established. The establishment
of different pH levels will allow for maximized
concentration of differing analytes that are pH
sensitive.
In Figure 3 an X-ray source 28 is located
adjacent to i~he trapping media 4 in situ. The X-rays 29
WO 94/245°' 216 0 2 3 5 ~T/~~4/00190
- 14 -
penetrate the media 4 and are reflected back into a
combined emitter/receiver 30 which detects the
fluorescence generated in the media 4 by the X-rays 29.
The receiver 30 provides an output 31, optionally at a
05 remote location 32 via a communication link 33, which
provides data on the identity and quantity of analytes,
typically heavy metals, present in the trapping media 4.
By including in the transmitted data sent to the
receiver 30 the output of the water meter 21,21A the
display at the output 31 can be formatted in terms of
the concentration of the measured analytes within the
sampled water stream 1.
In Figure 3, the X-ray source 28 may be provided
with a pre-set level for the concentration of a target
analyte before providing an output. This will ensure
that results are obtained with a desired level of
confidence and not prolonged unduly. This level may
typically be 10 times the threshold level for detection.
When the pre-set level of concentration in the
trapping media 4 has been reached, the X-ray source 28
may provide a signal that further sampling is no longer
required. The sampling procedure may then be
terminated. This may be effected by an automatic shut-
off device that closes the relevant valves 9, 10.
Figure 4 and 5 show a detail for a holder 30
that carries the column 2 containing the trapping media
4.
"' WO 94/24553 ,'~~ PCT/CA94/00190
- 15 -
The ~aedia 4, in the form of a compressed cake,
is placed between upper 31 and lower 32 screens
respectively of coarser and finer meshes. These
components a:re in turn contained within a portable
05 cartridge 33 container having a upper containment rim 34
with a centr~il hole, and a lower cup 35, also with a
central hole.
The :Lower cup 35 sits within a pressure cylinder
36. The assembly is compressed by a screw 37 between a
lower adjustable carrier plate 38 and an upper lid 39.
"O" rings 40 positioned at the interfaces provide
pressure resisting seals when the screw 37 is tightened.
Inleit 41 allows water to enter the assembly.
Bleed valve ~42 permits trapped air to be bled-off.
Outlet 43, conveniently mounted into the pressure
cylinder 36 through its flat lower surface, permits
water to escape. A notch 44 in the carrier plate 38
provides cle~~rance for the outlet 43.
The advantage of this assembly is that users in
the field can readily load and remove the cartridge 33
for transport to a remote location for analysis.
Turning to the performance of trapping media 4,
tests have b~sen carried-out using both uncoated CELITE
and CELITE wlZich has been treated by exposure to 0.4
millimoles o:E aluminum hydroxide per gram of CELITE.
Table 1 list;a the recovery on untreated CELITE of
amounts of grace metals from water to which the listed
WO 94/24553 PC'fICA94100190
16 -
metals have been added, or "spiked", at a concentration
of 20 parts per billion, with varying levels of pH.
TABLE 1
RECOVERY OF TRACE METALS BY UNCOATED CELITE
05 (20ppb Spiked Ultra Pure Water Sample)
(averaged recoveries over 6+ tests)
pH Pb Cu Cd Hg Cr As
6 14.3 0.0 13.4 11.0 1.9 2.4
7 10.1 0.0 6.9 7.9 3.0 10.7
8 7.2 0.0 7.0 9.4 2.0 15.3
9 14.8 0.2 9.1 5.7 6.0 6.9
10 19.6 19.7 14.2 16.2 8.0 10.0
11 19.9 19.4 19.6 12.3 0.0 12.1
12 19.2 14.1 19.3 19.3 0.0 11.2
From Table 1 it is apparent that differing pH levels are
suitable for maximizing the recovery of different
metals. Further, the recovery ratios can be calibrated
to permit projections to be made of the full content of
analyte within a sample, when a standard proportion is
recovered at a specific pH level.
WO 94/24553 ~ ~ PCT/CA94/00190
- 17 -
Table 2 lists similar results for treated CELITE.
TABLE 2
PERFORMANC1~ OF COATED CELITE WITH VARIOUS HYDROXIDES
(Percentages of Metals Trapped In The Filter)
05 Hydroxide
Treatment p1i Pb Cd As Cr Cu Hg
8 92.00 97.00 28.64 34.67 63.67 78.67
Mg 9 89.67 80.67 15.00 33.33 34.33 73.00
92.64 97.32 21.34 32.00 40.00 79.00
10 l:l 90.67 96.00 52.67 20.00 64.32 83.33
8 92.00 98.33 92.33 24.00 51.67 74.33
Fe 9 92.00 96.33 59.67 27.33 55.33 76.00
10 92.00 98.00 73.33 20.67 50.00 72.00
1~L 91.33 98.00 65.34 26.65 56.00 76.30
8 92.00 51.67 29.00 17.33 2.33 64.60
A1 9 91.67 82.67 22.00 10.65 9.67 63.67
1() 91.69 97.65 18.00 14.00 25.00 72.30
17L 92.00 98.64 99.30 16.00 81.00 82.33
Spiked with analytes at a level of 30.00 ppb
Measured Background Amount (ppb)
2.00 0.30 0.20 0.80 3.30 0.00
The c:oatirng procedure in respect of the ia
med
used to generate Table 2 was to dissolve an hydroxide,
such as aluminum magnesium or iron hydroxide in wat er
and then to impregnate the CELITE with the ater. The
w
CELITE is thE:n dried by a flow of air to remove
the
water and leave the pores impregnated with
the
hydroxide. 7a has been found preferable to use
a
freshly prepared h:Ydroxide solution, and not one at
th is
over 10 days old to obtain improved results.
WO 94/24553 PCTICA94/00190
- is -
Tests have been effected with manganese hydroxide -
Mg(OH)2. Other divalent metal ion hydroxides such as
iron, cobalt, nickel, copper and zinc are believed to be
suitable. Further, trivalent metal ion hydroxides such
05 as those of manganese, iron and chromium are also
believed to be suitable.
A pesticide spiking experiment was carried out
using 20L of ultra pure water. The spiking solution
contained 20 uL of SUPELCO PESTICIDE MIXTURE (trade
mark) and 1 ug of 4-4'dibromooctafluorobiphenyl
(surrogate compound) in 100 mL of distilled water. A
CELITE-based media was prepared as per the standard
aluminum hydroxide treatment procedure and 20L of spiked
water was allowed to pass through the system. The
extract was analysed using Gas Chromatograph-Electron
Capture Detector (GC-ECD) and Mass Spectrometer (GC-MS)
technology. The results are shown in Table 3.
~' WO 94/24553 PCT/CA94/00190
- 19
TABLE 3
EFF7:CIENC'.~C OF RECOVERY FOR PESTICIDES
PESTICIDE SPIKED RECOVERY RECOVERY
AMOUNT GC-ECD GC-MS
05 (PPt) (%) ($)
sxc l0 53 73
BHC 10 58 72
BHC 10 41 55
Lindane 10 52 65
Heptachlor 10 64 61
Heptachlor Ep~oxide 10 50 50
Aldrin 10 61 77
Endosulfan I 20 32 58
Endosulfan II 20 37 50
pp-DDE 20 62 73
Dieldrin 20 32 73
Endrin 20 50 75
pp-DDD 60 51 72
Endrin Aldehyde 60 70 59
Endosulfan Sulfate 60 193' 61
pp-DDT 60 56 80
Surrogate 50 56 61
' An unknown chlorocompound co-eluted
Table 4 shows the results of sampling 25 litres
of Rideau River water in the vicinity of Ottawa, Canada.
In this Table 4 the recovery is broken-down between the
proportions o:f analyte detected on the trapping media 4
and the resin column 19. The CELITE of Table 4, as in
the previous 'tests, had been treated with aluminum
hydroxide as described above.
WO 94/24553 2 ~ 6 0 2 ~T~CA94/00190
3 5
- 20 -
TABLE 4
ANALYSIS OF RIDEAU RIVERWATER FOR PESTICIDES
Using Hydroxide-Enhanced
CELITE
(parts per
trillion)]
05 TRAPPING RESIN
PESTICIDES MEDIA COLUMN TOTAL
DBCP 1.90 0 1.90
HCB 0.80 0.17 0.97
AIDRINE 0.21 0.17 0.97
op-DDE 0.50 0.76 1.26
CHLORDANE 0.00 0.00 0.00
pp-DDE 0.10 0.00 0.10
DIEDRIN + op-DD 0.13 0.00 0.13
pp-DDD + op-DDT 0.34 0.41 0.75
pp-DDT 0.00 1.16 1.16
PERMETHRINE 55.93 14.31 70.24
CYPERMETHRINE 3.31 1.57 4.88
Comment: On-site sampling of Rideau River water.
Sample volume = 25L
In all Tables the pesticides are identified
using the nomenclature of the United States
Environmental Protection Agency "Analytical Reference
Standards and Supplemental Data for Pesticides and Other
Organic Compounds", document EPA-600/9-78-012, May 1978.
Analysis was effected using gas chromatography for
separation and then either mass spectroscopy or an
electron capture detector for quantification.
- WO 94124553 ~ ~ PC'TlCA94100190
- 21 -
Table 5 snows the recovery of a series of
Polychlorodibenzo-~p-Dioxins from a combined organic and
inorganic analysi~~. A VARIANtm brand gas chromatograph
with an electron capture detector was used. The pH was
05 at the natural level (circa pH=7). As a source of
dioxins 10 litres of ultra pure water was spiked with 50
parts per trillion of dioxin mixtures (5 micrograms per
millilitre each intoluene) supplied by Chromatographic
Specialties Inc. of Brockville, Ontario, Canada,
Catalogue No. AM8280A. The components of the spiking
mixture are listed. in the first column of Table 5. The
second and third columns list percentage recoveries from
the filter a:nd column respectively, based on GC-ECD and
GC-MS respectively. The last two columns show results
from water samples taken from local rivers.
PCTICA94/00190
W0 94/2ds~' 216 0 2 3 5
- 22 -
TABLE 5
INVESTIGATION OF SYSTEM EFFICIENCY
FOR POLYCHLORODIBENZO-p-DIOXINS
(ON CPRT'S PLAIN CELITE FILTER AND RESIN COLUMN)1
05 TEST AMOUNT1 TOTAL PERCENT OTTAWA2 RIDEAU3
DIOXIN TRAPPED (ppt) RECOVERY RIVER RIVER
FILTER/COLUMN (ppt) (ppt)
TODD 14.6 24.9 39.5 78 20.8 15.1
PnCDD 4.5 35.7 40.2 80 1.2 2.1
HxCDD 24.5 129.4 153.9 308 3.2 0.8
HpCDD 6.8 29.0 35.8 72 0.4 2.3
OCDD 10.4 35.9 46.3 93 1.0 5.1
pH 7
1 lOL ultrapure water spiked at 50 ppt level
2 30L of river water was sampled at a site downstream of
the Eddy/Scott Paper Mills
3 A lOL Grab sample was pumped through the system at CPRT
Laboratories
Results obtained using Kieselghur that had been
impregnated with A1(OH)3 at a level of 0.2 mmole A1(OH)3
per gram of Kieselhur at pH 11 as the trapping media are
as follows in Table 6:
- 23 -
TABi.E 6
RECOVERY OF METALS
(KIESELGHURC AS TRAPPING MEDIA)
Metals Pb Cd Cr
05 Amount of spike (ppb) 20 20 20
Amount recovere~3 20 19 . 8 7 , 7
g Recovery 100 9g 3g,5
Table 7 shows. a comparison of the performance of
a series of filter media in terms of the volumes of
water trot may lbe sampled before significant back-
praacure Erom filter plugging develops.
TALE 7
FI: LTER PERFO~RNCE
Run ~ Flow Fil~tar Turbidity Prosaure Volume
Rate 4 . 7 ~cM dia, NTU gm/cm ( L
. 2 ps
i
)
mL/min Input/output
1. 200 W-934-AH l.5uia 0.15/0.13 l4Cb {20) 10
2. 200 W-GE/F 0.7um 0.15/0.08 1406 (20) 4
3. io0 CJ~:BRIpG~l.Oum 0.15/0.13 l4os (20) 40
4. 200 CAMBRIDGEI.Oum 5.0 /Z.0 1406 (20) 4
5. 100 ACTIYRT$D~ 10.C/1.45 1758 (25) 10
CARBON
6. 100 CELLUIDSE~ 10.0/0.6 1758 (25) 1
7. 100 SiL;IC~A GEL 5. o /<1. 1758 (z5) i
0
8. 100 GAC 5.0 /<1.0 1758 (25) Z
9. 100 SEP;H?~DEX 5.0 /<1.0 1758 (25) 1
10. 100 CELITE 0.15/O.IO 351 { 100
5)
- (unmodifi.erl)
11. 200 C:BLZTE 5.0 /0.a 70 { 20
1)
( ~unmodi, f
fed )
12. 200 C'ELITB 10.0/0.67 700 (10) 20
( unmod i. r
ied )
13. 200 CBLITE 10.0/0.50 633 ( 60
9)
l~f ied)
14. - 100 CELITE 10.0/0. 67 351
( 30
5)
(uamodi,fied)
~' WO 94124553 PCTICA94100190
- 24 -
1. W-934-AH :L.S um Filter Paper from the Cambridge
Filter Pa~~er Company, U.S.A.
2. W-GF/F 0.'7 um Filter Paper from the Cambridge Filter
Paper Company, U.S.A.
05 3. Cambridge 1.0 um Filter Paper from the Cambridge
Filter Paper Company, U.S.A.
4. Cambridge 1.0 um Filter Paper from the Cambridge
Filter Paper Company, U.S.A.
8. GAC Granular Activated Carbon supplied by
Aldrich Chemicals, U.S.A.
9. SEPHADEX Gel Filtration Resin supplied by Aldrich
chemicals, U.S.A.
Electron microscope scans of CELITE have been
taken at a ma~gnific.ation of 4000. These images show
uncoated CELI'TE, C'.ELITE coated with 0.2 mmoles per gram
of aluminum hvydroxide on CELITE and the same type of
treated sample of C'ELITE after it has been exposed to
water containing 20~ ppb/litre quantities each of
arsenic, copper, chromium,' cadmium, mercury and lead.
The degree of occlusion of the pores progressively with
the coating of the media, and after exposure to water
containing contaminents is apparent in these microscans.
While not wishing to be bound by the following,
Figure 6 depicts one theory of the operation of
the trapping media of this invention. In Figure 6
the wall 35 of a portion of microporous trapping media
4, such as diatomaceous earth, surrounding a pore 36 is
shown in cross-section. Along the inside surface 39 of
the pore 36, an hydroxide complex 37 presents an "active"
~1~'023
- 25 -
hydroxidQ group 38 outwardly from the inner surface 39.
In the adjacent, surrounding water 40, colloidal carriers
41 are present to which are adhered analytes 42. The
colloidal carrier 41.A upon entering tre pare 36, is
t» believed to form new bonds with the hydroxyl group 38,
releasing hydro~~en ions 43, converting the distributed
colloids into a gel. 'This irreversibly fixes the colloid
41A to the trap~~ing media 4 to a degree not present with
adsorbers.
The analyt~ss 42 are entrained within the colloids
41 and become similarly entrapped. They are then
available for analysis by the customary procedures.
It has also green found that CELITE (tm) in
particular wil3. trap bacteria and protozoa ~tnd, it is
believed, diato~~as and other taulticell rniCrobiota.
Samples of giax~dia azid cryptospirodiurn protozoa, and
salatonella bacteria have been concentrated in the CE?~ITE
filtor modium. The effectiveness of filter media of the
invention in effecting :uch a concentration may be due
in part to the pr~ser~ce of a charge distribution on such
organic micro-c=rgani~sias .
Once conci~ntratad in the filter medium by whatever
mechanism, a w~3shing solution taken from the medium was
able to innocttlate gel and produce cultures of these
bacteria. Acc~ordin~gly, the invention extends to the
concentration of such micro-organisms as a class of
analytes.
wWO 94124553 ~r PCT/CA94100190 ~~
_ _
26
Conclusion
The foregoing has constituted a description of
specific embodiments showing how the invention may be
applied and put into use. These embodiments are only
05 exemplary. The invc=ntion in its broadest, and more
specific aspects, ia~ further described and defined in
the claims which now follow.