Note: Descriptions are shown in the official language in which they were submitted.
W094/~68 ~ 6 ~ 5 7 S PCT/GB94/00802
METHOD FOR TREATING REINFORCED CONCRETE
AND/OR THE REINFORCEMENT THEREOF
BACKGROUND AND FIELD OF THE INVENTION
Embedded steel in reinforced concrete is normally5 protected against corrosion by virtue of a dense oxide
film which forms on the steel surface in alkaline
environments. This film acts as a barrier to aggressive
agents. However, when concrete becomes contaminated with
chloride ions or when its alkalinity is reduced by
absorption of carbon dioxide from the air, the passivating
oxide film may break down thus rendering the embedded
steel subject to corrosion.
Much research has been done to examine the causes and
mechanisms involved in the corrosion of steel
reinforcement in concrete. The general consensus today
is briefly that the corrosion process is electrochemical
in nature, in that sites where the passive oxide film is
broken form anodes, and the surrounding areas where the
film is intact form cathodes. The anodic and cathodic
areas together form corrosion cells leading to the
dissolution of iron at the anodic areas.
Various electro-chemical methods have been developed in an
effort to control this corrosion or to neutralise its
causes. One well known such method is that of cathodic
protection whereby the embedded steel is brought to and
maintained at an electrical potential at which it cannot
corrode. Cathodic protection installations have been
shown to be workable, but suffer from a number of adverse
factors, not the least of which is their necessarily being
permanent installations requiring ongoing monitoring and
W094/~68 PCT/GB94/00802
= -2-
2~a~
maintenance. Other disadvantages are high cost, the
extra structural loading introduced by heavy concrete
overlays and the difficulty of ensuring permanent correct
current distribution.
S Another such method is that of chloride extraction, in
which chloride ions are caused to migrate under the
influence of an electric field to an external electrolyte
where they accumulate in, and eventually are removed with
the electrolyte. The Vennesland et al. U.S. Patent No
10 4,032,803 is an example of such processes. The chloride
extraction process, though effective and less costly than
cathodic protection, and thus a substantial improvement
thereover, nevertheless suffers from the great and
economically expensive practical difficulty of predicting
15 the time necessary for the treatment to be completed.
Because of this, frequent sampling and analysis of the
concrete is required to determine remaining chloride
levels. This difficulty is compounded by there so far
being no residual chloride level which is generally
20 accepted by the industry as being safe with regard to
future chloride attack. These factors can make it
difficult to calculate the cost and time necessary to
reach a particular treatment target. In some cases, this
time can also be unacceptably long from a practical
25 aspect, especially since it is difficult to plan for in
advance.
A third such method, which is applied to carbonated
concretes, is the impregnation of the carbonated zones by
the electro-migration of alkaline substances from an
30 external source. The Miller et al U.S. Patent No
4,865,702 is illustrative of this process. This latter
method, though successful in carbonated concretes which
are low in chloride, can become inefficient or even fail,
when the concrete contains significant amounts of ionic
WOg4/~68 216 ~ 5 ~ PCT/GB94/00802
-3-
substances such as chlorides. Also when the concrete
contains blast furnace cement, or where pozzolans have
- been added to the mix, the treatment time can become
unreasonably long. Further problems arise where
chloride setting accelerators have been used in the
originating concrete mix whereby the chloride is
consequently distributed throughout the concrete mass.
In practice, it has been noted that it is difficult and
economically not cost effective for many treatment
10 situations to reduce the chloride content to below some
50% of the original content. Also, in practlce, the
documenting, monitoring and controlling of the chloride
removal entails the taking of numerous core samples by,
for example, diamond core drilling and then analysing the
15 cores for chloride content. As is well known concrete
is a notoriously inhomgeneous material so that
statistically significant numbers of core samples need to
be taken and analysed and documented to ensure effective
monitoring of the chloride removal. Furthermore, the
20 taking of a core sample leaves a hole which needs to be
filled.
Furthermore, since the removal of the chloride content of
concrete is never completely predictable there is always
the uncertainty of the time required to achieve a desired
25 chloride level. Similar considerations apply to the
realkalisaton of concrete in that core drilled samples are
required for phenolphthalin testing and sodium and
potassium determination.
.
It has additionally been found that in steel reinforced
structures it is desirable to be able to modify the bond
strength between the reinforeing steel and the set and
hardened concrete since in reinforced structures it has
been noted that over a period it is possible for such bond
W094/~68 ~ PCTIGB94/00802
strength to depart from a satisfactory bond.
The present invention overcomes the difficulties of the
above mentioned methods by be-ing highly predictable with
regard to treatment time, by eliminating the necessity for
sampling and chloride analysis, by being quicker and hence
more economical to apply, and by being equally applicable
to almost any kind of concrete, carbonated or not,
chloride contaminated or not, pozzolanic or not, and
whether or not blast furnace cement has been used.
SUMMARIES OF THE INVENTION
According to a first aspect of the invention there is
provided a process for the electrochemical treatment of
reinforcing steel in concrete having embedded steel
reinforcement, including applying an electroconductive
material to an exposed surface of the concrete to form a
distributed electrode, and applying a DC voltage to said
electroconductive material as a positive terminal, and to
said embedded steel reinforcement, as a negative terminal,
and characterised by effecting passivation of the embedded
steel and/or by imparting a predetermined modification to
the bond strength between said embedded steel and said
concrete including the steps of:-
(a) applying said DC voltage to the terminals so as toinpart a distributed current flow between said
electroconductive material, as an anode and said embedded
steel reinforcement as a cathode; and by
(b) continuing application of said DC voltage and said
distributed current flow in accordance with a
predetermined current flow/time treatment regime which i~
such that the flow of current per square metre of surface
area of said embedded steel reinforcement that passes
W094/~68 5?1 6 0 S 7~ PCT/GB94/00802
between said terminals within a predetermined time period
lies within predetermined limits.
In accordance with a particular aspect of the invention,
the application of said DC voltage and said distributed
current flow is continued until at least about lOO
ampere-hours of current, per square metre of surface area
of said embedded steel reinforcement, has passed between
said terminals; and said treatment is discontinued before
said current flow substantially exceeds 3000 ampere hours,
per square metre of surface area of the embedded steel
reinforcement, regardless of residual chloride levels and
residual alkali levels in the concrete.
Thus, the present invention is based upon the discovery
and recognition that the electrochemical treatment of
concrete does not have to be controlled, for example, as a
function of the chloride content or as a function of the
degree of carbonation. Rather, the invention is based
upon the recognition that the electrochemical processing
of concrete is optimally controlled as a function of the
surface area of the steel reinforcement. In a given
structure, the surface area of the embedded reinforcement
is either known from the construction records or is the
subject of a close approximation. Pursuant to the
invention, eletrochemical treatment can be set up more or
less in a known manner as is disclosed by the Vennesland
et al,. U.S. Patent No 4,032 ,803 or by the Miller U.S.
Patent No 5,228,959.
Significantly, however, instead of periodically taking
core samples of the concrete structure to evaluate
residual chloride levels, for example, the process is
controlled by reference to the accumulated current flow in
r~lation to the total surface area of the embedded
reinforcing steel. The process is continued until a
W094/~68 PCT/GB94/00802
2~&~ 6- ~
minimum of lO0 ampere-hours of current flow per square
metre of surface area of the embedded steel has been
realised. The process can be discontinued at that stage
(and preferably is discontinued before the current flow
significantly exceeds 300~ ampere hours per square metre
of surface area of the embedded steel), regardless of the
residual chloride level or carbonation level at various
points in the concrete.
The process may be discontinued at this stage with a high
level of confidence that the embedded reinforcing steel
will be protected for a significant period of time. As
compared with previously known procedures, processing
according to the present invention can be accomplished
with less than half the energy input and processing time
hitherto required.
For a more complete understanding of the invention,
reference should be made to the following detailed
description of a preferred embodiment of the invention and
to the accompanying drawings:
Figure l is a schematic illustration of reinforced
concrete set up for treatment in accordance with a first
aspect the present invention;
Figure 2 is a graphical representation illustrating the
increasing passivity (and therefore protection) of
embedded steel reinforcement over a period of time after
treatment in accordance with the first aspect of the
invention;
Figure 3 is a simplified cross sectional illustration of a
concrete structure illustrating the application of a
~0 second aspect of the invention; and
W094/~68 7 21 G ~ 5 7~ PCT/GB94/00802
Figure 4 is a representative graph illustrating the
relationships between treatment time according to the
invention and its effect upon the bond strength between
concrete and steel embedded therein.
.
DESCRIPTION OF PREFERRED EMBODIMENTS:
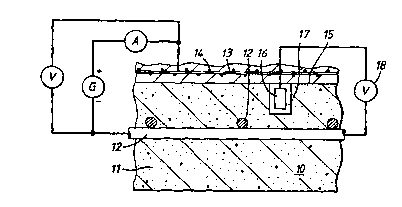
Referring now to the drawing lO represents a concrete,
comprised of set and hardened concrete ll in which is
embedded steel reinforcement 12, which can be of a known
and conventional type. Depending on the engineering
requirements for the structure, the amount of reinforcing
steel per unit of concrete may vary rather widely. For
the purposes of this invention, it is assumed that the
concrete structure is a mature installation, in which the
body of the concrete ll has become contaminated by
chloride ions, carbonation or other circumstances tending
to create conditions favouring corrosion of the steel
reinforcement l2.
To carry out the process of the invention, electrical
connections are made to the reinforcement steel to be
protected, and to a temporary anode placed externally in
an electrolytic mass or liquid in contact with the surface
of the concrete to be treated. In the illustated
arrangement a, D.C. power source, designated by the letter
"G", is connected at its positive side to a distributed
electrode structure 13, arranged in electrical
communication with an exposed surface of the concrete
structure lO, and at its negative side to the embedded
reinforcing steel. As many connecting points, as desired,
may be established with the objective of realising a
relatively uniformly distributed current flow between the
reinforcing steel and the distributed electrode.
W094/~68 PCT/GB94/00802
p~G~5 -8- ~
To advantage, the electrode structure 13 may comprise a
mesh like material of suitably conductive material, such
as steel wire mesh or titanium mesh, for example. As is
shown in Figure 1 the electrode structure is embedded in
an electrolytic medium 14 arranged in intimate contact
with the exposed surface 15 of the concrete structure lO.
In appropriate cases, when the surface 15 is upwardly
facing and horizontal (or nearly so), the electrolytic
medium can be a liquid, appropriately pooled to cover the
10 concrete surface. More preferably, the electrolytic
medium is a self adherent conductive mass, such as a
sprayed on mixture of cellulosic pulp fibre and water or
other electrolyte. The fibre mass is applied in a first
layer, prior to mounting the electrode structure 13 and in
15 a second layer thereafter to embed completely the
electrode structure within the conductive mass. It will
be understood that other suitable materials could be used
to form the requisite electrolytic mass.
A self-adherent electrolytic mass is desirable in many
20 cases, as where the exposed concrete is, for example,
vertical or downwardly facing or where the surface of the
concrete is convoluted or very rough.
Other arrangements of the distributed electrode are
possible, such as conductive surface coatings, foil layers
25 placed in direct contact with the concrete surface, spongy
blankets in certain cases etc. The particular form of
distributed surface electrode is not critical to the
invention, as long as it functions effectively to
distribute the current flow effectively over the surface
30 area of the embedded steel reinforcement. Generally this
objective is realised by distributing the current from the
externsl distributed electrode 13 relatively uniformly
over the exposed surface of the concrete structure.
WO 94124068 ~1 6 o~ 7~ PcT~GB94/00802
In carrying out the process of the invention, a direct
electric current of at least 0.1 amperes per square metre
of surface area of the embedded steel reinforcement 12 is
caused to flow between the reinforcement steel, which is
negatively connected, and the external electrode which is
positively connected to function as an anode. The output
voltage of the DC power source "G" may vary between wide
limits, but it should be designed to deliver sufficient
charge at the minimum current density mentioned above.
In practice, it has been found convenient to use a power
source "G" capable of being adjusted to between 5 and 40
volts DC output, and with sufficient current capacity to
deliver between 0.5 and 10 amperes per square metre of
surface area of the embedded steel. The output of the
power source can be monitored by suitable voltage and
current meters "V" and "A" as shown.
Pursuant to this aspect of the invention, the current is
passed for the time necessary to give a total charge of at
least about 100 amperes per square metre of surface area
20 of the embedded steel reinforcement 12. Preferably the
total charge should not exceed about 3000 ampere-hours per
square metre of steel surface area, because the energy
consumed is largely wasted and does not achieve
significant benefit. If the treatment of the invention is
25 required only to deal with chloride removal the total
charge can be in the order of 1000 amperehours per square
metre of steel surface area. Whilst higher charge levels
can be used it is useful to note that a total charge of as
high as 10,000 ampere-hours per square metre of steel
30 surface area could actually be detrimental, causing
degredation of the concrete.
The actual time taken to achieve the desired total charge
per unit of steel will of course depend on the available
W094/~68 PCT/GB94/00802
2~605~ -10-
DC power source and, within extremely wide limits, is not
significant.
After a sufficient total ch~rge has been passed to the
embedded reinforcing steel 12, the current is switched
off, the entire installation is removed, and the external
conductive material, if removable, is removed. The steel
will then have been given long term protection by being
conditioned to become strongly passivated.
An explanation of the treatment given to the steel by the
process of this aspect of the invention is as follows:
The application of a current charge at a density of not
less than O.l amperes per square metre of surface area of
the reinforcing steel results in a phenomenon known as
cathodic stripping. That is to say, any existing oxide
or other films present on the steel surface are completely
removed leaving a perfectly clean, active steel surface.
At the same time, since the steel in question is very
strongly charged negatively, chloride ions, if any are
present in the concrete, are strongly repelled from the
steel surface. This repulsion leaves the steel surface
chloride free. In addition, the surrounding concrete is
also rendered essentially chloride free to a distance of
usually at least lOmm. from the steel. Simultaneously,
the electrochemical cathodic reactions caused by the
25 action of the current at the steel surface lead to the
production of for instance sodium hydroxide which is
produced in sufficient quantities to impregnate the pores
of the concrete surrounding the steel and thus render the
environment highly alkaline.
30 These cathodic reactions are believed to be generally as
follows:
W094/~68 ~ 75 PCT/GB94/00802
(la) 2 + 2H2O + 4e --~40H
(lb) 2H2O + 2e~ --~H2 + 20H
(2a) Na~ ~ e~ --~Na
(2b) 2Na + 2H2O --~2NaOH + H2
When the current is then switched off, after a suitable
treatment charge has been delivered, the steel will begin
to repassivate by virtue of it now being in a clean active
condition in a chloride-free, highly alkaline environment.
Under these relatively ideal conditions, the steel will
oxidise to produce the dense oxide film necessary to
protect the steel from corrosion. This oxidation process
is actually a special form of corrosion which results in
the formation of the very dense protective oxide film
known as the passivating film.
If desired, the formation of this film is easily followed
by monitoring the electrical potential of the steel in
relation to a standard reference half cell 16, such as
silver/silver oxide, lead/lead oxide, copper/copper
sulphate, etc. The reference cell 16 should preferably,
though not necessarily, be installed in a fixed position
near to the steel to be monitored, for example, by
grouting into a drilled hole 17 in the concrete.
A diagram can then be drawn up showing the change in
potential with time, an example of which is shown in
Figure 2 of the drawings. Such a diagram will show that
the passivation process, which commences as soon as the
processing current is discontinued, extends over a long
period of time. If the reference cell monitoring is
sufficiently prolonged, it will show when the steel gains
the potential commonly considered as being safe from a
W094/~68 PCT/GB94/00802
~ 12-
corrosion point of view. Indeed, if sufficiently
prolonged, it can also show whether or not the steel ever
again becomes subject to corrosion, by noting whether the
potential again passes the value associated with
corrosion, but from the opposite direction.
As shown in Figure 2, the reference potential, measured
with a suitable volt meter 18, between the lead/lead oxide
half cell 16 and the steel reinforcement 12, increases
slowly, over a period of several months. Starting from an
initial potential of about 0 millivolts, the reference
potential gradually increases to about +500 millivolts
(considered relatively safe, from a corrosion standpoint),
in a period of around seven weeks. After a year, the
reference potential has continued to increase to a level
of around ~700 millivolts. It should be noted that
depending upon the nature of the concrete involved other
potential values could be applicable.
It has been found in practice that the corrosion
protection imparted this way is long lived, is robust
against new penetration by chloride ions, and even,
surprisingly, that the corrosion protection provided
eventually spreads to areas of the embedded steel in
concrete outside the treated area, but in metallic contact
with the steel within the treated zone, and tha~ this
occurs even after the current has been switched off and
the installation removed.
The process of the invention, while related to the
procedures described in the before mentioned Vennesland et
al and Miller patents, has surprisingly and unexpected
advantages in that by controlling the processing in
accordance with current flow in relation to surface area
of the embedded steel reinforcement, extraordinary
processing economies are realised. At the same time,
W094/24068 -13- 21 6 ~ ~ 7 ~ PcTlGB94loo8o2
there is greater assurance that the desired
protection/rehabilitation is effectively achieved within a
targeted processing period. ` Thus, processing in
accordance with the present invention may, in a typical
case, achieve reliable results in about half the time
required to achieve a chloride level which could be
regarded as reasonably safe,
The process of the present invention, involving processing
- of a concrete structure according to the surface area of
the embedded reinforcement, enables the processing time to
be accurately predicted in advance, whereas controlling in
accordance with the remaining chloride levels requires the
periodic taking and testing of core samples from the
material under treatment and cannot be predicted in
advance. Moreover, by the time the testing of the core
indicates that chloride levels have been reduced to
targeted levels, it can be expected that processing will
have been carried on for a time far beyond that required
to achieve the ampere-hour per square metre of surface
2~ levels known to be effective under the proposals of the
present invention.
As will be appreciated, concrete structures may vary
widely in the amount of internal embedded reinforcement
per unit of concrete. Depending upon engineering
requirements, steel to concrete ratios vary between 0.2
and 2 square metres of steel surface area per square metre
of concrete surface. A more typical range is between 0.3
and 1 square metre of steel surface area per square metre
of concrete surface. Accordingly, it will be appreciated
that controlling treatment time in accordance with the
surface area of the reinforcing steel can lead to
significantly different end results than those achieved
when controlling time in accordance with concrete core
samples.
W094/~68 ~ PCT/GB94/00802
~6~ -14- ~
This situation is further enhanced by the fact that, as
has been found in practice, it is not necessary to treat
the whole of a concrete structure subject to embedded
reinforcement corrosion in order to achieve an effective
treatment i.e., passivation of the reinforcing steel. It
has been found that the above mentioned lasting protection
can be achieved by subjecting only a part of the structure
to a predetermined current/time treatment regime.
In other words, satisfactory lasting protection can be
gained merely by subjecting a selected part of the
concrete structure to a said current flow/time treatmemt
regime by passing the direct current through the selected
part only.
In a practical instance, should the corrosion protection
given by a previous corrosion protection treatment by
chloride extraction or realkalisation breakdown, for
example, by the egress of new chlorides or by the washing
out of alkali, the required corrosion protection can be
achieved by treating the defective part of the structure
only.
A further feature of the process of the invention arises
in practical installations in which no part of the
structure is readily available for sufficient time for
adequate treatment to be effected (for example, as would
25 be the case of a bridge structure having to be closed to
traffic for the duration of a treatment lasting several
weeks). In such situations, in practicing the process
of the invention it is possible to construct a
prolongation to a part of a structure to be treated by,
30 for example, adding to a reinforced structure an
additional reinforced concrete part sufficiently in
physical contact which ensures that an adequate ion path
W094/~68 15 ~ 575 PCT/GB94/00802
is provided in the concrete across the join between the
concrete structure and the additional part and such that
effective electical connection is made with the embedded
steel to enable the required electrochemical treatment of
the actual concrete structure to take place.
..
As will be understood this aspect of the invention enables
a main structure to be given a lasting corrosion
protection by treatment of a part of the actual structure
it is required to treat or by treatment of an extension to
the actual structure it is required to treat.
As has been indicated above the amount of current flow
necessary to give a lasting protection is a function of
the area of a structure available for treatment, and that
it is desirable to restrict the maximum current to a level
that can safely be used without introducing any
undesirable side effects. Thus in general it has been
found advantageous, for the purposes of chloride removal
or realkalisation, to maintain current densities below 5
ampere/hours per square metre of concrete surface. It has
been noted that the use of e~cessively higher current
densities can cause detrimental effects. However, in the
case of any concrete part added to a structure for the
purposes above mentioned higher densities may be used.
This makes it possible to treat large areas of a structure
by the passage of higher current densities in the
reinforced prolongation part, i.e., outside the actual
main structure.
It has been noted that, in practice, in order to provide a
lasting protection i.e., five to ten years in a particular
application of the concepts of the invention, the steel
reinforcement needs to be charged at a level corresponding
to lOO0 ampere/hours per square metre thereof. This
-
W094/~68 6~$ 16- PCT/GB94/00802
to lO00 ampere/hours per square metre thereof. This
protection can for example, in practice, be achieved by
treating the whole area at one ampere/hour per square
metre for lO00 hours, by treating one half of the area at
2 ampere/hours per square metre for 500 hours, by treating
one tenth of the area at lO ampere/hours per square metre
for lO0 hours, or by any other proportional conbination.
It is to be noted that the above numerical data figures
are for guidance only since precise figures will depend
upon the geometry of the structure and other parameters
such as the chemical/physical nature of the concrete.
An additional aspect of the present invention relates to a
realisation that the application of the DC voltsge between
the embedded steel and the concrete can be used to modify
and establish targeted bond strengths between the concrete
and embedded steel. That is to say the process for the
electrochemical treatment of hardened concrete can be used
in order to modify (i.e., by increasing or decreasing) the
bond strength between hardened concrete and internally
embedded steel, particularly reinforcing bars,
pretensioning or post tensioning rods or cables.
Heretofore, this has been impossible, since there has been
no known procedure for controllably changing the
steel-to-concrete bond, in situ, in hardened concrete.
An additional aspect of the invention involves the
modification of the steel-to-concrete interface in a
hardened concrete structure to enhance the seal at such
interface. Frequently, the interface seal between
embedded steel reinforcing or tensioning elements is less
than perfect, due to accumulation of bleeding water at the
steel surface during the initial hardening of the
concrete, or possibly due to insufficient compaction of
W094/~68 -17_ 2 1 6 0 5 7 5 PCT/GB94/00802
the concrete when initially poured. Imperfections in the
seal at the steel-to-concrete interfaces can result in
seepages, in structures exposed to water pressure, or a
possible carbonation of the concrete surfaces adjacent to
the steel, with consequent corrosion of the steel.
The present aspect of the invention is based partly upon
the discovery that, during the electrochemical treatment
of concrete for the reasons so far discussed, by utilising
the internally embedded steel as a cathode, and a
10 distributed electrode structure structure spaced
therefrom, typically at an exposed surface of the concrete
as an anode, a marked change occurs in the bond between
the embedded steel and the surrounding concrete, as a
function of the electrical charge applied. During an
15 initial phase of the treatment, there is a progressive and
significant reduction in the bond strength to a level far
below the initial bond strength. This is followed, with
continued treatment, by a progressive and significant
increase in bond strength. It has been observed that this
2a variation in bond strength is both predictable and
repeatable for given types of concrete. Accordingly, by
establishing a simple database of relationships between a
given treatment time and its effect upon the
steel-to-concrete bond strength, it becomes possible
25 predictably to modify such bond strength in an existing
structure.
In the case of pre-tensioned or post-tensioned concrete
structures, for example, it may be desirable to decrease
the bond strength at the steel-to-concrete interface.
30 This would tend better to accommodate flexing of the
compressed concrete structural element. With static steel
reinforcing bars, on the other hand, it may be desirable
to effect an increased bond at the steel-to-concrete
interface.
W094/~68 PCT/GB94/00802
This aspect of the invention is not essentially a
rehabilitive process as has been previously discussed
above, but is to be considered as being directed to
controlling and modifying - the bond at the
steel-to-concrete interface.
In this aspect of the process of the present invention,
treatment conditions and controlling parameters are
different than for those involved in other aspects in the
treatment of a concrete structure.
10 For a better understanding of the invention of this aspect
of the invention reference should be made to the following
to Figures 3 and 4 of the accompanying drawings
Referring now to Figure 3, the bonding at the
steel-to-concrete interface is modified by passing an
15 electrical current between the embedded steel and a
distributed electrode associated with the concrete, at a
location spaced from the embedded steel. Figure 3 shows
a typical and advantageous arrangement for the
accomplishment of that objective. In Figure 3, the
20 reference numeral lO designates a reinforced (or
pre-tensioned of post-tensioned) concrete structure. In
the illustration, a concrete body is provided with a
plurality of reinforcing bars 12, which are embedded in
and surrounded by the concrete.
25 A source "G" of DC voltage is connected at its negative
side to the embedded steel elements 12 and at its negative
side to a distributed electrode element 13, which may be
in the form of a conductive wire mesh, for example, of
steel or titanium. In the illustrated system, the
30 electrode element 13 is embedded in an electrolytic mass
14, which advantangously may be a cellulosic pulp fibre,
WOg4/~68 21 6 0 5 7 5 PCT/GB94/00802
-19-
for example, maintained moist with water or electolytic
solution. Where the cellulosic pulp fibre is employed,
it typically is sprayed onto the outer surface 15 of the
concrete 11 in two layers. The fibrous material is
self-adherent to the surface of the concrete, and thus may
be applied to the vertical or even downwardly facing
surfaces. After applying the first layer, the mesh
electrode 13 is installed, and a second layer of the fibre
is applied over the top of the electrode substantially as
shown in Figure 1,
The particular form of the distributed electrode is not
significant to the invention. Where the character and
orientation of the concrete admits, the electrode 13 may
be submerged in a pool of liquid, or embedded in a wet
spongy mass or blanket, for example. Likewise, where
appropriate, the surface of the concrete may be coated
with a conductive layer (or placed in contact with a
conductive foil). The principal requirement, for the
purposes of the present invention, is to provide an
area-distributed electrode arrangement, to accommodate a
distributed flow of electricity between the internally
embedded steel elements 12 and the opposite electrode.
The operating capacity of the voltage source "~" is not
critical. Practical considerations, however, suggest
25 that DC voltage may be made available at from 5 to about
volts DC, preferably adjustable. 50 volts is a
~ convenient upper limit for safety purposes. The system
desirably has a sufficient current capacity to deliver
between 0.5 and 10 amps of current, per square metre of
30 surface area of embedded steel in the area being
processed.
With reference to Figure 4 of the drawings, there is shown
a typical curve of values of steel-to-concrete bond
W094/~68 ~6~ 20- PCT/GB94/0080
strength, in MPa (Megapascals) in relation to the total
electrical charge applied to the embedded steel, in terms
of ampere-hours per square metre of surface area of the
embedded steel. In the',illustration of Figure 4, the
solid line represents an ,average of values for a concrete
of typical composition. The upper and lower dotted lines
represent typical deviations from the average values
represented by the solid line.
As will be evident in Figure 4, during the first stages of
10 processing in accordance with the present invention, and
up to a point where between 4000 and 5000 ampere hours per
square metre of steel surface area have been caused to
flow, the bond strength between the embedded steel and the
~urrounding concrete progressively diminishes. In the
15 illustration, the starting bond strength is approximately
1.8 MPa, and this progressively reduces to a value of
around 0.6-0.7 MPa, after a current flow of around 3000
ampere hours per square metre of steel surface area.
Upon continued flow of current between the embedded steel
20 and the distributed anode, the bond strength between the
embedded steel and the surrounding concrete begins to
increase. With continued current flow, the bond strength
increases dramatically above initial levels finally
reaching a maximum limit. In the data illustrated in
25 Figure 4, maximum bond strength is reached at a level of
about 5.7 MPa, after current flow of approximately 12,000
ampere hours per square metre of surface area of embedded
steel.
After reaching its maximum values, bond s~rength again
30 begins to decrease with continued current flow, although
it ultimately levels off and becomes relatively stable at
current flow in the range 14000-lS000 ampere hours per
square metre of surface area. Normally, there would be no
-
~094/~68 ~ 6 ~ ~ ~ 5 PCT/GB94/00802
-21-
reason to carry the process beyond the point of maximum
bond strength. Indeed, it may be detrimental to do so.
.
The data reflected in Figure 4 of the drawings, represents
_ a smoothed-out curve based upon actual data readings from
a concrete of average quality. Similar databases can be
developed for any sp`ecific concrete mixture, although the
curve of Figure 4 is suitable for most practical cases.
In the course of treatment in accordance with this aspect
of the invention, it is observed that, when treatment has
10 continued to the point where bond strength has increased
above initial values, the interstices of the concrete, at
the steel-to-concrete interface and immediately adjacent
thereto, have been impregnated with substances produced by
the electrochemical reactions at the steel surface. These
15 are thought to be mixtures of various compounds, including
calcium hydroxide and calcium carbonate. This
impregnation with reaction compounds, renders the
interface zone impervious and sealed, for all practical
purposes.
20 The process of the invention achieves remarkable and
unexpected results in enabling for the first time, the in
situ modification of steel-to-concrete bond strength in a
hardened concrete structure. Depending upon the
requirements of a particular installation, the bond
strength may be controllably decreased, as may be desired
in installations utilising pre-tensioned or post tensioned
tendons, or increased, as in the case of standard static
reinforcing bars embedded in a typical concrete structure.
A database of values for a typical concrete composition is
30 easily produced and can serve acceptably for most types of
concrete. For particularly critical structures and/or
for unique concrete formulations, a relatively simple set
wo 94,24068 ~51~ -22- PCT/GB94/00802
of tests can be performed to establish a specific database
of values for a specific composition of concrete. These
values can then be followed in controlling the process as
applied to a particular structure utilising the special
composition.
In addition to providing for precise modification and
control of steel-to-concrete bond strength, the process of
the invention can also be utilised to seal effectively the
steel-to-concrete interface against the ingress of water
10 and atmosphere. This is the result of the precipitation
of reaction products in the interstices of the concrete at
and lmmediately surrounding the steel-to-concrete
interface, which makes the concrete in this area
relatively impenatrable to external liquids and gases.
15 The process of the invention is simple and economical to
apply, and utilises known technology and known equipment.
In a typical case, the external electrode means can be
installed on an exterior surface of the structure and then
washed away or otherwise removed upon completion of the
20 procedure.
It should be understood, of course, that the specific form
of the invention herein illustrated and described is
intended to be representative only, as certain changes may
be made therein without departing from the clear teachings
25 of the disclosure. Accordingly reference should be made
to the following appended claims in determinng the full
scope of the invention.