Note: Descriptions are shown in the official language in which they were submitted.
WO 94/27989 PCT/EP94/01613
216~
CARBAZOLE DERIVATIVES WITH 17,20-LYASE-INHIBITING ACTIYTY
- This invention relates to substituted carbazole derivatives, to processes for their
preparation, to phar",aceutical compositions containing them and to their use inmedicine for the reduction of oestrogen and/or androgen levels.
17,20-Lyase activity is responsible for the conversion of 17a-
hydroxyprogesterone to androsle,ledione and of 17a-hydroxypregnenolone to
dehydroepiandrosterone. This activity is critical for the biosynthesis of
androgens and estrogens, and inhibition of 17,20-lyase by ketoconazole (a
known antifungal agent) has been shown to reduce testosterone levels in
animals tEnglish, H.F. et al., Cancer Res. 47, 38-42 (1986) and men (Pont, A et
al., Arch. Intern. Med.142, 2137-2140 (1982).
High dose ketoconazole has been used in the treatment of prostate cancer
(Trachtenberg, J., J. Urol. 132, 61-63 (1984) and Pont, A., et al., Arch. Intern.
Med. 145, 1429-1431 (1985)). However, doses of ketoconazole that produce
inhibition of testosterone production are significantly more toxic than antifungal
doses, and more selective 17,20-lyase inhibitors would be expected to have a
much better therapeutic index.
Inhibition of 17,20-lyase, as an approach to inhibition of testosterone
biosynthesis, also has the advantage of blocking adrenal as well as testicular
derived androgens (English, H.F. et al., Cancer Res. 46, 38-42 (1986)), and
should be a more effective treatment for prostate cancer than that afforded by
LHRH agonists.
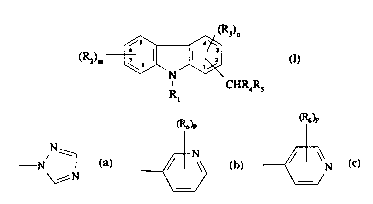
Thus the present invention provides compounds of general formula (I)
(R2)m ~ (R3)D (I)
R CHR4R5
wherein R1 and R4 each independently represent a hydrogen atom or a
C1 6alkyl group;
WO 94/27989 PCT/EP94/01613
~ ~ 6~9~ 1 2
each R2 may be the same or dirrerenl and represents an electron-withdrawing
group;
each R3 may be the same or different and represents an electron-withdrawing
group;
5 R5 is a group of formula
~)P ~
' ~ ~r ~ or ~~ N
\~N . = ~= / ;
R6 is a halogen atom, a C14alkyl group or a C14 alkoxy group;
m is zero or an integer 1 to 4;
n is zero or an integer 1 to 3; and
10 piszero, 1 or2
and pharmaceutically acceptable salts and solvates thereof.
The substituents R2 may each occupy any available position of the 5, 6, 7 or 8
posltlons.
The substituents R3 may each occupy any available position of the 1, 2, 3 or 4
positions, preferably the 1, 3 or 4 positions.
The group--CHR4R5 may occupy any of the 1, 2, 3 or 4 positions, for example,
the 1, 2 or 3 positions. Preferably the group--CHR4R5 will be in the 2 position.
Thus, in one aspect the invention provides compounds of formula (la)
N 1'' CHR4R5
R,
wherein R~, R2, R3, R4, R5, m and n are as hereinbefore defined.
WO 94/27989 2 1 ~ ~ g ~1 PCT/EP94/01613
-
The substituent R6 may be attached to any carbon atom in the pyridyl ring, but
preferably is attached in the 3,4 or 5 positions.
It will be appreciated by those skilled in the art that the numbering of the ring
system for individual co"lpounds within the scope of formula (I) will vary
5 according to the nature, number and position of substituents. For convenience
the numbering of ring atoms adopted herein is that shown in formula (I) above
except where individual compound names are given.
It will be appreciated that the compounds of formula (I) may contain a chiral
centre. It is to be understood that formula (I) is intended to encompass all
10 enantiomers and diastereoisomers of the compounds of the invention as well as mixtures thereof, including racemates.
Electron-withdrawing groups are well known to those skilled in the art and any
such group may be employed. Such groups include halogen atoms, such as
fluorine, chlorine and bromine atoms, nitrile groups, nitro groups, trifluoromethyl
15 groups, aldehydo groups, keto groups and carboxylic acid and ester groups andare preferably selected from fluorine atoms, chlorine atoms and nitrile groups.
Particularly preferred as compounds of formula (I) are those wherein each of R2
and R3 represents a fluorine atom.
Suitably R2 represents a fluorine atom and m is an integer 1 to 4.
When m is 1 R2 will preferably be in the 5 or 7 position, especially the 7-
position.
C1~alkyl and C1~alkoxy groups may contain straight or branched chain alkyl
groups, for example, methyl, ethyl, n-propyl, iso-propyl, n-butyl, t-butyl, pentyl or
hexyl groups, preferably C14alkyl groups. Thus, for example, each of R1 and
R4 may be a hydrogen atom or a methyl, ethyl, propyl or butyl group.
R1 and R4 are each preferably a hydrogen atom or a methyl group.
WO 94/2798g PCT/EP94/01613
~2~ 4
In one prefer,ed group of compounds of formula (I) R5 is a pyridin-3-yl or
pyridin4-yl group.
Suitably R6 represents a fluorine atom, a methyl group or a methoxy group.
In a preferred group of w,npounds of formula (I), R1 and R4 each represent a
hydrogen atom or a methyl group, R2 and R3 are fluorine atoms, R6 is a fluorine
atom, a methyl group or a methoxy group, m is an integer 1 to 4, n is zero or aninteger 1 to 3, and p is zero, 1 or 2.
In a particularly preferred group of compounds of formula (I), R1 and R4 each
represent a hydrogen atom, R2 and R3 are fluorine atoms, R6 is a fluorine atom
or a methyl or methoxy group and m, n and p each independently represent
zero, 1 or 2.
Specific compounds according to the invention include:
2-Fluoro-7-[1 ,2,4]triazol-1 ylmethyl-9H-carbazole
2-Fluoro-7-pyridin-3-ylmethyl-9H-carbazole
1 5 2-Fluoro-7-pyridin-4-ylmethyl-9H-carbazoie
2-Fluoro-7-(3-fluoropyridin-4-ylmethyl)-9H-carbazole
2-Fluoro-7-(3-methylpyridin4-ylmethyl)-9H-carbazole
2-Fluoro-7-(3-methoxypyridin-4-ylmethyl)-9H-carbazole
1 ,7-Difluoro-2-[1 ,2,4]triazol-1 -ylmethyl-9H-carbazole
2,4-Difluoro-7-[1,2,4]triazol-1-ylmethyl-9H-carbazole
1 ,4,7-Trifluoro-2-[1 ,2,4]triazol-1 -ylmethyl-9H-carL.a,ole
and pharmaceutically acceptable salts and solvates thereof.
Suitable pharmaceutically acceptable salts of the compounds of formula (I)
include acid addition salts derived from inorganic and organic acids, such as
25 hydrochlorides, hydrobromides, sulphates, phosphates, citrates, tartrates,
maleates, fumarates, succinates, p-toluenesulphonates and
methanesulphonates. Other suitable salts will be readily apparent to one skilledin the art. Hydrochloride, sulphate and phosphate salts are especially
preferred. Salts which are not pharmaceutically acceptable may be useful in the
WO 94/27989 PCT/EP94/01613
~162921
preparation of compounds of formula (I) and these form a further part of the
invention.
Compounds of the invention may be isolated in association with solvent
molecules by crystallisation from or evaporalion of an appropriate solvent. Such5 solvates of the compounds of formula (I) are included within the scope of the
present invention.
References hereinafter to a compound according to the invention includes both
the compounds of formula (I) and their pharmaceutically acceptable salts and
solvates.
10 The compounds according to the invention are potent and selective inhibitors of
the enzyme steroidal 1 7,20-lyase, which is a key enzyme involved in the
conversion of C21-steroids (e.g. pregnenolone) into androgens (e.g.
testosterone) and oeslroge"s (e.g. oestradiol).
The 1 7,20-lyase-inhibiting activity of the compounds of formu!a (I) was
15 demonstrated in vitro by their ability to inhibit the conversion of 1 7a-
hydroxypregnenolone into dehydroepiandrosterone by human testicular 17,20-
lyase and of 17a-hydroxyprogesterone into androstenedione by rat testicular
17,20-lyase. These assays were conducted according to a method based on
that of Ayub and Level, J. Steroid Biochem, 1987, 28, 521.
20 In in vivo tests the compounds of the invention were tested for their ability to
suppress the elevation of testosterone levels produced in male rats when
stimulated with human chorionic gonadotrophin (hCG).
Inhibitors of 17,20-lyase reduce circulating and local levels of androgens and
oestrogens. The compounds of the invention can thus be used in the treatment
25 of androgen- andtor oestrogen-dependant diseases such as malignant and
benign diseases of the breast, endometrium, ovary, prostate and pancreas.
These diseases include cancer of the prostate, breast and endometrium,
prostatic hype~ ll opl ~y and hyperplasia, fibrocystic breast disease, endometriosis
and polycystic ovarian dise~se. The compounds of formula (I) are also useful in
30 the treatment of Cushing's syndrome, gynecomastia, premalure labour,
precocious puberty, female hirsutism, premenstrual syndrome, male pattern
WO 94127989 PCT/EP94/01613
~6~ 6
baldness and acne. The compounds of the invention will be particularly useful
in the treatment of prostate cancer.
The invention thus further provides compounds of formula (I) and their
pharmaceutically acceptable salts and solvates for use as active therapeutic
5 agents in particular for the treatment of conditions whose underlying aetiology is
associated with elevated androgen and/or oestrogen levels in animals
(especially humans).
In a particular aspect of the present invention there is provided a compound of
formula (I) or a pharmaceutically acceptable salt or solvate thereof for use in the
10 treatment of prostate cancer.
In a further or alternative aspect there is provided a method for the lowering of
the levels of androgens and/or oestrogens in a mammal including a human
comprising administration of an effective amount of a compound of formula (I) ora pharmaceutically acceptable salt or solvate thereof.
15 There is also provided in a further or alternative aspect use of a compound of
formula (I) or a pharmaceutically acceptable salt or solvate thereof for the
manufacture of a medicament for the lowering of levels of androgens and/or
oestrogens.
It will be appreciated by those skilled in the art that reference herein to
20 treatment extends to prophylaxis as well as the treatment of established
s~" "~t~" ,s.
While it is possible that for use in therapy a compound of the invention may be
administered to a patient as the raw chemical it is preferable to present the
active ingredient as a pharmaceutical formulation.
25 The invention accordingly provides a pharmaceutical formulation comprising a
compound of formula (I) or a phar~naceutically acceptable salt or solvate thereof
together with one or more pharmaceutically acceptable carriers or excipients
and optionally other therapeutic and/or prophylactic ingredients. The carriers
must be "acceptable" in the sense of being compatible with the other ingredients30 of the formulation and not deleterious to the recipient thereof.
WO 94/27989 PCT/EP94/01613
2~16'~9~1
Pharmaceutical formulations include those suitable for oral, rectal, nasal,
topical, implant or parenteral (including intraml ~sculAr, subcutaneous and
intravenous) administration or in a form suitable for administration by inhalation
or insufflation. The formulations may, where appropriate, be conveniently
5 presented in discrete dos~ge units and may be prepared by any of the methods
well known in the art of pharmacy. All methods include the step of bringing intoassociation the active compound with liquid carriers or finely divided solid
carriers or both and then, if necessary, shaping the product into the desired
formulation.
10 For oral administration, the pharmaceutical compositions may take the form of,
for example, tablets or capsules prepared by conventional means with
pharmaceutically acceptable excipients such as binding agents (e.g.
pregelatinised maize starch, polyvinylpyrrolidone or hydroxypropyl
methylcellulose); fillers (e.g. Iactose, microcrystalline cellulose or calcium
15 phosphate); lubricants (e.g. magnesium stearate, talc or silica); disintegrants
(e.g. potato starch or sodium starch glycollate); or wetting agents (e.g. sodiumlauryl sulphate). The tablets may be coated by methods well known in the art.
Liquid preparations for oral administration may take the form of, for example,
solutions, syrups or suspensions, or they may be presented as a dry product for
20 constitution with water or other suitable vehicle before use. Such liquid
preparations may be prepared by conventional means with pharmaceutically
acceptable additives such as suspending agents (e.g. sorbitol syrup, methyl
cellulose or hydrogenated edible fats); emulsifying agents (e.g. Iecithin or
acacia); non-aqueous vehicles (e.g. almond oil, oily esters or ethyl alcohol); and
25 preservatives (e.g. methyl or propyl-c-hydroxybenzoates or sorbic acid).
For topical administration in the mouth, the pharmaceutical compositions may
take the form of buccal or sub-lingual tablets, drops or lozenges formulated in
conventional manner.
For topical administration to the epidermis the compounds of the invention may
30 be formulated as creams, gels, ointments or lotions or as transdermal patches.
Such compositions may, for example, be formulated with an aqueous or oily
WO 94/27989 PCT/EP94/01613
2~ 8
base with the addition of suitable thickening, gelling, emulsifying, stabilising,
dispersing, suspending, and/or colouring agents.
The compounds of the invention may also be formulated as depot preparations.
Such long acting formulations may be administered by implantation (for example
5 subcutaneously or intramuscularly) or by intramuscular injection. Thus, for
example, the compounds may be formulated with suitable polymeric or
hydrophobic materials (for example as an emulsion in an acceptable oil) or ion
exchange resins, or as sparingly soluble derivatives, for example as a sparinglysoluble salt.
10 The compounds of the invention may be formulated for parenteral administration
by injection, conveniently intravenous, intramuscular or subcutaneous injection,for example by bolus injection or continuous intravenous infusion. Formulations
for injection may be presented in unit dosage form e.g. in ampoules or in multi-dose containers, with an added preservative. The compositions may take such
15 forms as suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilising and/or
dispersing agents. Alternatively, the active ingredient may be in powder form for
constitution with a suitable vehicle, e.g. sterile pyrogen-free water, before use.
The compounds of the invention may also be formulated in rectal compositions
20 such as suppositories or retention enemas, e.g. containing conventional
suppository bases such as cocoa butter or other glyceride.
For intranasal administration the compounds of the invention may be used, for
example, as a liquid spray, as a powder or in the form of drops.
For administration by inhalation the compounds according to the invention are
25 conveniently delivered in the form of an aerosol spray presentation from
pressurised packs or a nebuliser, with the use of a suitable propellant, e.g.
dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane,
1,1,1,2-tetrafluoroethane, carbon dioxide or other suitable gas. In the case of a
pressurised aerosol the dosage unit may be determined by providing a valve to
30 deliver a metered amount. Capsules and cartridges of e.g. gelatin for use in an
WO 94/27989 PCT/EP94/01613
~62921
inhaler or insufflator may be formulated containing a powder mix of a compound
of the invention and a suitable powder base such as l~ctDse or starch.
Any of the pharmaceutical compositions described above may be presented in a
conventional manner ~ssoci~ted with controlled release forms.
5 Preferably the pharmaceutical compositions according to the invention are
suitable for oral, rectal or topical administration.
A convenient unit dose formulation contains the active ingredient in an amount
of from 0.1 to 200mg.
It will be appreciated that the amount of a compound of formula (I) required for10 use in treatment will vary not only with the particular compound selected, but
also with the route of administration, the nature of the condition being treatedand the age, weight and condition of the patient and will ultimately be at the
discretion of the attendant physician or veterinarian. In general, however, a
suitable dose will be in the range of from about 1 to about 500mg per day,
preferably in the range of 20 to 200mg per day, most preferably in the range of
50 to 120mg per day.
A suitable daily dose for use in prophylaxis will generally be in the range of
0.1 mg to 50mg.
The desired dose may conveniently be presented in a single dose or as divided
20 doses administered at appropriate intervals, for example as two, three, four or
more sub-doses per day. The compound is conveniently administered in unit
dosage form.
The compounds of the present invention may also be used in combination with
other therapeutic agents, for example, other androgen and/or oestrogen
25 lowering agents, or anticancer agents. In particular the compounds of the
invention may be employed together with known anticancer agents.
The invention thus provides, in a further aspect, a combination comprising a
compound of formula (I) as defined herein together with another therapeutically
active agent, in particular an anticancer agent.
WO 94/27g8g PCT/EP94/01613
~6`~
The combination referred to above may conveniently be presented for use in the
form of a phar",aceutical formulation and thus pharmaceutical formulations
comprising a combination as defined above together with a phar",aceutically
acceptable carrier therefor comprise a further aspect of the invention.
5 When compounds of formula (I) are used in combination with a second
therapeutic agent the compounds may be administered either sequentially or
simultaneously by any of the routes described above.
Suitable therapeutic agents for use in the combinations defined above include
for example cyproterone acetate flutamide and anandron.
10 When compounds of formula (I) are used in combination with a second
therapeutic agent effective to reduce levels of androgens and/or oestrogens in amammal including a human the dose of each compound may vary from that
when the compound is used alone. Thus when compounds of formula (I) are
used together with a second therapeutic agent the dose of each compound may
15 be the same or different to that employed when the compound is used alone.
Appropriate doses will be readily appreciated by those skilled in the art.
The compounds according to the invention may be prepared by any process
known in the art for the preparation of compounds of analogous structure. In
thefollowing description R1 R2 R3 R4 Rs R6 m n and p are as defined for
20 general formula (I) unless otherwise specified.
In one general process (A) a compound of general formula (I) wherein R1
represents a hydrogen atom may be prepared from an intermediate of formula
(Il)
(R3)D
(R2)., ~ CHR~R5
25 by cyclisation. The reaction is conveniently effected in the presence of a
suitable solvent such as a hydloca,bon solvent for example dodecane or a
W O 94/27989 PCT~EPg4/01613
_ ~1&2921
halogenated solvent, such as dichlorobenzene, preferably at elevated
- temperature, for example 100 to 300C, preferably 150 to 220C. General
process (A) is particularly useful for the preparation of compounds of formula (I)
wherein R5 is a triazole group.
5 Intermediates of formula (Il) may be prepared from the corresponding amines of formula (Ill)
(R3)~
(Tll)
(R ) " = '` ~ CHR4R5
by treatment with sodium nitrite in the presence of a mineral acid, e.g. sulphuric
acid, followed by sodium azide. The reaction is conveniently effected in
10 aqueous solution.
Compounds of formula (Ill) may be prepared from the corresponding nitro
compounds of formula (IV)
(R3)n
(~v)
(R2)m \ CHR4R5
NO2
by reduction using hydrogen or a hydrogen-donor, e.g. ammonium formate, in
15 the presence of a catalyst, such as a noble metal catalyst, e.g. platinum,
palladium, platinum oxide or rhodium, which may be supported, e.g. on
charcoal. The reduction may be carried out in a solvent such as an alcohol e.g.
methanol or ethanol (which may be aqueous), acetic acid, aqueous acetic acid,
an ether e.g. dioxan, an ester e.g. ethyl acetate or an amide e.g.
20 dimethylformamide, and conveniently at a temperature of from -10 to +50C,
preferably 20 to 30C.
WO 94/27989 PCT/EP94/01613
~&~6 ~9 12
Compounds of formula (IV) may be prepared from col"pounds of formula (V)
(R3)n
~ (v)
CHR4Br
NOl
by treatment with a compound of formula HR5 or the sodium salt thereof. The
reaction is conveniently effected in a suitable solvent, e.g dimethylformamide.
5 Intermediates of formula (V) may be prepared from compounds of formula (Vl)
(R3)n
(R2)m ` CH2R4 (Vl)
NO2
by free radical bromination, for example using N-bromosuccinimide in the
presence of an initiator, such as a peroxide and/or ultra-violet light. The
reaction is conveniently effected in a non-polar solvent, such as a halogenated
10 solvent, e.g. chloroform or tetrachloromethane, at a temperature of 20 to 80C.
Compounds of formula (Vl) may be prepared from compounds of formula (Vll)
~ ~L
(R2)m l~ ~1 ~ (VII)
NO2
(wherein L represents a readily displaceable atom or group) by reaction with a
compound of formula (Vlll)
(R3)n
, ~/3 B(OH)2 (VIII)
1 5 ~H2c
WO 94/27989 PCT/EP94/01613
21~2~1
13
in the presence of a suitable palladium(O) catalyst such as
tetrakis(triphenylphosphine)palladium(O) and a base, e.g. sodium carbonate, in
a suitable aqueous solvent such as an alcohol, e.g. ethanol, an aromatic
hydrocarbon, e.g. benzene, or an ether, e.g. dimethoxyethane, or an aqueous
5 mixture of solvents. Suitable atoms or groups represented by L include halogen atoms, e.g. bromine or iodine atoms, or a triflate group.
In another general process (B), a compound of formula (I) may be prepared
from a compound of formula (IX)
(R2)m ~ ¦ ~` ` (IX)
Rl C(H)R4Rs
10 by deoxygenation. The deoxygenation reaction is effected using a suitable
reducing agent such as hydrogen in the presence of a catalyst, such as a noble
metal catalyst, e.g. platinum, palladium, platinum oxide or rhodium, which may
be supported, e.g. on charcoal. The reaction may conveniently be carried out in
a solvent such as an alcohol, e.g. methanol or ethanol, which may be aqueous,
15 in the presence of an acid, e.g. hydrochloric acid, preferably at elevated
temperature, e.g. at the reflux temperature of the solvent or at elevated
pressure. General process (B) is particularly useful for the preparation of
compounds of formula (I) wherein R5 is a pyridyl group.
Intermediates of formula (IX) may be prepared from compounds of formula (X)
(R~)m ~\J~ J (X)
Rl
by reaction with compounds of formula (Xl)
Hal - R5 (Xl)
WO 94/27989 PCT/EP94/01613
2~ 1 4
in the presence of a suitable base, such as an alkyllithium, e.g. n-butyllithium.
The reaction is conveniently effected in the presence of a suitable solvent suchas an ether, e.g. diethyl ether, dimethoxyethane or tetrahydrofuran, or a mixture
of solvents, suitably at low temperature, e.g. -90 to - 50, preferably about
5 -70C.
Compounds of formula (X) may be prepared from compounds of formula (Xll)
~R3)o
(R2)m
`' N -- CH(OH)R4
R,
by oxidation. Suitable oxidising agents will be readily apparent to one skilled in
the art and include pyridinium chlorochromate, potassium dichromate in
10 sulphuric acid and barium manganate. The reaction may conveniently be
effected in the presence of a solvent, e.g. a halogenated solvent such as
dichloromethane.
Compounds of formula (Xll) may be prepared from compounds of formula (Xlll)
(R3)n
(R2)m ``~=\ // = \\ (XIII)
\~, CH(OH)R4
N3
15 by cyclisation. The reaction is conveniently effected in the presence of a
suitable solvent, such as a hydrocarbon solvent, e.g. dodecane, or a
halogenated solvent, e.g. dichloromethane, preferably at elevated temperature,
e.g. 100 to 300C, preferably 150 to 220C.
Compounds of formula (Xlll) may be prepared from compounds of formula (XIV)
WO 94/27989 PCT/EP94/01613
2~62`9~1
~{CH2R~
NH2
by treatment with sodium nitrite in the presence of a mineral acid, e.g. sulphuric
acid, followed by sodium azide. The reaction is conveniently effected in
aqueous solution.
5 Compounds of formula (XIV) may be prepared from compounds of formula (XV)
(R3)n
(R2~m CH(OG)R4
NO2
(wherein G represents a hydroxy protecting group) by reduction using hydrogen
or a hydrogen-donor, e.g. ammonium formate, in the presence of a catalyst,
such as a noble metal catalyst, e.g. platinum, palladium, platinum oxide or
10 rhodium, which may be supported, e.g. on charcoal and subsequent removal of
the protecting group G. The reduction may conveniently be carried out in a
solvent such as an alcohol, e.g. methanol or ethanol, which may be aqueous,
optionally in the presence of an acid, e.g. hydrochloric acid. Suitable hydroxy
protecting groups are described hereinafter.
15 Compounds of formula (XV) may be prepared from compounds of formula (XVI)
~ L
2 m ~\ ~\ (XVl)
NO2
(wherein L represents a readily displaceable atom or group) by reaction with a
compound of formula (XVII)
WO g4/27989 PCT/EP94/01613
9~ 16
(R3)D
, B(OH)2 (XVII)
R4(0G)CH
in the presence of a suitable palladium(O) catalyst such as
tetrakis(triphenylphosphine)palladium(O) and a base, e.g. sodium carbonate, in
a suitable aqueous solvent such as an alcohol, e.g. ethanol, an aromatic
5 hydrocarbon, e.g. benzene, or an ether, e.g. dimethoxyethane, or an aqueous
mixture of solvents prererably at elevated temperature. Suitable atoms or
groups represented by L include a halogen atom, e.g. a bromine or iodine atom,
and a triflate group.
Alternative synthetic routes to the intermediates of formula tX) will be readily10 apparent to those skilled in the art.
In another general process (C) a compound of formula (I) according to the
invention may be converted into another compound of the invention using
conventional procedures.
According to one embodiment of general process (C) a compound of formula (I)
15 wherein R1 represents a hydrogen atom may be alkylated using conventional
techniques. The reaction may be effected using a suitable alkylating agent
such as an alkyl halide, alkyl tosylate or dialkylsulphate. The reaction may
conveniently be carried out in an inert organic solvent such as an amide, e.g.
dimethylformamide, or an ether, e.g. tetrahydrofuran, preferably in the presence20 of a base. Suitable bases include, for example, alkali metal hydrides, e.g.
sodium hydride, alkali metal carbonates, e.g. sodium carbonate, or alkali metal
alkoxides, e.g. sodium or potassium, methoxide, ethoxide or t-butoxide. The
alkylation reaction is conveniently effected at a temperature of 25 to 1 00C.
According to another general process (D), a c~ pound of formula (I) according
25 to the invention, or a salt thereof may be prepared by subjecting a protectedderivative of formula (I) or a salt thereof to reaction to remove the protectinggroup or groups. Thus, at an earlier stage in the preparalion of a compound of
formula (I) or a salt thereof it may have been necessary and/or desirable to
WO 94/27989 PCT/EW4/01613
~162921
._
17
protect one or more sensitive groups in the molecule to prevent undesirable
side reactions. Such protection may be effected in conventional manner, for
example as described in 'Plotective Groups in Organic Chemistry' Ed.J.F.W.
McOmie (Plenum Press 1973) or 'Protective Groups in Organic Synthesis' by
5 T W Greene (John Wiley and Sons 1981).
In compounds of formula (I) wherein R1 represents hydrogen the group NR1
may be protected for example with a conventional amino protecting group.
Such groups may include for example aralkyl groups, such as benzyl,
diphenylmethyl or triphenylmethyl groups; and acyl groups such as tosyl, N-
10 benzyloxycarbonyl or t-butoxycarbonyl.
Removal of any amino protecting groups present may be achieved by
conventional procedures. Thus an aralkyl group such as benzyl, may be
cleaved by hydrogenolysis in the presence of a catalyst (e.g. palladium on
charcoal); an acyl group such as t-butoxycarbonyl may be removed by cleavage
15 with, for example, hydrogen chloride in dioxan or sodium methoxide in
methanol.
As will be appreciated, in some of the general processes (A) to (C) described
above it may be necessary or desired to protect any sensitive groups in the
molecule as just described. Thus, a reaction step involving deprotection of a
20 protected derivative of general formula (I) or a salt thereof may be carried out
subsequent to any of the above described processes (A) to (C).
Where it is desired to isolate a compound of the invention as a salt, for example
as an acid addition salt, this may be achieved by treating the free base of
general formula (I) with an appropriate acid, preferably with an equivalent
25 amount. Solvates of the compounds of the invention may be prepared by
crystallisation from or evaporation of an appropriate solvent solution of the
compounds of formula (I). Separation of enantiomers of formula (I) may be
carried out in conventional manner, for example by resolution of racemic
mixtures e.g. using chiral HPLC techniques or by stereospecific synthesis from
30 isomerically pure starting material or any convenient intermediate, for example
as described in Stereochemistry of Carbon Compounds by E.L. Eliel (McGraw
Hill, 1962) and Tables of Resolving Agents by S.H. Wilen.
WO 94/27989 PCT/EP94/01613
1 8
Thus, according to a further aspect of the invention, the following reactions may,
if necessary and/or desired be carried out in any a~propriate sequence
subsequent to any of the processes (A) to (C):
(i) removal of any protecting groups;
5 (ii) conversion of a compound of formula (I) or a salt or solvate thereof into a
pharmaceutically acceptable salt or solvate (for example, hydrate) thereof;
(iii) separation of a racemic mixture into individual enantiomers of formula (I).
As well as being employed as the last main step in the preparative sequence,
the general methods indicated above for the preparation of the compounds of
10 the invention may also be used for the introduction of the desired groups at an
intermediate stage in the preparation of the required compound. It should
ll,erefore be appreciated that in such multi-stage processes, the sequence of
reactions should be chosen in order that the reaction conditions do not affect
groups present in the molecule which are desired in the final product.
15 As indicated above the compounds of the invention are useful as 17,20-lyase
inhibitors. 1 7,20-Lyase inhibition may be demonstrated by the following tests:
ACTIVITY in vitro
Biological activity in vitro was determined by measuring the inhibition of 17,20-
lyase activity in a microsomal preparation from human testes. A range of
20 concentrations of each compound was incubated with the microsomal
preparation and 17~-hydroxy (21-14C) pregnenolone as substrate for 60 mins
at 37C. Radioactive product was assayed and enzyme inhibition determined
by comparison with uninhibited control samples.
ACTIVITY in vivo
25 Biological activity in vivo was determined in a rat model of testosterone
biosynthesis. Compounds were administered p.o. at a dose of 3mg/kg. One
hour later the rats were given human chorionic gonadotrophin (hCG) s.c. to
stimulate testosterone synthesis and two hours after hCG administration, blood
was taken and serum testosterone concenl,dlions measured. Inhibitory activity
30 was determined by comparison to values from rats receiving vehicle only.
WO 94/27989 PCT/EP94/01613
The results of the in vitro and in vivo assays for the compounds of the following
examples are shown in Table 1. The results for the known 17 20-lyase inhibitor
ketoconazole are included for comparison.
Table 1
Example In vitro assay In vivo assay
ICso conc~.~t-alion (nM) % Inhibition of serum
t~stost~r~. ~e
Ketoconazole 85 56
97 50
2 6 84
3 5 74
4 8 40
78
6 19 71
7 41 14
8 12 36
9 25 5
5 ~ The dose of ketoconazole was 30mg/kg.
Acute toxicity studies indicate that single doses of 3mg/kg p.o. of the compoundof Example 2 are well tolerated in rats and no overt toxicity was observed.
The invention is further illustrated by the following non-limiting examples. Alltemperatures are in C. Chromaloyraplly was performed on silica gel. Dried
10 means dried over anhydrous magnesium sulphate. DMSO means dimethyl
sulphoxide. DMF means dimethylformamide. THF means tetrahydrofuran.
DME means dimethoxyethane. IPE means isGpropyl ether.
Intermediate 1
4-(t-ButvldimethYlsilYloxYmethYl)phenylboronic acid
15 (i) 4-(t-ButYldimethYlsilYloxYmethvl)-bromobenzene
A solution of t-butyl~li",ell,ylsilyl chloride (21.19) in DMF (25ml) was treateddropwise with a solution of 4-bromo-benzyl alcohol (259) and imidazole (18.29)
in DMF (100ml). Resulting solution was left standing at room temperature for
WO 94/27989 PCT/EP94tO1613
2~
72h. Reaction mixture was added to ethyl acetate (2000ml) and organic layer
was washed with 2N HCI (2 x 250ml), water (4 x 250ml), brine (250ml), dried
and evaporated to give a yellow oil. Chromatography on silica gel (1 kg), eluting
with cyclohexane/ethyl acetate (10:1 v/v) gave the product as a pale yellow oil.
(ii) 4(t-ButYldimethvlsilvloxYmethvl)Phenvlboronic acid
A solution of (i) (32.37g) in dry tetrahydrofuran (250ml) was cooled to -70
under N2 n-Butyllithium (70ml of a 1.6M solution in hexane) was added
dropwise over 15 minutes maintaining temperature below ~5C. Resulting
yellow solution was stirred below -70C for 2h, then triisopropyl borate (75ml)
was added dropwise, maintaining temperature below-65. When addition was
complete, the reaction was allowed to warm to room temperature, then
quenched with water (550ml) and then ether (250ml).
The organic layer was separated off and the aqueous phase extracted with
ether (2 x 500ml). The combined organic layers were washed with brine
(200ml), dried and evaporated to give the product as a pale yellow
crystalline solid.
For the avoidance of doubt, in the compounds of Examples 1 to 7 and 9, R2 is in
the 7-position of formula (I), in Example 8 R2 is in the 5 and 7 positions, in
Example 7 R3 is in the 1-position and in Example 9 R3 is in the 1 and 4 positions
of formula (I). Individual compound names are in accordance with IUPAC
nomenclature.
Example 1
2-Fluoro-7-~1,2.41triazol-1-Ylmethvl-9H-carbazole
1(i) 4-Fluoro-4'-methYl-2-nitro-biphenyl
A solution of 2-bromo-5-fluoronitrobenzene(15g) in benzene (60ml) was rapidly
stirred under N2, and t~eated with tetrakistriphenylphosphine palladium (0)
(1.139), aq. 2M sodium carbonate (35.7ml) and a solution of p-tolylboronic acid
(10.2g) in 95% ethanol (25ml). Mixture was heated at 95C and rapidly stirred
under N2 for 16h. Reaction mixture was extracted with ethyl acetate (400ml)
then (2x 100ml). The combined extracts were washed with water (100ml), brine
(100ml), dried (MgSO4) and evaporated to give a brown oil. Chromatography
WO 94/27989 PCT/EP94/01613
216~g~l .
on silica gel (5009), eluting with cyclohexane/ethyl acetate (25:1v/v) gave the
title co,llpound.
1 (ii) 4'-Bromomethvl-4-fluoro-2-nitro-biPhenYl
A solution of 1 i) (2.049) in carbon tetrachloride (40ml) was treated with a trace
of benzoyl peroxide, and N-bromosuccinimide (1.819). Mixture was refluxed
over a 150W bulb for 1 h, then the precipitate of succinimide was filtered off, and
washed with CCI4 (2 x 5ml). The filtrate and washings were evaporated to give
the title compound as a yellow oil.
1(iii) 1-(4'-Fluoro-2'-nitro-biPhenYI-4-YlmethYl)-1H-~1~ 2. 41triazole
A solution of 1,2,4-triazole (0.6139) in DMF (5ml) was treated with sodium
hydride (0.2669 of an 80% dispersion. in oil). After 5 minutes when all
effervescence had ceased, mixture was treated with a solution of 1ii) (3.19) in
DMF(3ml). Resulting pale brown solution was stirred at room temperature for
20h. Mixture was added to ethyl acetate (500ml) and the organic phase was
washed with water (4 x 50ml) then extracted with 2N HCI (6 x 80ml), then
washed with brine (100ml), dried (MgSO4) and evaporated. The residue was
purified by chromatography on silica gel (1509) eluting with CHCI3 to give a
yellow crystalline solid. Elution with CHCI3/MeOH (19:1 v/v) gave the title
compound as a yellow gum.
1 (iv) 4-Fluoro-4'-~1.2.41triazol-1 -vlmethYl-biPhenyl-2-ylamine
A solution of 1 iii) (1.209) in acetic acid (5ml) was treated with 10% Pd/C catalyst
(400 mg) and stirred under H2 for 3h. The catalyst was filtered off through a
Kieselguhr pad, and washed with acetic acid (2 x 5 ml), then the filtrate and
washings were evaporated to a yellow gum. This was dissolved in
dichloromethane (100ml) and was carefully washed with sat. aq. NaHCO3
(100ml), then the organic layer was dried (MgSO4) and evaporated to give the
- 30 title cor"pound as a brownish crystalline solid.
- 1 (v) 1 -(2'-Azido-4'-fluoro-biPhenyl-4-ylmethyl)-1 H-~1.2.41triazole
A solution of 1 iv) (970mg) in water (9ml) and conc. sulphuric acid (0.75ml) wascooled in an ice-bath. A solution of sodium nitrite (261mg) in water (3ml) was
added dropwise. Resulting yellow solution was stirred at ice-bath temperature
WO 94/27989 PCT/EP94/01613
22
for 30 minutes. A solution of sodium azide (243mg) in water (6ml) was added
dropwise. Rapid effervescence of N2 gas was observed. After 30 minutes the
mixture was neutralized with sat. aq. NaHCO3, then extracted with
dichloromethane (4 x 50ml). The combined extracts were dried (MgSO4), and
evaporated to give the title compound as a pale brown crystalline solid.
1 (vi) 2-Fluoro-7-~1.2,41triazol-1 -vlmethvl-9H-carbazole
A solution of 1v) (200mg) in 1,2-dichlorobenzene (5ml) was heated at 170C for
4h. The reaction mixture was diluted with ethyl acetate (200ml) and extracted
with 2N HCI (3 x 60ml). The organic layer was then washed with brine (50ml),
dried (MgSO4) and evaporated to give the impure product plus
dichlorobenzene. Impure product was added to cyclohexane (100ml) and the
solid filtered off and dried to give crystals of the title compound.
NMR ~ (DMSO-d6) 5.57 s (2H), 6.99 t (1H), 7.12 d (1H), 7.25 dd (1H), 7.38 s
(1H), 8.00 s (1H), 8.05 d (1H), 8.10 dd (1H), 8.70 s (1H), 11.42 s (1H).
Example 2
2-Fluoro-7-pvridin-3-ylmethyl-9H-carbazole
2(i) 4-t-ButyldimethYlsilYloxYmethYl-4~-fluoro-2l-nitrodiphenyl
20 A solution of 4-(t-butyldimethylsilyloxymethyl)-phenylboronic acid
(Intermediate 1) (259) and 2-bromo-5-fluoro-nitrobenzene (15.59) in dry
dimethoxyethane (120ml) was treated with tetrakistriphenylphosphine palladium
(O) (1.19) and 2M aq sodium carbonate (40ml). Resulting mixture was refluxed
under N2 for 16h. Reaction mixture was diluted with ethyl acetate (600ml) and
washed with water (2 x 100ml), brine (100ml), dried (MgSO4) and evaporated to
give a brown oil. Chro,ndlography on silica gel (7509), eluting with cyclohexane~ cyclohexane/ethyl acetate (15:1 ) gave the title compound as a yellow oil.
2(ii) 2-Amino-4'-t-butYldimethYlsilYloxvmethYl-4-fluorodiphenyl
A solution of 2(i) (219) in methanol (300ml) was treated with 10% Pd/C catalyst.(3.49) and dry a"""onium for~"2le (16.489). Mixture was stirred under N2 at
room temperature for 24h. The catalyst was filtered off, washed with methanol
(2 x 25ml), under a blanket of N2, then the filtrate and washings evaporated to
give a brownish residue. This was dissolved in water (200ml) and extracted
WO 94/27989 PCT/EP94/01613
2~6~21
.
23
with dichloromethane (3 x 200ml). Combined exl,acts were dried (MgSO4) and
- evaporated to give the title co",pound as a brown oil.
2(iii) (2-Azido4-fluoro-biPhenyl-4-yl)-methanol
A solution of 2(ii) (18.4089) in dioxan (200ml) and water (200ml) was treated
with conc.sulphuric acid (11.47ml) then cooled to 5C. A solution of sodium
nitrite (4.009) in water (15ml) was added dropwise. The resulting orange
solution was stirred at 5C for 30 minutes then a solution of sodium azide
(3.759) in water (20ml) was added carefully avoiding over-effervescence of N2
gas. When addition was complete reaction was stirred at 5C for 30 minutes
then carefully treated with sat. aq NaHCO3 solution until neutral. Mixture was
extracted with dichloromethane (5 x 200ml) and the extracts were dried
(MgSO4) and evaporated to give the title compound as a brown oil.
2(iv) (7-Fluoro-9H-carba,ol-2-yl)-methanol
A solution of 2(iii) (13.99) in 1 2-dichlorobenzene (200ml) was refluxed under N2
for 3h. Mixture was allowed to cool and then was added to cyclohexane
(100ml). Solid material was filtered off and washed with cyclohexane (2 x
100ml) then dried in vacuo to give the title compound as a pale brown solid.
2(v) 2-Fluoro-7-formyl-carbazole-9-carboxylic acid tert-butvl ester
A solution of 2(iv) (7.189) in dichloromethane (1500ml) was treated with barium
manganate (54.59) and stirred under N2 for 5h. The solid was filtered off and
washed with dichloro" ,etl)ane (4 x 100ml). Filtrate and washings were
evaporated to give a green-brown solid. This was suspended in
dichloromethane (100ml) and treated with 4-dimethylaminopyridine (3.789) and
di-tbutyldicarbonate (7.439). Resulting brown solution was stood at room
temperature for 30 minutes then diluted with ethyl acetate. Organic solution
was washed with 2N HCI (2 x 400ml) water (200ml) brine (2 x 200ml) then
dried (MgSO4) and evaporated to give impure product. This was purified by
chro",atography on silica gel (5009) eluting with cyclohexane/ethyl acetate
- (15:1 v/v) to give the title co",pound as a pale cream solid.
2(vi)2-Fluoro-7-(hYdroxY-PYridin-3-yl-methyl)-carbazole-9-cal L,oxylic acid tert-
35 butyl ester
WO 94/27989 PCT/EP94/01613
216~ 24
A solution of 3-bror"opyridine (0.306ml) in ether (10ml) was cooled to 45C
under N2. A solution of "butyllithium (2ml of 1.6M sol. in hexane) was added
dropwise. Resulting brownish suspension was warmed to 0C, then cooled
again to -45C and left for 15 minutes. A sol. of 2(v) (0.59) in dry THF (10ml)
5 was added. Mixture was stirred at -40C for 1 h, then quenched with ammonium
chloride solution (20ml). Mixture was extracted with ethyl acetate (3 x 30ml) and
the combined exlracts were washed with brine (200ml), dried (MgSO4) and
evaporated to give the title compound as a yellow foam.
2(vii) 2-Fluoro-7-Pvridin-3-vlmethYl-9H-carbazole
A solution of 2(vi) (670mg) in methanol (20ml) was treated under N2 with
palladium black catalyst (300mg) and conc. HCI (0.8ml). Mixture was stirred
under H2 for 3h at 50C, then a further 300mg of catalyst was added and
reaction continued for 3h. Catalyst was filtered off, and washed with methanol
15 (2 x 10ml), then the filtrate and washings were evaporated to give a sticky
yellow foam. Chro",atography on silica gel (1009), eluting with CHCI3/lPE (50:1
20:1 v/v) gave the title compound.
NMR â (DMSO-d6) 4.12 s (2H), 6.97 t (1H), 7.08 d (1H), 7.22 d (1H), 7.32 m
(1 H), 7.68 d (1H), 8.05 m (2H), 8.41 d (1 H), 8.58 s (1 H), 11.31 s (1 H).
20 ExamPle 3
2-Fluoro-7-pyridin-4-ylmethyl-9H-carbazole
i) 2-Fluoro-7-thvdroxY-PYridin-4-Yl-methyl)-carbazole-9-carboxylic acid, tert butyl
ester
A solution of 4-bromopyridine (253mg) in ether (10ml) was cooled to -75 under
25 N2. A solution of "butyllithium (1ml of 1.6M sol. in hexane) was added. Afterstirring for 10 minutes at -75 a solution of 2(v) (0.59) in dry THF (Sml) was
added. After stirring the reaction at -70 for 1 h, a further mixture of 4-
bromopyridine (506mg) and 2ml of "butyllithium (2ml of 1.6M sol.) in ether
(10ml) was added (premixed at -70). After a further 1h at -70, aqueous
30 ammonium chloride (10ml) was added. The mixture was extracted with ether (4
x 30ml). The combined ether extracts were then extracted with 2N HCI (3 x
30ml). These combined acid extracts were treated with 40% aqueous sodium
hydroxide to pH 11, then extracted with dichloromethane (4 x 40ml), then the
WO 94/27989 PCT/EP94/01613
21~2921
combined extracts were dried (MgSO4) and evaporated to give a yellow solid.
Preparative t.l.c. gave the title compound as a yellow solid.
ii) 2-Fluoro-7-Pvridin4-vlmethvl-9H-carbazole
A solution of 3i) (164mg) in methanol (5ml) was treated under N2 with palladium
5 black catalyst (40mg) and conc. HCI (0.2ml). Mixture was stirred under H2 for
1.5h at 50, then a further charge of catalyst (80mg) was added and then the
reaction stirred under H2 at 50 for 3h. The catalyst was filtered off, washed
with methanol (2 x 5ml) then the filtrate and washings evaporated to a gum.
This was dissolved in 4M HCI in dioxan (5ml) for 30 minutes then evaporated to
10 give a yellow gum. This was dissolved in water (10ml), treated with 40%
aqueous sodium hydroxide to pH 11 then the mixture was extracted with
dichloromethane (4 x 20ml). The combined extracts were dried (MgSO4) and
evaporated to give a yellow solid. Preparative t.l.c. gave the title compound asa yellow solid.
NMR (DMSOd6). 4.12~ s (2H), 6.97~ t (1H), 7.06~ d (1H), 7.2-7.4~ m (4H), 8.0-
8.1~m(2H),8.46~d(1H),11.33~s(1H).
Example 4
2-Fluoro-7-(3-fluoroPvridin4-vl-methvl)-9H-carbazole
i) 2-Fluoro-7-(hvdroxv-(3-fluoroPvridin4-vl)-methyl)-carbazole-9-carboxylic acid.
20 tert butvl ester
A solution of N,N,N',N'-tetramethylethylenediamine (0.48ml) in dry THF (5ml)
was cooled to -70 under N2. A solution of "butyllithium (2ml of 1.6M sol. in
hexane) was added. The resulting solution was stirred at -20 for 1h then
cooled to -70. A solution of 3-fluoropyridine (0.273ml) in dry THF (3ml) was
25 added. The resulting suspension was stirred at 40 for 1h, then a solution of2(v) (500mg) in dry THF (10ml) was added. over 2 minutes. The reaction was
stirred at -70 for 1h then quenched with aqueous ammonium chloride (20ml).
The mixture was extracted with ethyl acetate (3 x 30ml), then the combined
extracts were washed with water (4 x 10ml ) and brine (10ml), then dried
30 (MgSO4) and evaporatecl to give a yellow solid. Chromatography on silica gel
(1009), eluting with chlorofcr"l/isopropanol (50:1 ~ 20:1 v/v) gave the title
compound as a white solid.
ii) 2-Fluoro-7-(3-fluoroPvridin-4-vl-methvl)-9H-ca~ba~ole
WO 94127989 PCT/EP94/01613
~ 6~9'1~ 26
A solution of 4i) (328mg) in methanol (10ml) was treated with palladium black
catalyst (320mg) and conc. HCI (0.4ml). Mixture was stirred under H2 for 6h at
50. The catalyst was filtered off and washed with methanol (3 x 5ml). The
filtrate and washings were evaporated to give a yellow solid. This was
dissolved in water (20ml) and treated with 40% aqueous sodium hydroxide to
pH 11. The mixture was extracted with dichloromethane (4 x 30ml), then the
combined e)~lracts were dried (MgSO4) and evaporated to give a cream solid.
Chromatography on silica gel (509), eluting with chloroform:isopropanol (50:1
20: 1 v/v) gave the title compound as a white solid.
NMR (DMSOd6). 4.19~ s (2H), 6.97â t (1H), 7.07~ d (1H), 7.23~ d (1H), 7.40~ m
(2H), 8.05â m (2H), 8.35~ d (1 H), 8.52~ s (1 H),11.32~ s (1 H).
Example 5
2-Fluoro-7-(3-methYiPvridin4-vl-methvl)-9H-carbazole
i) 2-Fluoro-7-(hvdroxv-(3-methvlpvridin-4-vl)-methvl)-carbazole-9-carboxylic
acid, tert butyl ester
A solution of 4-bromo-3-methylpyridine (549mg) in ether (10ml) was cooled to
-70 under N2. A solution of "butyllithium (3.2ml of 1.6M sol. in hexane) was
added. The resulting suspension was stirred at -70 for 30 minutes, then a
solution of 2(v) (500mg)in dry THF (10ml) was added. The solution was stirred
at -70 for 1h then quenched with aqueous ammonium chloride (20ml). The
mixture was extracted with ethyl acetate (3 x 30ml) then the combined extracts
were washed with brine (10ml), dried (MgSO4) and evaporated to give a yellow
gum. Chromatography on silica gel (909), eluting with chloroform:isopropanol
(50:1 ~ 20: 1 v/v) gave the title compound as a pale yellow foam.
ii) 2-Fluoro-7-(3-methvlPvridin-4-vl-methvl)-9H-carbazole
A solution of 5i) (267mg) in methanol (5ml) was treated with palladium black
catalyst (100mg) and conc. HCI (0.25ml). Mixture was stirred under H2 for 14h
30 with periodic additions of further batches of catalyst. The catalyst was filtered
off and washed with methanol (3 x 2ml) and acetic acid (2 x 1ml). The filtrate
and washings were evaporated to give a pale yellow gum. This was dissolved
in water (1 Oml) and treated with 40% aqueous sodium hydroxide to pH 11. The
mixture was extracted with dichloromethane (4 x 15ml), then the combined
35 extracts were dried (MgSO4) and evaporated to give a pale cream foam.
WO 94/2798g PCT/EP94/01613
- ~162g21
27
Chromatography on silica gel (509) eluting with chlorofo"":isopropanol (50:1
20:1 v/v) gave the title co",pound as a white solid.
NMR (DMSOd6). 2.25~ s (3H) 4.14~ s (2H) 6.97~ t (1H) 7.01~ d (1H) 7.13~ d
(1h) 7.23~ m (2H) 8.0-8.1~ m (2H) 8.33~ m (2H) 11.27~ s (1H).
ExamPle 6
2-Fluoro-7-(3-methoxvPvridin-4-yl-methvl)-9H-carbazole
i) 2-Fluoro-7-(hYdroxv-(3-methoxypyridin-4-yl)-methyl)-carbazole-9-carboxylic
acid. tert butyl ester
A solution of 4-bromo-3-methoxypyridine (530mg) in ether (10ml) was cooled to
-70 under N2. A solution of "butyllithium (1.76ml of 1.6M sol. in hexane) was
added. The resulting suspension was stirred at -70 for 30 minutes then a
solution of 2(v) (442mg)in dry THF (10ml) was added. The solution was stirred
at -70 for 1h then quenched with aqueous a"""onium chloride (20ml). The
15 mixture was extracted with ethyl acetate (3 x 50ml) then the combined extracts
were washed with water (30ml) brine (30ml) dried (MgSO4) and evaporated to
give a yellow solid. Chromatography on silica gel (909) eluting with
chlorofor",:isopropanol (50:1 ~ 20:1 v/v) gave the title compound as a white
solid.
ii) 2-Fluoro-7-(3-methoxYpyridin-4-yl-methyl)-9H-callJa~ole
A solution of 6i) (200mg) in methanol (5ml) was treated under N2 with palladium
black catalyst (100mg) and conc. HCI (0.25ml). Mixture was stirred under H2 for
8h at 50. The catalyst was filtered off washed with methanol and acetic acid
25 then the filtrate and washings evaporaled to a white solid. This was dissolved
in 4M HCI in dioxan (15ml) and dichloromethane (50ml) and stirred at room
temperature for 6h then evaporated to give a gum. This was dissolved in water
(10ml) treated with 40% aqueous sodium hydroxide to pH 11 then the mixture
was extracted with dichloromethane (4 x 20ml). The combined extracts were
30 dried (MgSO4) and evaporated to give a yellow solid. Chlo",atograplly on silica
gel (509) eluting with chlorofor~ isopropanol (50:1 ~ 20:1 v/v) gave the title
compound as a white solid.
NMR (DMSOd6). 3.93~ s (3H) 4.07~ s (2H) 6.96~ t (1H) 7.04â d (1H) 7.13~ d
(1H) 7.21~ d (1H) 7.29~ s (1H) 7.95-8.15~ m (3H) 8.33â s (1H) 11.28~ s (1H).
WO 94t27g8g PCT/EP94/01613
28
ExamPles 7 to 9
The following compounds were prepared by methods analogous to those
described in Examples 1 to 6:
7. 1,7-Difluoro-2-~1,2,41triazol-1-vlmethyl-9H~arbazole
NMR ~ (DMSO-d6) 5.63 s (2H), 7.10 m (2H), 7.26 dd (1H), 7.92 d (1H), 7.98
s (1H), 8.15 dd (1H), 8.69 s (1H),11.90 s (1H).
8. 2,4-Difluoro-7-~1,2,41triazol-1-vlmethYI-9H-carbazole
NMR ~ (DMSO-d6) 5.59 s (2H), 6.96 t (1H), 7.19 m (2H), 7.44 s (1H), 7.99 m
(2H), 8.72 s (1 H).
9. 1,4,7-Trifluoro-2-~1,2,41triazol-1-ylmethyl-9H-carbazole.
NMR â (DMSO-d6) 5.62 s (2H), 6.96 dd (1H), 7.11 t (1H), 7.31 dd (1H), 8.02
m (2H), 8.72 s (1H), 12.25 s (1H).
ExamPle 10
i) 4-FluoroPhenvl boronic acid
A solution of 1-bromo-4-fluorobenzene (318.29) in THF (3000ml) was cooled to
-52 under N2. "Butyllithium (2.5M in hexane, 800ml) was added by cannula
over 5 minutes, temperature rising to -44. After addition was complete, the
reaction was allowed to store at -52 for 15 minutes, then tri-isopropyl borate
(1240ml) was added over 10 minutes. Cooling was removed and the reaction
allowed to warm to -15. It was quenched by adding water (2000ml). The
mixture was then partitioned between a further 2000ml of water, and ethyl
acetate (2000ml). The gelatinous white material remaining in the flask was
discarded.
The aqueous phase was further extracted with ethyl acetate (2000ml), then the
combined ethyl acetate layers were washed with brine (1500ml), dried (MgSO4)
and evaporated to give a pale cream solid, (228.129). The solid was
crystallized from boiling water (1100ml) to give the title compound (152.189).
ii) m-Nitro-p-bro"~obenzaldehyde
Conc. sulphuric acid (3125ml) and conc. nitric acid (1250ml) were mixed
together (conc. nitric acid added in -100ml portions) and subsequently cooled
WO 94/27989 PCT/EP94/01613
2162921
29
to 5C, in an ice/salt bath. p-Bromobenzaldehyde (5009) was then added
portion-wise, maintaining the temperature below 10C. Once the addition was
complete, the resulting mixture was stored at room temperature for -3 hrs (untilall p-bromobenzaldehyde dissolved), then carefully poured over ice. The
5 precipitate formed was collected by filtration and washed thoroughly with water,
until washings ~pH6, then dried in vacuo at 40C to give the title comPound as
white powder (607.639).
iii) 4'-Fluoro-2-nitrobiPhenvl-4-carbaldehyde
A mixture of 10i) (3009), 10ii) (4489), tetratristriphenyl phosphine palladium (0)
(159) and aq. 2M sodium carbonate (1.07L) in DME (3.2L) was refluxed under
N2 for 16h. After cooling, the reaction mixture was extracted with ethyl acetate(2 x 2000ml). The combined extracts were washed with brine (1000ml), dried
(MgSO4) and evaporated. Before complete evaporation, the product
15 crystallized. The crystals were filtered off, washed with IPE, then dried to give
the title compound (244.39).
The washings and mother liquors were concentrated. The residue crystaliized
and was triturated with ether, then the solid filtered and dried to give a further
20 crop of the title comPound (1209).
iv) 2-(4'-Fluoro-2-nitrobiPhenyl-4-yl)-f1~3l-dioxolane
A solution of 10iii) (357.49) in isopropyl acetate (4000ml) was treated with p-
toluene sulphonic acid, monohydrate (279) and ethylene glycol (750ml). The
25 mixture was refluxed with a Dean-Stark trap for 2 hours. After cooling, the
solution was washed with water (2000ml, sat aq. NaHCO3 (2000ml), water (2 x
2000ml), brine (1000ml), then dried (MgSO4) and evaporated to give the title
compound as a yellowish syrup (520.969).
30 Toluene has also been found to be a suitable solvent for this reaction.
v) 2-f1.31-Dioxolan-2-YI-7-fluoro-9H-carbazole
Xylene (1250ml) and triethylpl,ospl,ite (1250ml) were mechanically stirred underN2 and heated to reflux (-142). A solution of 10iv) (514.769) in xylene
(3750ml) was added over 2h, maintaining reflux. Reflux was continued for a
WO 94/27989 PCT/EP94/01613
2~6~9~
further 51/2h, and then further triethylphosphite (500ml) was added dropwise.
Reflux was continued for 11/2h, then reaction was left to cool to room
temperature overnight. After cooling in icelwater for 30 minutes, crystals were
filtered off, washed with xylene and cyclohexane, then dried in vacuo at 40 to
give the title comPound (171.759).
Further crops were obtained from the cyclohexane washes and the rest of the
mother liquors.
Neat triethylphosphite has also been found to be a suitable solvent for this
reaction.
vi) 7-Fluoro-9H-carbazole-2-carbaldehYde
A suspension of 10v) (209.6269) in acetone (5000ml) was treated with 2N HCI
(1250ml) and the resulting yellow brine solution was stored for 30 minutes. The
acetone was evaporated off, and the aqueous layer and solid was extracted with
ethyl acetate (7000ml). The organic solution was washed with water (1000ml),
dried (MgSO4) and evaporated to give the title compound as a pale
apricoVmango solid (171.599).
vii) 2-Fluoro-7-formYI-carbazole-9-carboxYlic acid tert-butyl ester
A suspension of 10vi) (168.259) in ethyl acetate (300ml) was stirred with 4-
dimethylaminopyridine (96.419) at room temperature under N2. A solution of di-
butyldicarbonate (172.29) in ethyl acetate (1000ml) was added over 30 minutes,
so CO2 effervescence was only at a slow rate and then further BOC anhydride
(259) was added to complete reaction. A white solid crystallized out. After
stirring for -30 minutes, this was filtered off, washed with 2N HCI (2 x 500ml),then water until neutral, then dried in vacuo to give the title compound
(187.449).
Further crops were obtained from the mother liquors.
viii) 2-Fluoro-7-(hYdroxY-Pvridin-3-Ylmethyl)-9H-carba~ole
A solution of 3-bromopyridine (16.6ml) in THF (500ml) was cooled to -70C
under N2. This solution was added dropwise over 10 minutes to a solution of n-
WO 94/27g89 PCT/EP94/01613
21629`~1
31
butyllithium (220ml of a 1.6M solution in hexanes) that was cooled to -70C.
After 10 minutes, a solution of 10vii) (279) in THF (700ml) that was cooled to
-70C was added dropwise over 20 minutes. The mixture was stirred at -70C
for 40 minutes, then quenched with ammonium chloride solution (300ml). The
mixture was exl,acted with ethyl acetate (2 x 500ml), and the combined organic
layers washed with brine (500ml), dried (MgSO4) and evaporated to give a
mixture of 2-fluoro-7-(hydroxy-pyridin-3-ylmethyl)-carbazole-9-carboxylic acid
tert-butyl ester and 2-fluoro-7-(hydroxy-pyridin-3-ylmethyl)-9H-carbazole as an
amber oil.
This oil was dissolved in methanol (500ml) and treated with sodium methoxide
(379 of a 25% solution in methanol). After stirring for 24 hours, the reaction was
concentrated to 50ml, then water (250ml) was added and the mixture was
extracted with ethyl acetate (3 x 250ml). The combined organic layers were
extracted with HCI (20% aqueous solution, 3 x 250ml). The combined HCI
layers were washed with ethyl acetate (250ml). The aqueous layer was then
neutralized to pH10 with NaOH pellets, then was extracted with methylene
chloride (2 x 500ml). The combined methylene chloride layers were washed
with brine (500ml), dried (MgSO4), and evaporated to 100ml. Toluene (250ml)
was added, and the suspension concentrated to 150ml. Collection of the
resulting precipitate afforded, upon drying, the title comPound as an off white
powder.
ix) 2-Fluoro-7-PYridin-3-Ylmethyl-9H-carbazole
A solution of 10viii) in 50% methanol/THF (200ml) was treated with
concentrated HCI (5ml) and palladium on carbon catalyst (10% active catalyst,
50% water wet). The suspension was placed in a Parr hydrogenation bottle and
L,eated with hydrogen gas at 10 PSI, 25C for 3 hours. The catalyst was filteredoff, the catalyst cake was washed with HCI (10%, 100ml), water (100ml), and
10% NaOH (100ml). The filtrate was concentrated to 200ml, then neutralized
with NaOH pellets to pH9. The solution was then extracted with ethyl acetate (2
x 250ml), and the combined organic layers washed with brine (250ml), dried
(Na2SO4) and co"c~nl~aled to give a white solid. The crude product was
dissolved in methanol (400ml) and treated dropwise with water (200ml). The
WO 94/27989 PCT/EP94/01613
p~6?,9~l 32
resulting precipitate was collected and dried to give the title comPound as a
white crystalline solid.
Melting point: 196-197C.
Mass Spectrum (FAB+): 277
Elemental analysis:
% C H N
Calculated: 78.24 4.74 10.13
Found: 78.254.80 10.18
The following examples illustrate pharmaceutical formulations according to the
invention containing 2-fluoro-7-pyridin-3-ylmethyl-9H-ca, L a~ole as the active
ingredient. Other compounds of the invention may be formulated in a similar
1 0 manner.
Tablets for oral administration
a) Direct ComPression Tablet
Component Co .r" osition (%)
Active ingredient 20.0
Microcrystalline cellulose 50.0
Spray dried lactose 24.2
Sodium starch glycolate 5.0
Colloidal silicon dioxide 0.3
Magnesium stearate 0.5
All materials except magnesium stearate are blended until sufficiently mixed.
Magnesium stearate is screened and added to the mixture which is blended
15 thoroughly. The resultant mix is compressed to predetermined tablet size and
weight.
WO 94/27989 PCT/EP94/01613
2I 6~921
33
b) Wet Granulation Tablet
Component Co"",osilion (%)
Active ingredient 30.0
Microcrystalline cellulose55.2
Starch 1500 6.0
Sodium starch glycolate 5.0
10% polyvinylpyrrolidone 3 0
in water
Magnesium stearate 0.5
Colloidal silicon dioxide 0.3
All of the ingredients except for the polyvinylpyrrolidone solution and
magnesium stearate are blended in a fluidized air bed. Polyvinylpyrrolidone
solution is added to the blended powders with constant mixing until uniformly
5 moist. After drying, the granules are milled to reduce particle size and increase
size uniformity and blended with the magnesium stearate. The granules are
then compressed to predetermined tablet size and weight.
Tablets of other strengths may be prepared by altering the ratio of active
ingredient e.g. to lactose or altering the compression weight.
10 The tablets may be film-coated with suitable film-forming materials such as
hydroxypropyl methylcellulose using standard techniques. Alternatively the
tablets may be sugar coated or enteric coated.
SYruP for oral administration
Component Composition (%)
Active ingredient, micronized 5.00
Magnesium aluminum silicate 0.50
Sodium carboxymethylcellulose 0.80
WO 94/27989 PCT/EP94/01613
Z16~g~
34
Component Composition (%)
Sodium lauryl sulphate 0.01
Sorbitol solution, USP 26.0
Methylparaben 0.20
Propylparaben 0.04
Flavour 0 50
Purified water 66.95
The sodium carboxymethylcellulose and magnesium aluminium silicate is
hydrated in a solution of sodium lauryl sulphate in water for 24 hours. Active
ingredient is suspended in the vehicle with the aid of a mixer. The
preservatives are dissolved in the remaining water by heating and after cooling
5 to room temperature, the sorbitol solution is added. The solution is added to the
suspension, flavour mixed in and the pH adjusted as needed. The final
suspension is mixed in a homogenizer.
Soft qelatin caPsules for oral administration
Component Composition (%)
Active ingredient, micronized 5.0
Polyethylene glycol 47.5
Propylene glycol 47 5
The glycols are blended with warming until homogeneous. Active ingredient is
10 added and the mixture homogenised and filled into an appropriate gelatin mass to give soft gelatin capsules containing the appro~,, iate fill weight.
SuPpositorv for rectal administration
Component Composition (%)
Active ingredient, micronized 2
Witepsol W32, hard fat 98
A slurry of the active ingred;cn~ in a portion of molten Witepsol (approximately36C) is prepared using a high speed mixer and is then evenly dispersed in the
WO 94/27g8g PCTtEP94tO1613
21~2!~21
.
remaining molten hard fat. The suspension is filled, using suitable machinery,
into 1 or 29 size s~ ~ppository moulds and allowed to cool.
TransJen"al sYstem
Component Composition (%)
Active ingredient 5
Silicone fluid 90
Colloidal silicon dioxide 5
The silicone fluid and active ingredient are mixed together and the colloidal
5 silicon dioxide is added to increase viscosity. The material is then dosed into a
subsequently heat sealed polymeric laminate comprised of the following:
polyester release liner, skin conta.;l adhesive composed of silicone or acrylic
polymers, a control me"lbrane which is a polyolefin (e.g. polyethylene or
polyvinyl acetate) or polyurethane, and an impermeable backing membrane
10 made of a polyester multilaminate. The laminated sheet is then cut into
patches.
Formulations for parenteral administration
a) Intravenous solution
Component cG.~ sition (%)
Active ingredient 5.0
Sodium chloride USP 0.9
Phosphate buffer (monobasic 7 0
and dibasic potassium
phospl ,ate)
Water for Injection USP 87.1
Active ingredient is dissolved in the water with the remaining components and
15 sterile filtered (0.22~m filter). The solution is filled into glass vials, stoppered
and sealed before autoclaving.
WO 94127989 PCT/EP94/01613
2~9~ ~ -
36
b) LvoPhilised Product
Component Composition (%)
Active ingredient 2.5
Mannitol 5.0
Phosphate buffer (monoL,asic 7.0
and dibasic potassium
phosphate)
Water for Injection USP 85.5
Active ingredient is dissolved in the water with the remaining components and
sterile filtered (0.22~1m filter). The solution is filled into glass vials, stoppered
and Iyophilised before sealing. The Iyophilised product is reconstituted with
5 saline prior to administration.